Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula angustifolia Mill.) from In Vitro Cultures
Abstract
1. Introduction
2. Results
2.1. Antioxidative Enzyme Activity
2.1.1. APX
2.1.2. POX
2.1.3. SOD
2.1.4. CAT
2.2. Free Radical Scavenging Activity and Total Polyphenols Content
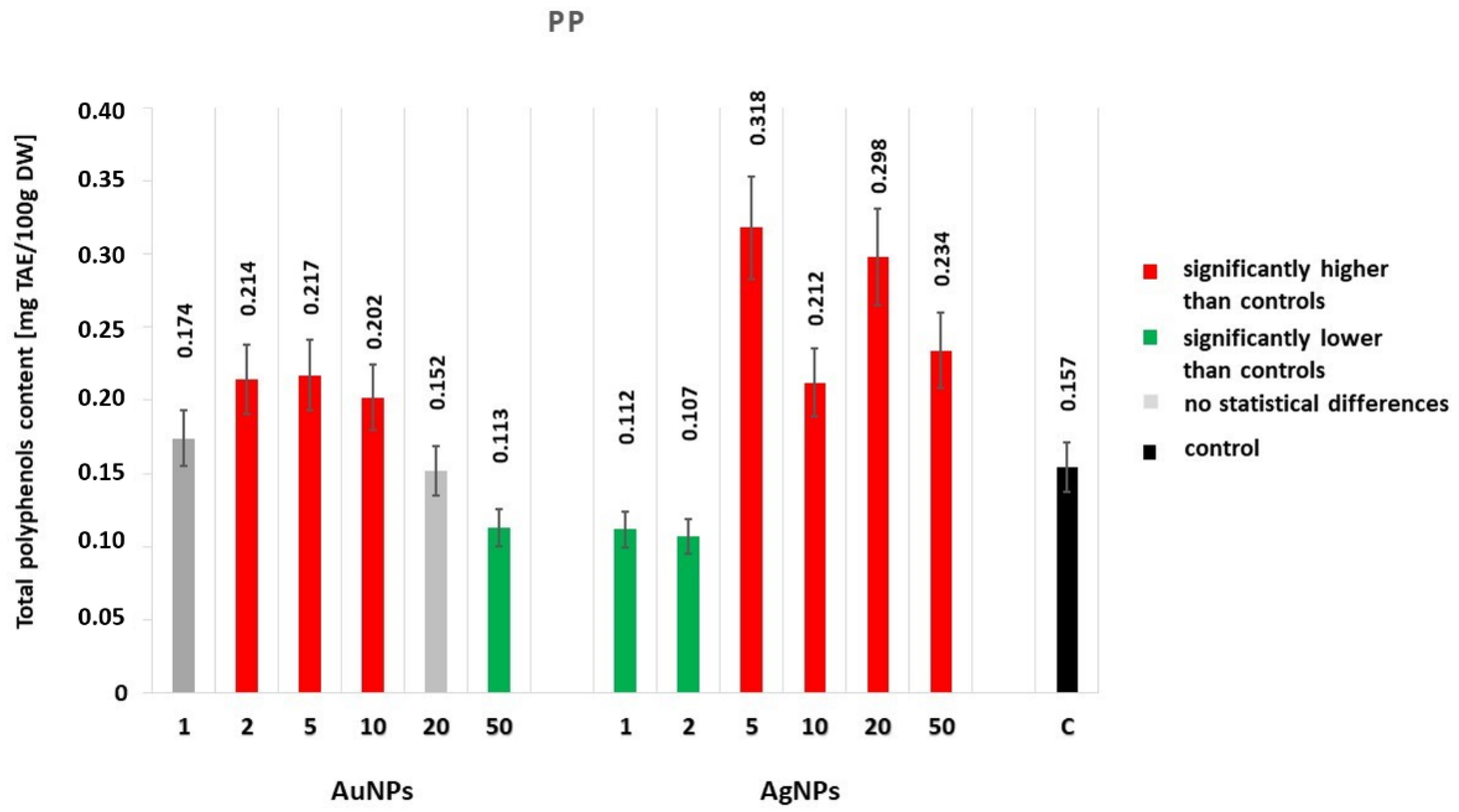
2.2.1. The Total Polyphenol Content
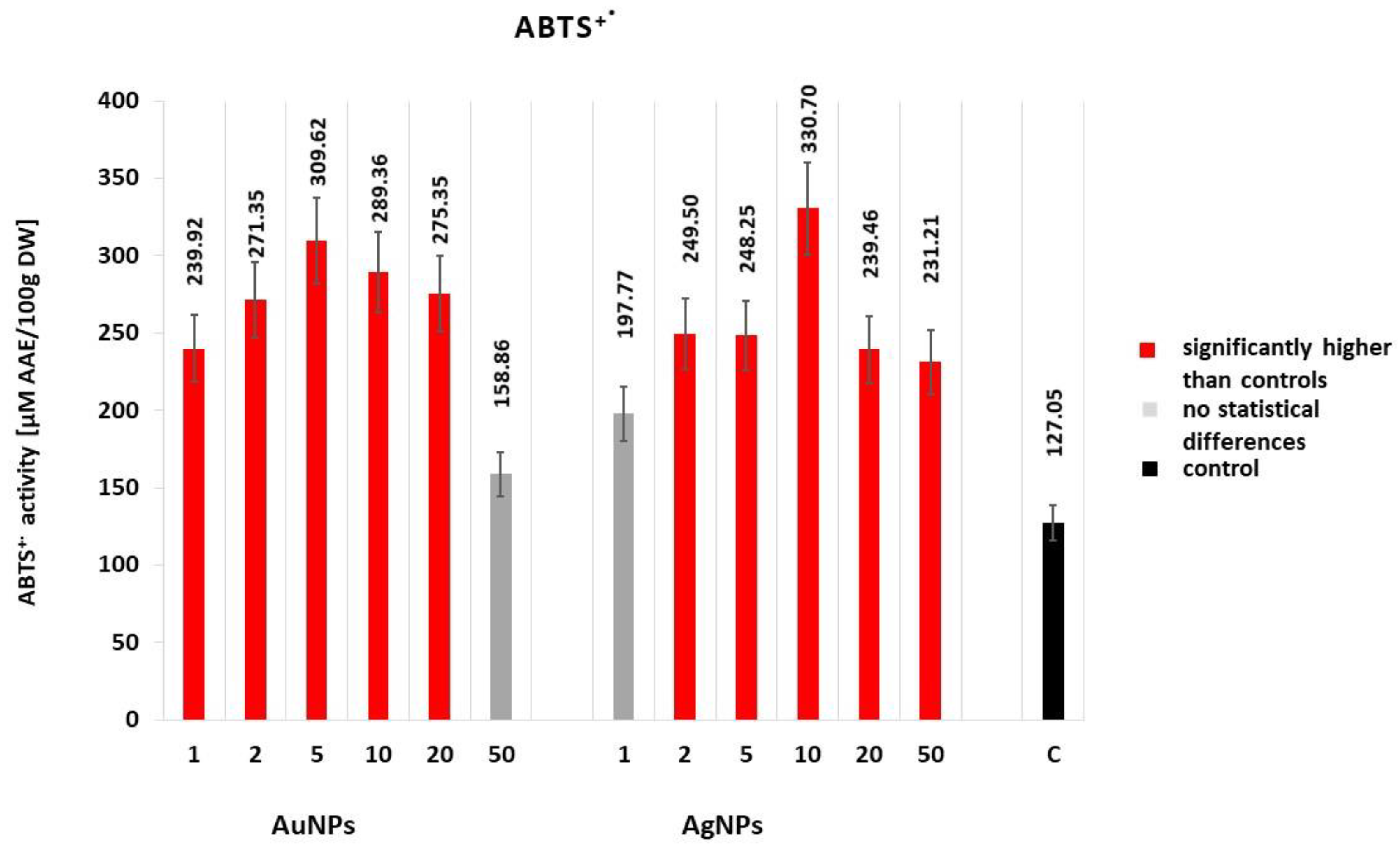
2.2.2. Free Radical Scavenging Activity (ABTS•+)
3. Discussion
4. Materials and Study Methods
4.1. Plant Culture under In Vitro Conditions
4.2. Nanoparticles
4.3. Antioxidant Enzymes Activity Assay
4.3.1. Sample Preparation
4.3.2. Guaiacol Peroxidase Assay (POX)
4.3.3. Ascorbate Peroxidase Assay (APX)
4.3.4. Dismutase Assay (SOD)
4.3.5. Catalase Assay (CAT)
4.4. Total Phenol Content and Free-Radical ABTS•+ Scavenging Ability Assay
4.4.1. Tissue Extract Preparation
4.4.2. Total Polyphenol Content Assay
4.4.3. Free Radical ABTS•+ Scavenging Ability Assay
4.5. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Cavanagh, H.M.A.; Wilkinson, J.M. Lavender essential oil: A review. Healthc. Infect. 2005, 10, 35–38. [Google Scholar] [CrossRef]
- Verma, R.S.; Rahman, L.U.; Chanotiya, C.S.; Verma, R.K.; Chauhan, A.; Yadav, A.; Singh, A.; Yadav, A.K. Essential oil composition of Lavandula angustifolia Mill. Cultivated in mild hills of Uttarahand, India. J. Serb. Chem. Soc. 2010, 75, 343–348. [Google Scholar] [CrossRef]
- Brailko, V.A.; Mitrofanova, O.; Lesnikowa-Sedoshenko, N.; Chelombit, S.; Mitrofanova, I.V. Anatomy features of Lavandula angustifolia Mill. and Lavandula hybrida rev. plants in vitro. J. Agric. Food Chem. 2017, 63, 111–117. [Google Scholar] [CrossRef][Green Version]
- Da Porto, C.; Decorti, D.; Kikic, I. Flavour compounds of Lavandula angustifolia L. to use in food manufacturing: Comparison of three different extraction methods. Food Chem. 2009, 112, 1072–1078. [Google Scholar] [CrossRef]
- Hamad, K.J.; Al-Shaheen, S.J.A.; Kaskoos, R.A.; Ahamad, J.; Jameel, M.; Mir, S.R. Essential oil composition and antioxidant activity of Lavandula angustifolia from Iraq. Int. Res. J. Pharm. 2013, 4, 117–120. [Google Scholar]
- Waithaka, P.; Gathuru, E.; Githaiga, B.; Kwoko, J. Making of perfumes from essential oils extracted from lavender plant collected from Egerton University, Main Campus Njoro, Kenya. Afr. J. Biomed. Res. 2016, 2, 35–40. [Google Scholar]
- Landmann, C.; Fink, B.; Festner, M.; Dregus, M.; Engel, K.H.; Schwab, W. Cloning and functional characterization of three terpene sythases from lavender (Lavandula angustifolia). Arch. Biochem. Biophys. 2007, 465, 417–429. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Nostratpour, S. Teuricum polium L. essential oil: Phytochemical component and antioxidant properties. Int. Food. Res. J. 2013, 4, 1697–1701. [Google Scholar]
- Gören, A.; TopÇu, G.; Bilsel, M.; AydoĞmuŞ, Z.; Pezzuto, J.M. The chemical contituents and biological activity of Lavandula stocheas ssp. stocheas. Z. Nat. C 2002, 57, 797–800. [Google Scholar]
- Hajhashemi, V.; Ghannadi, A.; Sharif, B. Anti-inflammatory and analgesic properties of the leaf extracts and essential oil of Lavandula angustifolia Mill. J. Ethnopharmacol. 2003, 89, 67–71. [Google Scholar] [CrossRef]
- Raut, S.J.; Karruppayil, M.S. A status review on the medicinal properties of essential oils. Ind. Crops. Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Prasad, A.; Shukla, S.P.; Mathur, A.; Singh, C.C.; Kumar, A.M. Genetic fidelity of long-term micropropagated Lavandula officinalis Chaix: An important aromatic plant. Plant. Cell Tiss. Org. 2015, 120, 803–811. [Google Scholar] [CrossRef]
