New Trends in C–C Cross-Coupling Reactions: The Use of Unconventional Conditions
Abstract
1. Introduction
2. Alternative Activation Methods for Cross-Coupling Reactions
2.1. Microwave Irradiation
2.2. Sonochemistry
2.3. Mechanochemistry
3. Unconventional Media for Cross-Coupling Reactions
3.1. Solvent-Free
3.2. Water as Solvent
3.3. Poly(Ethylene Glycol)
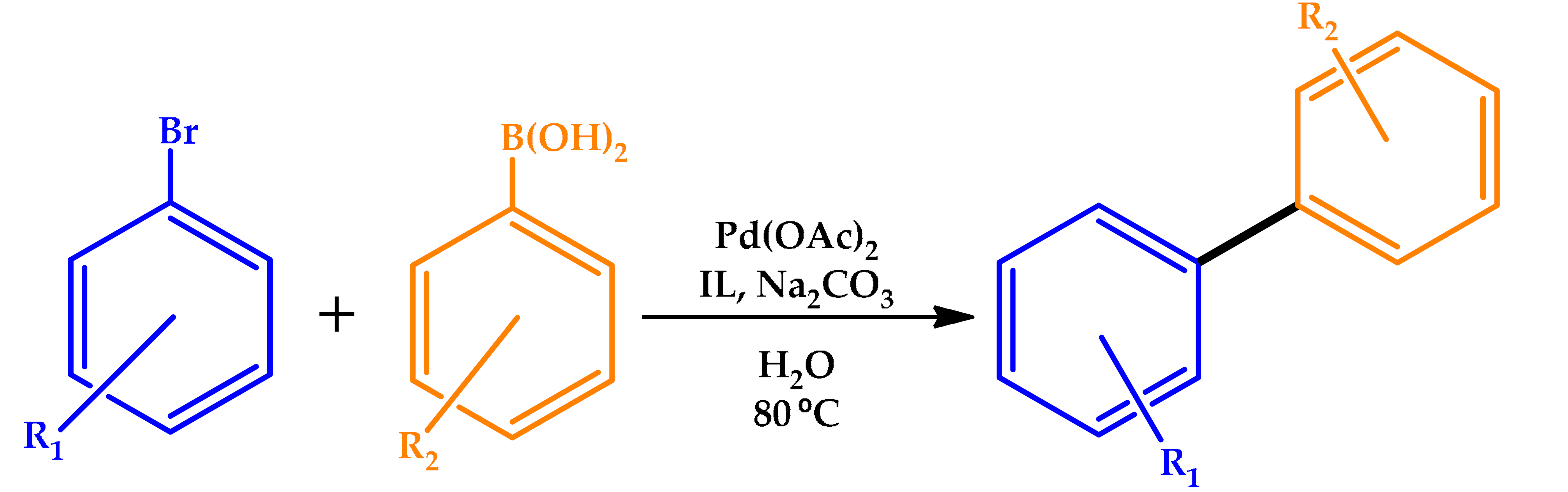
3.4. Ionic Liquids
3.5. Deep Eutectic Solvents
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Dieck, H.A.; Heck, F.R. Palladium catalyzed synthesis of aryl, heterocyclic and vinylic acetylene derivatives. J. Organomet. Chem. 1975, 93, 259–263. [Google Scholar] [CrossRef]
- Sonogashira, K.; Tohda, Y.; Hagihara, N. A convenient synthesis of acetylenes: Catalytic substitutions of acetylenic hydrogen with bromoalkenes. Tetrahedron Lett. 1975, 50, 4467–4470. [Google Scholar] [CrossRef]
- Chen, X.; Engle, K.M.; Wang, D.H.; Jin-Quan, Y. Palladium(II)-cataIyzed C-H aetivation/C-C cross-coupling reactions: Versatility and practicality. Angew. Chem. Int. Ed. 2009, 48, 5094–5115. [Google Scholar] [CrossRef] [PubMed]
- de Barros, S.D.T.; Senra, J.D.; Lachter, E.R.; Malta, L.F.B. Metal-catalyzed cross-coupling reactions with supported nanoparticles: Recent developments and future directions. Catal. Rev. Sci. Eng. 2016, 58, 439–496. [Google Scholar] [CrossRef]
- Molnár, Á. Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef]
- Buskes, M.J. Impact of Cross-Coupling Reactions in Drug Discovery and Development. Molecules 2020, 25, 3493. [Google Scholar] [CrossRef]
- Floris, B.; Sabuzi, F.; Galloni, P.; Conte, V. The beneficial sinergy of MW irradiation and ionic liquids in catalysis of organic reactions. Catalysts 2017, 7. [Google Scholar] [CrossRef]
- Sherwood, J.; Clark, J.H.; Fairlamb, I.J.S.; Slattery, J.M. Solvent effects in palladium catalysed cross-coupling reactions. Green Chem. 2019, 21, 2164–2213. [Google Scholar] [CrossRef]
- Hooshmand, S.E.; Heidari, B.; Sedghi, R.; Varma, R.S. Recent advances in the Suzuki-Miyaura cross-coupling reaction using efficient catalysts in eco-friendly media. Green Chem. 2019, 21, 381–405. [Google Scholar] [CrossRef]
- Yousaf, M.; Zahoor, A.F.; Akhtar, R.; Ahmad, M.; Naheed, S. Development of green methodologies for Heck, Chan–Lam, Stille and Suzuki cross-coupling reactions. Mol. Divers. 2020, 24, 821–839. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, S.E.; Afshari, R.; Ramón, D.J.; Varma, R.S. Deep eutectic solvents: Cutting-edge applications in cross-coupling reactions. Green Chem. 2020, 22, 3668–3692. [Google Scholar] [CrossRef]
- Santoro, S.; Ferlin, F.; Luciani, L.; Ackermann, L.; Vaccaro, L. Biomass-derived solvents as effective media for cross-coupling reactions and C-H functionalization processes. Green Chem. 2017, 19, 1601–1612. [Google Scholar] [CrossRef]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: Mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef] [PubMed]
- Philippot, K.; Serp, P. Concepts in Nanocatalysis. In Nanomaterials in Catalysis, 1st ed.; Wiley-VCH: Weinheim, Germany, 2012; pp. 1–54. ISBN 9783527331246. [Google Scholar]
