Advances in Nanodelivery of Green Tea Catechins to Enhance the Anticancer Activity
Abstract
1. Introduction
2. Phenolics in Green Teas
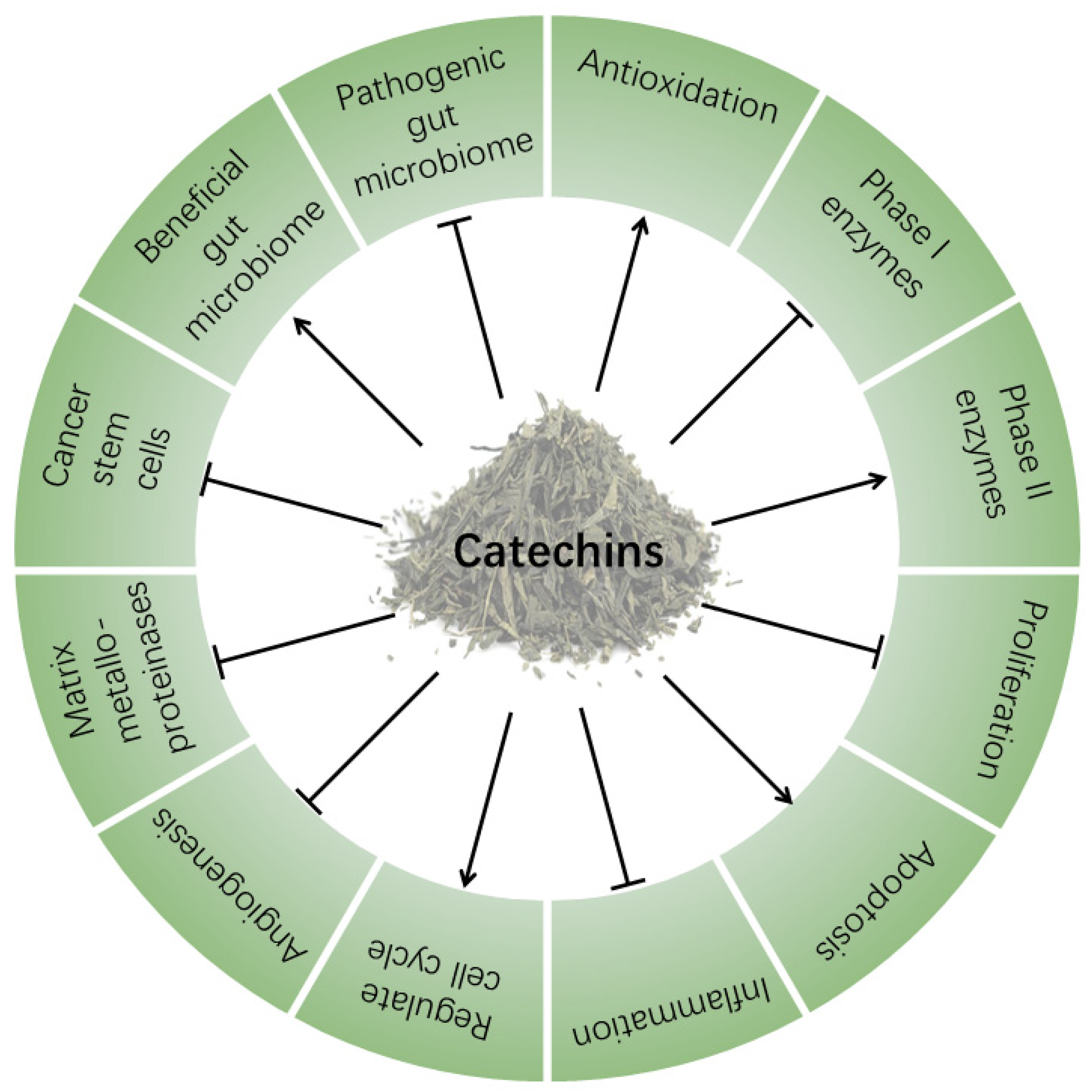
3. Anticancer Activity of Green Tea Catechins
3.1. Antioxidation
3.2. Regulation of Drug Metabolizing Enzymes
3.3. Inhibition of Proliferation
3.4. Induction of Apoptosis
3.5. Antiinflammation
3.6. Regulation of Cell Cycle
3.7. Inhibition of Cancer Metastasis
3.8. Inhibition of Cancer Stem Cells
3.9. Regulation of Gut Microbiota
4. Oral Bioavailability of Catechins
5. Nanodelivery of Green Tea Catechins
5.1. Nanocarriers for Encapsulation and Delivery of Green Tea Catechins
5.1.1. Polymer-Based NPs
5.1.2. Lipid-Based NPs
5.2. Encapsulation Methods
5.3. Delivery Strategies
5.3.1. Non-Invasive Delivery Strategies
Oral Delivery
Topical and Transdermal Delivery
5.3.2. Invasive Delivery Strategies
Intravenous Delivery
Other Delivery Routes
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Full Name | |
| 5-FU | 5-Fluorouracil |
| AP | Apical |
| AP-1 | Activator protein-1 |
| ARE | Antioxidant response element |
| AUC | Area under the curve |
| BSA | Bovine serum albumin |
| BL | Basolateral |
| C | (+)-catechin |
| CDKs | Cyclins-dependent kinases |
| CG | Catechin gallate |
| Cmax | Maximum plasma concentration |
| COMT | Catechol-O-methyltransferase |
| CYP450 | Cytochrome P450 |
| CYP1A1 | CYP450 1A1 |
| CS | Chitosan |
| CSCs | Cancer stem cells |
| DMBA | 7,12-Dimethylbenz [α]anthouracene |
| EC | (−)-Epicatechin |
| ECDG | Epicatechin digallates |
| ECG | (−)-Epicatechin gallate |
| EGC | (−)-Epigallocatechin |
| EGCDG | Epigallocatechin digallates |
| EGCG | (−)-Epigallocatechin gallate |
| EMT | Epithelial-mesenchymal transition |
| FD | Fucoidan |
| GC | Gallocatechin |
| GCG | Gallocatechin gallate |
| GI | Gastrointestinal |
| HA | Hyaluronic acid |
| HDL | High-density lipoprotein |
| IL | Interleukin |
| i.p. | Intraperitoneal |
| i.t. | Intratumoral |
| i.v. | Intravenous |
| Keap1 | Kelch-like ECH-associated protein 1 |
| LDL | Low-density lipoprotein |
| LOX | Lipoxygenases |
| MMP | Matrix metalloproteinase |
| M.W. | Molecular weight |
| NF-E2 | Nuclear factor-erythroid 2p45 |
| NF-κB | Nuclear factor-κB |
| NPs | Nanoparticles |
| Nrf2 | NF-E2-related factor 2 |
| PAA | Polyaspartic acid |
| Papp | Apparent permeability coefficients |
| PARP | Poly (ADP-ribose) polymerase |
| PCNA | Proliferating cell nuclear antigen |
| pDNA | Plasmid DNA |
| PDX | Patient-derived xenograft |
| PEG | Polyethylene glycol |
| PEI | Polyethylenimine |
| PEVs | Penetration enhancer-containing vesicles |
| γ-PGA | Poly(γ-glutamic acid) |
| PI3K | Phosphoinositide-3-kinase |
| PLGA | Poly(lactic-co-glycolic acid) |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SLNs | Solid lipid nanoparticles |
| TEs | Transethosomes |
| TNF | Tumor necrosis factor |
| TPGS | D-α-tocopheryl polyethylene glycol 1000 succinate |
| TPP | Tripolyphosphate |
| VEGF | Vascular endothelial growth factor |
| VEGFR | Vascular endothelial growth factor receptor |
References
- Ho, C.T.; Lin, J.K.; Shahidi, F. Tea and Tea Products: Chemistry and Health-Promoting Properties; CRC: Boca Raton, FL, USA, 2008; Volume 8. [Google Scholar]
- Sang, S.; Lambert, J.D.; Ho, C.-T.; Yang, C.S. The chemistry and biotransformation of tea constituents. Pharmacol. Res. 2011, 64, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, S.; Zhou, H.; Hanson, T.; Yang, L.; Chen, Z.; Zhou, M. Association of green tea consumption with mortality from all-cause, cardiovascular disease and cancer in a Chinese cohort of 165,000 adult men. Eur. J. Epidemiol. 2016, 31, 853–865. [Google Scholar] [CrossRef] [PubMed]
- Abe, S.K.; Inoue, M. Green tea and cancer and cardiometabolic diseases: A review of the current epidemiological evidence. Eur J. Clin. Nutr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Pan, M.-H.; Chiou, Y.-S.; Wang, Y.-J.; Ho, C.-T.; Lin, J.-K. Multistage carcinogenesis process as molecular targets in cancer chemoprevention by epicatechin-3-gallate. Food Funct. 2011, 2, 101–110. [Google Scholar] [CrossRef]
- Yang, C.S.; Hong, J. Prevention of Chronic Diseases by Tea: Possible Mechanisms and Human Relevance. Annu. Rev. Nutr. 2013, 33, 161–181. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Zhang, Y.; Wang, J.; Han, J.; Shao, Z.; Sun, Y.; Liang, J. Bioavailability enhancement of EGCG by structural modification and nano-delivery: A review. J. Funct. Foods 2020, 65, 103732. [Google Scholar] [CrossRef]
- Ting, Y.; Jiang, Y.; Ho, C.-T.; Huang, Q. Common delivery systems for enhancing in vivo bioavailability and biological efficacy of nutraceuticals. J. Funct. Foods 2014, 7, 112–128. [Google Scholar] [CrossRef]
- Balentine, D.A.; Wiseman, S.A.; Bouwens, L.C. The chemistry of tea flavonoids. Crit. Rev. Food Sci. Nutr. 1997, 37, 693–704. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef]
- Tsujimura, M. Isolation of a New Catechin, Tea Catechin II or Gallo-Catechin from Green Tea. Bull. Agric. Chem. Soc. Jpn. 1934, 10, 140–147. [Google Scholar] [CrossRef]
- Tsujimura, M. On Tea Tannin Isolated from Green Tea. Bull. Agric. Chem. Soc. Jpn. 1930, 6, 70–75. [Google Scholar] [CrossRef][Green Version]
- Perkin, A.G.; Yoshitake, E. CXV.—Constituents of acacia and gambier catechus. Part I. J. Chem. Soc. Trans. 1902, 81, 1160–1173. [Google Scholar] [CrossRef][Green Version]
- Bradfield, A.E.; Penney, M. 456. The catechins of green tea. Part II. J. Chem. Soc. 1948, 2249–2254. [Google Scholar] [CrossRef]
- Coxon, D.T.; Holmes, A.; Ollis, W.D.; Vora, V.C.; Grant, M.S.; Tee, J.L. Flavanol digallates in green tea leaf. Tetrahedron 1972, 28, 2819–2826. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, Z.; Han, Y.; Wang, J.; Wang, Y.; Chen, X.; Shao, Y.; Cheng, Y.; Zhou, W.; Lu, X.; et al. A review on anti-cancer effect of green tea catechins. J. Funct. Foods 2020, 74, 104172. [Google Scholar] [CrossRef]
- Ursini, F.; Maiorino, M.; Forman, H.J. Redox homeostasis: The Golden Mean of healthy living. Redox Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, X.; Cueto, R.; Effi, C.; Zhang, Y.; Tan, H.; Qin, X.; Ji, Y.; Yang, X.; Wang, H. Biochemical basis and metabolic interplay of redox regulation. Redox Biol. 2019, 26, 101284. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef]
- Procházková, D.; Boušová, I.; Wilhelmová, N. Antioxidant and prooxidant properties of flavonoids. Fitoterapia 2011, 82, 513–523. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting Multiple Signaling Pathways by Green Tea Polyphenol (−)-Epigallocatechin-3-Gallate. Cancer Res. 2006, 66, 2500–2505. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Structure−Activity Relationships of Flavonoids in the Cellular Antioxidant Activity Assay. J. Agric. Food Chem. 2008, 56, 8404–8411. [Google Scholar] [CrossRef]
