Src Mediates Epigallocatechin-3-O-Gallate-Elicited Acid Sphingomyelinase Activation
Abstract
1. Introduction
2. Results
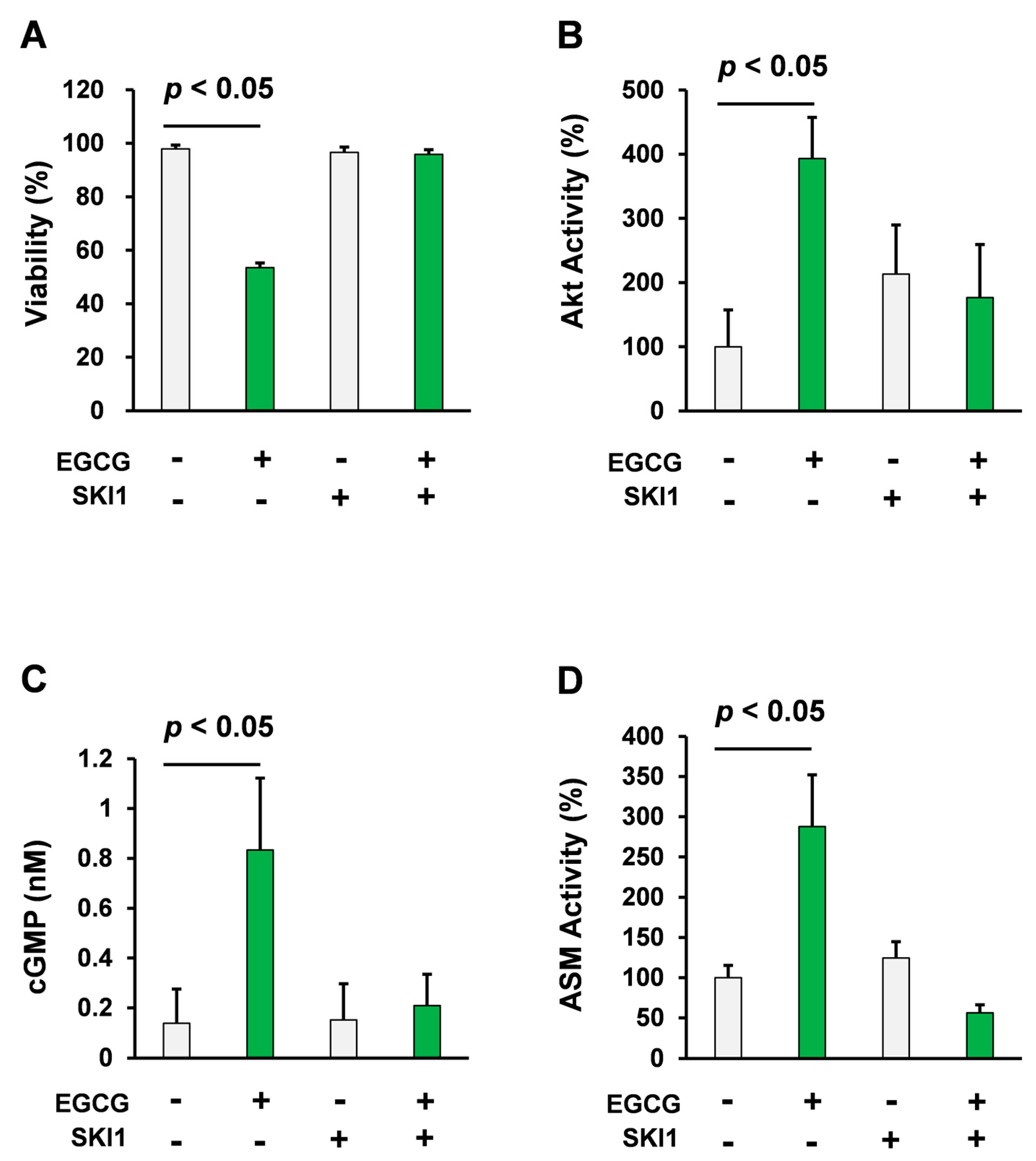
2.1. Pharmacological Inhibition of Src Attenuated Cell Death Inducing Effect of EGCG
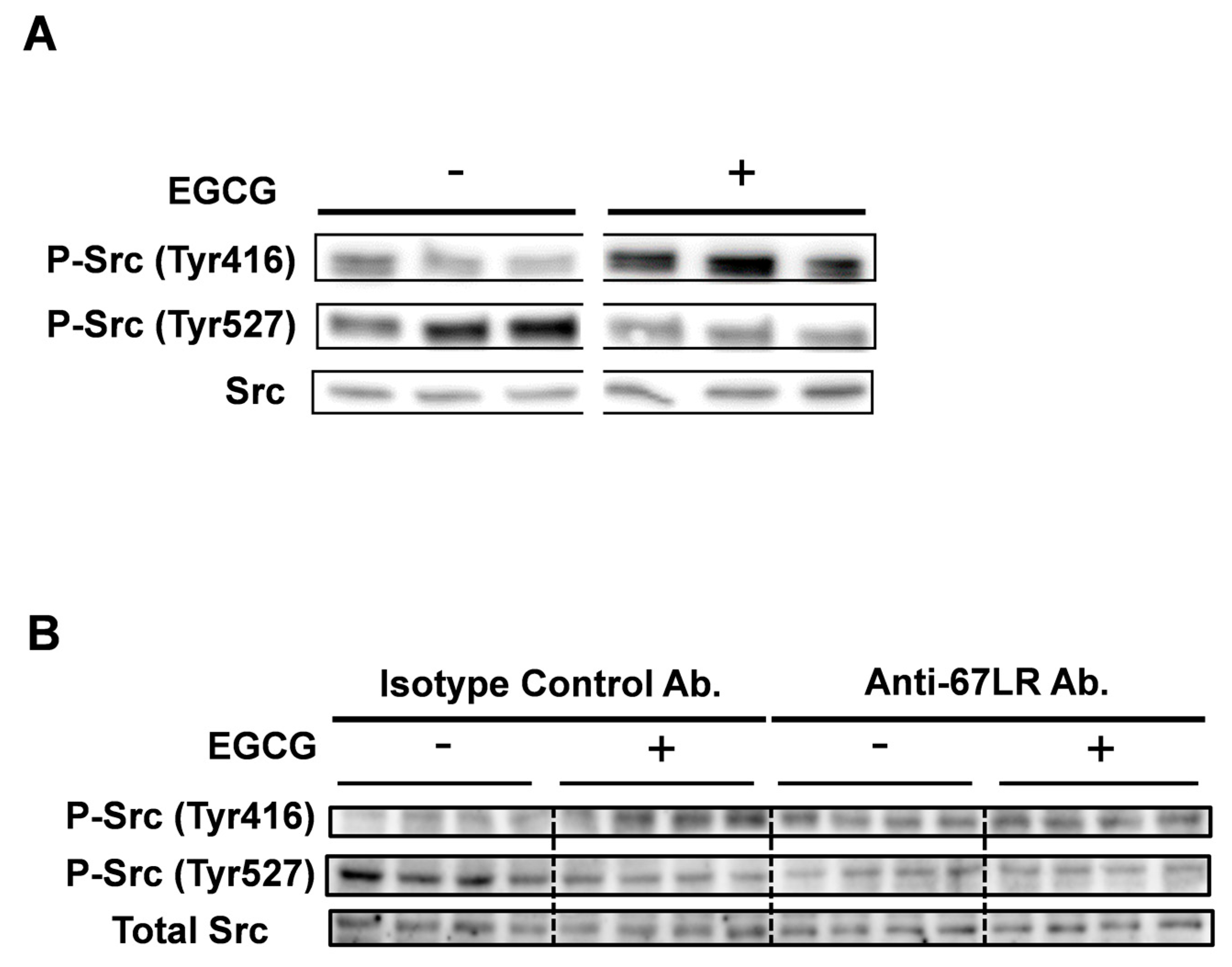
2.2. EGCG Induced Src Phosphorylation of Tyr 416 in Multiple Myeloma Cells
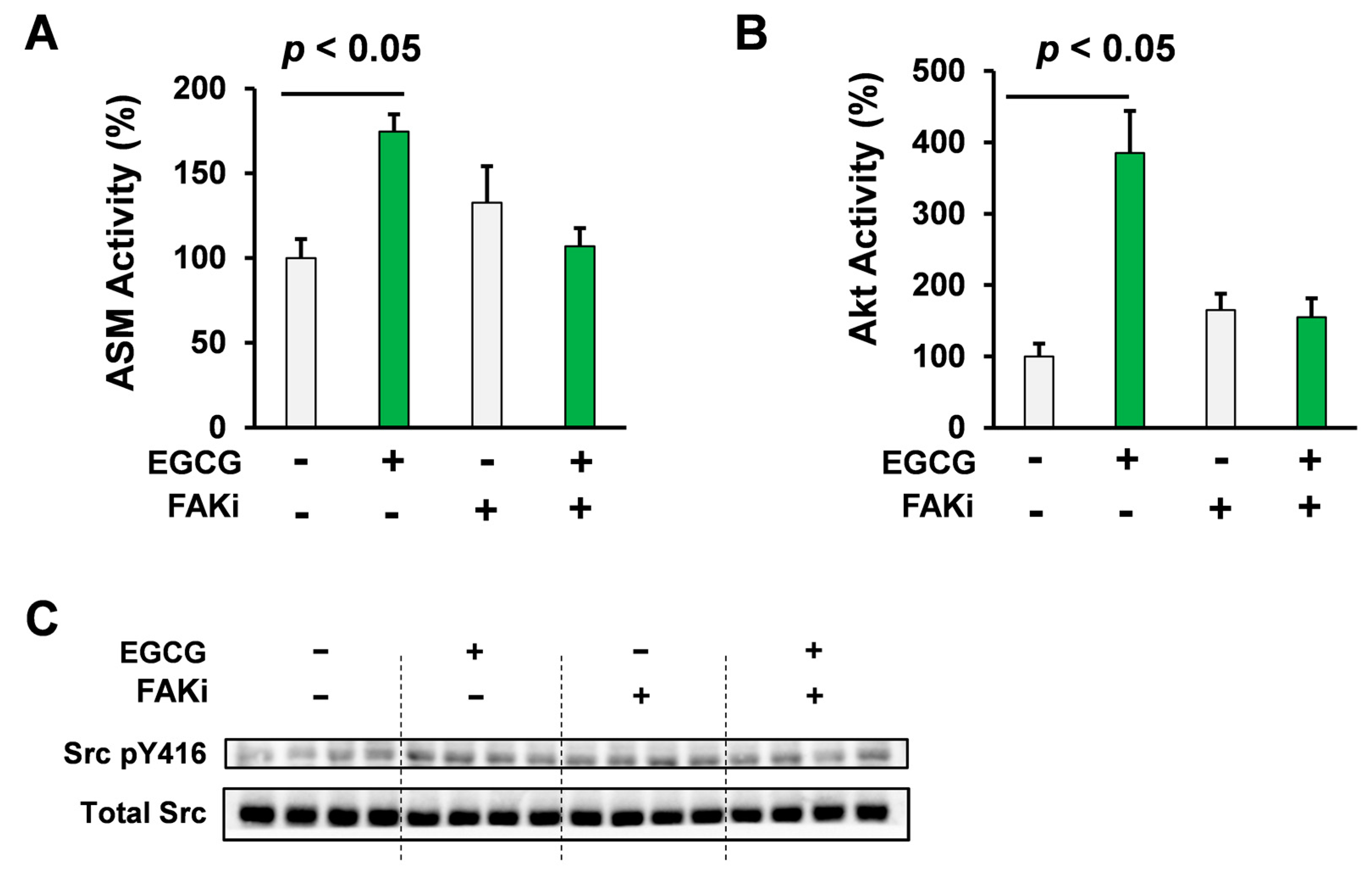
2.3. Pharmacological Inhibition of FAK Attenuated the Akt/ASM Axis Elicited by EGCG
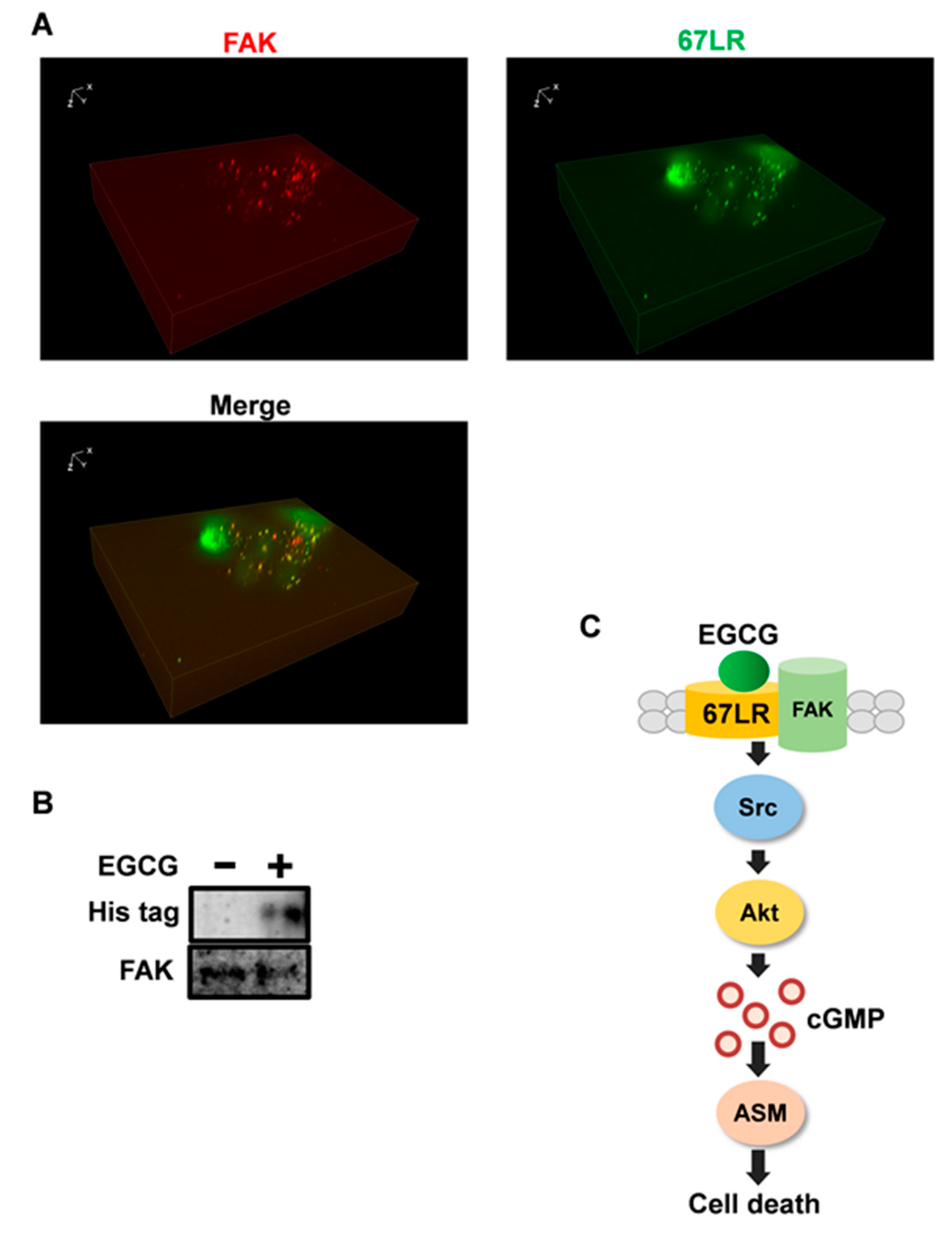
2.4. FAK Is the Adaptor Protein of 67LR
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Evaluation of Akt Activity
4.2. ASM Activity Evaluation and Western Blotting
4.3. Immunoprecipitation and Immunofluorescence Staining
4.4. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Röllig, C.; Knop, S.; Bornhäuser, M. Multiple myeloma. Lancet 2015, 30, 2197–2208. [Google Scholar] [CrossRef]
- Naganuma, T.; Kuriyama, S.; Kakizaki, M.; Sone, T.; Nakaya, N.; Ohmori-Matsuda, K.; Hozawa, A.; Nishino, Y.; Tsuji, I. Green Tea Consumption and Hematologic Malignancies in Japan: The Ohsaki Study. Am. J. Epidemiology 2009, 170, 730–738. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takada, M.; Yamagishi, K.; Iso, H.; Tamakoshi, A. Green tea consumption and risk of hematologic neoplasms: The Japan Collaborative Cohort Study for Evaluation of Cancer Risk (JACC Study). Cancer Causes Control. 2019, 30, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sun, Z.; Han, C.; Chen, J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc. Soc. Exp. Biol. Med. 1999, 220, 218–224. [Google Scholar] [PubMed]