- Kandathil, V.; Koley, T.S.; Manjunatha, K.; Dateer, R.B.; Keri, R.S.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. A new magnetically recyclable heterogeneous palladium(II) as a green catalyst for Suzuki-Miyaura cross-coupling and reduction of nitroarenes in aqueous medium at room temperature. Inorg. Chim. Acta 2018, 478, 195–210. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Sadeghi, A.R.; Khorsandi, Z. Pd nanoparticles immobilized on magnetic chitosan as a novel reusable catalyst for green Heck and Suzuki cross-coupling reaction: In water at room temperature. Appl. Organomet. Chem. 2018, 32, 1–11. [Google Scholar] [CrossRef]
- Madrahalli Bharamanagowda, M.; Panchangam, R.K. Fe3O4-Lignin@Pd-NPs: A highly efficient, magnetically recoverable and recyclable catalyst for Mizoroki-Heck reaction under solvent-free conditions. Appl. Organomet. Chem. 2020, 34, 1–18. [Google Scholar] [CrossRef]
- García-Álvarez, J.; Hevia, E.; Capriati, V. Reactivity of Polar Organometallic Compounds in Unconventional Reaction Media: Challenges and Opportunities. Eur. J. Org. Chem. 2015, 2015, 6779–6799. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S.; Warren, P. Practical Microwave Synthesis for Organic Chemists; KGaA: Weinheim, Germany, 2009; ISBN 9783527314522. [Google Scholar]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and heterogeneous catalysis: A review on selected catalytic processes. Catalysts 2020, 10. [Google Scholar] [CrossRef]
- Kappe, C.O. Microwave-assisted chemistry. In Comprehensive Medicinal Chemistry II; Elsevier: Amsterdam, The Netherlands, 2006; Volume 3, pp. 837–860. [Google Scholar] [CrossRef]
- Chatel, G.; Varma, R.S. Ultrasound and microwave irradiation: Contributions of alternative physicochemical activation methods to Green Chemistry. Green Chem. 2019, 21, 6043–6050. [Google Scholar] [CrossRef]
- De La Hoz, A.; Díaz-Ortiz, Á.; Moreno, A. Microwaves in organic synthesis. Thermal and non-thermal microwave effects. Chem. Soc. Rev. 2005, 34, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Gawande, M.B.; Bonifácio, V.D.B.; Luque, R.; Branco, P.S.; Varma, R.S. Solvent-free and catalysts-free chemistry: A benign pathway to sustainability. ChemSusChem 2014, 7, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Martínez, A.V.; Invernizzi, F.; Leal-Duaso, A.; Mayoral, J.A.; García, J.I. Microwave-promoted solventless Mizoroki-Heck reactions catalysed by Pd nanoparticles supported on laponite clay. RSC Adv. 2015, 5, 10102–10109. [Google Scholar] [CrossRef]
- Shah, D.; Kaur, H. Supported palladium nanoparticles: A general sustainable catalyst for microwave enhanced carbon-carbon coupling reactions. J. Mol. Catal. A Chem. 2016, 424, 171–180. [Google Scholar] [CrossRef]
- Massaro, M.; Schembri, V.; Campisciano, V.; Cavallaro, G.; Lazzara, G.; Milioto, S.; Noto, R.; Parisi, F.; Riela, S. Design of PNIPAAM covalently grafted on halloysite nanotubes as a support for metal-based catalysts. RSC Adv. 2016, 6, 55312–55318. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, T.; Wu, X.; Wu, Y.; Xiang, H.; Sun, H.; You, Q.; Zhang, X. Microwave-assisted copper- and palladium-catalyzed sonogashira-type coupling of aryl bromides and iodides with trimethylsilylacetylene. Tetrahedron Lett. 2016, 57, 1100–1103. [Google Scholar] [CrossRef]
