(E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives as Tyrosinase Inhibitors and Melanogenesis Inhibition: An In Vitro and In Silico Study
Abstract
1. Introduction
2. Results and Discussion
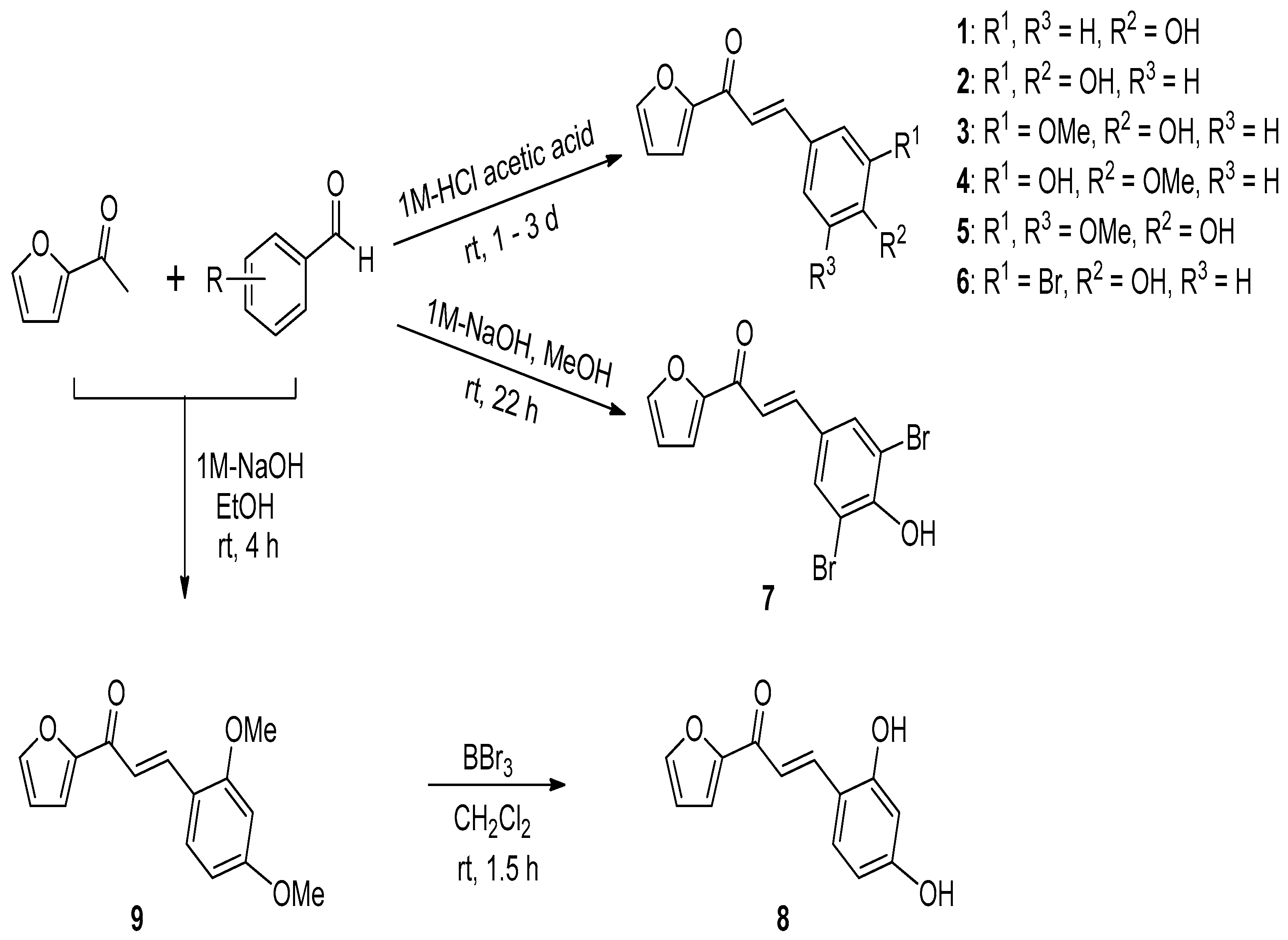