- Shimizu, M.; Fukutomi, Y.; Ninomiya, M.; Nagura, K.; Kato, T.; Araki, H.; Suganuma, M.; Fujiki, H.; Moriwaki, H. Green Tea Extracts for the Prevention of Metachronous Colorectal Adenomas: A Pilot Study. Cancer Epidemiology Biomarkers Prev. 2008, 17, 3020–3025. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of Human Prostate Cancer by Oral Administration of Green Tea Catechins in Volunteers with High-Grade Prostate Intraepithelial Neoplasia: A Preliminary Report from a One-Year Proof-of-Principle Study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef]
- Shanafelt, T.D.; Call, T.G.; Zent, C.S.; Leis, J.F.; LaPlant, B.; Np, D.A.B.; Roos, M.; Laumann, K.; Ghosh, A.K.; Lesnick, C.; et al. Phase 2 trial of daily, oral polyphenon E in patients with asymptomatic, Rai stage 0 to II chronic lymphocytic leukemia. Cancer 2013, 119, 363–370. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Katiyar, S.K. Emerging Phytochemicals for the Prevention and Treatment of Head and Neck Cancer. Molecules 2016, 21, 1610. [Google Scholar] [CrossRef]
- Miyoshi, N.; Tanabe, H.; Suzuki, T.; Saeki, K.; Hara, Y. Applications of a Standardized Green Tea Catechin Preparation for Viral Warts and Human Papilloma Virus-Related and Unrelated Cancers. Molecules 2020, 25, 2588. [Google Scholar] [CrossRef] [PubMed]
- Minnelli, C.; Laudadio, E.; Mobbili, G.; Galeazzi, R. Conformational Insight on WT- and Mutated-EGFR Receptor Activation and Inhibition by Epigallocatechin-3-Gallate: Over a Rational Basis for the Design of Selective Non-Small-Cell Lung Anticancer Agents. Int. J. Mol. Sci. 2020, 21, 1721. [Google Scholar] [CrossRef]
- Hayakawa, S.; Saito, K.; Miyoshi, N.; Ohishi, T.; Oishi, Y.; Miyoshi, M.; Nakamura, Y. Anti-Cancer Effects of Green Tea by Either Anti- or Pro-Oxidative Mechanisms. Asian Pac. J. Cancer Prev. 2016, 17, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.; Gao, Z.; Li, X.; Yan, X.; Xiong, Y.; Wang, S. 67-kDa Laminin Receptor in Human Bile Duct Carcinoma. Eur. Surg. Res. 2009, 42, 168–173. [Google Scholar] [CrossRef]