- Savitha, B.; Sajith, A.M.; Reddy, E.K.; Kumar, C.S.A.; Padusha, M.S.A. Suzuki-Miyaura cross–coupling reaction in water: Facile synthesis of (hetero) aryl uracil bases using potassiumorganotrifluoroborates under microwave irradiation. ChemistrySelect 2016, 1, 4721–4725. [Google Scholar] [CrossRef]
- Baran, T. A new chitosan Schiff base supported Pd(II) complex for microwave-assisted synthesis of biaryls compounds. J. Mol. Struct. 2017, 1141, 535–541. [Google Scholar] [CrossRef]
- Verbitskiy, E.V.; Baranova, A.A.; Khokhlov, K.O.; Yakovleva, Y.A.; Chuvashov, R.D.; Kim, G.A.; Moiseykin, E.V.; Dinastiya, E.M.; Rusinov, G.L.; Chupakhin, O.N.; et al. New push–pull system based on 4,5,6-tri(het)arylpyrimidine containing carbazole substituents: Synthesis and sensitivity toward nitroaromatic compounds. Chem. Heterocycl. Compd. 2018, 54, 604–611. [Google Scholar] [CrossRef]
- Elazab, H.A.; Sadek, M.A.; El-Idreesy, T.T. Microwave-assisted synthesis of palladium nanoparticles supported on copper oxide in aqueous medium as an efficient catalyst for Suzuki cross-coupling reaction. Adsorpt. Sci. Technol. 2018, 36, 1352–1365. [Google Scholar] [CrossRef]
- Zakharchenko, B.V.; Khomenko, D.M.; Doroshchuk, R.O.; Raspertova, I.V.; Starova, V.S.; Trachevsky, V.V.; Shova, S.; Severynovska, O.V.; Martins, L.M.D.R.S.; Pombeiro, A.J.L.; et al. New palladium(ii) complexes with 3-(2-pyridyl)-5-alkyl-1,2,4-triazole ligands as recyclable C-C coupling catalysts. New J. Chem. 2019, 43, 10973–10984. [Google Scholar] [CrossRef]
- Lupacchini, M.; Mascitti, A.; Giachi, G.; Tonucci, L.; D’Alessandro, N.; Martinez, J.; Colacino, E. Sonochemistry in non-conventional, green solvents or solvent-free reactions. Tetrahedron 2017, 73, 609–653. [Google Scholar] [CrossRef]
- Tabatabaei Rezaei, S.J. PEDOT nanofiber/Pd(0) composite-mediated aqueous Mizoroki–Heck reactions under ultrasonic irradiation: An efficient and green method for the C–C cross-coupling reactions. J. Iran. Chem. Soc. 2017, 14, 585–594. [Google Scholar] [CrossRef]
- Panahi, L.; Naimi-Jamal, M.R.; Mokhtari, J. Ultrasound-assisted Suzuki-Miyaura reaction catalyzed by Pd@Cu2(NH2-BDC)2(DABCO). J. Organomet. Chem. 2018, 868, 36–46. [Google Scholar] [CrossRef]
- Sancheti, S.V.; Gogate, P.R. Intensification of heterogeneously catalyzed Suzuki-Miyaura cross-coupling reaction using ultrasound: Understanding effect of operating parameters. Ultrason. Sonochem. 2018, 40, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Baran, T. Ultrasound-accelerated synthesis of biphenyl compounds using novel Pd(0) nanoparticles immobilized on bio-composite. Ultrason. Sonochem. 2018, 45, 231–237. [Google Scholar] [CrossRef]
- Naeimi, H.; Kiani, F. Hexamethylenetetramine Copper Diiodide Immobilized on Graphene Oxide Nanocomposite as Recyclable Catalyst for Sonochemical Green Synthesis of Diarylethynes. ChemistrySelect 2018, 3, 13311–13318. [Google Scholar] [CrossRef]