- Akhondzadeh, S.; Kashan, L.; Fotouhi, A.; Jarvandi, S.; Mobaseri, M.; Moin, M.; Khani, M.; Amir, H.J.; Baghalian, K.; Taghizadeh, M. Comparison of Lavandula angustifolia Mill. tincture and imipramine in the treatment of mild to moderate depression: A double-blind, randomized trial. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 123–127. [Google Scholar] [CrossRef]
- Al-Qudah, T.; Shibli, R.A.; Alali, F.Q. In vitro propagation and secondary metabolites production in wild germander (Teucrium polium L.). Vitr. Cell. Dev. Biol. -Plant 2011, 47, 496–505. [Google Scholar] [CrossRef]
- Frabetti, M.; Gutiérrez-Pesce, P.; Mendoza-de, G.E.; Rugini, E. Micropropagation of Teucrium fruticans L. an ornamental and medicinal plant. Vitr. Cell. Dev. Biol. -Plant. 2009, 45, 129–134. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. In vitro culture of lavenders (Lavandula spp.) and the production of secondary metabolites. Biotechnol. Adv. 2013, 31, 166–174. [Google Scholar]
- Amoo, S.O.; Aremu, A.O.; Van Staden, J. In vitro plant regeneration, secondary metabolite production and antioxidant activity of micropropagated Aloe arborescens Mill. Plant Cell Tiss. Org. 2012, 111, 345–358. [Google Scholar] [CrossRef]
- Jakovljević, D.Z.; Vasić, S.M.; Stanković, M.S.; Čomić, L.R.; Topuzović, M.D. Secondary metabolite content and in vitro biological effects of Ajuga chamaepitys (L.) Schreb subsp. chamaepitys. Arch. Biol. Sci. 2015, 67, 1195–1202. [Google Scholar]
- Makowczyńska, J.; Sliwinska, E.; Kalemba, D.; Piątczak, E.; Wysokińska, H. In vitro propagation, DNA content and essential oil composition of Teucrium scorodonia L. ssp. scorodonia. Plant Cell Tiss. Org. 2016, 127, 1–13. [Google Scholar]
- Vanisree, M.; Hsin-Sheng, T. Plant cell cultures—An alternative and efficient source for the production of biologically important secondary metabolites. Int. J. Appl. Eng. Res. 2004, 2, 29–48. [Google Scholar]
- Shakeran, Z.; Keyhanfar, M.; Asghari, G.; Ghanadian, M. Improvement of atropine production by different biotic and abiotic elicitors in hairy root cultures of Datura metel. Turk. J. Biol. 2015, 39, 111–118. [Google Scholar] [CrossRef]
- Rajak, A. Nanotechnology and its application. J. Nanomed. Nanotechnol. 2018, 9, 502. [Google Scholar] [CrossRef]
- Das, M.; Shim, K.H.; An, S.S.A.; Yi, D. Review on gold nanoparticles and their applications. J. Toxiol. Env. 2012, 3, 193–205. [Google Scholar] [CrossRef]
- Roco, M.C. Nanotechnology: Convergence with modern biology and medicine. Curr. Opin. Biotechnol. 2003, 3, 337–346. [Google Scholar] [CrossRef]
- Nowack, B.; Bucheli, T.D. Occurrence, behavior and effects of nanoparticles in the environment. Env. Pollut. 2007, 1, 5–22. [Google Scholar] [CrossRef]
- Rajmedevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar]
- Ahmed, J.; Gultekinoglu, M.; Edirsinghe, M. Bacterial cellulose micro-nano fibres for wound healing applications. Biotechnol. Adv. 2020, 41, 107549. [Google Scholar] [CrossRef]
- Matharu, R.K.; Porwal, H.; Chen, B.; Ciric, L.; Edirsinghe, M. Viral filtration using carbon-based materials. Med. Devices. Sens. 2020, 3, e10107. [Google Scholar] [CrossRef]
- Matharu, R.K.; Ciric, L.; Ren, G.; Edirsinghe, M. Comparative study of the antimicrobial effects of tungsten nanoparticles and tungsten nanocomposite fibres on hospital acquired bacterial and viral pathogens. J. Nanomater. 2020, 10, 1017. [Google Scholar] [CrossRef]