- Westhouse, R.A.; Car, B.D. Chapter 13—Pharmacokinetics and Safety Assessment. In Cancer Immunotherapy, 2nd ed.; Prendergast, G.C., Jaffee, E.M., Eds.; Academic Press: San Diego, CA, USA, 2013; pp. 187–206. [Google Scholar] [CrossRef]
- Schwarz, D.; Roots, I. In vitro assessment of inhibition by natural polyphenols of metabolic activation of procarcinogens by human CYP1A1. Biochem. Biophys. Res. Commun. 2003, 303, 902–907. [Google Scholar] [CrossRef]
- Muto, S.; Fujita, K.-I.; Yamazaki, Y.; Kamataki, T. Inhibition by green tea catechins of metabolic activation of procarcinogens by human cytochrome P450. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2001, 479, 197–206. [Google Scholar] [CrossRef]
- Khan, S.G.; Katiyar, S.K.; Agarwal, R.; Mukhtar, H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: Possible role in cancer chemoprevention. Cancer Res. 1992, 52, 4050–4052. [Google Scholar]
- Hayes, J.D.; McMahon, M. Molecular basis for the contribution of the antioxidant responsive element to cancer chemoprevention. Cancer Lett. 2001, 174, 103–113. [Google Scholar] [CrossRef]
- Surh, Y.-J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-S.; Surh, Y.-J. Nrf2 as a novel molecular target for chemoprevention. Cancer Lett. 2005, 224, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-K.; Kim, E.-H.; Jung, J.-H.; Lee, H.-H.; Hyun, J.-W.; Surh, Y.-J. (−)-Epigallocatechin gallate induces Nrf2-mediated antioxidant enzyme expression via activation of PI3K and ERK in human mammary epithelial cells. Arch. Biochem. Biophys. 2008, 476, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Na, H.-K.; Surh, Y.-J. Modulation of Nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol EGCG. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef]
- Thangapazham, R.L.; Singh, A.K.; Sharma, A.; Warren, J.; Gaddipati, J.P.; Maheshwari, R.K. Green tea polyphenols and its constituent epigallocatechin gallate inhibits proliferation of human breast cancer cells in vitro and in vivo. Cancer Lett. 2007, 245, 232–241. [Google Scholar] [CrossRef]
- Manson, M.M. Cancer prevention—the potential for diet to modulate molecular signalling. Trends Mol. Med. 2003, 9, 11–18. [Google Scholar] [CrossRef]
- Nomura, M.; Kaji, A.; He, Z.; Ma, W.-Y.; Miyamoto, K.-i.; Yang, C.S.; Dong, Z. Inhibitory Mechanisms of Tea Polyphenols on the Ultraviolet B-activated Phosphatidylinositol 3-Kinase-dependent Pathway. J. Biol. Chem. 2001, 276, 46624–46631. [Google Scholar] [CrossRef]
- Hwang, J.-T.; Ha, J.; Park, I.-J.; Lee, S.-K.; Baik, H.W.; Kim, Y.M.; Park, O.J. Apoptotic effect of EGCG in HT-29 colon cancer cells via AMPK signal pathway. Cancer Lett. 2007, 247, 115–121. [Google Scholar] [CrossRef]
- Manna, S.; Banerjee, S.; Mukherjee, S.; Das, S.; Panda, C. Epigallocatechin gallate induced apoptosis in Sarcoma180 cells in vivo: Mediated by p53 pathway and inhibition in U1B, U4-U6 UsnRNAs expression. Apoptosis 2006, 11, 2267–2276. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Lu, H.; Ouyang, W.; Huang, C. Inflammation, a Key Event in Cancer Development. Mol. Cancer Res. 2006, 4, 221–233. [Google Scholar] [CrossRef]
- Mackenzie, G.G.; Carrasquedo, F.; Delfino, J.M.; Keen, C.L.; Fraga, C.G.; Oteiza, P.I. Epicatechin, catechin, and dimeric procyanidins inhibit PMA-induced NF-κB activation at multiple steps in Jurkat T cells. FASEB J. 2004, 18, 167–169. [Google Scholar] [CrossRef]
- Kühn, H.; O’Donnell, V.B. Inflammation and immune regulation by 12/15-lipoxygenases. Prog. Lipid Res. 2006, 45, 334–356. [Google Scholar] [CrossRef]
- Schewe, T.; Kuhn, H.; Sies, H. Flavonoids of cocoa inhibit recombinant human 5-lipoxygenase. J. Nutr. 2002, 132, 1825–1829. [Google Scholar] [CrossRef]
- Ahmad, N.; Cheng, P.; Mukhtar, H. Cell Cycle Dysregulation by Green Tea Polyphenol Epigallocatechin-3-Gallate. Biochem. Biophys. Res. Commun. 2000, 275, 328–334. [Google Scholar] [CrossRef]
- Peng, G.; Wargovich, M.J.; Dixon, D.A. Anti-proliferative effects of green tea polyphenol EGCG on Ha-Ras-induced transformation of intestinal epithelial cells. Cancer Lett. 2006, 238, 260–270. [Google Scholar] [CrossRef]
- Sah, J.F.; Balasubramanian, S.; Eckert, R.L.; Rorke, E.A. Epigallocatechin-3-gallate Inhibits Epidermal Growth Factor Receptor Signaling Pathway: Evidence for direct inhibition of erk1/2 and akt kinases. J. Biol. Chem. 2004, 279, 12755–12762. [Google Scholar] [CrossRef]
- Chaffer, C.L.; Weinberg, R.A. A Perspective on Cancer Cell Metastasis. Science 2011, 331, 1559–1564. [Google Scholar] [CrossRef]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of Vascular Endothelial Growth Factor-Induced Angiogenesis Suppresses Tumour Growth In Vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef]
- Shankar, S.; Ganapathy, S.; Hingorani, S.R.; Srivastava, R.K. EGCG inhibits growth, invasion, angiogenesis and metastasis of pancreatic cancer. Front. Biosci. A J. Virtual Libr. 2008, 13, 440–452. [Google Scholar] [CrossRef]
- Kondo, T.; Ohta, T.; Igura, K.; Hara, Y.; Kaji, K. Tea catechins inhibit angiogenesis in vitro, measured by human endothelial cell growth, migration and tube formation, through inhibition of VEGF receptor binding. Cancer Lett. 2002, 180, 139–144. [Google Scholar] [CrossRef]
- Cao, Y.; Cao, R. Angiogenesis inhibited by drinking tea. Nature 1999, 398, 381. [Google Scholar] [CrossRef]
- Deryugina, E.; Quigley, J. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar] [CrossRef]
- Isaacson, K.J.; Martin Jensen, M.; Subrahmanyam, N.B.; Ghandehari, H. Matrix-metalloproteinases as targets for controlled delivery in cancer: An analysis of upregulation and expression. J. Control Release 2017, 259, 62–75. [Google Scholar] [CrossRef]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. Mech. Dis. 2018, 13, 395–412. [Google Scholar] [CrossRef]
- Ko, H.; So, Y.; Jeon, H.; Jeong, M.-H.; Choi, H.-K.; Ryu, S.-H.; Lee, S.-W.; Yoon, H.-G.; Choi, K.-C. TGF-β1-induced epithelial–mesenchymal transition and acetylation of Smad2 and Smad3 are negatively regulated by EGCG in Human A549 lung cancer cells. Cancer Lett. 2013, 335, 205–213. [Google Scholar] [CrossRef]
- Liu, L.-C.; Tsao, T.C.-Y.; Hsu, S.-R.; Wang, H.-C.; Tsai, T.-C.; Kao, J.-Y.; Way, T.-D. EGCG Inhibits Transforming Growth Factor-β-Mediated Epithelial-to-Mesenchymal Transition via the Inhibition of Smad2 and Erk1/2 Signaling Pathways in Nonsmall Cell Lung Cancer Cells. J. Agric. Food Chem. 2012, 60, 9863–9873. [Google Scholar] [CrossRef]