- Sanjuan, X.; Fernandez, P.L.; Miquel, R.; Munoz, J.; Castronovo, V.; Menard, S.; Palacin, A.; Cardesa, A.; Campo, E. Overexpression of the 67-kD laminin receptor correlates with tumor progression in human colorectal carcinoma. J. Pathol. 1996, 179, 376–380. [Google Scholar] [CrossRef]
- Al-Saleh, W.; Delvenne, P.; van den Brule, F.A.; Menard, S.; Boniver, J.; Castronovo, V. Expression of the 67 KD laminin receptor in human cervical preneoplastic and neoplastic squamous epithelial lesions: An immunohistochemical study. J. Pathol. 1997, 181, 287–293. [Google Scholar] [CrossRef]
- Viacava, P.; Naccarato, A.G.; Collecchi, P.; Ménard, S.; Castronovo, V.; Bevilacqua, G. The Spectrum of 67-Kd Laminin Receptor Expression in Breast Carcinoma Progression. J. Pathol. 1997, 182, 36–44. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Huang, Y.; Umeda, D.; Yamada, S.; Yamashita, S.; Kumazoe, M.; Kim, Y.; Murata, M.; Yamada, K.; Tachibana, H. 67-kDa Laminin Receptor-dependent Protein Phosphatase 2A (PP2A) Activation Elicits Melanoma-specific Antitumor Activity Overcoming Drug Resistance. J. Biol. Chem. 2014, 289, 32671–32681. [Google Scholar] [CrossRef]
- Shammas, M.A.; Neri, P.; Koley, H.; Batchu, R.B.; Bertheau, R.C.; Munshi, V.; Prabhala, R.; Fulciniti, M.; Tai, Y.T.; Treon, S.P.; et al. Specific killing of multiple myeloma cells by (-)-epigallocatechin-3-gallate extracted from green tea: Biologic activity and therapeutic implications. Blood 2006, 108, 2804–2810. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Hirotsu, K.; Kumazoe, M.; Goto, Y.; Sugihara, K.; Suda, T.; Tsurudome, Y.; Suzuki, T.; Yamashita, S.; Kim, Y.; et al. Green tea polyphenol EGCG induces lipid-raft clustering and apoptotic cell death by activating protein kinase Cδ and acid sphingomyelinase through a 67 kDa laminin receptor in multiple myeloma cells. Biochem. J. 2012, 443, 525–534. [Google Scholar] [CrossRef]
- Britschgi, A.; Simon, H.-U.; Tobler, A.; Fey, M.F.; Tschan, M.P. Epigallocatechin-3-gallate induces cell death in acute myeloid leukaemia cells and supports all-transretinoic acid-induced neutrophil differentiation via death-associated protein kinase 2. Br. J. Haematol. 2010, 149, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Tsukamoto, S.; Lesnick, C.; Kay, N.E.; Yamada, K.; Shanafelt, T.D.; Tachibana, H. Vardenafil, a clinically available phosphodiesterase inhibitor, potentiates the killing effect of EGCG on CLL cells. Br. J. Haematol. 2014, 168, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kumazoe, M.; Bae, J.; Yamada, S.; Takai, M.; Hidaka, S.; Yamashita, S.; Kim, Y.; Won, Y.; Murata, M.; et al. Green tea polyphenol epigallocatechin-O-gallate induces cell death by acid sphingomyelinase activation in chronic myeloid leukemia cells. Oncol. Rep. 2015, 34, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Sugihara, K.; Tsukamoto, S.; Huang, Y.; Tsurudome, Y.; Suzuki, T.; Suemasu, Y.; Ueda, N.; Yamashita, S.; Kim, Y.; et al. 67-kDa laminin receptor increases cGMP to induce cancer-selective apoptosis. J. Clin. Investig. 2013, 123, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Kim, Y.; Bae, J.; Takai, M.; Murata, M.; Suemasu, Y.; Sugihara, K.; Yamashita, S.; Tsukamoto, S.; Huang, Y.; et al. Phosphodiesterase 5 inhibitor acts as a potent agent sensitizing acute myeloid leukemia cells to 67-kDa laminin receptor-dependent apoptosis. FEBS Lett. 2013, 587, 3052–3057. [Google Scholar] [CrossRef]