- Naeimi, H.; Kiani, F. Magnetically thiamine palladium complex nanocomposites as an effective recyclable catalyst for facile sonochemical cross coupling reaction. Appl. Organomet. Chem. 2019, 33, 1–12. [Google Scholar] [CrossRef]
- Stolle, A.; Szuppa, T.; Leonhardt, S.E.S.; Ondruschka, B. Ball milling in organic synthesis: Solutions and challenges. Chem. Soc. Rev. 2011, 40, 2317–2329. [Google Scholar] [CrossRef]
- Jiang, Z.J.; Li, Z.H.; Yu, J.B.; Su, W.K. Liquid-Assisted Grinding Accelerating: Suzuki-Miyaura Reaction of Aryl Chlorides under High-Speed Ball-Milling Conditions. J. Org. Chem. 2016, 81, 10049–10055. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Yu, J.; Jiang, Z.; Shao, Q.; Su, W. Encaging palladium(0) in layered double hydroxide: A sustainable catalyst for solvent-free and ligand-free Heck reaction in a ball mill. Beilstein J. Org. Chem. 2017, 13, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Howard, J.L.; Wheatley, E.; Browne, D.L. Mechanochemical Activation of Zinc and Application to Negishi Cross-Coupling. Angew. Chem. Int. Ed. 2018, 57, 11339–11343. [Google Scholar] [CrossRef] [PubMed]
- Seo, T.; Ishiyama, T.; Kubota, K.; Ito, H. Solid-state Suzuki-Miyaura cross-coupling reactions: Olefin-accelerated C-C coupling using mechanochemistry. Chem. Sci. 2019, 10, 8202–8210. [Google Scholar] [CrossRef]
- Vogt, C.G.; Grätz, S.; Lukin, S.; Halasz, I.; Etter, M.; Evans, J.D.; Borchardt, L. Direct Mechanocatalysis: Palladium as Milling Media and Catalyst in the Mechanochemical Suzuki Polymerization. Angew. Chem. Int. Ed. 2019, 58, 18942–18947. [Google Scholar] [CrossRef]
- Pentsak, E.O.; Ananikov, V.P. Pseudo-Solid-State Suzuki–Miyaura Reaction and the Role of Water Formed by Dehydration of Arylboronic Acids. Eur. J. Org. Chem. 2019, 2019, 4239–4247. [Google Scholar] [CrossRef]
- Soliman, M.M.A.; Peixoto, A.F.; Ribeiro, A.P.C.; Kopylovich, M.N.; Alegria, E.C.B.A.; Pombeiro, A.J.L. Mechanochemical preparation of Pd(II) and Pt(II) composites with carbonaceous materials and their application in the Suzuki-Miyaura reaction at several energy inputs. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Gallou, F.; Handa, S. Evolution of solvents in organic chemistry. ACS Sustain. Chem. Eng. 2016, 4, 5838–5849. [Google Scholar] [CrossRef]
- Dumonteil, G.; Hiebel, M.A.; Berteina-Raboin, S. Solvent-freesolvent-free mizoroki-heckmizoroki-heck reaction applied to the synthesis of abscisic acid and some derivatives. Catalysts 2018, 8. [Google Scholar] [CrossRef]
- Baran, T.; Yılmaz Baran, N.; Menteş, A. Highly active and recyclable heterogeneous palladium catalyst derived from guar gum for fabrication of biaryl compounds. Int. J. Biol. Macromol. 2019, 132, 1147–1154. [Google Scholar] [CrossRef]
- Gribanov, P.S.; Chesnokov, G.A.; Dzhevakov, P.B.; Kirilenko, N.Y.; Rzhevskiy, S.A.; Ageshina, A.A.; Topchiy, M.A.; Bermeshev, M.V.; Asachenko, A.F.; Nechaev, M.S. Solvent-free Suzuki and Stille cross-coupling reactions of 4- and 5-halo-1,2,3-triazoles. Mendeleev Commun. 2019, 29, 147–149. [Google Scholar] [CrossRef]
- Chatterjee, A.; Ward, T.R. Recent Advances in the Palladium Catalyzed Suzuki-Miyaura Cross-Coupling Reaction in Water. Catal. Lett. 2016, 146, 820–840. [Google Scholar] [CrossRef]