- Wei, R.; Cortez Penso, N.E.; Hackman, R.M.; Wang, Y.; Mackenzie, G.G. Epigallocatechin-3-Gallate (EGCG) Suppresses Pancreatic Cancer Cell Growth, Invasion, and Migration partly through the Inhibition of Akt Pathway and Epithelial–Mesenchymal Transition: Enhanced Efficacy When Combined with Gemcitabine. Nutrients 2019, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef] [PubMed]
- Dean, M.; Fojo, T.; Bates, S. Tumour stem cells and drug resistance. Nat. Rev. Cancer 2005, 5, 275–284. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.L.; Xiang, D.; Shigdar, S.; Macdonald, J.; Li, Y.; Wang, T.; Pu, C.; Wang, Z.; Qiao, L.; Duan, W. Cancer stem cells: A contentious hypothesis now moving forward. Cancer Lett. 2014, 344, 180–187. [Google Scholar] [CrossRef]
- Visvader, J.E.; Lindeman, G.J. Cancer Stem Cells: Current Status and Evolving Complexities. Cell Stem Cell 2012, 10, 717–728. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal. Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-catenin pathway mediates (−)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef]
- Lee, S.H.; Nam, H.J.; Kang, H.J.; Kwon, H.W.; Lim, Y.C. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur. J. Cancer 2013, 49, 3210–3218. [Google Scholar] [CrossRef]
- Sun, X.; Song, J.; Li, E.; Geng, H.; Li, Y.; Yu, D.; Zhong, C. (−)-Epigallocatechin-3-gallate inhibits bladder cancer stem cells via suppression of sonic hedgehog pathway. Oncol. Rep. 2019, 42, 425–435. [Google Scholar] [CrossRef]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef]
- Schwabe, R.F.; Jobin, C. The microbiome and cancer. Nat. Rev. Cancer 2013, 13, 800–812. [Google Scholar] [CrossRef]
- Roy, S.; Trinchieri, G. Microbiota: A key orchestrator of cancer therapy. Nat. Rev. Cancer 2017, 17, 271–285. [Google Scholar] [CrossRef]
- Tao, J.; Li, S.; Gan, R.-Y.; Zhao, C.-N.; Meng, X.; Li, H.-B. Targeting gut microbiota with dietary components on cancer: Effects and potential mechanisms of action. Crit. Rev. Food Sci. Nutr. 2020, 60, 1025–1037. [Google Scholar] [CrossRef]
- Chen, H.; Sang, S. Biotransformation of tea polyphenols by gut microbiota. J. Funct. Foods 2014, 7, 26–42. [Google Scholar] [CrossRef]
- Monagas, M.; Khan, N.; Andrés-Lacueva, C.; Urpí-Sardá, M.; Vázquez-Agell, M.; Lamuela-Raventós, R.M.; Estruch, R. Dihydroxylated phenolic acids derived from microbial metabolism reduce lipopolysaccharide-stimulated cytokine secretion by human peripheral blood mononuclear cells. Br. J. Nutr. 2009, 102, 201–206. [Google Scholar] [CrossRef]
- Abreu, M.T.; Peek, R.M. Gastrointestinal Malignancy and the Microbiome. Gastroenterology 2014, 146, 1534–1546.e1533. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, H. Cancer Preventive Activities of Tea Catechins. Molecules 2016, 21, 1679. [Google Scholar] [CrossRef]
- Du, G.-J.; Zhang, Z.; Wen, X.-D.; Yu, C.; Calway, T.; Yuan, C.-S.; Wang, C.-Z. Epigallocatechin Gallate (EGCG) Is the Most Effective Cancer Chemopreventive Polyphenol in Green Tea. Nutrients 2012, 4, 1679–1691. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Bausch, J.; Edwards, J.A.; Wolz, E. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 1: Genotoxicity. Food Chem. Toxicol. 2006, 44, 626–635. [Google Scholar] [CrossRef]
- Isbrucker, R.A.; Edwards, J.A.; Wolz, E.; Davidovich, A.; Bausch, J. Safety studies on epigallocatechin gallate (EGCG) preparations. Part 2: Dermal, acute and short-term toxicity studies. Food Chem. Toxicol. 2006, 44, 636–650. [Google Scholar] [CrossRef]
- Lambert, J.D.; Sang, S.; Yang, C.S. Possible Controversy over Dietary Polyphenols: Benefits vs Risks. Chem. Res. Toxicol. 2007, 20, 583–585. [Google Scholar] [CrossRef]
- Deng, Y.T.; Lin, J.K. EGCG inhibits the invasion of highly invasive CL1-5 lung cancer cells through suppressing MMP-2 expression via JNK signaling and induces G2/M arrest. J. Agric. Food Chem. 2011, 59. [Google Scholar] [CrossRef]
- Yamauchi, R.; Sasaki, K.; Yoshida, K. Identification of epigallocatechin-3-gallate in green tea polyphenols as a potent inducer of p53-dependent apoptosis in the human lung cancer cell line A549. Toxicol. Vitr. 2009, 23, 834–839. [Google Scholar] [CrossRef]
- Kim, J.; Zhang, X.; Rieger-Christ, K.M.; Summerhayes, I.C.; Wazer, D.E.; Paulson, K.E.; Yee, A.S. Suppression of Wnt Signaling by the Green Tea Compound (–)-Epigallocatechin 3-Gallate (EGCG) in Invasive Breast Cancer Cells: REQUIREMENT OF THE TRANSCRIPTIONAL REPRESSOR HBP1*. J. Biol. Chem. 2006, 281, 10865–10875. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Moulik, S.; Dutta, A.; Choudhury, P.R.; Banerji, A.; Das, S.; Roy, M.; Chatterjee, A. Multifunctional effect of epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A (MMP-2) in human breast cancer cell line MCF-7. Life Sci. 2009, 84, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Van Aller, G.S.; Carson, J.D.; Tang, W.; Peng, H.; Zhao, L.; Copeland, R.A.; Tummino, P.J.; Luo, L. Epigallocatechin gallate (EGCG), a major component of green tea, is a dual phosphoinositide-3-kinase/mTOR inhibitor. Biochem. Biophys. Res. Commun. 2011, 406, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Han, Z.; Li, X.; Xie, H.-H.; Zhu, S.-S. Mechanism of EGCG promoting apoptosis of MCF-7 cell line in human breast cancer. Oncol. Lett. 2017, 14, 3623–3627. [Google Scholar] [CrossRef]
- Hong, O.Y.; Noh, E.M.; Jang, H.Y.; Lee, Y.R.; Lee, B.K.; Jung, S.H.; Kim, J.S.; Youn, H.J. Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol. Lett. 2017, 14, 441–446. [Google Scholar] [CrossRef]
- Huh, S.W.; Bae, S.M.; Kim, Y.-W.; Lee, J.M.; Namkoong, S.E.; Lee, I.P.; Kim, S.H.; Kim, C.K.; Ahn, W.S. Anticancer effects of (−)-epigallocatechin-3-gallate on ovarian carcinoma cell lines. Gynecol. Oncol. 2004, 94, 760–768. [Google Scholar] [CrossRef]
- Yan, C.; Yang, J.; Shen, L.; Chen, X. Inhibitory effect of Epigallocatechin gallate on ovarian cancer cell proliferation associated with aquaporin 5 expression. Arch. Gynecol. Obstet. 2012, 285, 459–467. [Google Scholar] [CrossRef]
- Rao, S.D.; Pagidas, K. Epigallocatechin-3-gallate, a Natural Polyphenol, Inhibits Cell Proliferation and Induces Apoptosis in Human Ovarian Cancer Cells. Anticancer Res. 2010, 30, 2519–2523. [Google Scholar]
- Zhu, B.-H.; Zhan, W.-H.; Li, Z.-R.; Wang, Z.; He, Y.-L.; Peng, J.-S.; Cai, S.-R.; Ma, J.-P.; Zhang, C.-H. (−)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J. Gastroenterol. 2007, 13, 1162–1169. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Jiang, J.; Wan, X.; Wang, Y.; Xu, P. Epigallocatechin gallate reverses gastric cancer by regulating the long noncoding RNA LINC00511/miR-29b/KDM2A axis. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165856. [Google Scholar] [CrossRef]