2.1. Procedure for the Synthesis of (E)-1-(Furan-2-yl)-3-(substituted phenyl)prop-2-en-1-one Derivatives 1–9
2.2. Mushroom Tyrosinase Inhibitory Activities of (E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives (Compounds 1–8)
2.3. Enzyme Kinetics Mechanism Study
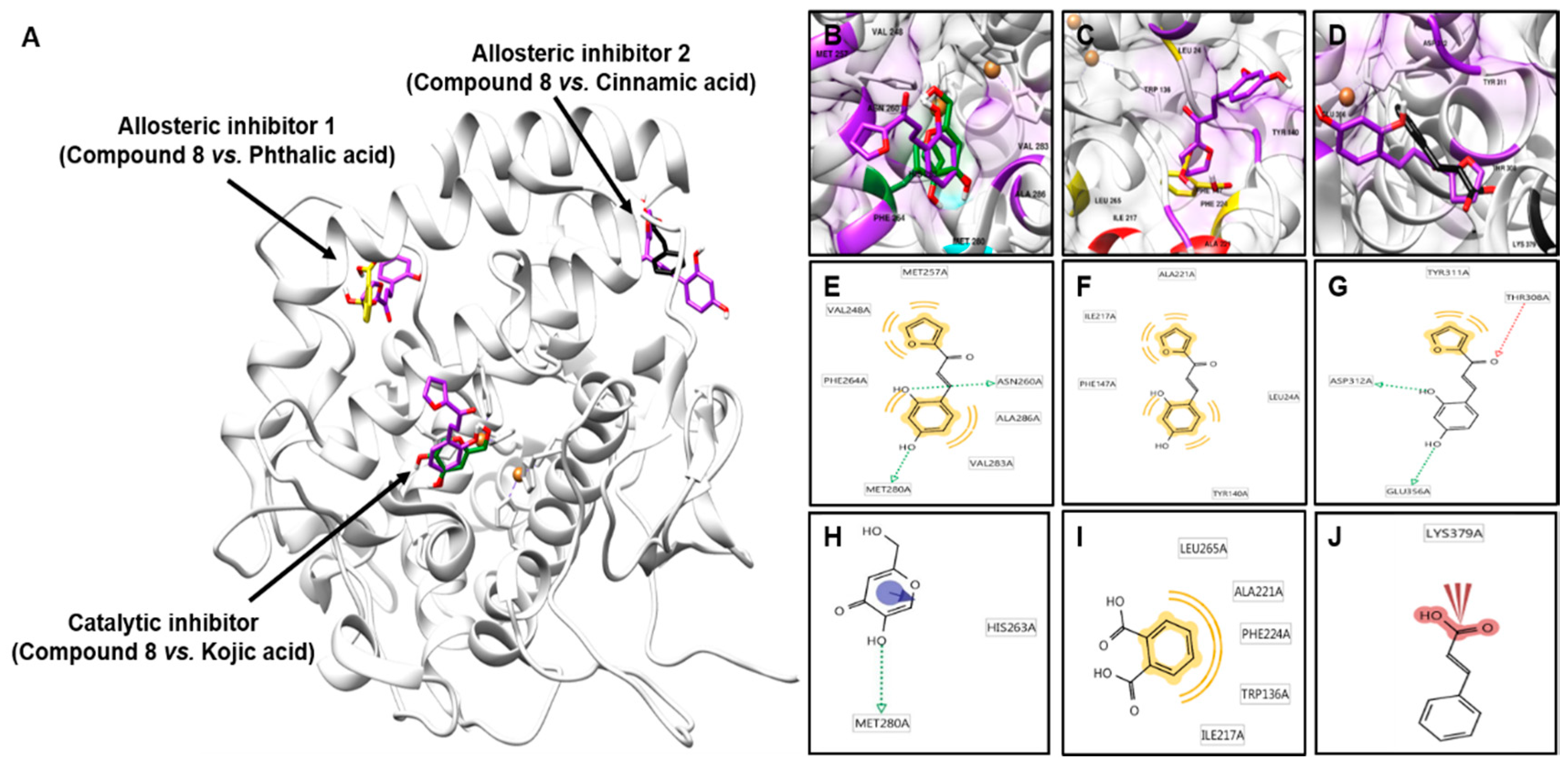
