Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H2O2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway
Abstract
:1. Introduction
2. Results
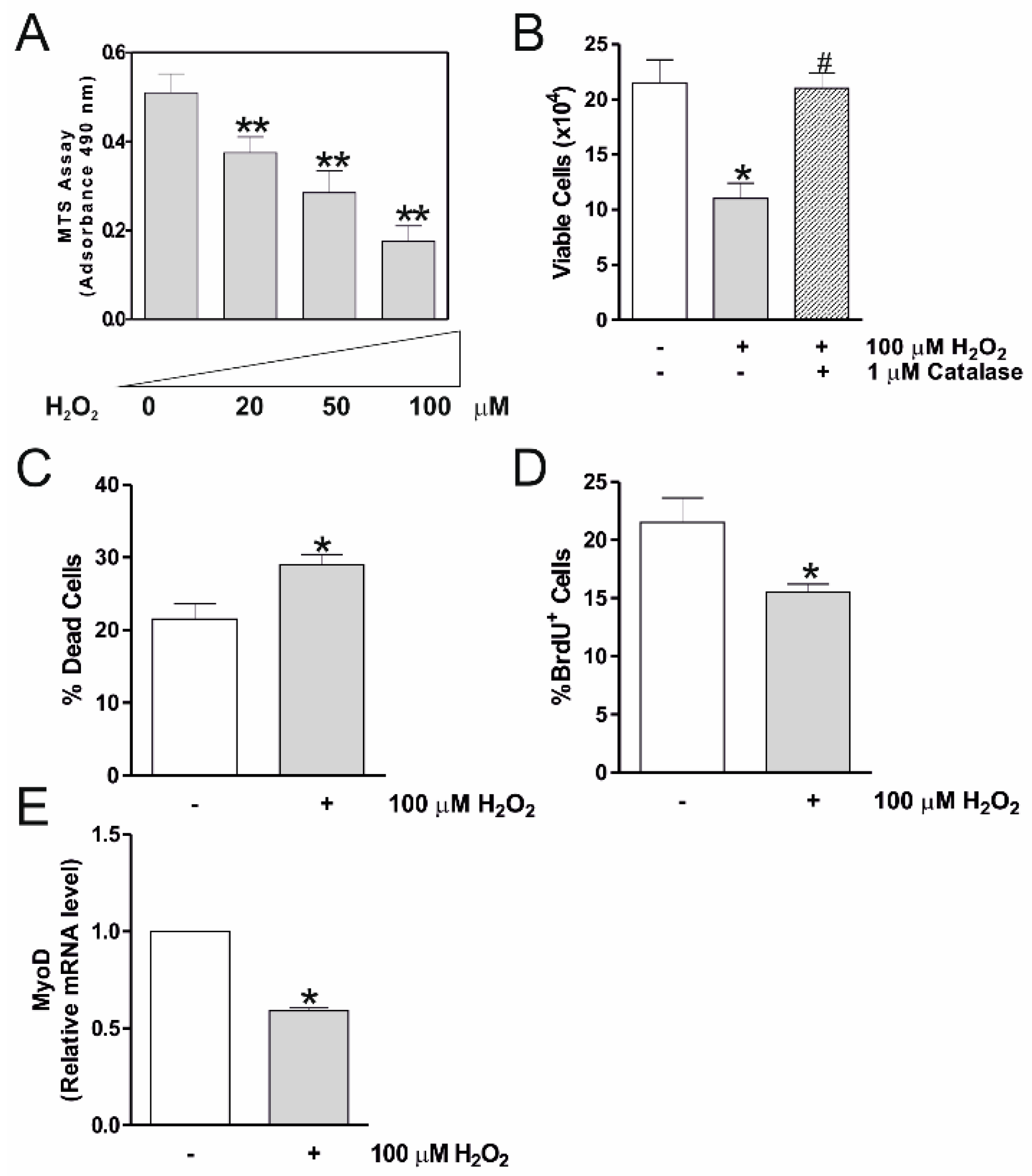
2.1. H2O2 Reduces C2C12 Myocytes Viability and Myogenesis
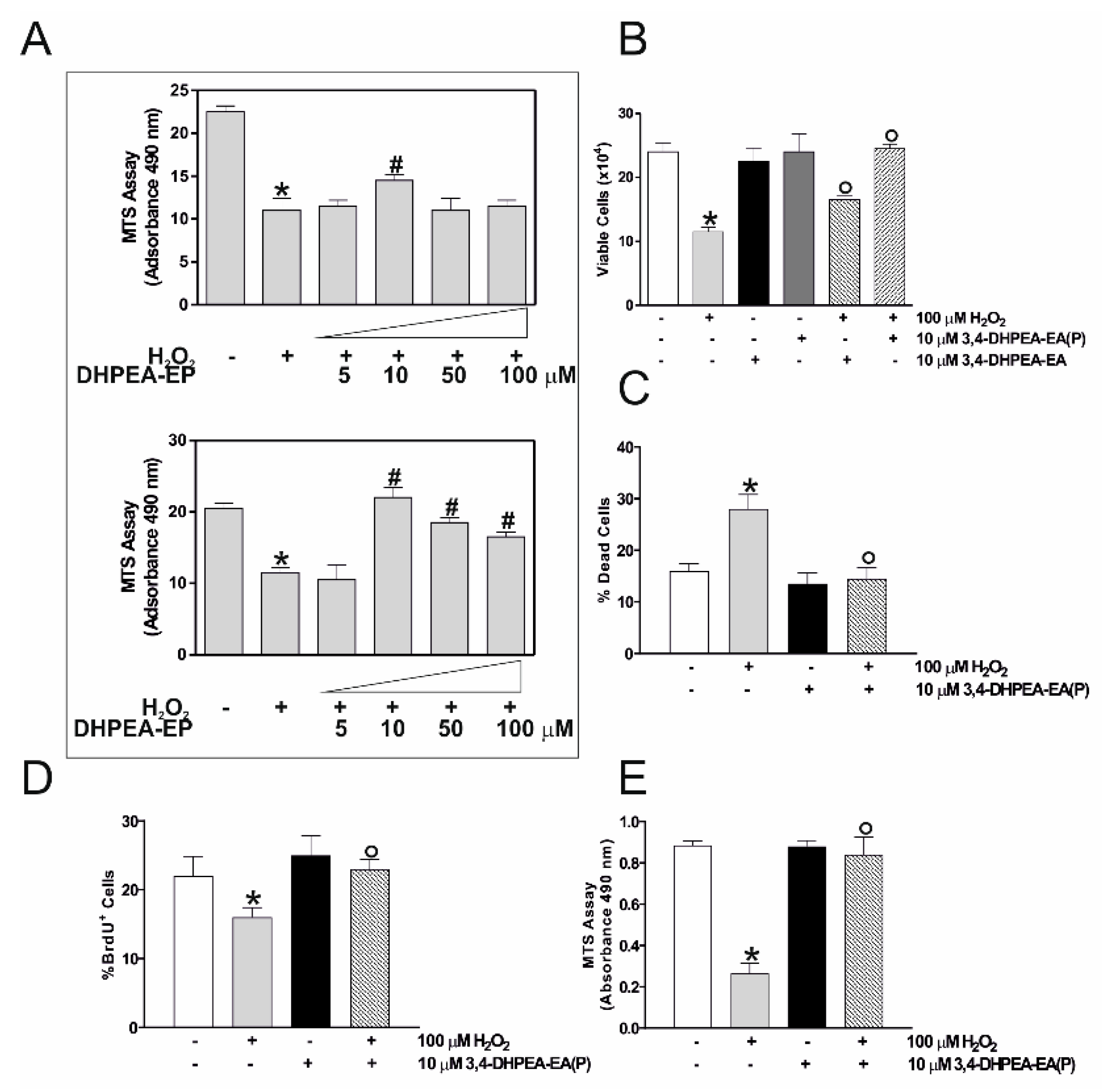
2.2. 3,4-DHPEA-EA(P) Prevents the Cytotoxic Effect of H2O2 in C2C12 Myocytes
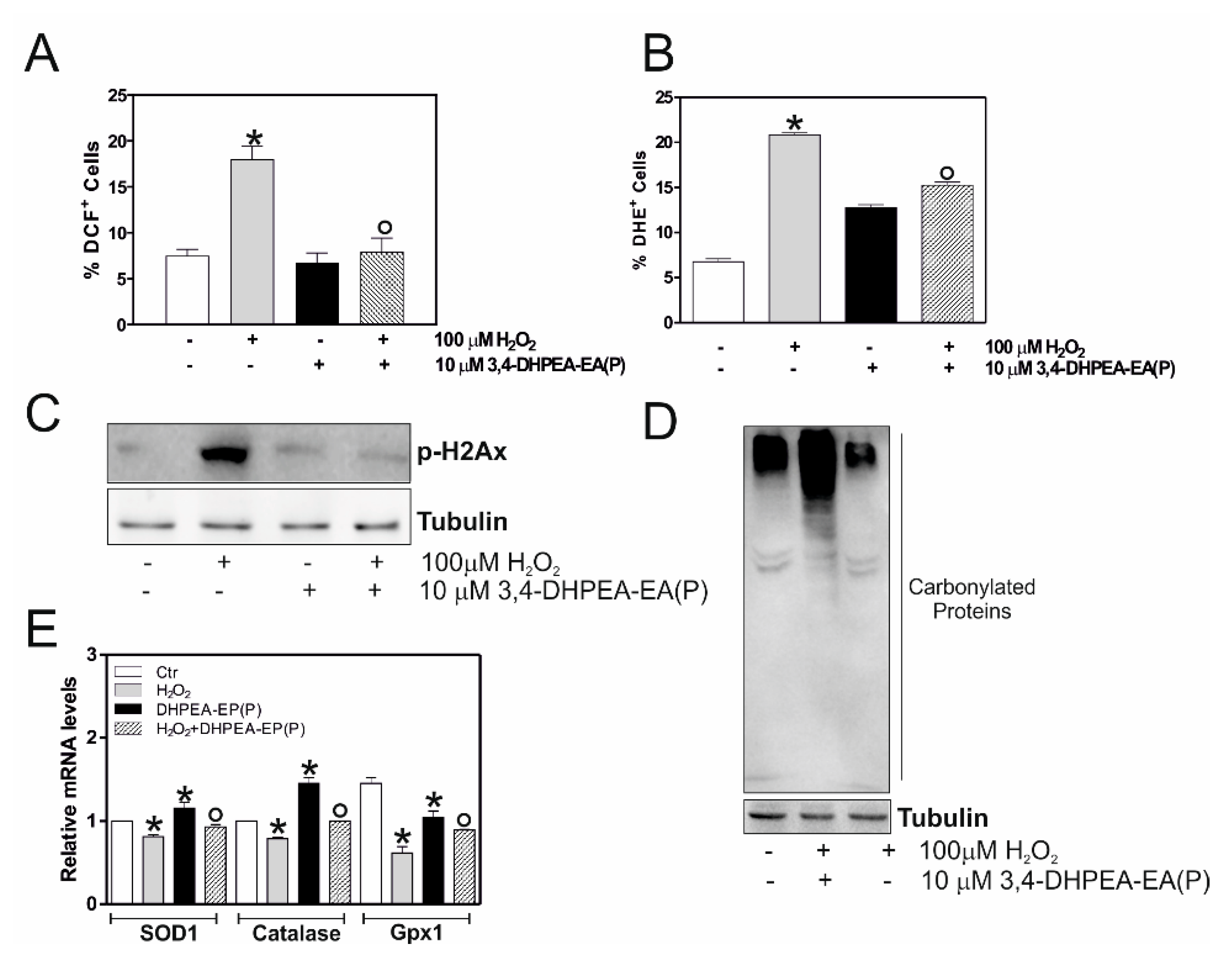
2.3. 3,4-DHPEA-EA(P) Counteracts H2O2-Mediate Oxidative Stress Damage in C2C12 Myocytes
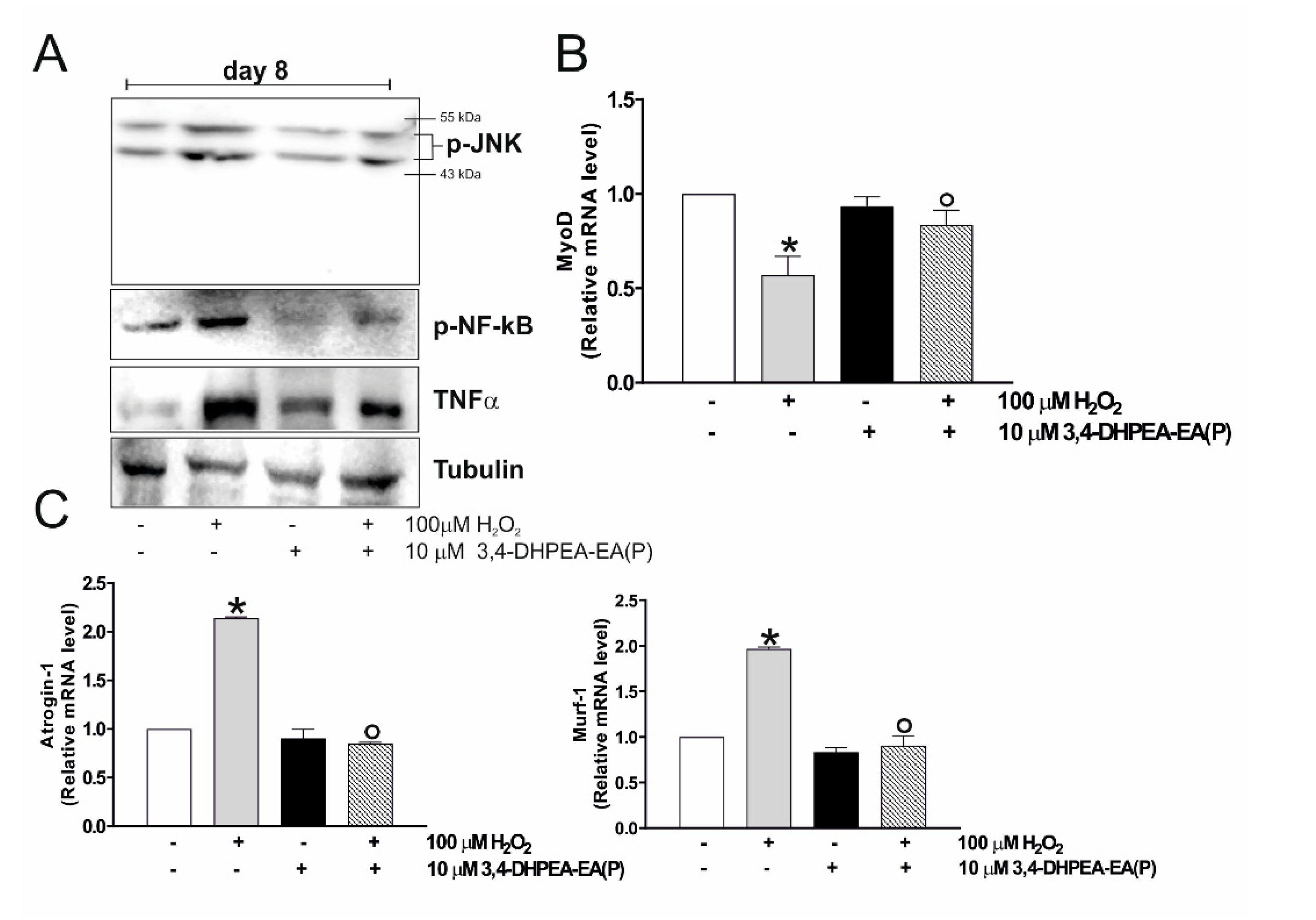
2.4. 3,4-DHPEA-EA(P) Inhibits H2O2-Mediated Decrease of C2C12 Myocytes Viability by Inhibiting p-JNK Signaling Pathway
2.5. 3,4-DHPEA-EA(P) Prevents C2C12 Atrophy Mediated by H2O2 Treatment
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Treatments
4.2. Analysis of Cell Viability and Proliferation
4.3. RT-qPCR Analysis
4.4. Preparation of Cell Lysates and Western Blot Analyses
4.5. Determination of Protein Carbonylation
4.6. Determination of ROS
4.7. Statistical Analysis
4.8. Microwave-Assisted Extraction of Oleuropein from Olive Leaves
4.9. Synthesis of 3,4-DHPEA-EA and 3,4-DHPEA-EA (P)
Author Contributions
Funding
Conflicts of Interest
References
- Barrajón-Catalán, E.; Taamalli, A.; Quirantes-Piné, R.; Roldan-Segura, C.; Arráez-Román, D.; Segura-Carretero, A.; Micol, V.; Zarrouk, M. Differential metabolomic analysis of the potential antiproliferative mechanism of olive leaf extract on the JIMT-1 breast cancer cell line. J. Pharm. Biomed. Anal. 2015, 105, 156–162. [Google Scholar] [PubMed]
- Bulotta, S.; Cellano, M.; Lepore, S.M.; Montalcini, T.; Puija, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [PubMed] [Green Version]
- Visioli, F.; Galli, C. Antiatherogenic components of olive oil. Curr. Atheroscler. Rep. 2001, 3, 64–67. [Google Scholar] [PubMed]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Morittu, V.M.; Procopio, A.; Britti, D.; Cuzzocrea, S. The effects of oleuropein aglycone, an olive oil compound, in a mouse model of carrageenan-induced pleurisy. Clin. Nutr. 2011, 30, 533–540. [Google Scholar] [PubMed]