- Kumazoe, M.; Hiroi, S.; Tanimoto, Y.; Miyakawa, J.; Yamanouchi, M.; Suemasu, Y.; Yoshitomi, R.; Murata, M.; Fujimura, Y.; Takahashi, T.; et al. Cancer cell selective probe by mimicking EGCG. Biochem. Biophys. Res. Commun. 2020, 525, 974–981. [Google Scholar] [CrossRef]
- Bae, J.; Kumazoe, M.; Takeuchi, C.; Hidaka, S.; Fujimura, Y.; Tachibana, H. Epigallocatechin-3-O-gallate induces acid sphingomyelinase activation through activation of phospholipase C. Biochem. Biophys. Res. Commun. 2019, 520, 186–191. [Google Scholar] [CrossRef]
- An, E.J.; Kim, Y.; Lee, S.H.; Ko, H.M.; Chung, W.S.; Jang, H.J. Anti-Cancer Potential of Oxialis obtriangulata in Pancreatic Cancer Cell through Regulation of the ERK/Src/STAT3-Mediated Pathway. Molecules 2020, 25, 2301. [Google Scholar] [CrossRef]
- Chhikara, B.S.; Ashraf, S.; Mozaffari, S.; Jeans, N.S.; Mandal, D.; Tiwari, R.K.; Ul-Haq, Z.; Parang, K. Phenylpyrazalopyrimidines as Tyrosine Kinase Inhibitors: Synthesis, Antiproliferative Activity, and Molecular Simulations. Molecules 2020, 25, 2135. [Google Scholar] [CrossRef]
- Murata, M.; Shimizu, Y.; Marugame, Y.; Nezu, A.; Fujino, K.; Yamada, S.; Kumazoe, M.; Fujimura, Y.; Tachibana, H. EGCG down-regulates MuRF1 expression through 67-kDa laminin receptor and the receptor signaling is amplified by eriodictyol. J. Nat. Med. 2020, 74, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Fujimura, Y.; Hidaka, S.; Kim, Y.; Murayama, K.; Takai, M.; Huang, Y.; Yamashita, S.; Murata, M.; Miura, D.; et al. Metabolic Profiling-based Data-mining for an Effective Chemical Combination to Induce Apoptosis of Cancer Cells. Sci. Rep. 2015, 5, 9474. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Takai, M.; Hiroi, S.; Takeuchi, C.; Yamanouchi, M.; Nojiri, T.; Onda, H.; Bae, J.; Huang, Y.; Takamatsu, K.; et al. PDE3 inhibitor and EGCG combination treatment suppress cancer stem cell properties in pancreatic ductal adenocarcinoma. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Nakamura, Y.; Yamashita, M.; Suzuki, T.; Takamatsu, K.; Huang, Y.; Bae, J.; Yamashita, S.; Murata, M.; Yamada, S.; et al. Green Tea Polyphenol Epigallocatechin-3-gallate Suppresses Toll-like Receptor 4 Expression via Up-regulation of E3 Ubiquitin-protein Ligase RNF216. J. Biol. Chem. 2017, 292, 4077–4088. [Google Scholar] [CrossRef] [PubMed]
- Kumazoe, M.; Yamashita, M.; Nakamura, Y.; Takamatsu, K.; Bae, J.; Yamashita, S.; Yamada, S.; Onda, H.; Nojiri, T.; Kangawa, K.; et al. Green Tea Polyphenol EGCG Upregulates Tollip Expression by Suppressing Elf-1 Expression. J. Immunol. 2017, 199, 3261–3269. [Google Scholar] [CrossRef]
- Smart, J.E.; Oppermann, H.; Czernilofsky, A.P.; Purchio, A.F.; Erikson, R.L.; Bishop, J.M. Characterization of sites for tyrosine phosphorylation in the transforming protein of Rous sarcoma virus (pp60v-src) and its normal cellular homologue (pp60c-src). Proc. Natl. Acad. Sci. USA 1981, 78, 6013–6017. [Google Scholar] [CrossRef]
- Fujii, M.; Shalloway, D.; Verma, I.M. Gene regulation by tyrosine kinases: Src protein activates various promoters, including c-fos. Mol. Cell. Biol. 1989, 9, 2493–2499. [Google Scholar] [CrossRef]
- Guan, J.-L. Role of focal adhesion kinase in integrin signaling. Int. J. Biochem. Cell Biol. 1997, 29, 1085–1096. [Google Scholar] [CrossRef]
- Huveneers, S.; Danen, E.H.J. Adhesion signaling—Crosstalk between integrins, Src and Rho. J. Cell Sci. 2009, 122, 1059–1069. [Google Scholar] [CrossRef]