- Panyala, N.R.; Pena-Mendez, E.M.; Havel, J. Gold and nano-gold in medicine: Overview, toxicology and perspectives. J. Appl. Biomed. 2009, 7, 75–91. [Google Scholar] [CrossRef]
- Neira, T.R.; Carmora, E.; Recio, G.; Nesi, A.N.; Diaz, M.R.; Alberdi, M.; Rengel, Z.; Blancheteau, A.I. Metallic nanoparticles influence the structure and function of the photosynthetic apparatus in plants. Plant. Psychiol. Biochem. 2018, 130, 408–417. [Google Scholar] [CrossRef]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affectin the clearance and biodistibution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef]
- Attarad, A.; Hira, Z.; Muhammad, Z.; Ihsan, U.H.; Abdul, R.P.; Joham, S.A.; Altaf, H. Sythesis, characterization, applications and challenges of iron oxide nanoparticles. Nanotechnol. Sci. Appl. 2019, 9, 49–67. [Google Scholar]
- Jain, P.K.; Lee, K.S.; El-Sayed, I.H.; El-Sayed, M.A. Calculated absorption and acattering properties of gold nanoparticles of different size, shape, and composition: Applications in biological imaging and biomedicine. J. Phys. Chem. B 2006, 110, 7238–7248. [Google Scholar] [CrossRef]
- Cheun-Yi, Y.; Ceran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties and applications in biotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug. Deliv. Rev. 2008, 11, 1289–1306. [Google Scholar] [CrossRef]
- Abdi, G.; Selehi, H.; Kosh-Khui, M. Noano silver: A novel nanomaterial for removal of bacterial contaminations in valerian (Valeriana officinalis L.) tissue culture. Acta Physiol. Plant. 2008, 30, 709–714. [Google Scholar] [CrossRef]
- Mahna, N.; Vahed, S.Z.; Khani, S. Plant in vitro culture goes nano: Nanosilver-mediated decontamination of ex vitro explants. J. Nanomed. Nanotechnol. 2013, 4, 1. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant. Sci. 2016, 21, 699–721. [Google Scholar] [CrossRef]
- Hussain, M.; Raja, N.I.; Mashwani, Z.; Iqbal, M.; Sabir, S.; Yasmeen, F. In vitro seed germination and biochemical profiling of Artemisia absinthium exposed to various metallic nanoparticles. 3 Biotech 2018, 130, 408–417. [Google Scholar] [CrossRef]
- Lee, W.M.; An, Y.J.; Yoo, H.; Kweon, H.S. Toxicity and bioavailibity of copper nanoparticles to the terrestrial plants mung been (Phaseolus radiatus) and wheat (Triticum aestivum): Plant agar test for water-insoluble nanoparticles. Env. Toxicol. Chem. 2008, 27, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Javed, R.; Yucesan, B.; Zia, M.; Gurel, E. Elicitation of secondary metabolites in callus cultures of Stevia rebaudiana Bertoni grown under ZnO and CuO nanoparticles stress. Sugar Tech. 2018, 20, 194–201. [Google Scholar] [CrossRef]
- Asztemborska, M.; Steborowski, R.; Kowalska, J.; Bystrzejewska-Piotrowska, G. Accumulation of platinum nanoparticles by Sinapis alba and Lepidium sativum plants. Water Air Soil Pollut. 2015, 226, 126. [Google Scholar] [CrossRef] [PubMed]
- Burman, U.; Saini, M.; Kumar, P. Effect of zinc oxide nanoparticles on growth and antioxidant system of chickpea seedlings. Toxicol. Env. Chem. 2013, 95, 605–612. [Google Scholar] [CrossRef]
- Fraizer, T.P.; Burklew, C.E.; Zhang, B. Titanium dioxide nanoparticles affect the growth and microRNA expression of tobacco (Nicotiana Tab.). Funct. Integr. Genom. 2014, 14, 75–83. [Google Scholar] [CrossRef]
- Sarmast, M.K.; Salehi, H. Silver nanoparticles: An influential element in plant nanobiotechnology. Mol. Biotechnol. 2016, 58, 441–449. [Google Scholar] [CrossRef]
- Homaee, M.B.; Ehsanpour, A.A. Physiological and biochemical responses of potato (Solanum tuberosum) to silver nanoparticles and silver nitrate treatments under in vitro conditions. Ind. J. Plant. Physiol. 2015, 20, 353–359. [Google Scholar] [CrossRef]