- Fu, J.D.; Yao, J.J.; Wang, H.; Cui, W.G.; Leng, J.; Ding, L.Y.; Fan, K.Y. Effects of EGCG on proliferation and apoptosis of gastric cancer SGC7901 cells via down-regulation of HIF-1α and VEGF under a hypoxic state. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 155–161. [Google Scholar] [CrossRef]
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem. Toxicol. 2015, 84, 125–132. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Yang, J.; Yi, P.; Xu, P.; Yi, M.; Peng, W. Integrated transcriptomic and metabolomic analyses to characterize the anti-cancer effects of (−)-epigallocatechin-3-gallate in human colon cancer cells. Toxicol. Appl. Pharmacol. 2020, 401, 115100. [Google Scholar] [CrossRef]
- Shimizu, M.; Deguchi, A.; Hara, Y.; Moriwaki, H.; Weinstein, I.B. EGCG inhibits activation of the insulin-like growth factor-1 receptor in human colon cancer cells. Biochem. Biophys. Res. Commun. 2005, 334, 947–953. [Google Scholar] [CrossRef]
- Hoffmann, J.; Junker, H.; Schmieder, A.; Venz, S.; Brandt, R.; Multhoff, G.; Falk, W.; Radons, J. EGCG downregulates IL-1RI expression and suppresses IL-1-induced tumorigenic factors in human pancreatic adenocarcinoma cells. Biochem. Pharmacol. 2011, 82, 1153–1162. [Google Scholar] [CrossRef]
- Kürbitz, C.; Heise, D.; Redmer, T.; Goumas, F.; Arlt, A.; Lemke, J.; Rimbach, G.; Kalthoff, H.; Trauzold, A. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Sci. 2011, 102, 728–734. [Google Scholar] [CrossRef]
- Nishikawa, T.; Nakajima, T.; Moriguchi, M.; Jo, M.; Sekoguchi, S.; Ishii, M.; Takashima, H.; Katagishi, T.; Kimura, H.; Minami, M.; et al. A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J. Hepatol. 2006, 44, 1074–1082. [Google Scholar] [CrossRef]
- Shirakami, Y.; Shimizu, M.; Adachi, S.; Sakai, H.; Nakagawa, T.; Yasuda, Y.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin gallate suppresses the growth of human hepatocellular carcinoma cells by inhibiting activation of the vascular endothelial growth factor–vascular endothelial growth factor receptor axis. Cancer Sci. 2009, 100, 1957–1962. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, J.; Cai, Z.; An, H.; Li, Y.; Peng, Y.; Chen, N.; Luo, A.; Tao, H.; Li, K. Epigallocatechin gallate induces chemopreventive effects on rats with diethylnitrosamine-induced liver cancer via inhibition of cell division cycle 25A. Mol. Med. Rep. 2020, 22, 3873–3885. [Google Scholar] [CrossRef]
- Luo, K.-W.; Wei, C.; Lung, W.-Y.; Wei, X.-Y.; Cheng, B.-H.; Cai, Z.-M.; Huang, W.-R. EGCG inhibited bladder cancer SW780 cell proliferation and migration both in vitro and in vivo via down-regulation of NF-κB and MMP-9. J. Nutr. Biochem. 2017, 41, 56–64. [Google Scholar] [CrossRef]
- Albrecht, D.S.; Clubbs, E.A.; Ferruzzi, M.; Bomser, J.A. Epigallocatechin-3-gallate (EGCG) inhibits PC-3 prostate cancer cell proliferation via MEK-independent ERK1/2 activation. Chem. Biol. Interact. 2008, 171, 89–95. [Google Scholar] [CrossRef]
- Chuu, C.-P.; Chen, R.-Y.; Kokontis, J.M.; Hiipakka, R.A.; Liao, S. Suppression of androgen receptor signaling and prostate specific antigen expression by (−)-epigallocatechin-3-gallate in different progression stages of LNCaP prostate cancer cells. Cancer Lett. 2009, 275, 86–92. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Lambert, J.D.; Yang, C.S. Cancer chemopreventive activity and bioavailability of tea and tea polyphenols. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2003, 523–524, 201–208. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.; Chow, M.S.S.; Zuo, Z. Investigation of intestinal absorption and disposition of green tea catechins by Caco-2 monolayer model. Int. J. Pharm. 2004, 287, 1–12. [Google Scholar] [CrossRef]
- Zhu, Q.Y.; Zhang, A.; Tsang, D.; Huang, Y.; Chen, Z.-Y. Stability of Green Tea Catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [Google Scholar] [CrossRef]
- Lun Su, Y.; Leung, L.K.; Huang, Y.; Chen, Z.-Y. Stability of tea theaflavins and catechins. Food Chem. 2003, 83, 189–195. [Google Scholar] [CrossRef]
- Sang, S.; Lee, M.-J.; Hou, Z.; Ho, C.-T.; Yang, C.S. Stability of Tea Polyphenol (−)-Epigallocatechin-3-gallate and Formation of Dimers and Epimers under Common Experimental Conditions. J. Agric. Food Chem. 2005, 53, 9478–9484. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Lambert, J.D.; Lee, S.-H.; Sinko, P.J.; Yang, C.S. Involvement of multidrug resistance-associated proteins in regulating cellular levels of (−)-epigallocatechin-3-gallate and its methyl metabolites. Biochem. Biophys. Res. Commun. 2003, 310, 222–227. [Google Scholar] [CrossRef]
- Yang, C.S.; Sang, S.; Lambert, J.D.; Lee, M.-J. Bioavailability issues in studying the health effects of plant polyphenolic compounds. Mol. Nutr. Food Res. 2008, 52, S139–S151. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Maliakal, P.; Chen, L.S.; Meng, X.F.; Bondoc, F.Y.; Prabhu, S.; Lambert, G.; Mohr, S.; Yang, C.S. Pharmacokinetics of tea catechins after ingestion of green tea and (−)-Epigallocatechin-3-gallate by humans: Formation of different metabolites and individual variability. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1025–1032. [Google Scholar]
- Myung, S.K.; Bae, W.K.; Oh, S.M.; Kim, Y.; Ju, W.; Sung, J.; Lee, Y.J.; Ko, J.A.; Song, J.I.; Choi, H.J. Green tea consumption and risk of stomach cancer: A meta-analysis of epidemiologic studies. Int. J. Cancer 2009, 124, 670–677. [Google Scholar] [CrossRef]
- Ogunleye, A.A.; Xue, F.; Michels, K.B. Green tea consumption and breast cancer risk or recurrence: A meta-analysis. Breast Cancer Res. Treat. 2009, 119, 477. [Google Scholar] [CrossRef]
- Tanaka, K.; Tamakoshi, A.; Sugawara, Y.; Mizoue, T.; Inoue, M.; Sawada, N.; Matsuo, K.; Ito, H.; Naito, M.; Nagata, C.; et al. Coffee, green tea and liver cancer risk: An evaluation based on a systematic review of epidemiologic evidence among the Japanese population. Jpn. J. Clin. Oncol. 2019, 49, 972–984. [Google Scholar] [CrossRef]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Wu, M.; Jin, J.; Jin, P.; Xu, Y.; Yin, J.; Qin, D.; Wang, K.; Du, Q. Epigallocatechin gallate-β-lactoglobulin nanoparticles improve the antitumor activity of EGCG for inducing cancer cell apoptosis. J. Funct. Foods 2017, 39, 257–263. [Google Scholar] [CrossRef]
- Li, B.; Du, W.; Jin, J.; Du, Q. Preservation of (−)-Epigallocatechin-3-gallate Antioxidant Properties Loaded in Heat Treated β-Lactoglobulin Nanoparticles. J. Agric. Food Chem. 2012, 60, 3477–3484. [Google Scholar] [CrossRef]
- Kulandaivelu, K.; Mandal, A.K.A. Improved bioavailability and pharmacokinetics of tea polyphenols by encapsulation into gelatin nanoparticles. IET Nanobiotechnology 2017, 11, 469–476. [Google Scholar] [CrossRef]