- Shen, Y.; Schaller, M.D. Focal Adhesion Targeting: The Critical Determinant of FAK Regulation and Substrate Phosphorylation. Mol. Biol. Cell. 2017, 10. [Google Scholar] [CrossRef]
- Hsia, D.A.; Mitra, S.K.; Hauck, C.R.; Streblow, D.N.; Nelson, J.A.; Ilic, D.; Huang, S.; Li, E.; Nemerow, G.R.; Leng, J.; et al. Differential regulation of cell motility and invasion by FAK. J. Cell Biol. 2003, 160, 753–767. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Gao, M.; Ma, Y.; Wang, L.; Shen, Y.; Liu, X. Inhibition of cell migration by focal adhesion kinase: Time-dependent difference in integrin-induced signaling between endothelial and hepatoblastoma cells. Int. J. Mol. Med. 2018, 41, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Gowda, M.; Sharad, Y.; A Singh, S.; Sesti, F. Oxidation of KCNB1 potassium channels triggers apoptotic integrin signaling in the brain. Cell Death Dis. 2017, 8, e2737. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.; Kumazoe, M.; Murata, K.; Fujimura, Y.; Tachibana, H. Procyanidin C1 Inhibits Melanoma Cell Growth by Activating 67-kDa Laminin Receptor Signaling. Mol. Nutr. Food. Res 2020, 64, e1900986. [Google Scholar] [CrossRef] [PubMed]
- Luk, C.T.; Shi, S.Y.; Cai, E.P.; Sivasubramaniyam, T.; Krishnamurthy, M.; Brunt, J.J.; Schroer, S.A.; Winer, D.A.; Woo, M. FAK signalling controls insulin sensitivity through regulation of adipocyte survival. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef]
- Nagao, T.; Meguro, S.; Hase, T.; Otsuka, K.; Komikado, M.; Tokimitsu, I.; Yamamoto, T.; Yamamoto, K. A Catechin-rich Beverage Improves Obesity and Blood Glucose Control in Patients with Type 2 Diabetes. Obesity 2009, 17, 310–317. [Google Scholar] [CrossRef]