- Rani, P.U.; Yasur, J.; Loke, K.S.; Dutta, D. Effect of synthetic and biosynthesized silver nanoparticles on growth, physiology and oxidative stress of water hyacinth: Eichhornia crassipes (Mart) Solms. Acta Physiol. Plant. 2016, 38, 58. [Google Scholar] [CrossRef]
- Arora, S.; Sharma, P.; Kumar, S.; Nayan, R.; Khanna, P.K.; Zaidi, M.G.H. Gold-nanoparticle induced enhancement in growth and seed yield of Brassica Juncea. Plant. Growth Regul. 2012, 66, 303–310. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level studies on the toxicity of silver nanoparticles in germinating seedlings of mung bean (Vigna Radiata L.). Acta Physiol. Plant. 2015, 37, 1719. [Google Scholar] [CrossRef]
- Vecerova, K.; Vecera, Z.; Docekal, B.; Oravec, M.; Pompeiano, A.; Tríska, J.; Urban, O.; Pompiano, J. Changes of primary and secondary metabolites in barley plants exposed to CdO nanoparticles. Environ. Pollut. 2016, 218, 207–218. [Google Scholar] [CrossRef]
- Golkar, P.; Moradi, M.; Garousi, A.G. Elicitation of stevia glycosides using salicylic acid and silver nanoparticles under callus culture. Sugar Tech. 2018, 21, 569–577. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghanti, F.; Razaei, A.; Bemani, E. Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotechnology 2014, 68, 525–530. [Google Scholar] [CrossRef]
- Javed, R.M.; Usman, M.; Tabassum, S.; Zia, M. Effect of capping agents: Structural, optical and biological properties of ZnO nanoparticles. Appl. Surf. Sci. 2016, 386, 319–326. [Google Scholar] [CrossRef]
- Lu, C.M.; Zhang, C.Y.; Wen, J.Q.; Tao, M.X. Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Mater. Sci. 2002, 21, 168–171. [Google Scholar]
- Dimkpa, C.O.; McLean, J.E.; Latta, D.E.; Manangón, E.; Britt, D.W.; Johnson, W.P.; Boyanov, M.I.; Anderson, A.J. Cuo and ZnO nanoparticles: Phytotoxicity, metal speciation, and induction of oxidative stress in sand-grown wheat. J. Nanopart. Res. 2012, 14, 1–15. [Google Scholar] [CrossRef]
- Shaw, A.K.; Hossain, Z. Impact of nano-Cuo stress on rice (Oryza sativa L.) seedlings. Chemosphere 2013, 96, 906–915. [Google Scholar] [CrossRef]
- Gopinath, K.; Gowri, S.; Karthika, V.; Arumugam, A. Green synthesis of gold nanoparticles from fruit extract of Terminalia arjuna for the enhanced seed germination activity of Gloriosa superb. J. Nanostructure Chem. 2014, 4, 1–11. [Google Scholar] [CrossRef]
- Ghosh, M.; Bandyopadhyay, M.; Mukherjee, A. Genotoxicity of titanium dioxide (TiO2) nanoparticles at two trophic levels: Plant and human lymphocytes. Chemosphere 2010, 81, 1253–1262. [Google Scholar] [CrossRef]
- Kumbhakar, D.V.; Datta, A.K.; Mandal, A.; Das, D.; Gupta, S.; Ghosh, B.; Halder, S.; Dey, S. Effectivity of copper and cadmium sulfide nanoparticles in mitotic and meiotic cells of Nigella sativa L. (black cumin)–can nanoparticles act as mutagenic agents? J. Exp. Nanosci. 2016, 11, 823–829. [Google Scholar] [CrossRef][Green Version]
- Pramanik, A.; Datta, A.K.; Gupta, S.; Ghosh, B.; Das, D.; Kumbhakar, D.V. Assessment of genotoxicity of engineered nanoparticles (cadmium sulphide-CdS and copper oxide-CuO) using plant model (Coriandrum sativum L.). Int. J. Res. Pharm. Sci. 2017, 8, 741–753. [Google Scholar]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Env. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Qiu, X.N.; Li, G.B.; Li, W.; Yin, L.Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Env. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant. Physiol. Bioch. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hatami, M.; Ghorbanpour, M. Defense enzymes activity and biochemical variations of Pelargonium zonale in response to nanosilver particles and dark storage. Turk. J. Biol. 2014, 38, 130–139. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Env. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef]
- Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. Vitr. 2011, 25, 1097–1105. [Google Scholar] [CrossRef]
- De, A.; Chakrabarti, M.; Ghosh, I. Evaluation of genotoxicity and oxidative stress of aluminium oxide nanoparticles and its bulk form in Allium cepa. Nucleus 2016, 59, 219–225. [Google Scholar] [CrossRef]
- Rao, S.; Shekhawat, G.S. Phytotoxicity and oxidative stress perspective of two selected nanoparticles in Brassica juncea. 3 Biotech 2016, 6, 244. [Google Scholar] [CrossRef]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 2012, 167, 2225–2233. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, H.; Ye, S.; Chen, W.P.; Liang, W.; Xu, Y. The Arabidopsis P450 protein CYP82C2 modulates jasmonate-induced root growth inhibition, defense gene expression and indole glucosinolate biosynthesis. Cell Res. 2010, 20, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, Z.; Zhao, J.; Xu, L.; Yu, X.; Wei, Y.; Xing, B. Interaction of CuO nanoparticles with plant cells: Internalization, oxidative stress, electron transport chain disruption, and toxicogenomic responses. Environ. Sci. Nano 2018, 5, 2269–2281. [Google Scholar] [CrossRef]
- Moharrami, F.; Hosseini, B.; Sharafi, A.; Farjaminezhad, M. Enhanced production of hyoscyamine and scopolamine from genetically trans-formed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. Vitr. Cell. Dev. Biol.-Plant 2017, 53, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zheng, L.P.; Li, W.Y.; Wang, J.W. Stimulation of artemisinin production in Artemisia annua hairy roots by Ag-SiO2 core-shell nanoparticles. Curr. Nanosci. 2013, 9, 363–370. [Google Scholar] [CrossRef]
- Sharafi, E.; Nekoei, S.M.K.; Fotokian, M.H.; Davoodi, D.; Mirzaei, H.H.; Hasanloo, T. Improvement of hypericin and hyperforin production using zinc and iron nano-oxides as elicitors in cell suspension culture of St John’s wort (Hypericum perforatum L.). J. Med. Plants By-Prod. 2013, 2, 177–184. [Google Scholar]
- Oloumi, H.; Soltaninejad, R.; Baghizadeh, A. The comparative effects of nano and bulk size particles of CuO and ZnO on glycyrrhizin and phenolic compounds contents in Glycyrrhiza glabra L. seedlings. Indian J. Plant. Physiol. 2015, 20, 157–161. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmed, N.; Ali, S.S.; Shujait, A.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B Bio. 2016, 159, 1–7. [Google Scholar] [CrossRef]
- Chung, I.; Rajakumar, G.; Thiruvengadam, M. Effect of silver nanoparticles on phenolic compounds production and biological activities in hairy root cultures of Cucumis Anguria. Acta Biol. Hung. 2018, 69, 97–109. [Google Scholar] [CrossRef]
- Ali, A.; Mohamed, S.; Khan, M.A.; Raja, N.I.; Arif, M.; Kamil, A.; Mashwani, Z. Silver nanoparticles elicited in vitro callus cultures for accumulation of biomass and secondary metabolites in Caralluma Tuberculate. Artif. Cells Nanomed. Biotechnol. 2019, 47, 715–724. [Google Scholar] [CrossRef]
- Kruszka, D.; Sawikowska, A.; Selvakesavan, R.K.; Krajewski, P.; Kachlicki, P.; Franklin, G. Silver nanoparticles affect phenolic and phytoalexin composition of Arab Thaliana. Sci. Total Env. 2020, 716, 135361. [Google Scholar] [CrossRef] [PubMed]