- Impellizzeri, D.; Esposito, E.; Mazzon, E.; Paterniti, I.; Di Paola, R.; Bramanti, P.; Morittu, V.M.; Procopio, A.; Britti, D.; Cuzzocrea, S. Oleuropein aglycone, an olive oil compound, ameliorates development of arthritis caused by injection of collagen type II in mice. J. Pharmacol. Exp. Ther. 2011, 339, 859–869. [Google Scholar]
- Pantano, D.; Luccarini, I.; Nardiello, P.; Servili, M.; Stefani, M.; Casamenti, F. Oleuropein aglycone and polyphenols from olive mill waste water ameliorate cognitive deficits and neuropathology. Br. J. Clin. Pharmacol. 2017, 83, 54–62. [Google Scholar]
- Procopio, A.; Alcaro, S.; Nardi, M.; Oliverio, M.; Ortuso, F.; Sacchetta, P.; Pieragostino, P.; Sindona, G. Synthesis, biological evaluation, and molecular modeling of oleuropein and its semisynthetic derivatives as cyclooxygenase inhibitors. Synthesis, biological evaluation, and molecular modeling of oleuropein and its semisynthetic derivatives as cyclooxygenase inhibitors. J. Agric. Food Chem. 2009, 57, 11161–11167. [Google Scholar]
- Bonacci, S.; Paonessa, R.; Costanzo, P.; Salerno, R.; Maiuolo, J.; Nardi, M.; Procopio, A.; Oliverio, M. Peracetylation as a strategy to improve oleuropein stability and its affinity to fatty foods. Food Funct. 2018, 9, 5759–5767. [Google Scholar]
- Sindona, G.; Caruso, A.; Cozza, A.; Fiorentini, S.; Lorusso, B.; Marini, E.; Nardi, M.; Procopio, A.; Zicari, S. Anti-inflammatory effect of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl)ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate] on primary human vascular endothelial cells. Curr. Med. Chem. 2012, 19, 4006–4013. [Google Scholar]
- Nardi, M.; Bonacci, S.; De Luca, G.; Maiuolo, J.; Oliverio, M.; Sindona, G.; Procopio, A. Biomimetic synthesis and antioxidant evaluation of 3,4-DHPEA-EDA [2-(3,4-hydroxyphenyl) ethyl (3S,4E)-4-formyl-3-(2-oxoethyl)hex-4-enoate]. Food Chem. 2014, 162, 89–93. [Google Scholar]
- Fujiwara, Y.; Tsukahara, C.; Ikeda, N.; Sone, Y.; Ishikawa, T.; Ichi, I.; Koike, T.; Aoki, Y. Oleuropein improves insulin resistance in skeletal muscle by promoting the translocation of GLUT4. J. Clin. Biochem. Nutr. 2017, 61, 196–202. [Google Scholar] [PubMed] [Green Version]