- He, A.; Guan, X.; Song, H.; Li, S.; Huang, K. Encapsulation of (−)-Epigallocatechin-gallate (EGCG) in hordein nanoparticles. Food Biosci. 2020, 37, 100727. [Google Scholar] [CrossRef]
- Dube, A.; Ng, K.; Nicolazzo, J.A.; Larson, I. Effective use of reducing agents and nanoparticle encapsulation in stabilizing catechins in alkaline solution. Food Chem. 2010, 122, 662–667. [Google Scholar] [CrossRef]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Bharali, D.J.; Nihal, M.; Adhami, V.M.; Khan, N.; Chamcheu, J.C.; Khan, M.I.; Shabana, S.; Mousa, S.A.; Mukhtar, H. Excellent anti-proliferative and pro-apoptotic effects of (−)-Epigallocatechin-3-gallate encapsulated in chitosan nanoparticles on human melanoma cell growth both in vitro and in vivo. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1619–1626. [Google Scholar] [CrossRef]
- Zeng, L.; Yan, J.; Luo, L.; Ma, M.; Zhu, H. Preparation and characterization of (−)-Epigallocatechin-3-gallate (EGCG)-loaded nanoparticles and their inhibitory effects on Human breast cancer MCF-7 cells. Sci. Rep. 2017, 7, 15. [Google Scholar] [CrossRef]
- Dai, W.; Ruan, C.; Sun, Y.; Gao, X.; Liang, J. Controlled release and antioxidant activity of chitosan and β-lactoglobulin complex nanoparticles loaded with epigallocatechin gallate. Colloids Surf. B Biointerfaces 2020, 188, 110802. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, Z.-R.; Lai, C.-H.; Hsieh, C.-H.; Feng, C.-L. Active Targeted Nanoparticles for Oral Administration of Gastric Cancer Therapy. Biomacromolecules 2015, 16, 3021–3032. [Google Scholar] [CrossRef]
- Gao, J.; Mao, Y.; Xiang, C.; Cao, M.; Ren, G.; Wang, K.; Ma, X.; Wu, D.; Xie, H. Preparation of β-lactoglobulin/gum arabic complex nanoparticles for encapsulation and controlled release of EGCG in simulated gastrointestinal digestion model. Food Chem. 2021, 354, 129516. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gu, L. Fabrication of Self-Assembled (−)-Epigallocatechin Gallate (EGCG) Ovalbumin–Dextran Conjugate Nanoparticles and Their Transport across Monolayers of Human Intestinal Epithelial Caco-2 Cells. J. Agric. Food Chem. 2014, 62, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Meena, R.; Rajamani, P. Fabrication of BSA–Green Tea Polyphenols–Chitosan Nanoparticles and Their Role in Radioprotection: A Molecular and Biochemical Approach. J. Agric. Food Chem. 2016, 64, 6024–6034. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ha, J.; Zou, T.; Gu, L. Fabrication of coated bovine serum albumin (BSA)-epigallocatechin gallate (EGCG) nanoparticles and their transport across monolayers of human intestinal epithelial Caco-2 cells. Food Funct. 2014, 5, 1278–1285. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Cao, L.; Zhang, L.; Wan, X.-C. Preparation, characterization, and in vitro antitumor activity of folate conjugated chitosan coated EGCG nanoparticles. Food Sci. Biotechnol. 2014, 23, 569–575. [Google Scholar] [CrossRef]
- Hong, Z.; Xu, Y.; Yin, J.-F.; Jin, J.; Jiang, Y.; Du, Q. Improving the Effectiveness of (−)-Epigallocatechin Gallate (EGCG) against Rabbit Atherosclerosis by EGCG-Loaded Nanoparticles Prepared from Chitosan and Polyaspartic Acid. J. Agric. Food Chem. 2014, 62, 12603–12609. [Google Scholar] [CrossRef]
- Tang, D.-W.; Yu, S.-H.; Ho, Y.-C.; Huang, B.-Q.; Tsai, G.-J.; Hsieh, H.-Y.; Sung, H.-W.; Mi, F.-L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Chu, P.-Y.; Tsai, S.-C.; Ko, H.-Y.; Wu, C.-C.; Lin, Y.-H. Co-Delivery of Natural Compounds with a Dual-Targeted Nanoparticle Delivery System for Improving Synergistic Therapy in an Orthotopic Tumor Model. ACS Appl. Mater. Interfaces 2019, 11, 23880–23892. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kulhari, H.; Pooja, D.; Gudem, S.; Bhargava, S.; Shukla, R.; Sistla, R. Encapsulation of biophenolic phytochemical EGCG within lipid nanoparticles enhances its stability and cytotoxicity against cancer. Chem. Phys. Lipids 2016, 198, 51–60. [Google Scholar] [CrossRef]
- Shtay, R.; Keppler, J.K.; Schrader, K.; Schwarz, K. Encapsulation of (−)-Epigallocatechin-3-gallate (EGCG) in solid lipid nanoparticles for food applications. J. Food Eng. 2019, 244, 91–100. [Google Scholar] [CrossRef]
- Zou, L.-Q.; Liu, W.; Liu, W.-L.; Liang, R.-H.; Li, T.; Liu, C.-M.; Cao, Y.-L.; Niu, J.; Liu, Z. Characterization and Bioavailability of Tea Polyphenol Nanoliposome Prepared by Combining an Ethanol Injection Method with Dynamic High-Pressure Microfluidization. J. Agric. Food Chem. 2014, 62, 934–941. [Google Scholar] [CrossRef]
- De Pace, R.C.C.; Liu, X.; Sun, M.; Nie, S.; Zhang, J.; Cai, Q.; Gao, W.; Pan, X.; Fan, Z.; Wang, S. Anticancer activities of (−)-Epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013, 23, 187–196. [Google Scholar] [CrossRef]
- Zhang, J.; Nie, S.; Wang, S. Nanoencapsulation Enhances Epigallocatechin-3-gallate Stability and Its Antiatherogenic Bioactivities in Macrophages. J. Agric. Food Chem. 2013, 61, 9200–9209. [Google Scholar] [CrossRef]
- Hajipour, H.; Hamishehkar, H.; Nazari Soltan Ahmad, S.; Barghi, S.; Maroufi, N.F.; Taheri, R.A. Improved anticancer effects of epigallocatechin gallate using RGD-containing nanostructured lipid carriers. Artif. Cells Nanomed. Biotechnol. 2018, 46, 283–292. [Google Scholar] [CrossRef]
- Liao, B.; Ying, H.; Yu, C.; Fan, Z.; Zhang, W.; Shi, J.; Ying, H.; Ravichandran, N.; Xu, Y.; Yin, J.; et al. (−)-Epigallocatechin gallate (EGCG)-nanoethosomes as a transdermal delivery system for docetaxel to treat implanted human melanoma cell tumors in mice. Int. J. Pharm. 2016, 512, 22–31. [Google Scholar] [CrossRef]
- Park, S.J.; Garcia, C.V.; Shin, G.H.; Kim, J.T. Fabrication and optimization of EGCG-loaded nanoparticles by high pressure homogenization. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Rocha, S.; Generalov, R.; Pereira, M.d.C.; Peres, I.; Juzenas, P.; Coelho, M.A. Epigallocatechin gallate-loaded polysaccharide nanoparticles for prostate cancer chemoprevention. Nanomedicine 2011, 6, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Antoniou, J.; Li, Y.; Majeed, H.; Liang, R.; Ma, Y.; Ma, J.; Zhong, F. Chitosan/sulfobutylether-β-cyclodextrin nanoparticles as a potential approach for tea polyphenol encapsulation. Food Hydrocoll. 2016, 57, 291–300. [Google Scholar] [CrossRef]
- Hu, B.; Xie, M.; Zhang, C.; Zeng, X. Genipin-Structured Peptide–Polysaccharide Nanoparticles with Significantly Improved Resistance to Harsh Gastrointestinal Environments and Their Potential for Oral Delivery of Polyphenols. J. Agric. Food Chem. 2014, 62, 12443–12452. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chen, W.; Tu, G.; Chen, X.; Lu, Y.; Wu, L.; Zheng, D. Enhanced Chemotherapeutic Efficacy of PLGA-Encapsulated Epigallocatechin Gallate (EGCG) Against Human Lung Cancer. Int. J. Nanomed. 2020, 15, 4417–4429. [Google Scholar] [CrossRef]