- Shirai, Y.; Kuriki, K.; Otsuka, R.; Kato, Y.; Nishita, Y.; Tange, C.; Tomida, M.; Imai, T.; Ando, F.; Shimokata, H. Association between green tea intake and risk of cognitive decline, considering glycated hemoglobin level, in older Japanese adults: The NILS-LSA study. Nagoya J. Med. Sci. 2019, 81, 655–666. [Google Scholar]
- Suzuki, T.; Kumazoe, M.; Kim, Y.; Yamashita, S.; Nakahara, K.; Tsukamoto, S.; Sasaki, M.; Hagihara, T.; Tsurudome, Y.; Huang, Y.; et al. Green Tea Extract Containing a Highly Absorbent Catechin Prevents Diet-Induced Lipid Metabolism Disorder. Sci. Rep. 2013, 3, 2749. [Google Scholar] [CrossRef]
- Scheiman, J.; Tseng, J.C.; Zheng, Y.; Meruelo, D. Multiple functions of the 37/67-kd laminin receptor make it a suitable target for novel cancer gene therapy. Mol. Ther. 2009, 18, 63–74. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kumazoe, M.; Kadomatsu, M.; Bae, J.; Otsuka, Y.; Fujimura, Y.; Tachibana, H. Src Mediates Epigallocatechin-3-O-Gallate-Elicited Acid Sphingomyelinase Activation. Molecules 2020, 25, 5481. https://doi.org/10.3390/molecules25225481
Kumazoe M, Kadomatsu M, Bae J, Otsuka Y, Fujimura Y, Tachibana H. Src Mediates Epigallocatechin-3-O-Gallate-Elicited Acid Sphingomyelinase Activation. Molecules. 2020; 25(22):5481. https://doi.org/10.3390/molecules25225481
Chicago/Turabian StyleKumazoe, Motofumi, Mai Kadomatsu, Jaehoon Bae, Yushi Otsuka, Yoshinori Fujimura, and Hirofumi Tachibana. 2020. "Src Mediates Epigallocatechin-3-O-Gallate-Elicited Acid Sphingomyelinase Activation" Molecules 25, no. 22: 5481. https://doi.org/10.3390/molecules25225481
APA StyleKumazoe, M., Kadomatsu, M., Bae, J., Otsuka, Y., Fujimura, Y., & Tachibana, H. (2020). Src Mediates Epigallocatechin-3-O-Gallate-Elicited Acid Sphingomyelinase Activation. Molecules, 25(22), 5481. https://doi.org/10.3390/molecules25225481