- Alkhateeb, H.; Al-Duais, M.; Qnais, E. Beneficial effects of oleuropein on glucose uptake and on parameters relevant to the normal homeostatic mechanisms of glucose regulation in rat skeletal muscle. Phytother. Res. 2018, 32, 651–656. [Google Scholar] [PubMed]
- Rodriguez, V.M.; Portillo, M.P.; Pico, C.; Macarulla, M.T.; Palou, A. Olive oil feeding up-regulates uncoupling protein genes in rat brown adipose tissue and skeletal muscle. Am. J. Clin. Nutr. 2002, 75, 213–220. [Google Scholar]
- Baldelli, S.; Ciccarone, F.; Limongi, D.; Checconi, P.; Palmara, A.T.; Ciriolo, M.R. Glutathione and Nitric Oxide: Key Team Players in Use and Disuse of Skeletal Muscle. Nutrients 2019, 11, 2318. [Google Scholar]
- Powers, S.K.; Ji, L.L.; Kavazis, A.N.; Jackson, M.J. Reactive oxygen species: Impact on skeletal muscle. Compr. Physiol. 2011, 1, 941–969. [Google Scholar]
- Powers, S.K.; Jackson, M.J. Exercise-induced oxidative stress: Cellular mechanisms and impact on muscle force production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [PubMed] [Green Version]
- Fulle, S.; Protasi, F.; Di Tano, G.; Pietrangelo, T.; Beltramin, A.; Boncompagni, S.; Vecchiet, L.; Fanò, G. The contribution of reactive oxygen species to sarcopenia and muscle ageing. Exp. Gerontol. 2004, 39, 17–24. [Google Scholar]
- Muller, F.L.; Song, W.; Jang, Y.C.; Liu, Y.; Sabia, M.; Richardson, A.; Van Remmen, H. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1159–R1168. [Google Scholar]
- Muhammad, M.H.; Allam, M.M. Resveratrol and/or exercise training counteract aging-associated decline of physical endurance in aged mice; targeting mitochondrial biogenesis and function. J. Physiol. Sci. 2018, 68, 681–688. [Google Scholar]
- Pierno, S.; Tricarico, D.; Liantonio, A.; Mele, A.; Digennaro, C.; Rolland, J.F.; Bianco, G.; Villanova, L.; Marendino, A.; Camerino, G.; et al. An olive oil-derived antioxidant mixture ameliorates the age-related decline of skeletal muscle function. Age Dordr. 2014, 36, 73–88. [Google Scholar]