- Jadczak, P.; Kulpa, D.; Bihun, M.; Przewodowski, W. Positive effect of AgNps and AuNps in in vitro cultures of Lavandula angustifolia Mill. Tissue Organ. Cult. 2019, 139, 191–197. [Google Scholar] [CrossRef]
- Wesołowska, A.; Jadczak, P.; Kulpa, D.; Przewodowski, W. Gas chromatography mass spectrometry (GC-MS) analysis of essential oils from AgNps and AuNps elicited Lavandula angustifolia in vitro cultures. Molecules 2019, 24, 606. [Google Scholar] [CrossRef] [PubMed]
- Akasaka-Kennedy, Y.; Yoshida, H.; Takathaka, Y. Efficient plant regeneration from leaves of rapeseed (Brassica napus L.): The influence of AgNO3 and genotype. Plant. Cell Rep. 2006, 24, 649–654. [Google Scholar] [CrossRef]
- Devi, G.H.; Suruthi, P.; Veerakumar, R.; Vinoth, S.; Subbaiya, R.; Chozhavendahn, S. A review on metallic gold and silver nanoparticles. J. Pharm. Tech. 2019, 12, 935–943. [Google Scholar] [CrossRef]
- Aziz, S.G.G.; Aziz, S.G.G.; Akbarzadeh, A. Advances in silver nanotechnology: An update on biomedical applications and future perspectives. Drug Res. 2017, 67, 198–203. [Google Scholar] [CrossRef]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef]
- Austin, L.A.; Mackey, M.A.; Dreaden, E.C.; El-Sayed, A.M. The optical, photothermal, and facile surface chemical properties of gold and silver nanoparticles in biodiagnostics, therapy, and drug delivery. Arch. Toxicol. 2014, 88, 1391–1417. [Google Scholar] [CrossRef]
- Prociak, J.; Grabowska, A.; Chwastowski, J.; Majka, T.; Banach, M. Safety of the application of nanosilver and nanogold in topical cosmetic preparations. Colloids Surf. B Biointerfaces 2019, 183, 110416. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Ayaz, M.; Saravanan, M.; Ali, M.; Ahmed, I.; Shahid, M.; Shinwari, Z.K. Multifunctional theranostic applications of biocompatible green-synthesized colloidal nanoparticles. Appl. Microbiol. Biotechnol. 2018, 102, 4393–4408. [Google Scholar] [CrossRef]
- Dhas, S.P.; Anbarasan, S.; Mukherjee, A.; Chandrasekaran, N. Biobased silver nanocolloid coating on silk fibers for prevention of post-surgical wound infections. Int. J. Nanomed. 2015, 10, 159–170. [Google Scholar]
- Baygar, T. Characterization of silk sutures coated with propolis and biogenic silver nanoparticles (AgNPs); an eco-friendly solution with wound healing potential against surgical site infections (SSIs). Turk. J. Med. Sci. 2020, 13, 258–266. [Google Scholar]
- Doughton, J.A.; Hofman, M.S.; Eu, P.; Hicks, R.J.; Williams, S. A first-in-human study of 68Ga-nanocolloid PET/CT sentinel lymph node imaging in prostate cancer demonstrates aberrant lymphatic drainage pathways. J. Nucl. Med. 2018, 59, 1837–1842. [Google Scholar] [CrossRef] [PubMed]
- Rasilla, J.M.; Balbín, L.F.; Arboniés, J.C.; Elola-Olaso, A.M.; Delgado-Bolton, R.; Pereyra, L.I.; Rey, C.R.; Gutiérrez, L.L.; Maté, A.G.; Santamaría, J.M.R.; et al. SPECT-TAC: A new tool for localisation of sentinel lymph nodes in breast cancer patients. Rev. Española Med. Nucl. 2008, 27, 183–190. [Google Scholar]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.J.; Cho, M.; Lee, S.M.; Park, J.H.; Oh, S.G.; Bang, K.-S.; Oh, B.T. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess. Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef]
- Carrillo-Inungaray, M.L.; Trejo-Ramirez, J.A.; Reyes-Munguia, A.; Carranza-Alvarez, C. Use of nanoparticles in the food industry: Advances and perspectives. In Impact of Nanoscience in the Food Industry; Academic Press: Cambridge, MA, USA, 2018; pp. 419–444. [Google Scholar]