- Handa, S.; Smith, J.D.; Hageman, M.S.; Gonzalez, M.; Lipshutz, B.H.; Handa, S.; Smith, J.D.; Hageman, M.S.; Gonzalez, M.; Lipshutz, B.H. Synergistic and Selective Copper/ppm Pd-Catalyzed Suzuki-Miyaura Couplings: In Water, Mild Conditions, with Recycling. ACS Catal. 2016, 6, 8179–8183. [Google Scholar] [CrossRef]
- Guarnizo, A.; Angurell, I.; Muller, G.; Llorca, J.; Seco, M.; Rossell, O.; Rossell, M.D. Highly water-dispersible magnetite-supported Pd nanoparticles and single atoms as excellent catalysts for Suzuki and hydrogenation reactions. RSC Adv. 2016, 6, 68675–68684. [Google Scholar] [CrossRef]
- Ghazali-Esfahani, S.; PǍunescu, E.; Bagherzadeh, M.; Fei, Z.; Laurenczy, G.; Dyson, P.J. A simple catalyst for aqueous phase Suzuki reactions based on palladium nanoparticles immobilized on an ionic polymer. Sci. China Chem. 2016, 59, 482–486. [Google Scholar] [CrossRef]
- Ahadi, A.; Rostamnia, S.; Panahi, P.; Wilson, L.D.; Kong, Q.; An, Z.; Shokouhimehr, M. Palladium comprising dicationic bipyridinium supported periodic mesoporous organosilica (PMO): Pd@Bipy-PMO as an efficient hybrid catalyst for suzuki-miyaura cross-coupling reaction in water. Catalysts 2019, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Zhang, W.Q.; Sun, H.; Zhang, K.; Jian, Y.; Gu, Q.; Zhang, G.; Li, J.; Gao, Z. Sustainable Ligand-Free, Palladium-Catalyzed Suzuki–Miyaura Reactions in Water: Insights into the Role of Base. ChemSusChem 2019, 12, 5265–5273. [Google Scholar] [CrossRef]
- Lambert, R.; Wirotius, A.L.; Vignolle, J.; Taton, D. C-C couplings in water by micellar catalysis at low loadings from a recyclable polymer-supported Pd(ii)-NHC nanocatalyst. Polym. Chem. 2019, 10, 460–466. [Google Scholar] [CrossRef]
- Jakobi, M.; Gallou, F.; Sparr, C.; Parmentier, M. A General Protocol for Robust Sonogashira Reactions in Micellar Medium. Helv. Chim. Acta 2019, 102. [Google Scholar] [CrossRef]
- Pires, M.J.D.; Purificação, S.I.; Santos, A.S.; Marques, M.M.B. The Role of PEG on Pd- and Cu-Catalyzed Cross-Coupling Reactions. Synthesis 2017, 49, 2337–2350. [Google Scholar] [CrossRef]
- Soni, J.; Sahiba, N.; Sethiya, A.; Agarwal, S. Polyethylene glycol: A promising approach for sustainable organic synthesis. J. Mol. Liq. 2020, 315, 113766. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, T.; Huang, B.; Tuo, Y.; Cai, M. Recyclable and reusable NiCl2(PPh3)2/CuI/PEG-400/H2O system for the sonogashira coupling reaction of aryl iodides with alkynes. Appl. Organomet. Chem. 2015, 29, 846–849. [Google Scholar] [CrossRef]
- Qi, X.; Jiang, L.B.; Wu, X.F. Manganese-catalyzed Sonogashira coupling of aryl iodides. Tetrahedron Lett. 2016, 57, 1706–1710. [Google Scholar] [CrossRef]
- Gautam, P.; Bhanage, B.M. Oxime Palladacycle Catalyzed Carbonylative Sonogashira Cross-Coupling with High Turnovers in PEG as a Benign and Recyclable Solvent System. ChemistrySelect 2016, 1, 5463–5470. [Google Scholar] [CrossRef]