- Hadrich, F.; Garcia, M.; Maalej, A.; Moldes, M.; Isoda, H.; Feve, B.; Sayadi, S. Oleuropein activated AMPK and induced insulin sensitivity in C2C12 muscle cells. Life Sci. 2016, 151, 167–173. [Google Scholar] [PubMed] [Green Version]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid Based Complement. Alternat. Med. 2014, 971890, 1–12. [Google Scholar]
- Cao, K.; Xu, J.; Zou, X.; Li, Y.; Chen, C.; Zheng, A.; Li, H.; Szeto, I.M.Y.; Shi, Y.; Long, J.; et al. Hydroxytyrosol prevents diet-induced metabolic syndrome and attenuates mitochondrial abnormalities in obese mice. Free Radic. Biol. Med. 2014, 67, 396–407. [Google Scholar] [PubMed]
- Bosutti, A.; Degens, H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci. Rep. 2015, 5, 1–13. [Google Scholar]
- Siu, P.M.; Wang, Y.; Alway, S.E. Apoptotic signaling induced by H2O2-mediated oxidative stress in differentiated C2C12 myotubes. Life Sci. 2009, 84, 468–481. [Google Scholar]
- Caporossi, D.; Ciaffrè, S.A.; Pittaluga, M.; Farace, M.G. Cellular responses to H2O2 and bleomycin-induced oxidative stress in L6C5 rat myoblasts. Free Radic. Bio. Med. 2003, 35, 1355–1364. [Google Scholar]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the olive-derived polyphenol oleuropein on human health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar]
- Nardi, M.; Bonacci, S.; Cariati, L.; Costanzo, P.; Oliverio, M.; Sindona, G.; Procopio, A. Synthesis and antioxidant evaluation of lipophilic oleuropein aglycone derivatives. Food Funct. 2017, 8, 4684–4692. [Google Scholar]
- Wilson, E.M.; Rotwein, P. Control of MyoD function during initiation of muscle differentiation by an autocrine signaling pathway activated by insulin-like growth factor-II. J. Biol. Chem. 2006, 281, 29962–29971. [Google Scholar]
- Aquilano, K.; Baldelli, S.; Cardaci, S.; Rotilio, G.; Ciriolo, M.R. Nitric oxide is the primary mediator of cytotoxicity induced by GSH depletion in neuronal cells. J. Cell Sci. 2011, 124, 1043–1054. [Google Scholar]
- Demontis, F.; Piccirillo, R.; Goldberg, A.L.; Perrimon, N. Mechanisms of skeletal muscle aging: Insights from Drosophila and mammalian models. Dis. Models Mech. 2013, 6, 1339–1352. [Google Scholar]