- Kazi, J.; Sen, R.; Ganguly, S.; Jha, T.; Ganguly, S.; Chatterjee Debnath, M. Folate decorated epigallocatechin-3-gallate (EGCG) loaded PLGA nanoparticles; in-vitro and in-vivo targeting efficacy against MDA-MB-231 tumor xenograft. Int. J. Pharm. 2020, 585, 119449. [Google Scholar] [CrossRef]
- Chen, M.-L.; Lai, C.-J.; Lin, Y.-N.; Huang, C.-M.; Lin, Y.-H. Multifunctional nanoparticles for targeting the tumor microenvironment to improve synergistic drug combinations and cancer treatment effects. J. Mater. Chem. B 2020, 8, 10416–10427. [Google Scholar] [CrossRef]
- Shan, L.; Gao, G.; Wang, W.; Tang, W.; Wang, Z.; Yang, Z.; Fan, W.; Zhu, G.; Zhai, K.; Jacobson, O.; et al. Self-assembled green tea polyphenol-based coordination nanomaterials to improve chemotherapy efficacy by inhibition of carbonyl reductase 1. Biomaterials 2019, 210, 62–69. [Google Scholar] [CrossRef]
- Liang, K.; Bae, K.H.; Lee, F.; Xu, K.; Chung, J.E.; Gao, S.J.; Kurisawa, M. Self-assembled ternary complexes stabilized with hyaluronic acid-green tea catechin conjugates for targeted gene delivery. J. Control Release 2016, 226, 205–216. [Google Scholar] [CrossRef]
- Das, A.; Haque, I.; Ray, P.; Ghosh, A.; Dutta, D.; Quadir, M.; De, A.; Gunewardena, S.; Chatterjee, I.; Banerjee, S.; et al. CCN5 activation by free or encapsulated EGCG is required to render triple-negative breast cancer cell viability and tumor progression. Pharmacol. Res. Perspect. 2021, 9, e00753. [Google Scholar] [CrossRef]
- Ramesh, N.; Mandal, A.K.A. Pharmacokinetic, toxicokinetic, and bioavailability studies of epigallocatechin-3-gallate loaded solid lipid nanoparticle in rat model. Drug Dev. Ind. Pharm. 2019, 45, 1506–1514. [Google Scholar] [CrossRef]
- Wu, Z.; Li, H.; Ming, J.; Zhao, G. Optimization of Adsorption of Tea Polyphenols into Oat β-Glucan Using Response Surface Methodology. J. Agric. Food Chem. 2011, 59, 378–385. [Google Scholar] [CrossRef]
- Tang, H.R.; Covington, A.D.; Hancock, R.A. Structure–activity relationships in the hydrophobic interactions of polyphenols with cellulose and collagen. Biopolymers 2003, 70, 403–413. [Google Scholar] [CrossRef]
- Shpigelman, A.; Israeli, G.; Livney, Y.D. Thermally-induced protein–polyphenol co-assemblies: Beta lactoglobulin-based nanocomplexes as protective nanovehicles for EGCG. Food Hydrocoll. 2010, 24, 735–743. [Google Scholar] [CrossRef]
- Shpigelman, A.; Cohen, Y.; Livney, Y.D. Thermally-induced β-lactoglobulin–EGCG nanovehicles: Loading, stability, sensory and digestive-release study. Food Hydrocoll. 2012, 29, 57–67. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Yang, X.; Tang, W.; Zeng, X.; Huang, Q. Nanochemoprevention by encapsulation of (−)-Epigallocatechin-3-gallate with bioactive peptides/chitosan nanoparticles for enhancement of its bioavailability. Chem. Commun. 2012, 48, 2421–2423. [Google Scholar] [CrossRef]
- Zhang, K.; Tang, X.; Zhang, J.; Lu, W.; Lin, X.; Zhang, Y.; Tian, B.; Yang, H.; He, H. PEG–PLGA copolymers: Their structure and structure-influenced drug delivery applications. J. Control Release 2014, 183, 77–86. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Bhatnagar, P.; Singh, M.; Mishra, S.; Kumar, P.; Shukla, Y.; Gupta, K.C. Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int. J. Nanomed. 2013, 8, 1451–1462. [Google Scholar] [CrossRef]
- Duan, Y.; Dhar, A.; Patel, C.; Khimani, M.; Neogi, S.; Sharma, P.; Siva Kumar, N.; Vekariya, R.L. A brief review on solid lipid nanoparticles: Part and parcel of contemporary drug delivery systems. RSC Adv. 2020, 10, 26777–26791. [Google Scholar] [CrossRef]
- Moreno-Vásquez, M.J.; Plascencia-Jatomea, M.; Sánchez-Valdes, S.; Tanori-Córdova, J.C.; Castillo-Yañez, F.J.; Quintero-Reyes, I.E.; Graciano-Verdugo, A.Z. Characterization of Epigallocatechin-Gallate-Grafted Chitosan Nanoparticles and Evaluation of Their Antibacterial and Antioxidant Potential. Polymers 2021, 13, 1375. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ghosh, S.; Das, D.K.; Chakraborty, P.; Choudhury, S.; Gupta, P.; Adhikary, A.; Dey, S.; Chattopadhyay, S. Gold-conjugated green tea nanoparticles for enhanced anti-tumor activities and hepatoprotection—synthesis, characterization and in vitro evaluation. J. Nutr. Biochem. 2015, 26, 1283–1297. [Google Scholar] [CrossRef]
- Sogias, I.A.; Williams, A.C.; Khutoryanskiy, V.V. Why is Chitosan Mucoadhesive? Biomacromolecules 2008, 9, 1837–1842. [Google Scholar] [CrossRef]
- Menchicchi, B.; Fuenzalida, J.P.; Bobbili, K.B.; Hensel, A.; Swamy, M.J.; Goycoolea, F.M. Structure of Chitosan Determines Its Interactions with Mucin. Biomacromolecules 2014, 15, 3550–3558. [Google Scholar] [CrossRef]
- Sonaje, K.; Chuang, E.-Y.; Lin, K.-J.; Yen, T.-C.; Su, F.-Y.; Tseng, M.T.; Sung, H.-W. Opening of Epithelial Tight Junctions and Enhancement of Paracellular Permeation by Chitosan: Microscopic, Ultrastructural, and Computed-Tomographic Observations. Mol. Pharm. 2012, 9, 1271–1279. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (−)-Epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef]
- Wang, R.; Huang, J.; Chen, J.; Yang, M.; Wang, H.; Qiao, H.; Chen, Z.; Hu, L.; Di, L.; Li, J. Enhanced anti-colon cancer efficacy of 5-fluorouracil by epigallocatechin-3- gallate co-loaded in wheat germ agglutinin-conjugated nanoparticles. Nanomed.: Nanotechnol. Biol. Med. 2019, 21, 102068. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, W.; Jia, L.; Sun, X.; Chen, G.; Zhao, X.; Li, X.; Meng, X.; Kong, L.; Xing, L.; et al. Phase I study of topical epigallocatechin-3-gallate (EGCG) in patients with breast cancer receiving adjuvant radiotherapy. Br. J. Radiol. 2016, 89, 20150665. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Gokarn, Y.; Mitragotri, S. Non-invasive delivery strategies for biologics. Nat. Rev. Drug Discov. 2019, 18, 19–40. [Google Scholar] [CrossRef]
- El-Kayal, M.; Nasr, M.; Elkheshen, S.; Mortada, N. Colloidal (−)-Epigallocatechin-3-gallate vesicular systems for prevention and treatment of skin cancer: A comprehensive experimental study with preclinical investigation. Eur. J. Pharm. Sci. 2019, 137, 104972. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Park, M.R.; Kim, M.S.; Kwon, O.H. Polyphenol-loaded polycaprolactone nanofibers for effective growth inhibition of human cancer cells. Mater. Chem. Phys. 2012, 133, 674–680. [Google Scholar] [CrossRef]
- Li, Y.; Lim, L.-T.; Kakuda, Y. Electrospun Zein Fibers as Carriers to Stabilize (−)-Epigallocatechin Gallate. J. Food Sci. 2009, 74, C233–C240. [Google Scholar] [CrossRef] [PubMed]