- Khanmoradi, M.; Nikoorazm, M.; Ghorbani-Choghamarani, A. Synthesis and Characterization of Pd Schiff Base Complex Immobilized onto Functionalized Nanoporous MCM-41 and its Catalytic Efficacy in the Suzuki, Heck and Stille Coupling Reactions. Catal. Lett. 2017, 147, 1114–1126. [Google Scholar] [CrossRef]
- Zeynizadeh, B.; Mousavi, H.; Sepehraddin, F. A green and efficient Pd-free protocol for the Suzuki–Miyaura cross-coupling reaction using Fe3O4@APTMS@Cp2ZrClx(x = 0, 1, 2) MNPs in PEG-400. Res. Chem. Intermed. 2020, 46, 3361–3382. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef]
- Patil, J.D.; Korade, S.N.; Patil, S.A.; Gaikwad, D.S.; Pore, D.M. Dual functionalized task specific ionic liquid promoted in situ generation of palladium nanoparticles in water: Synergic catalytic system for Suzuki-Miyaura cross coupling. RSC Adv. 2015, 5, 79061–79069. [Google Scholar] [CrossRef]
- Hejazifar, M.; Earle, M.; Seddon, K.R.; Weber, S.; Zirbs, R.; Bica, K. Ionic Liquid-Based Microemulsions in Catalysis. J. Org. Chem. 2016, 81, 12332–12339. [Google Scholar] [CrossRef]
- Taskin, M.; Cognigni, A.; Zirbs, R.; Reimhult, E.; Bica, K. Surface-active ionic liquids for palladium-catalysed cross coupling in water: Effect of ionic liquid concentration on the catalytically active species. RSC Adv. 2017, 7, 41144–41151. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, S.; Ferlin, N.; Bouquillon, S. High catalytic and recyclable systems for heck reactions in biosourced ionic liquids. Mol. Catal. 2017, 437, 121–129. [Google Scholar] [CrossRef]
- Choudhary, H.; Berton, P.; Gurau, G.; Myerson, A.S.; Rogers, R.D. Ionic liquids in cross-coupling reactions: “liquid” solutions to a “solid” precipitation problem. Chem. Commun. 2018, 54, 2056–2059. [Google Scholar] [CrossRef] [PubMed]
- Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. New C-scorpionate nickel(II) catalyst for Heck C–C coupling under unconventional conditions. J. Organomet. Chem. 2019, 896, 32–37. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. Metal-Mediated and Metal-Catalyzed Reactions under Mechanochemical Conditions. ACS Catal. 2020, 10, 8344–8394. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents—Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Marset, X.; Pérez, J.M.; Ramón, D.J. Cross-dehydrogenative coupling reaction using copper oxide impregnated on magnetite in deep eutectic solvents. Green Chem. 2016, 18, 826–833. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Wu, F.S.; Yang, L.; Liang, Y.; Cao, X.L.; Wang, H.S.; Pan, Y.M. Catalyst- and solvent-free approach to 2-arylated quinolines via [5 + 1] annulation of 2- methylquinolines with diynones. RSC Adv. 2018, 8, 4584–4587. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, I.; Sharma, R.; Sharma, U. Catalyst and solvent-free alkylation of quinoline N-oxides with olefins: A direct access to quinoline-substituted α-hydroxy carboxylic derivatives. Org. Biomol. Chem. 2016, 14, 2613–2617. [Google Scholar] [CrossRef]
- Boess, E.; Schmitz, C.; Klussmann, M. A comparative mechanistic study of Cu-catalyzed oxidative coupling reactions with N-phenyltetrahydroisoquinoline. J. Am. Chem. Soc. 2012, 134, 5317–5325. [Google Scholar] [CrossRef] [PubMed]