- Barreiro, E.; Hussain, S.N.A. Protein Carbonylation in Skeletal Muscles: Impact on Function. Antioxid. Redox Signal 2010, 12, 417–429. [Google Scholar] [PubMed]
- Xie, S.J.; Li, J.H.; Chen, H.F.; Tan, Y.Y.; Liu, S.R.; Zheng, L.L.; Huang, M.B.; Guo, Y.H.; Zhang, Q.; Zhou, H.; et al. Inhibition of the JNK/MAPK signaling pathway by myogenesis-associated miRNAs is required for skeletal muscle development. Cell Death Differ 2018, 25, 1581–1597. [Google Scholar] [PubMed] [Green Version]
- Bogoyevitch, M.A.; Kobe, B. Uses for JNK: The many and varied substrates of the c-Jun N-terminal kinases. Microbiol. Mol. Biol. Rev. 2006, 70, 1061–1095. [Google Scholar] [PubMed] [Green Version]
- Limongi, D.; Bldelli, S.; Checconi, P.; Marcocci, M.E.; De Chiara, G.; Fraternale, A.; Magnani, M.; Ciriolo, M.R.; Palamara, A.T. Corrigendum: GSH-C4 Acts as Anti-inflammatory Drug in Different Models of Canonical and Cell Autonomous Inflammation Through NF kappa B Inhibition. Front Immunol. 2019, 10, 1–14. [Google Scholar]
- Baldelli, S.; Ciriolo, M.R. Altered S-nitrosylation of p53 is responsible for impaired antioxidant response in skeletal muscle during aging. Aging 2016, 8, 3450–3462. [Google Scholar]
- Michaelson, L.P.; Iler, C.; Ward, C.W. ROS and RNS signaling in skeletal muscle: Critical signals and therapeutic targets. Annu. Rev. Nurs. Res. 2013, 31, 367–387. [Google Scholar]
- Powers, S.K.; Talbert, E.E.; Adhihetty, P.J. Reactive oxygen and nitrogen species as intracellular signals in skeletal muscle. J. Physiol. 2011, 589, 2129–2138. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar]
- McCalley, A.E.; Kaja, S.; Payne, A.J.; Koulen, P. Resveratrol and calcium signaling: Molecular mechanisms and clinical relevance. Molecules 2014, 19, 7327–7340. [Google Scholar]
- Burattini, S. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar]
- Vlavcheski, F.; Young, M.; Tsiani, E. Antidiabetic Effects of Hydroxytyrosol: In Vitro and In Vivo Evidence. Antioxidants 2019, 8, 188. [Google Scholar]