- Forouzideh, N.; Nadri, S.; Fattahi, A.; Abdolahinia, E.D.; Habibizadeh, M.; Rostamizadeh, K.; Baradaran-Rafii, A.; Bakhshandeh, H. Epigallocatechin gallate loaded electrospun silk fibroin scaffold with anti-angiogenic properties for corneal tissue engineering. J. Drug Deliv. Sci. Technol. 2020, 56, 101498. [Google Scholar] [CrossRef]
- Hsieh, D.-S.; Wang, H.; Tan, S.-W.; Huang, Y.-H.; Tsai, C.-Y.; Yeh, M.-K.; Wu, C.-J. The treatment of bladder cancer in a mouse model by epigallocatechin-3-gallate-gold nanoparticles. Biomaterials 2011, 32, 7633–7640. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhang, Y.S.; Pang, B.; Hyun, D.C.; Yang, M.; Xia, Y. Engineered Nanoparticles for Drug Delivery in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 12320–12364. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2016, 17, 20. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef]
- O’Dwyer, J.; O’Cearbhaill, R.E.; Wylie, R.; O’Mahony, S.; O’Dwyer, M.; Duffy, G.P.; Dolan, E.B. Enhancing Delivery of Small-Molecule- and Cell-Based Therapies for Ovarian Cancer Using Advanced Delivery Strategies. Adv. Ther. 2020, 3, 2000144. [Google Scholar] [CrossRef]
- Rossi, S.M.; Murray, T.; McDonough, L.; Kelly, H. Loco-regional drug delivery in oncology: Current clinical applications and future translational opportunities. Expert Opin. Drug Deliv. 2020, 1–17. [Google Scholar] [CrossRef]
- Hallaj-Nezhadi, S.; Dass, C.R.; Lotfipour, F. Intraperitoneal delivery of nanoparticles for cancer gene therapy. Future Oncol. 2013, 9, 59–68. [Google Scholar] [CrossRef]
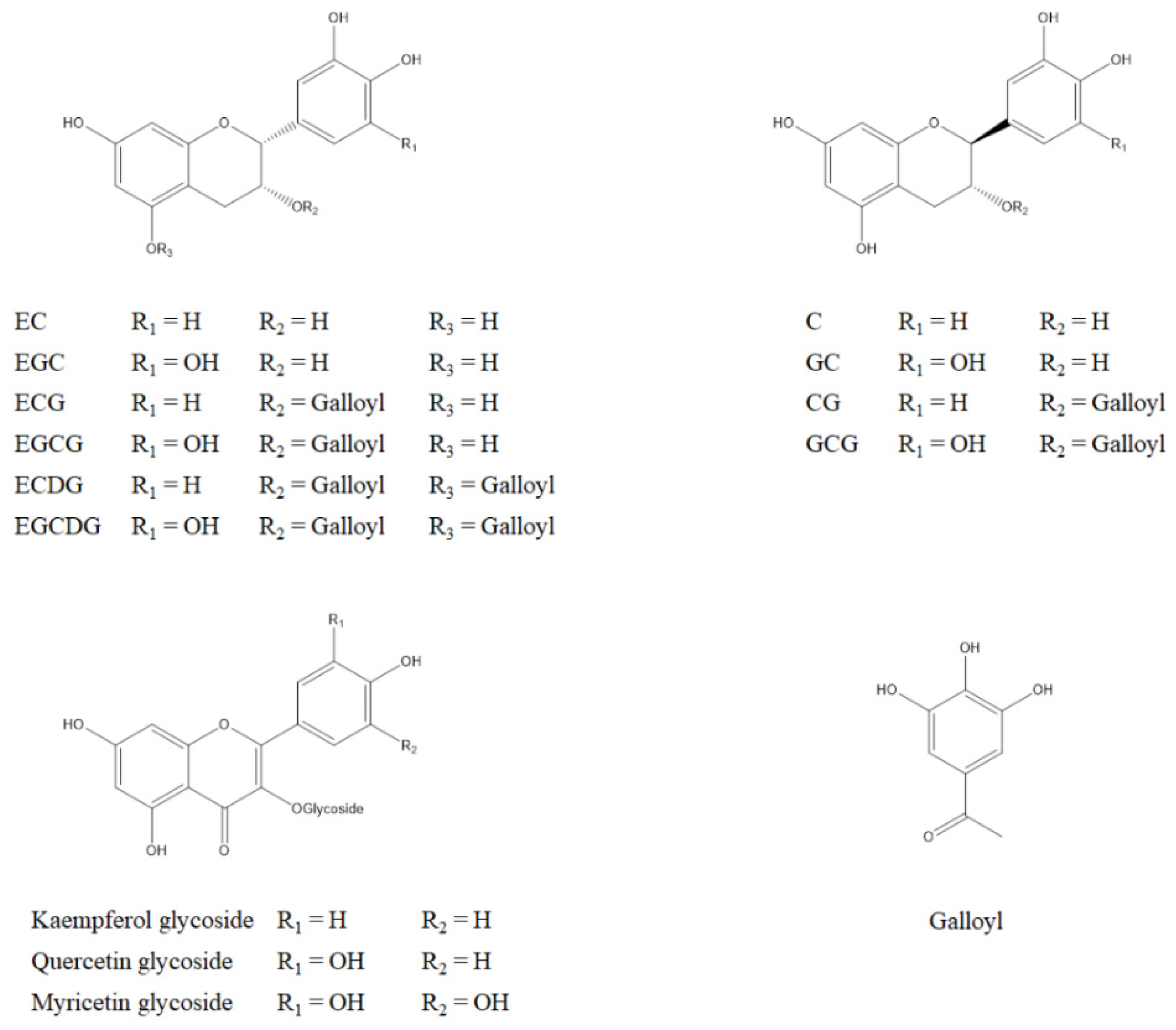



| Type of Cancer | Catechin | Experimental Models | Inhibition Mechanisms | Ref. |
|---|---|---|---|---|
| Lung Cancer | EGCG | A549 cell | Inhibit activation of p300/CBP in TGF-β1-induced EMT by deacetylation of Smad2 and Smad3 | [58] |
| EGCG | A549 and H1299 cells | Inhibit TGF-β-induced EMT through downregulation of phosphorylated Smad2 and Erk1/2 | [59] | |
| EGCG | CL1-5 cell and CL1-5 tumor-bearing nude mice | Downregulate the expression of MMP-2 via the JNK pathway, induce G2/M arrest | [81] | |
| EGCG | Tumorspheres derived from A549 and H1299 cells | Inhibit CSC through suppression of the Wnt/β-catenin pathway | [66] | |
| C, EC, ECG, EGC, EGCG, and mixture | A549 cell | EGCG induces apoptosis through a p53-dependent pathway | [82] | |
| Breast Cancer | EGCG | MDA-MB-231 cell | Block the Wnt pathway by inducing the HBP1 transcriptional repressor | [83] |
| EGCG | MCF-7 cell | Inhibit the gelatinolytic activity and expression of MMP-2 by downregulating MT1-MMP, VEGF, NF-κB, FAK, αvβ3, and α5β1 integrin receptors | [84] | |
| EGCG | MDA-MB-231 cell | Inhibit proliferation by inactivation of the PI3K/AKT/mTOR pathway | [85] | |
| EGCG | MCF-7 cell | Inhibit proliferation and promote apoptosis via p53/Bcl-2 pathway | [86] | |
| EGCG | MDA-MB-231 cell | Inhibit cell growth via inactivation of β-catenin pathway | [87] | |
| Ovarian Cancer | EGCG | SKOV-3, OVCAR-3, PA-1 cells | Induce apoptosis and arrest cell cycle | [88] |
| EGCG | SKOV3 cell | Inhibit proliferation and induce apoptosis, downregulate of aquaporin 5 via inactivation of NF-κB | [89] | |
| EGCG | SKOV-3 cell | Inhibit proliferation via DNA synthesis reduction, induce apoptosis via DNA damage, arrest cell cycle | [90] | |
| Gastric Cancer | EGCG | SGC-7901 cell and SGC-7901 tumor-bearing nude mice | Inhibit angiogenesis and the expression of VEGF, reduce the activation of Stat3 | [91] |
| EGCG | AGS and SGC7901 cells | Inhibit proliferation by regulating the long non-coding RNA LINC00511/miR-29b/KDM2A axis | [92] | |
| EGCG | SGC7901 cell | Induce apoptosis under hypoxia via downregulation of HIF-1α and VEGF | [93] | |
| Colon Cancer | EGCG | HT-29 cell | Inhibit COX-2 through activation of the AMPK pathway | [40] |
| EGCG | HT-29 cell | Induce apoptosis through Akt, ERK1/2, and alternative p38MAPK pathways | [94] | |
| EGCG | HT-29 cell | Induce cell cycle arrest, apoptosis, and autophagy | [95] | |
| EGCG | Caco2, HCT116, HT29, SW480, SW837 cells | Inactivation of the insulin-like growth factor-1 receptor | [96] | |
| Pancreatic Cancer | EGCG | Colo357 cell | Inhibit IL-1-induced secretion of IL-6, IL-8, VEGF, and PGHS-2, reduce the level of MMP-2, activate caspase-3, downregulate the expression of IL-1 receptor type I via inhibition of NF-κB | [97] |
| CG, ECG, EGCG | PancTu-I, Panc1, Panc89, BxPC3 cells | Arrest cell cycle, inhibit TNF-α-mediated activation of NF-κB and secretion of IL-8 and uPA | [98] | |
| EGCG | Panc-1, MIA PaCa-2, BxPC-3, HPAF-II, CFPAC-1, Su.86.86, FC1245 cells, and FC1245 tumor-bearing mice | Inhibit migration and invasion by suppressing EMT via inhibition of the Akt pathway | [60] | |
| Liver Cancer | EGCG | HLE, HepG2, HuH-7, PLC/PRF/5 cells, HLE tumor-bearing nude mice | Induce apoptosis, downregulate Bcl-2α and Bcl-xl via inactivation of NF-κB | [99] |
| EGCG | HLF, PLC/PRF/5, HepG2, HLE, Hep3B, HuH-7 cells, and HuH-7 tumor-bearing nude mice | Inhibit the VEGF-VEGFR axis and downstream signaling molecules (ERK, Akt), downregulate the expression of Bcl-xL | [100] | |
| EGCG | HepG2 and Huh7 cell, diethylnitrosamine-induced liver cancer rat model | Inhibit proliferation, downregulate the expression of cell division cycle 25A, upregulate the expression of p21waf1/Cip1 | [101] | |
| Bladder Cancer | EGCG | SW780 cell and SW780 tumor-bearing nude mice | Inhibit proliferation and migration via downregulation of NF-κB and MMP-9 | [102] |
| EGCG | Tumorspheres derived from EJ and UM-UC-3 cells | Inhibit CSC through suppression of the Hedgehog pathway | [68] | |
| Prostate Cancer | EGCG | PC-3 cell | Inhibit proliferation by activation of ERK1/2 via a MEK-independent, PI3K-dependent pathway | [103] |
| EGCG | LNCaP cell sublines and LNCaP104-R1 tumor-bearing nude mice | Suppress cell proliferation, prostate-specific antigen expression, and androgen receptor transcriptional activity | [104] |
| Catechins | M.W. (g/mol) | Log P a | H-Bond Donor | H-Bond Acceptor | Papp × 10−7 (cm/s) b | ||
|---|---|---|---|---|---|---|---|
| AP to BL | BL to AP | Efflux Ratio | |||||
| EC | 290 | 1.5 | 5 | 6 | 1.39 | 29.96 | 21.55 |
| EGC | 306 | 1.11 | 6 | 7 | 1.49 | 7.72 | 5.18 |
| ECG | 442 | 2.46 | 7 | 10 | 0.96 | 3.86 | 4.02 |
| EGCG | 458 | 2.07 | 8 | 11 | 0.83 | 1.52 | 1.83 |
| Nanocarriers | Catechins | Improvements | Ref. |
|---|---|---|---|
| β-lactoglobulin NPs | EGCG | Preserved the antioxidant activity of EGCG at neutral pH | [119] |
| β-lactoglobulin NPs | EGCG | Enhanced anticancer activity of EGCG in vitro | [118] |
| Gelatin NPs | Tea polyphenols | Enhanced sustained release of tea polyphenols in vitro, significantly improved the pharmacokinetic profiles and oral bioavailability of tea polyphenols in vivo | [120] |