- Baslé, O.; Li, C.J. Copper catalyzed oxidative alkylation of sp3 C-H bond adjacent to a nitrogen atom using molecular oxygen in water. Green Chem. 2007, 9, 1047–1050. [Google Scholar] [CrossRef]
- Dilauro, G.; García, S.M.; Tagarelli, D.; Vitale, P.; Perna, F.M.; Capriati, V. Ligand-Free Bioinspired Suzuki–Miyaura Coupling Reactions using Aryltrifluoroborates as Effective Partners in Deep Eutectic Solvents. ChemSusChem 2018, 11, 3495–3501. [Google Scholar] [CrossRef] [PubMed]
- Messa, F.; Dilauro, G.; Perna, F.M.; Vitale, P.; Capriati, V.; Salomone, A. Sustainable Ligand-Free Heterogeneous Palladium-Catalyzed Sonogashira Cross-Coupling Reaction in Deep Eutectic Solvents. ChemCatChem 2020, 12, 1979–1984. [Google Scholar] [CrossRef]
- Saavedra, B.; González-Gallardo, N.; Meli, A.; Ramón, D.J. A Bipyridine-Palladium Derivative as General Pre-Catalyst for Cross-Coupling Reactions in Deep Eutectic Solvents. Adv. Synth. Catal. 2019, 361, 3868–3879. [Google Scholar] [CrossRef]
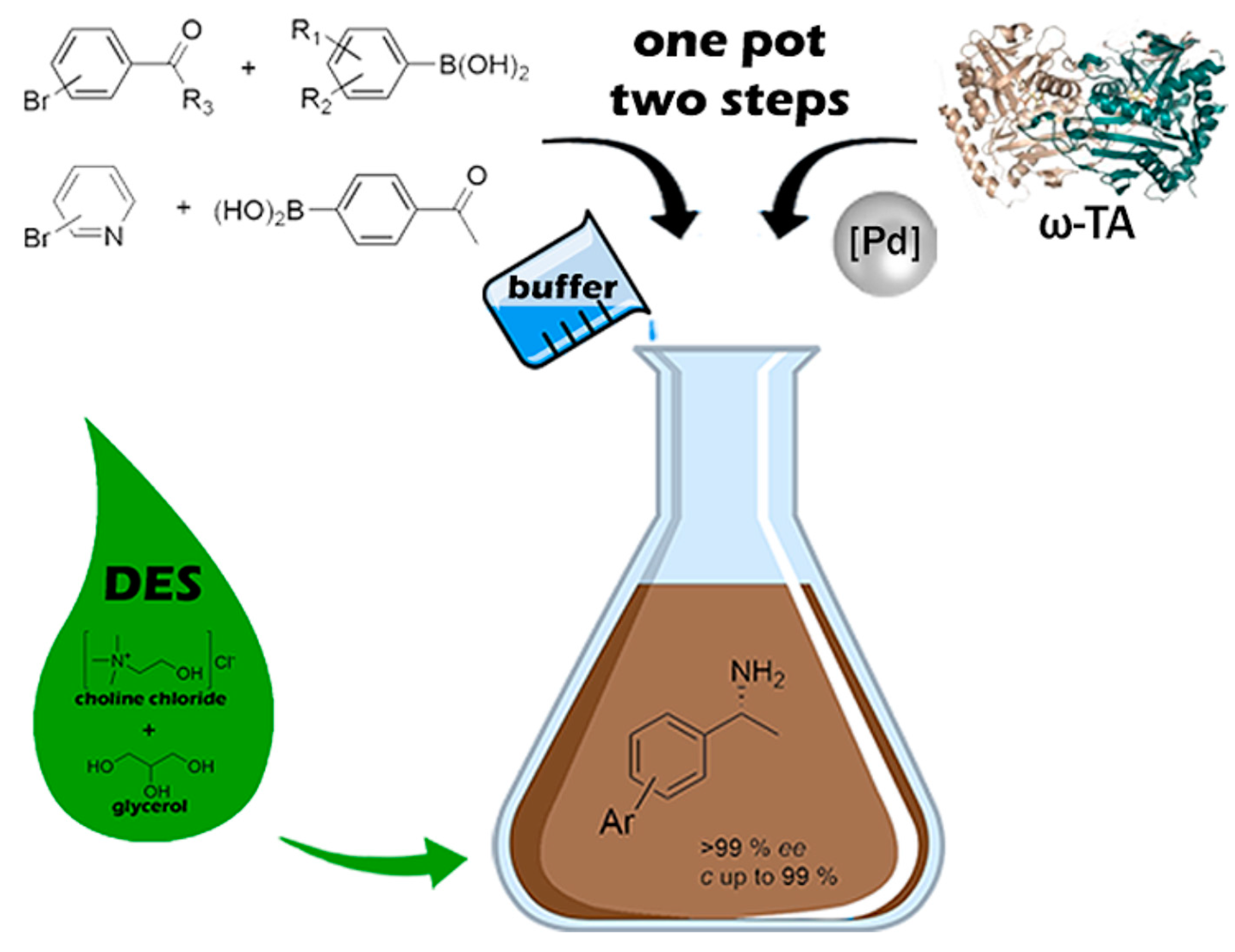
- Paris, J.; Telzerow, A.; Ríos-Lombardía, N.; Steiner, K.; Schwab, H.; Morís, F.; Gröger, H.; González-Sabín, J. Enantioselective One-Pot Synthesis of Biaryl-Substituted Amines by Combining Palladium and Enzyme Catalysis in Deep Eutectic Solvents. ACS Sustain. Chem. Eng. 2019, 7, 5486–5493. [Google Scholar] [CrossRef]
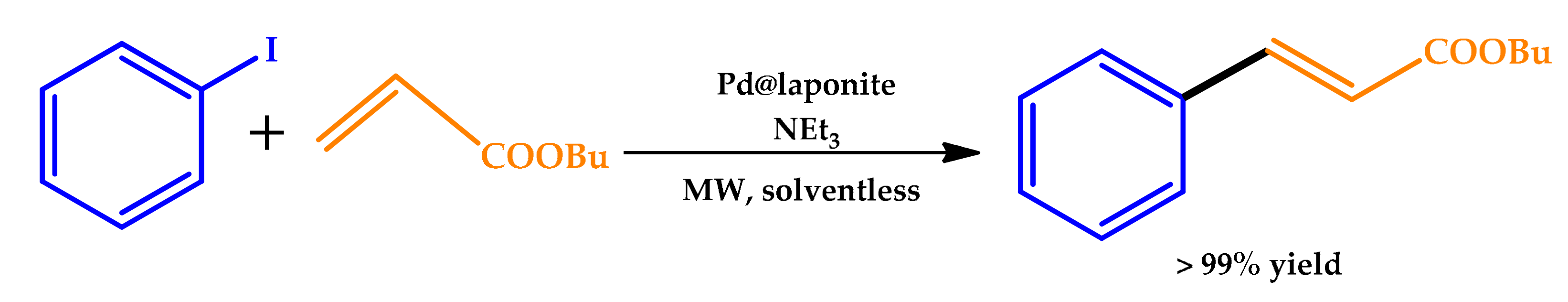
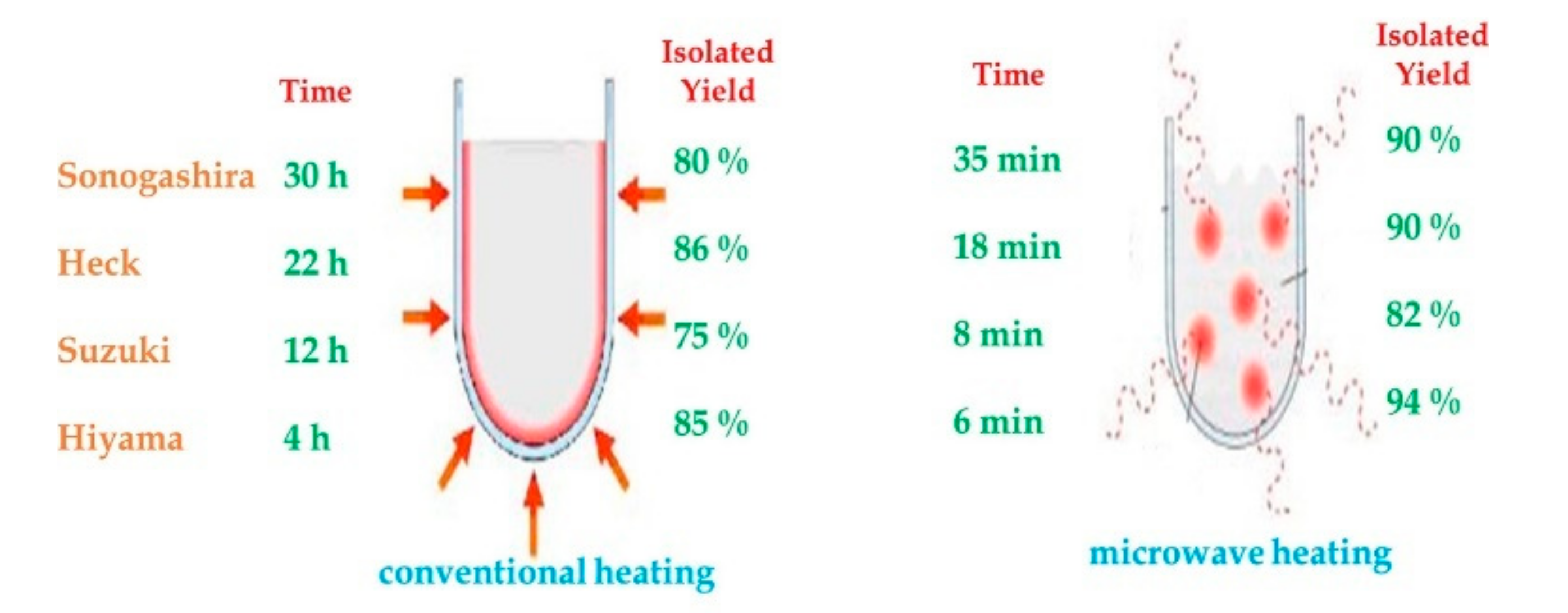
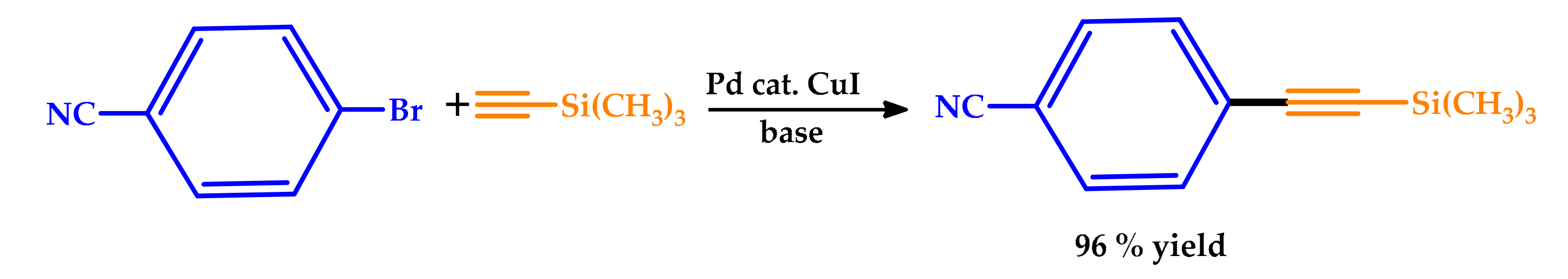

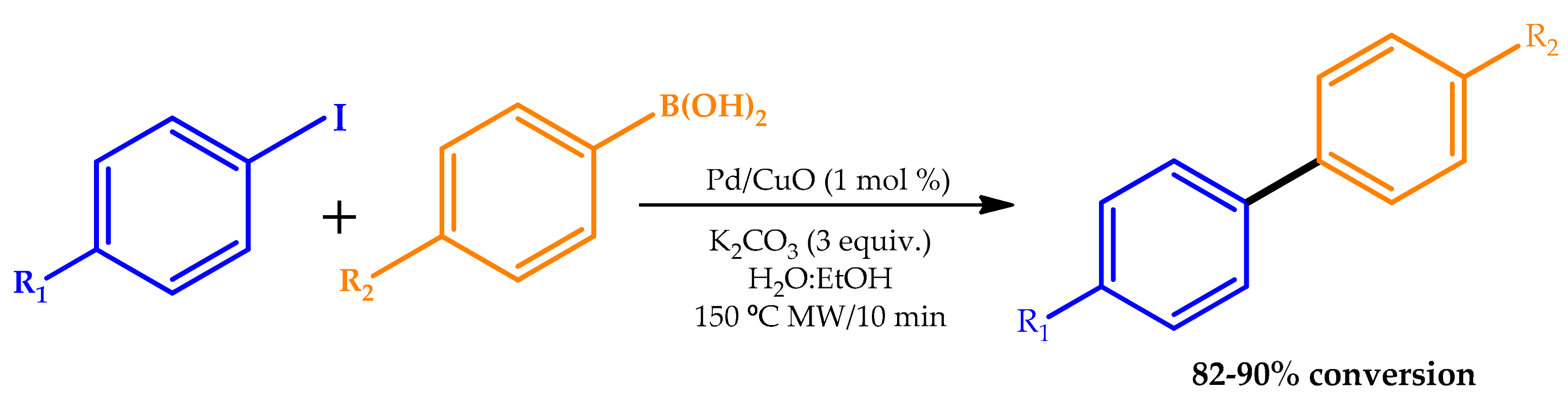

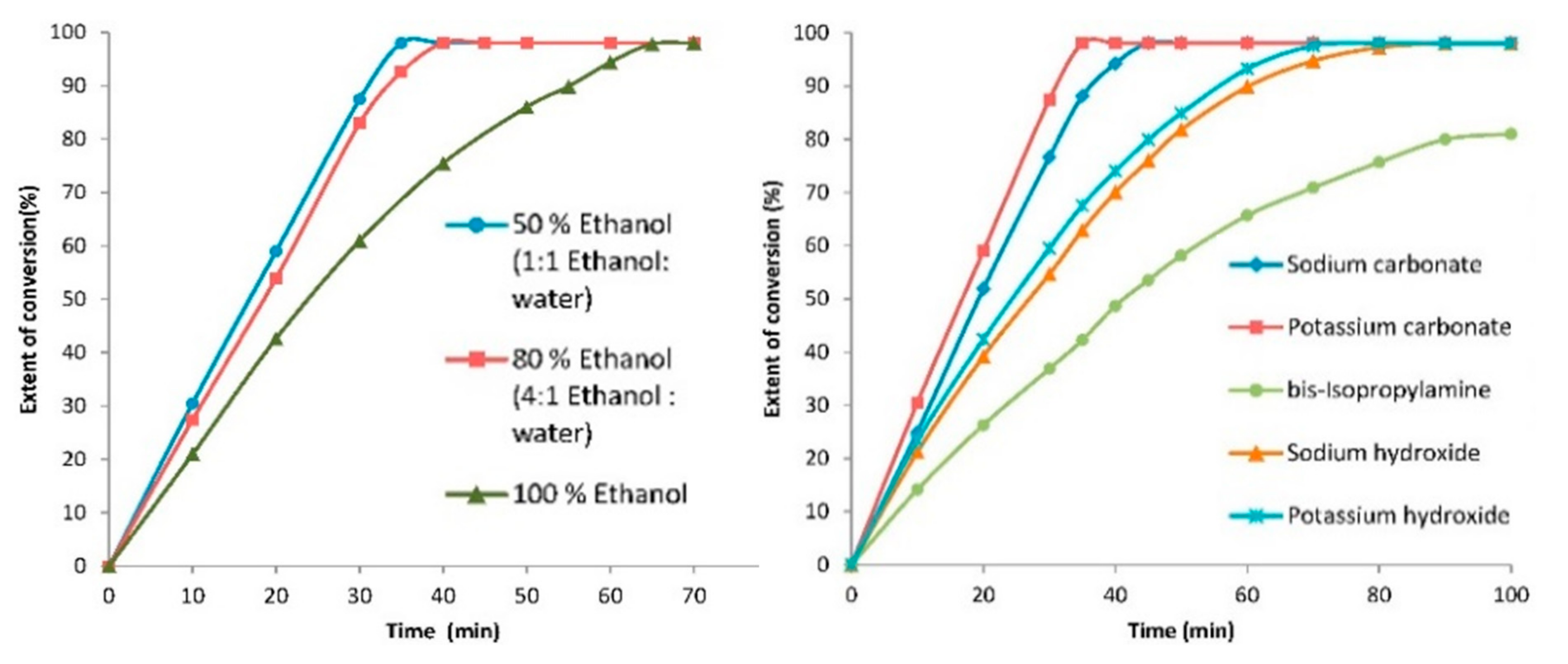
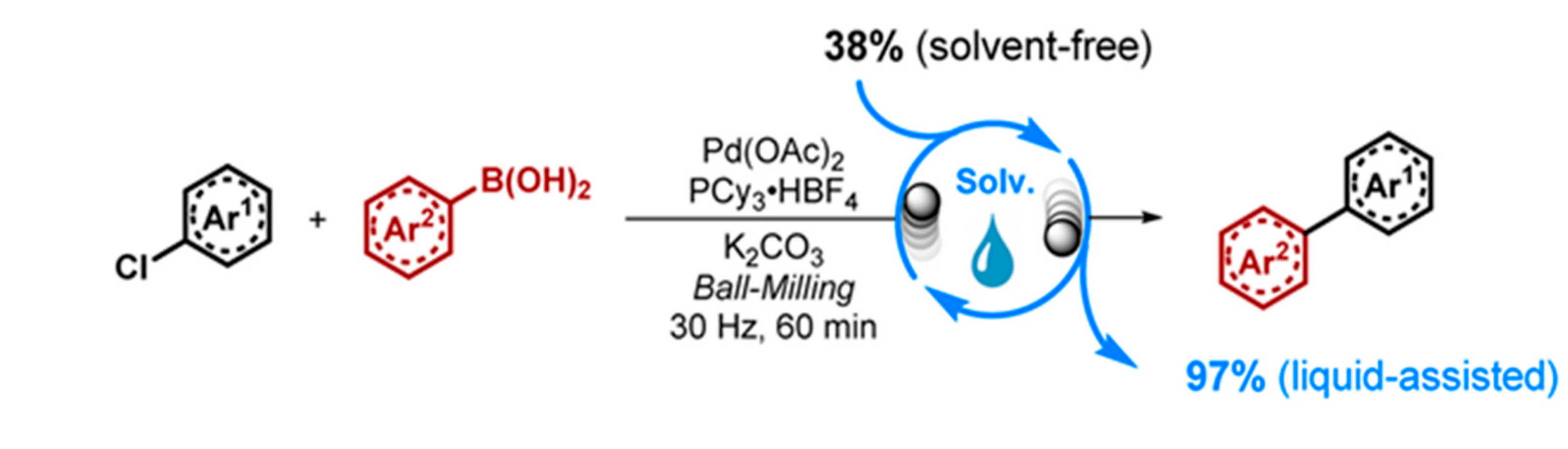
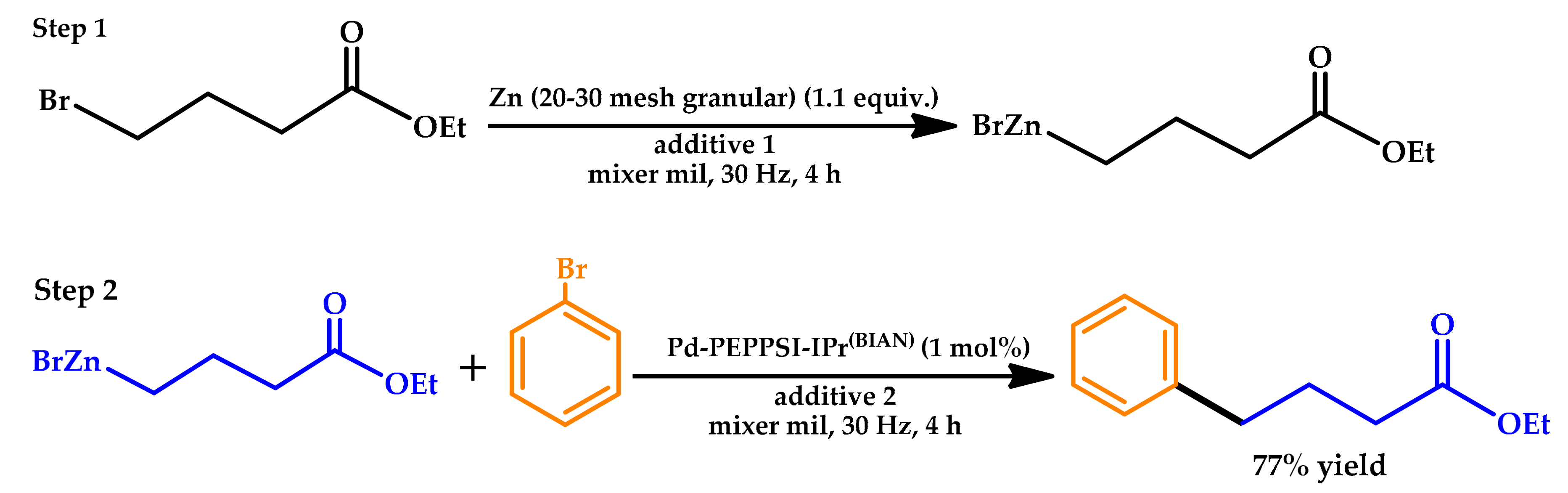

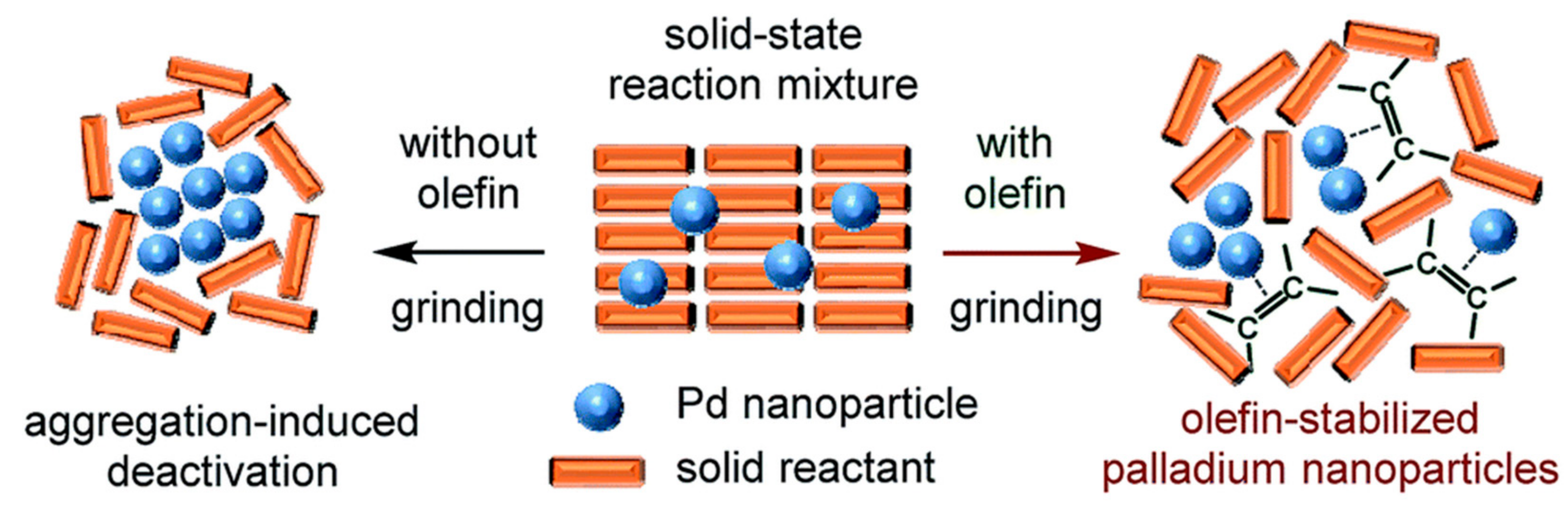



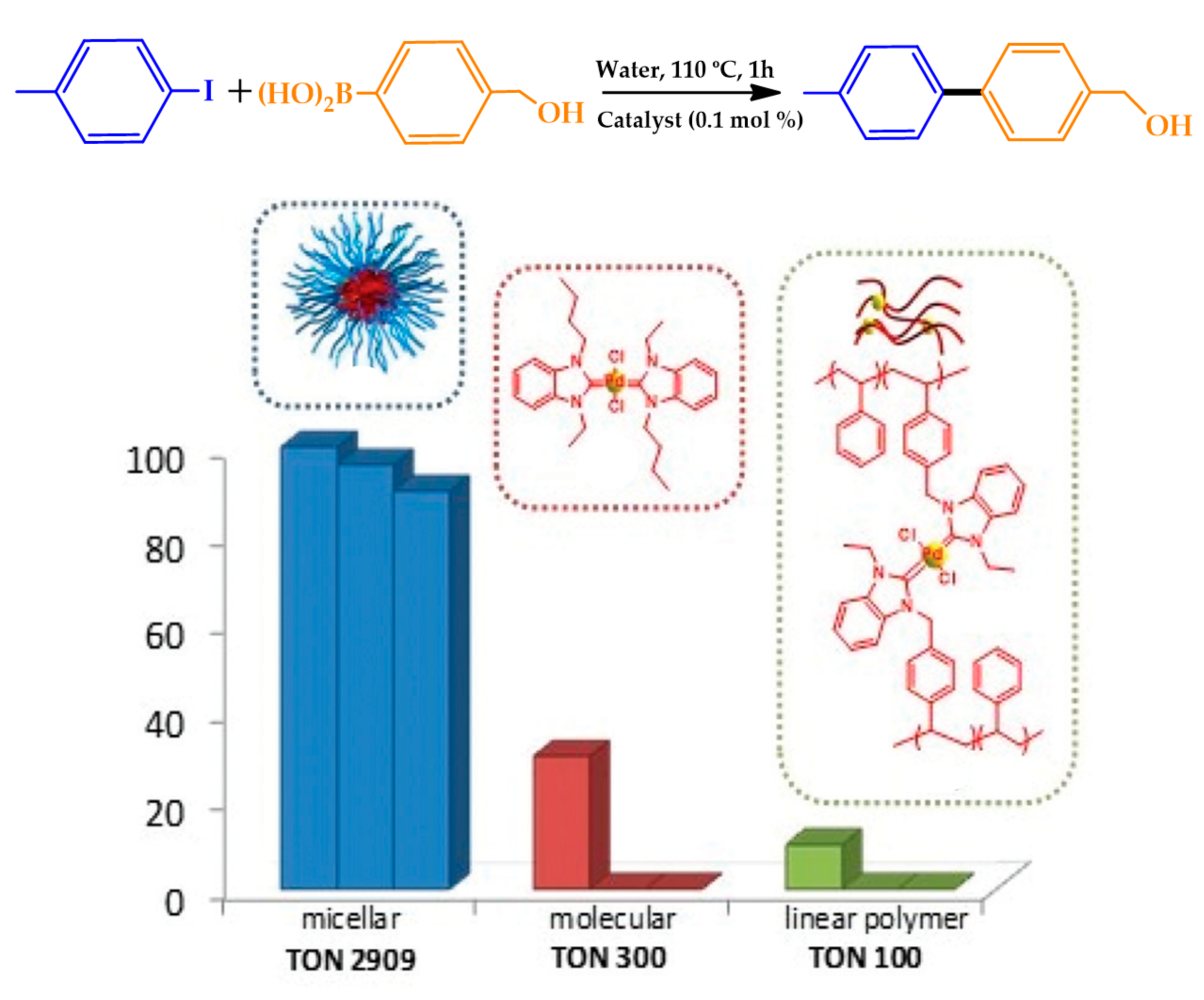



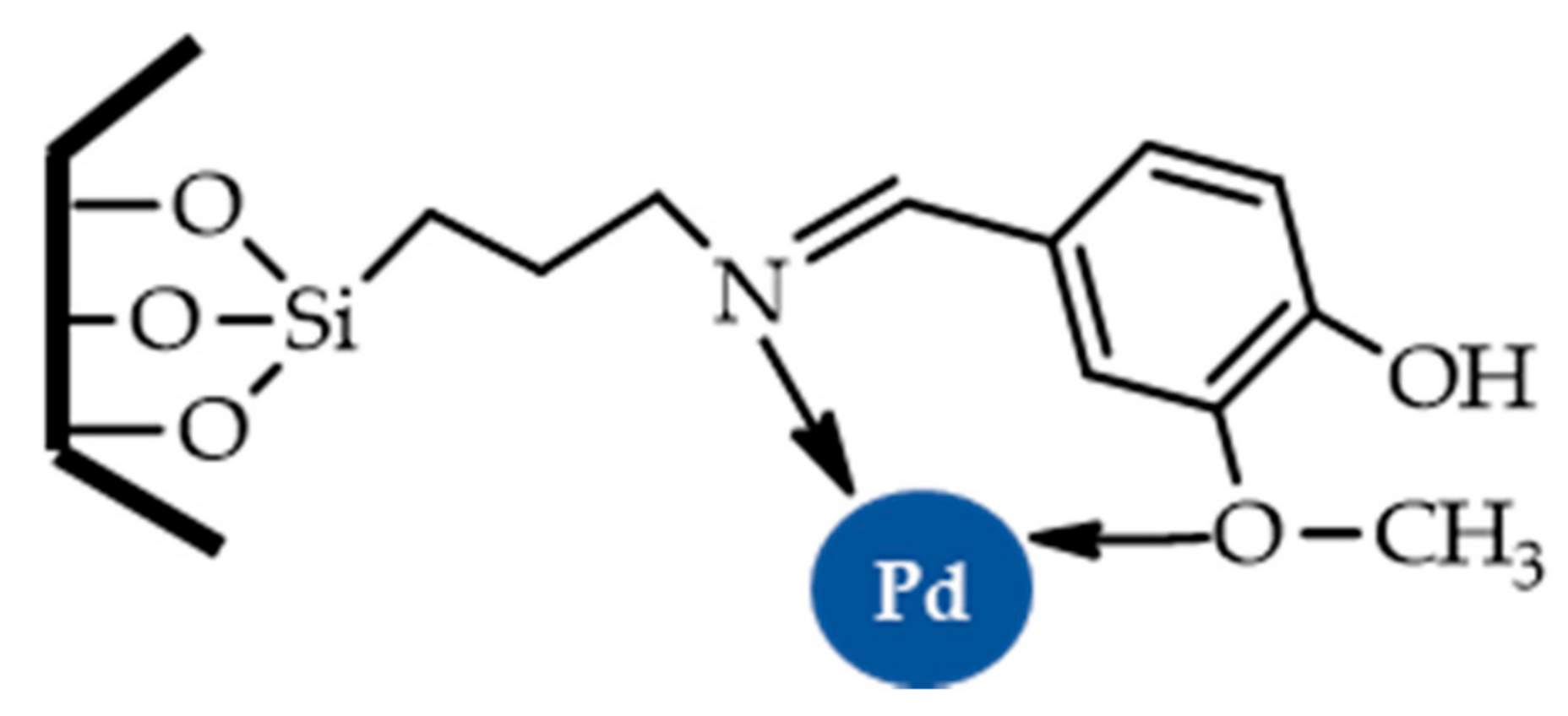

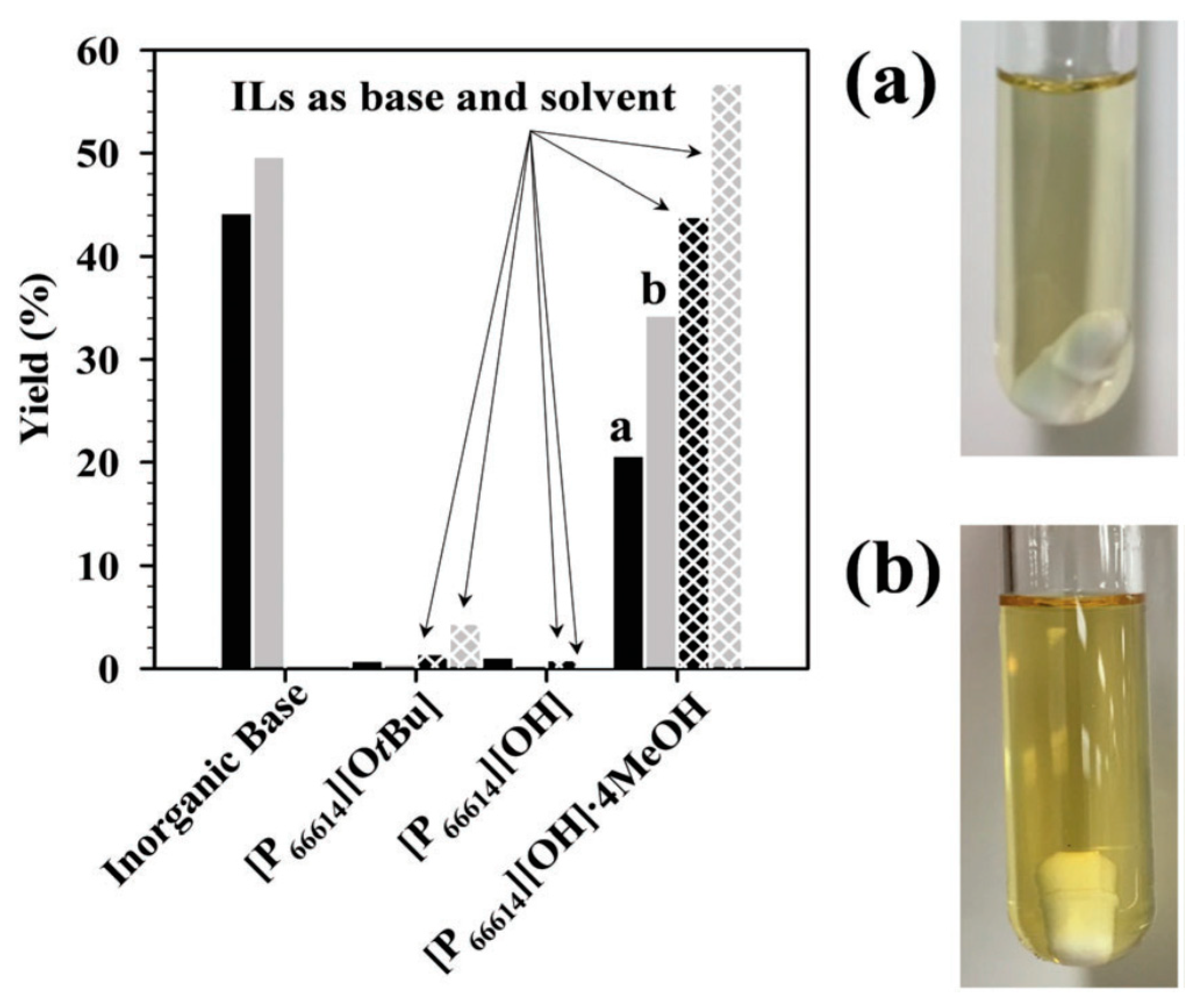

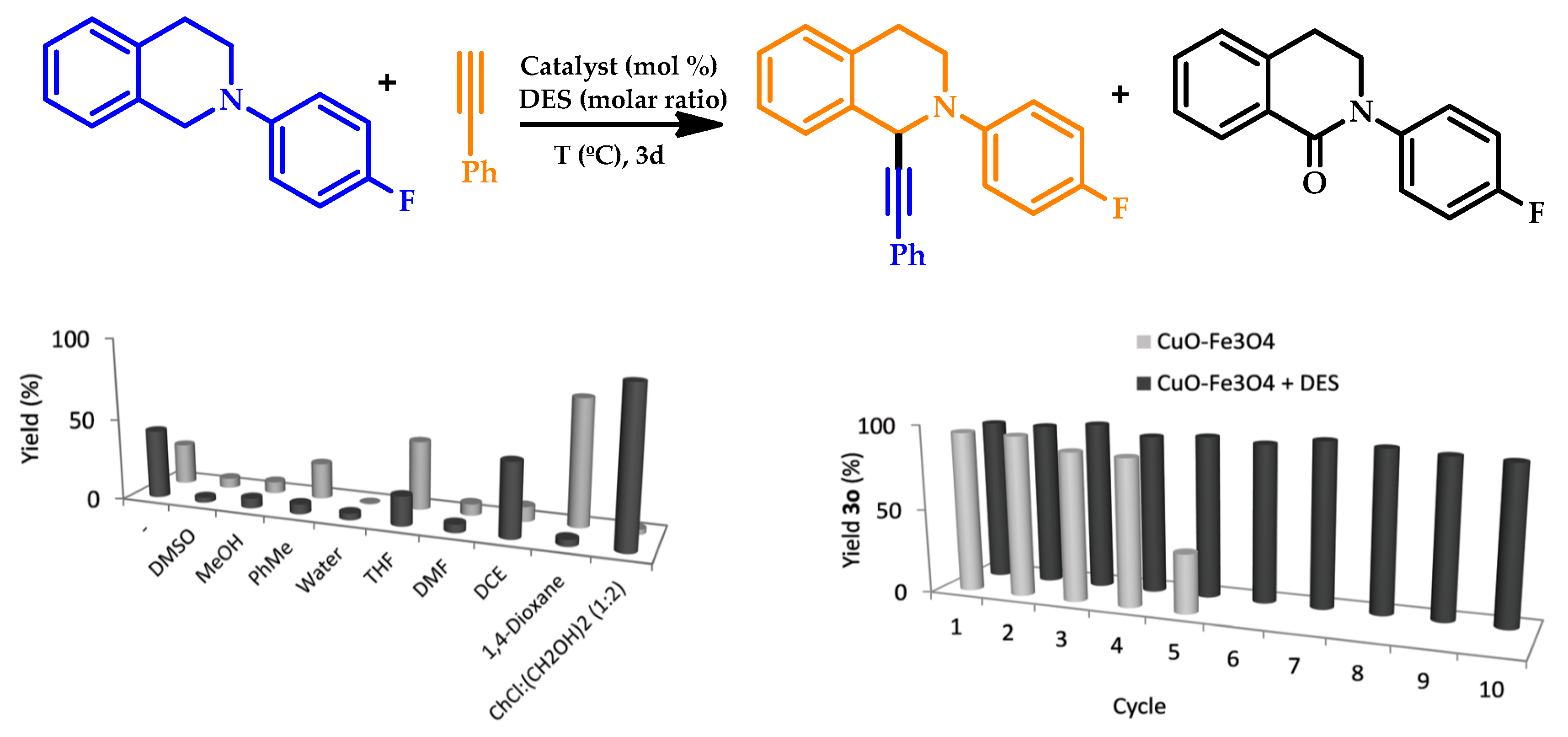

 | |||
|---|---|---|---|
| Ar-X | Conversion (%) | TON | TOF (h−1) |
| 4-bromoacetophenone | >95 | 6250 | 37,500 |
| 4-iodoanisole | 85 | 5310 | 31,870 |
| 4-bromobenzaldheyde | 94 | 5880 | 35,250 |
| 4-bromoanisole | 85 | 5310 | 31,880 |
| 3-bromobenzaldheyde | 82 | 5130 | 30,750 |
| 4-iodoacetophenone | 94 | 5880 | 35,250 |
| 2-iodotoluene | 73 | 4560 | 27,380 |
 | ||
|---|---|---|
| Power (W) | Time (min) | Yield (%) |
| Silent | 120 | - |
| 35 | 60 | 81 |
| 40 | 50 | 87 |
| 45 | 35 | 93 |
| 50 | 25 | 98 |
| 55 | 25 | 98 |
 | ||
|---|---|---|
| Power (W) | Time (min) | Yield (%) |
| 25 | 30 | 61 |
| 30 | 26 | 73 |
| 35 | 21 | 85 |
| 40 | 15 | 91 |
| 45 | 8 | 99 |
| 50 | 8 | 99 |
 | |||
|---|---|---|---|
| Frequency (Hz) | Number of Balls | Ball Size` (mm) | Yield (%) |
| 10 | 1 | 5 | 70 |
| 15 | 1 | 5 | 75 |
| 20 | 1 | 5 | 70 |
| 25 | 1 | 5 | 97 |
| 30 | 1 | 5 | 99 |
| 25 | 1 | 3 | 90 |
| 25 | 2 | 5 | 97 |
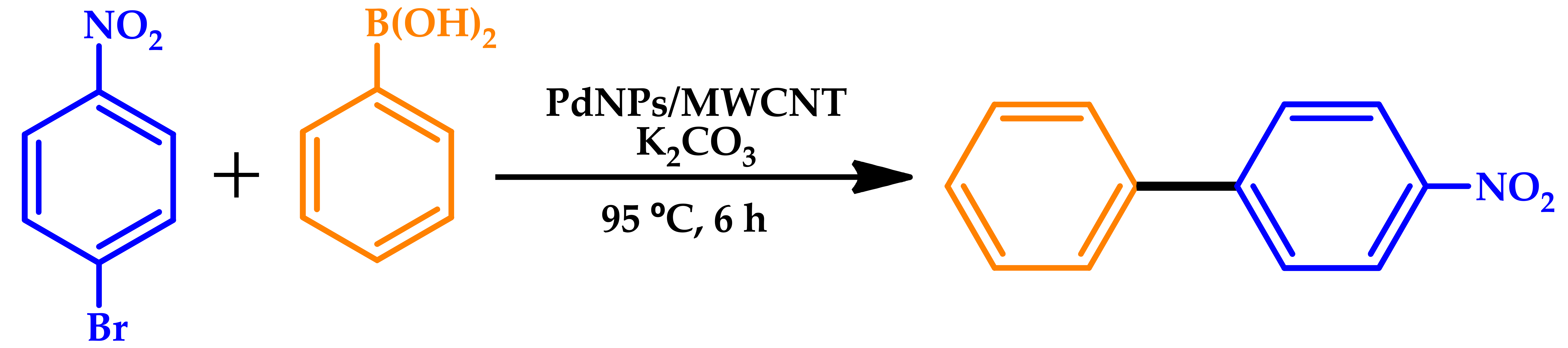 | |||||||
|---|---|---|---|---|---|---|---|
| Conversion% | Cycle | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 0.1 mol% of the catalyst | 95 | 75 | 94 | 84 | 100 | 98 | 100 |
| 0.5 mol% of the catalyst | 79 | 80 | 88 | 86 | 96 | 97 | 93 |
 | ||
|---|---|---|
| Catalyst | Time | Yield (%) |
| AC-Pd1 | 10 | 27 |
| 30 | 30 | |
| AC-Pd2 | 10 | 41 |