- Kikusato, M.; Muroi, H.; Uwabe, Y.; Furukawa, K.; Toyomuzu, M. Oleuropein induces mitochondrial biogenesis and decreases reactive oxygen species generation in cultured avian muscle cells, possibly via an up-regulation of peroxisome proliferator-activated receptor gamma coactivator-1alpha. Anim. Sci. J. 2016, 87, 1371–1378. [Google Scholar] [PubMed]
- Zhang, S.; Lin, Y.; Kim, Y.S.; Hande, M.P.; Liu, Z.G.; Shen, H.M. c-Jun N-terminal kinase mediates hydrogen peroxide-induced cell death via sustained poly(ADP-ribose) polymerase-1 activation. Cell Death Differ. 2007, 14, 1001–1010. [Google Scholar]
- Pagliei, B.; Aquilano, K.; Baldelli, S.; Ciriolo, M.R. Garlic-derived diallyl disulfide modulates peroxisome proliferator activated receptor gamma co-activator 1 alpha in neuroblastoma cells. Biochem. Pharmacol. 2013, 85, 335–344. [Google Scholar] [PubMed]
- Kim, H.; Lee, K., II; Jang, M.; Namkoong, S.; Park, R.; Ju, H.; Choi, I.; Oh, W.K.; Park, J. Conessine Interferes with Oxidative Stress-Induced C2C12 Myoblast Cell Death through Inhibition of Autophagic Flux. PLoS ONE 2016, 11, e0157096. [Google Scholar]
- Santa-Gonzalez, G.A.; Gomez-Molina, A.; Arcos-Burgos, M.; Meyer, J.N.; Camargo, M. Distinctive adaptive response to repeated exposure to hydrogen peroxide associated with upregulation of DNA repair genes and cell cycle arrest. Redox Biol. 2016, 9, 124–133. [Google Scholar]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.M.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX phosphorylation: A marker for DNA damage. Methods Mol. Biol. 2012, 920, 613–626. [Google Scholar]
- Berkes, C.A.; Tapscott, S.J. MyoD and the transcriptional control of myogenesis. Semin. Cell Dev. Biol. 2005, 16, 585–595. [Google Scholar]
- Mitchelson, K.R.; Qin, W.Y. Roles of the canonical myomiRs miR-1, -133 and -206 in cell development and disease. World J. Biol. Chem. 2015, 6, 162–208. [Google Scholar] [PubMed]
- Karkovic Markovic, A.; Toric, J.; Barbaric, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 17–24. [Google Scholar]
- Li, G.; Luo, W.; Abdalla, B.A.; Ouyang, H.; Yu, J.; Hu, F.; Nie, Q.; Zhang, X. miRNA-223 upregulated by MYOD inhibits myoblast proliferation by repressing IGF2 and facilitates myoblast differentiation by inhibiting ZEB1. Cell Death Dis. 2017, 8, 3094–3107. [Google Scholar]