- Connor, E.E.; Mwamuka, J.; Gole, A.; Murphy, J.M.; Wyatt, M.D. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small 2005, 1, 325–327. [Google Scholar] [CrossRef]
- Tiedemann, D.; Taylor, U.; Rehbock, C.; Jakobi, J.; Klein, S.; Kues, W.A.; Barcikowski, S.; Rath, D. Reprotoxicity of gold, silver, and gold-silver alloy nanoparticles on mammalian gametes. Analyst 2014, 7, 931–942. [Google Scholar] [CrossRef]
- Sung, J.H.; Ji, J.H.; Park, J.D.; Song, M.Y.; Song, K.S.; Ryu, H.R.; Yoon, J.U.; Jeon, K.S.; Jeong, J.; Han, B.S.; et al. Subchronic inhalation toxicity of gold nanoparticles. Part. Fibre Toxicol. 2011, 8, 16. [Google Scholar] [CrossRef]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Harish, K.K.; Nagasmay, V.; Himangshu, B.; Anuttam, K. Metallic nanoparticles: A review. Biomed. J. Sci. Tech. Res. 2018, 4, 3765–3775. [Google Scholar]
- Mehrian, S.K.; Heidari, R.; Rahmani, F. Effect of chemical synthesis silver nanoparticles on germination indices and seedlings growth in seven varieties of Lycopersicon esculentum mill (tomato) plants. J. Clust. Sci. 2016, 27, 327–340. [Google Scholar] [CrossRef]
- Barbasz, A.; Kreczmer, B.; Ocwieja, M. Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO3). Acta Physiol. Plant. 2016, 38, 76. [Google Scholar] [CrossRef]
- Iqbal, M.; Raja, N.I.; Ali, A.; Rashid, H.; Hussain, M.; Ejaz, M.; Iqbal, R.; Khan, U.A.; Shaheen, N.; Rauf, A.; et al. Effect of silver nanoparticles on growth of wheat under heat stress. Iran. J. Sci. Technol. Trans. A Sci. 2017, 43, 387–395. [Google Scholar] [CrossRef]
- Kim, S.; Lee, I. Alteration of phytotoxicity and oxidant stress potential by metal oxide nanoparticles in Cucumis sativus. Water Air Soil Pollut. 2012, 223, 2799–2806. [Google Scholar] [CrossRef]
- Gunjan, B.; Zaidi, M.G.H.; Sandeep, A. Impact of gold nanoparticles on physiological and biochemical characteristics of Brarica juncea. J. Plant. Physiol. Biochem. 2014, 2, 133. [Google Scholar]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S. Nitric oxide alleviates silver nanoparticles (AgNps)-induced phytotoxicity in Pisum sativum seedlings. Plant. Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Demissie, Z.A.; Sarker, L.S.; Mahmoud, S.S. Cloning and functional characterization of β-phellandrene synthase from Lavandula angustifolia. Planta 2011, 233, 685–696. [Google Scholar] [CrossRef]
- Wornouk, G.; Demisse, Z.; Rheut, M.; Mahmoud, S. Biosythesis and therapuetic properties of lavandula essential oil constituent. Planta Med. 2011, 77, 7–15. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.; Gillespie, K. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- Sanchezz-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velazquez, D.A. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Tian, H.; Ghorbanpour, M.; Kariman, K. Manganese oxide nanoparticle-induced changes in growth, redox reactions and elicitation of antioxidant metabolites in deadly nightshade (Atropa Belladonna L.). Ind. Crop. Prod. 2018, 126, 403–414. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Geneshan, J.E.; Rajan, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. Plant growth metabolism. Process. Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Homaee, M.B.; Ehsanpour, A.A. Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic. Environ. Biotechnol. 2015, 57, 544–553. [Google Scholar] [CrossRef]
- Yasur, J.; Rani, P.U. Environmental effects of nanosilver: Impact on castor seed germination, seedling growth, and plant physiology. Environ. Sci. Pollut. Res. 2013, 20, 8636–8648. [Google Scholar] [CrossRef]