| 30 | 49 | |
| 180 | 69 | |
| CNT-Pd1 | 10 | 37 |
| 30 | 29 | |
| CNT-Pd2 | 10 | 32 |
| 30 | 50 | |
| Pd(OAc)2 | 30 | 75 |
 | |||
|---|---|---|---|
| R | X | ||
| I | Br | Cl | |
| 4-OCH3 | 98 | 88 | 56 |
| 3-NO2 | 87 | 85 | 70 |
| 3-CH3 | 62 | 59 | 33 |
| 4-CH3 | 71 | 69 | 42 |
 | ||
|---|---|---|
| R | X | Yield (%) |
| H | I | 98 |
| Me | I | 93 |
| MeO | I | 99 |
| NO2 | I | 99 |
| CN | I | 97 |
| Me | Br | 70 |
| MeO | Br | 99 |
| NO2 | Br | 92 |
| CN | Br | 98 |
| Me | Cl | 23 |
| MeO | Cl | 17 |
| CN | Cl | 16 |
 | |||||
|---|---|---|---|---|---|
| Pd Loading (ppm) | TBAOH (equiv.) | t (h) | Yield (%) | TON | TOF (h−1) |
| 30 | 2 | 2 | >99 | 33,000 | 16,500 |
| 30 | 1 | 2 | 90 | 30,000 | 15,000 |
| 30 | 4 | 6 | 75 | 25,000 | 4100 |
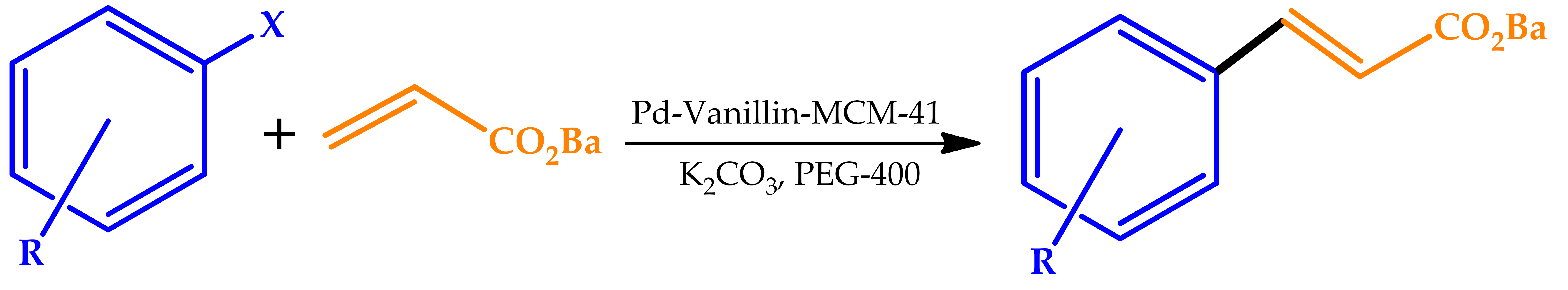 | |||
|---|---|---|---|
| Solvent | Base (eq.) | Catalyst (mol%) | Yield (%) |
| PEG-400 | K2CO3 | 0.53 | 72 |
| PEG-400 | K2CO3 | 0.71 | 91 |
| PEG-400 | K2CO3 | 0.88 | 91 |
| EtOH | K2CO3 | 0.71 | - |
| H2O | K2CO3 | 0.71 | 75 |
| DMF | K2CO3 | 0.71 | 83 |
| PEG-400 | Et3N | 0.71 | 90 |
| PEG-400 | NaHCO3 | 0.71 | 75 |
| PEG-400 | NaHCO3 | 0.71 | 88 |
| PEG-400 | NaHCO3 | 0.71 | 35 |
 | |||
|---|---|---|---|
| R | Yield (%) | TON | TOF (h−1) |
 | 98 | 481 | 1424 |
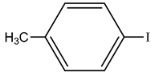 | 96 | 471 | 628 |
 | 83 | 407 | 444 |
 | 95 | 466 | 1398 |
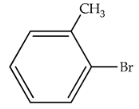 | 80 | 392 | 336 |
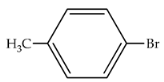 | 83 | 407 | 543 |
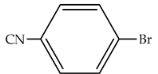 | 97 | 476 | 1903 |
 | |||
|---|---|---|---|
| IL | Loading (mol%) | Time (h) | Isolated Yield (%) |
| [BMIM][Cl] | 20 | 2 | 65 |
| [BMIM][BF4] | 20 | 1 | 76 |
| [BMIM][Pro] | 20 | 1 | 80 |
| [HEMPy][Cl] | 20 | 2 | 80 |
| [HEMPy][BF4] | 20 | 1 | 84 |
| [HEMPy][Pro] | 20 | 1 | 98 |
| 1-proline | 20 | 2 | 50 |
| -------- | 20 | 2 | 40 |
| [HEMPy][Pro] | 10 | 1 | 84 |
| [HEMPy][Pro] | 15 | 1 | 90 |
| [HEMPy][Pro] | 25 | 1 | 98 |
| [HEMPy][Pro] | 30 | 1 | 98 |
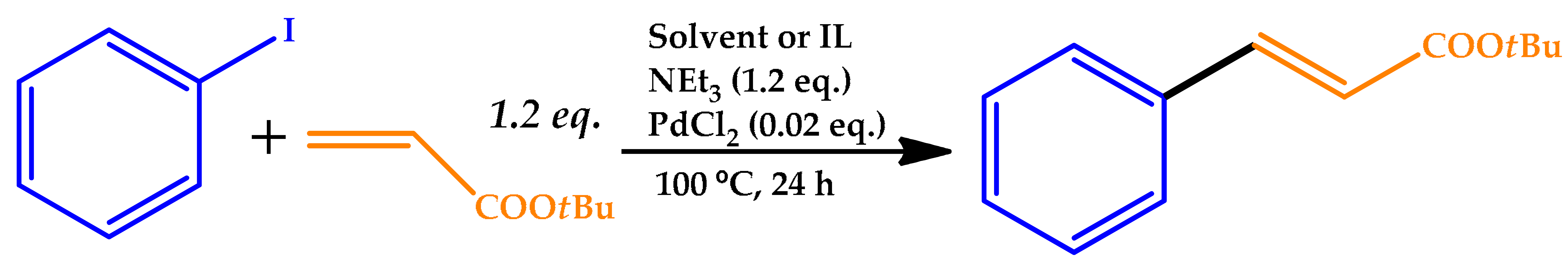 | |||
|---|---|---|---|
| Solvent or ILs | Cycle | Conversion (%) | Selectivity (%) |
| toluene | 1 | 1 | 100 |
| water | 1 | 30 | 100 |
| DMSO | 1 | 76 | 80 |
| TBP Br | 1 | 47 | 100 |
| TBA Cl | 1 | 99 | 100 |
| 2 | 100 | 100 | |
| 3 | 61 | 100 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, M.A.; Martins, L.M.D.R.S. New Trends in C–C Cross-Coupling Reactions: The Use of Unconventional Conditions. Molecules 2020, 25, 5506. https://doi.org/10.3390/molecules25235506
Andrade MA, Martins LMDRS. New Trends in C–C Cross-Coupling Reactions: The Use of Unconventional Conditions. Molecules. 2020; 25(23):5506. https://doi.org/10.3390/molecules25235506
Chicago/Turabian StyleAndrade, Marta A., and Luísa M. D. R. S. Martins. 2020. "New Trends in C–C Cross-Coupling Reactions: The Use of Unconventional Conditions" Molecules 25, no. 23: 5506. https://doi.org/10.3390/molecules25235506
APA StyleAndrade, M. A., & Martins, L. M. D. R. S. (2020). New Trends in C–C Cross-Coupling Reactions: The Use of Unconventional Conditions. Molecules, 25(23), 5506. https://doi.org/10.3390/molecules25235506