| Hordein NPs | EGCG | Protected EGCG from degradation | [121] |
| CS/TPP complex NPs | C and EGCG | Enhanced stability of C and EGCG in pH 7.4 buffer | [122] |
| CS/TPP complex NPs | EGCG | Significantly inhibited prostate tumor growth in vivo | [123] |
| CS/TPP complex NPs | EGCG | Enhanced the anti-melanoma effect of EGCG in vitro and in vivo | [124] |
| CS/TPP complex NPs modified with PEG and folate | EGCG | Enhanced anticancer effect of EGCG against MCF-7 cells by regulating the PI3K/Akt pathway | [125] |
| CS/β-lactoglobulin complex NPs | EGCG | Enhanced cellular antioxidant activity of EGCG | [126] |
| CS/gelatin complex NPs | EGCG | Significantly decreased the expression of VEGF in gastric cancer cells and significantly inhibited gastric tumor growth in vivo | [127] |
| β-lactoglobulin/gum Arabic complex NPs | EGCG | Enhanced antioxidant activity of EGCG | [128] |
| Ovalbumin-dextran conjugate NPs | EGCG | Significantly enhanced the Papp of EGCG in vitro | [129] |
| CS-coated BSA NPs | Tea polyphenols | Significantly enhanced the radioprotective effect in vivo | [130] |
| Poly-ε-lysine- or CS-coated BSA NPs | EGCG | Significantly enhanced the Papp of EGCG in the CS-coated EGCG-loaded BSA NPs compared to the EGCG solution | [131] |
| Folate conjugated CS NPs | EGCG | Effectively enhanced anticancer effect in 3 cancer cell lines, especially in the folate receptor-overexpressing cell line | [132] |
| CS/PAA NPs | EGCG | Increased the stability of EGCG in simulated gastric and intestinal conditions, significantly enhanced the oral anti-atherosclerosis effect in rabbit in vivo through the reduction of serum levels of triglyceride, total cholesterol, HDL cholesterol, and LDL cholesterol | [133] |
| CS/γ-PGA | Tea catechins | Effectively enhanced the in vitro transport of tea catechins through Caco-2 monolayer | [134] |
| Hyaluronic acid/fucoidan/PEG-gelatin NPs | EGCG | Significantly enhanced the inhibitory effect against prostate cancer in vitro and in vivo | [135] |
| SLN | EGCG | Significantly enhanced in vitro cytotoxicity against human breast cancer cells MDA-MB-231 and human prostate cancer cells DU-145 through apoptosis | [136] |
| SLN | EGCG | Enhanced stability of EGCG | [137] |
| Nanoliposome | Tea polyphenol | Enhanced tea polyphenol stability in pH 7.4 buffer | [138] |
| CS-coated nanoliposome | EGCG | Significantly enhanced EGCG stability, improved sustained-release, increased intracellular level of EGCG in MCF-7 cells, induced apoptosis, and inhibited proliferation of MCF-7 cells | [139] |
| Nanostructured lipid carriers and CS-coated nanostructured lipid carriers | EGCG | Significantly enhanced the stability of EGCG at pH 7.4, increased the sustained release of EGCG and the content of EGCG in the macrophage, enhanced the anti-atherogenic activity of EGCG by the reduction of the cholesteryl ester content in the macrophage, and significant inhibition of inflammatory factor secretion | [140] |
| RGD peptide-modified nanostructured lipid carriers | EGCG | Enhanced in vitro cytotoxicity of EGCG against breast cancer cells | [141] |
| Nanoethosomes | EGCG | Significantly enhanced melanoma growth-inhibition in vivo when being topically delivered | [142] |
| Delivery Strategy | Nanocarrier | Targeting Molecule | Receptor | Catechin | Co-Delivered Drug | Cancer Type | Models | Efficacies | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| i.v. | PLGA NPs | Folate peptide | Folate receptor | EGCG | - | Breast cancer | MDA-MB-231 and MCF-7 cell lines, SD rats and MDA-MB-231 tumor-bearing nude mice | ↑ In vitro cellular uptake of NPs in the folate receptor-expressing breast cancer cell lines ↑ In vitro cytotoxicity against the folate receptor-expressing breast cancer cell lines compared to free EGCG and non-targeting counterpart ↑ Percentage of early and late apoptotic cells in vitro, which was related to an elevated level of ROS, loss of mitochondrial membrane potential, downregulation of Bcl-2, and upregulation of Bax ↑ In vivo pharmacokinetic profiles and tumor accumulation ↑ Inhibition of breast tumor growth in vivo | [148] |
| i.v. | TPGS-g-HA/FD/PEG-g-gelatin NPs | HA and FD | CD44 and L- or P-selectins | EGCG | Docetaxel | Prostate cancer | PC3 cell line and PC3 tumor-bearing mice | ↑ Anticancer effect in vitro ↑ Cell cycle arrest ↑ Inhibition of tumor growth in vivo | [149] |
| i.v. | Fe3+-doxorubicin@EGCG-PEG NPs | - | - | EGCG | Doxorubicin | Glioma | U87MG and 293T cell lines, Balb/C mice, and U87MG tumor-bearing mice | ↓ Expression of carbonyl reductase 1 and generation of doxorubicinol ↑ in vitro cytotoxicity against cancer cells, and penetration into multicellular spheroids ↑ Tumor accumulation and blood circulation ↓ Hematotoxicity and cardiac toxicity in vivo ↑ Inhibition of tumor growth in vivo | [150] |
| i.t. | PEI/pDNA/HA-EGCG ternary complexes | HA | CD44 | EGCG | pDNA | Colon cancer | HCT-116 and HEK293 cell lines and HCT-116 tumor-bearing mice | ↑ Gene transfection efficiency in vitro ↑ Cellular uptake in CD44-overexpressing cells ↑ Tumor accumulation in vivo | [151] |
| i.p. | PLGA NPs | - | - | EGCG | - | Lung cancer | A549 and H1299 cell lines and patient-derived xenograft bearing nude mice | ↑ In vitro cellular uptake in lung cancer cells ↑ In vitro cytotoxicity against lung cancer cells ↑ Lung cancer cell apoptosis by suppression of NF-κB ↑ Inhibition of lung cancer PDX growth in vivo by enhancing apoptosis, inhibiting proliferation, and downregulation of phosphor-NF-κB | [147] |
| i.p. | Folate and PEG mod ied CS/TPP complex NPs | Folate | Folate receptor | EGCG | - | Breast cancer | MCF-7, MDA-MB-231, HCC-70, 4T1, Panc-1 cell lines, MDA-MB-231 mammosphere, and MDA-MB-231 tumor-bearing nude mice | ↑ In vitro cellular uptake ↑ Expression of CCN5 in vitro ↑ Tumor growth in vivo | [152] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Jiang, Z.; Ma, L.; Huang, Q. Advances in Nanodelivery of Green Tea Catechins to Enhance the Anticancer Activity. Molecules 2021, 26, 3301. https://doi.org/10.3390/molecules26113301
Jiang Y, Jiang Z, Ma L, Huang Q. Advances in Nanodelivery of Green Tea Catechins to Enhance the Anticancer Activity. Molecules. 2021; 26(11):3301. https://doi.org/10.3390/molecules26113301
Chicago/Turabian StyleJiang, Yike, Ziyi Jiang, Lan Ma, and Qingrong Huang. 2021. "Advances in Nanodelivery of Green Tea Catechins to Enhance the Anticancer Activity" Molecules 26, no. 11: 3301. https://doi.org/10.3390/molecules26113301
APA StyleJiang, Y., Jiang, Z., Ma, L., & Huang, Q. (2021). Advances in Nanodelivery of Green Tea Catechins to Enhance the Anticancer Activity. Molecules, 26(11), 3301. https://doi.org/10.3390/molecules26113301