- Iannone, F.; Montesanto, A.; Cione, E.; Crocco, P.; Caroleo, M.C.; Dato, S.; Rose, G.; Passarino, G. Expression Patterns of Muscle-Specific miR-133b and miR-206 Correlate with Nutritional Status and Sarcopenia. Nutrients 2020, 12, 297–311. [Google Scholar]
- Aquilano, K.; Baldelli, S.; Pagliei, B.; Cannata, S.M.; Rotilio, G.; Ciriolo, M.R. p53 orchestrates the PGC-1alpha-mediated antioxidant response upon mild redox and metabolic imbalance. Antioxid. Redox Signal 2013, 18, 386–399. [Google Scholar]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Sign. 2018, 29, 1727–1745. [Google Scholar]
- Dong, J.; Sulik, K.K.; Chen, S.Y. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: Implications for the prevention of fetal alcohol spectrum disorders. Antioxid. Redox Sign. 2008, 10, 2023–2033. [Google Scholar]
- Zou, Y.; Wang, J.; Peng, J.; Wei, H.K. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxid. Med. Cell Longev. 2016, 1–16. [Google Scholar] [CrossRef]
- Aquilano, K.; Baldelli, S.; La Barbera, L.; Lettieri Barbato, D.; Tatulli, G.; Ciriolo, M.R. Adipose triglyceride lipase decrement affects skeletal muscle homeostasis during aging through FAs-PPARalpha-PGC-1alpha antioxidant response. Oncotarget 2016, 7, 23019–23032. [Google Scholar]
- Filomeni, G.; Aquilano, K.; Civitareale, P.; Rotilio, G.; Ciriolo, M.R. Activation of c-Jun-N-terminal kinase is required for apoptosis triggered by glutathione disulfide in neuroblastoma cells. Free Radic. Biol. Med. 2005, 39, 345–354. [Google Scholar]
- Baldelli, S.; Aquilano, K.; Rotilio, G.; Ciriolo, M.R. Glutathione and copper, zinc superoxide dismutase are modulated by overexpression of neuronal nitric oxide synthase. Int. J. Biochem. Cell Biol. 2008, 40, 2660–2670. [Google Scholar] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Bandall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Mazzei, R.; Piacentini, E.; Nardi, M.; Poerio, T.; Bazzarelli, F.; Procopio, A.; Di Gioia, M.L.; Rizza, P.; Ceraldi, R.; Morelli, C.; et al. Production of Plant-Derived Oleuropein Aglycone by a Combined Membrane Process and Evaluation of Its Breast Anticancer Properties. Front. Bioeng. Biotechnol. 2020, 8, 908–933. [Google Scholar]