- Jamshidi, M.; Ghanati, F. Taxanes content and cytotoxicity of hazel cells extract after elicitation with silver nanoparticles. Plant. Physiol. Biochem. 2017, 110, 178–184. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Hadian, J. Multi-walled carbon nanotubes stimulate callus induction, secondary metabolites biosynthesis and antioxidant capacity in medicinal plant Satureja khuzestanica grown in vitro. Carbon 2015, 94, 749–759. [Google Scholar] [CrossRef]
- Saha, N.; Gupta, S.D. Promotion of shoot regeneration of Swertia chirata by biosynthesized silver nanoparticles and their involvement in ethylene interceptions and activation of antioxidant activity. Plant. Cell Tissue Organ. Cult. 2018, 134, 89–300. [Google Scholar] [CrossRef]
- García-Sánchez, S.; Bernales, I.; Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signaling. BMC Genom. 2015, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, R.; Li, Y.S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 18, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Andrys, D.; Kulpa, D. In vitro propagation affects the composition of narrow-leaved lavender essential oils. Acta Chromat. 2018, 30, 225–230. [Google Scholar] [CrossRef]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Liu, F.K.; Ker, C.J.; Chang, Y.C.; Ko, F.H.; Chu, T.C.; Dai, B.T. Microwave heating for the preparation of nanometer gold particles. J. Appl. Physic. 2003, 42, 4152–4158. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, A.C. Assay of catalase and peroxidases. Method Enzym. 1995, 2, 764–775. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant. Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assay and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Li, Y.; Schellhorn, H.E. Rapid kinetic microassay for catalase activity. J. Biomol. Tech. 2007, 18, 185–187. [Google Scholar] [PubMed]
- Anastasiadi, M.; Pratsinis, H.; Kletsas, D.; Skaltsounis, A.L.; Haroutounian, S.A. Bioactive non-coloured polyphenols content of grapes, wines and vinification by-products: Evaluation of the antioxidant activities of their extracts. Food Res. Int. 2010, 43, 805–813. [Google Scholar] [CrossRef]
- Shi, F.; Jia, X.; Zhao, C.; Chen, Y. Antioxidant activities of various extracts from Artemisisa selengensis turcz (LuHao). Molecules 2010, 15, 4934–4946. [Google Scholar] [CrossRef] [PubMed]

Sample Availability: Samples of the compounds are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jadczak, P.; Kulpa, D.; Drozd, R.; Przewodowski, W.; Przewodowska, A. Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula angustifolia Mill.) from In Vitro Cultures. Molecules 2020, 25, 5511. https://doi.org/10.3390/molecules25235511
Jadczak P, Kulpa D, Drozd R, Przewodowski W, Przewodowska A. Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula angustifolia Mill.) from In Vitro Cultures. Molecules. 2020; 25(23):5511. https://doi.org/10.3390/molecules25235511
Chicago/Turabian StyleJadczak, Paula, Danuta Kulpa, Radosław Drozd, Włodzimierz Przewodowski, and Agnieszka Przewodowska. 2020. "Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula angustifolia Mill.) from In Vitro Cultures" Molecules 25, no. 23: 5511. https://doi.org/10.3390/molecules25235511
APA StyleJadczak, P., Kulpa, D., Drozd, R., Przewodowski, W., & Przewodowska, A. (2020). Effect of AuNPs and AgNPs on the Antioxidant System and Antioxidant Activity of Lavender (Lavandula angustifolia Mill.) from In Vitro Cultures. Molecules, 25(23), 5511. https://doi.org/10.3390/molecules25235511