| Genes | Sequences |
|---|---|
| mMyoD FW mMyoD RV | 5′-GGGGCCGCTGTAATCCATCATGC-3′ 5′-GGAGATCCTGCGCAACGCCA-3′ |
| mAtrogin-1 FW mAtrogin-1 RV | 5′-GCGACCTTCCCCAACGCCTG-3′ 5′-GGCGACCGGGACAAGAGTGG-3′ |
| mMurf-1 FW mMurf-1 RV | 5′-AGGGGCTACCTTCCTCTAAGTG-3′ 5′-TCTTCCCCAGCTGGCAGCCC-3′ |
| mRPL FW mRPL RV | 5′-GTACGACCACCACCTTCCGGC-3′ 5′-ATGGCGGAGGGGCAGGTTCTG-3′ |
| mGpx1 FW mGpx1 RV | 5′-CAGCCGGAAAGAAAGCGATG-3′ 5′-TTGCCATTCTGGTGTCCGAA-3′ |
| mCatalase FW mCatalase RV | 5′-CCGACCAGGGCATCAAAA-3′ 5′-GAGGCCATAATCCGGATCTTC-3′ |
| mSOD1 FW mSOD1 RV | 5′-TCTTCAGCCTGCACTGAAGT-3′ 5′-ACTGAAGGTAGTAAGCGTGC-3′ |
Sample Availability: Samples of the compounds: 3,4-DHPEA-EA and 3,4-DHPEA-EA (P) are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nardi, M.; Baldelli, S.; Ciriolo, M.R.; Costanzo, P.; Procopio, A.; Colica, C. Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H2O2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway. Molecules 2020, 25, 5472. https://doi.org/10.3390/molecules25225472
Nardi M, Baldelli S, Ciriolo MR, Costanzo P, Procopio A, Colica C. Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H2O2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway. Molecules. 2020; 25(22):5472. https://doi.org/10.3390/molecules25225472
Chicago/Turabian StyleNardi, Monica, Sara Baldelli, Maria Rosa Ciriolo, Paola Costanzo, Antonio Procopio, and Carmela Colica. 2020. "Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H2O2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway" Molecules 25, no. 22: 5472. https://doi.org/10.3390/molecules25225472
APA StyleNardi, M., Baldelli, S., Ciriolo, M. R., Costanzo, P., Procopio, A., & Colica, C. (2020). Oleuropein Aglycone Peracetylated (3,4-DHPEA-EA(P)) Attenuates H2O2-Mediated Cytotoxicity in C2C12 Myocytes via Inactivation of p-JNK/p-c-Jun Signaling Pathway. Molecules, 25(22), 5472. https://doi.org/10.3390/molecules25225472