2.4. Molecular Docking Simulation of Compound 8 with Mushroom Tyrosinase
2.5. Molecular Dynamics (MD) Simulation Analyses
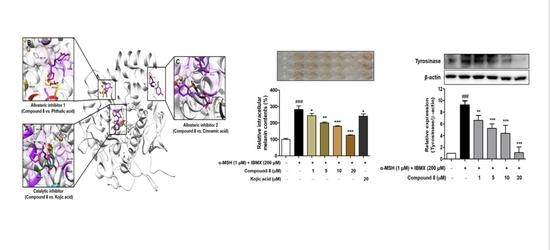
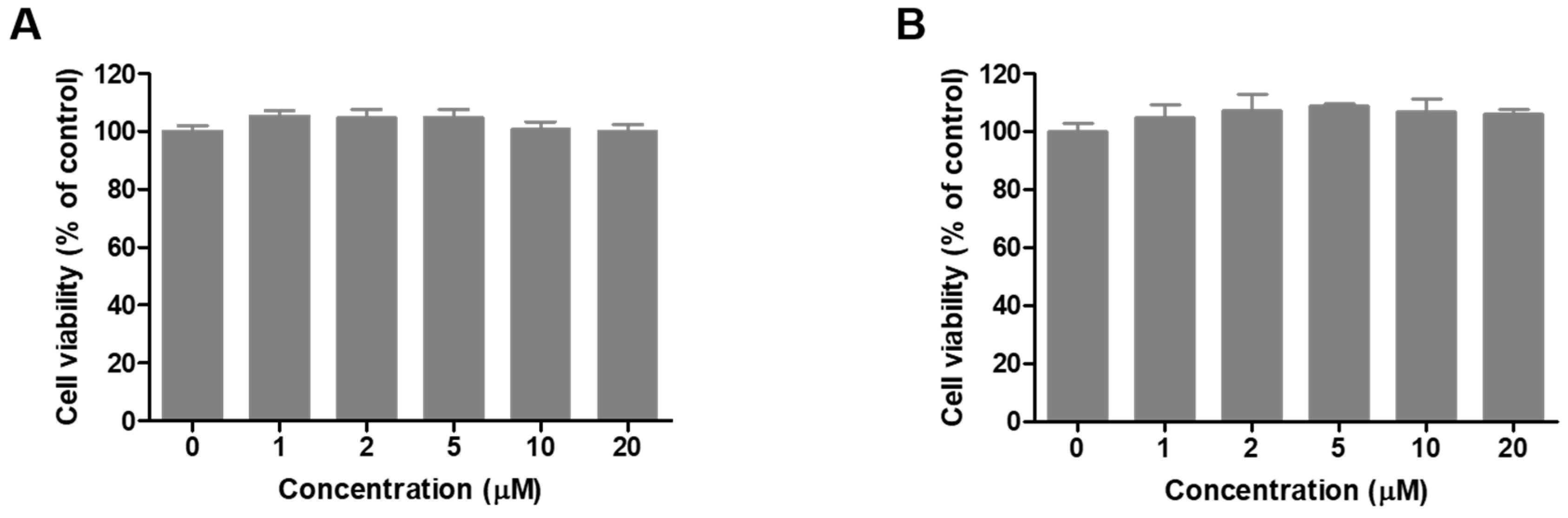
2.6. Cell Viability of Compound 8 in B16F10 Cells
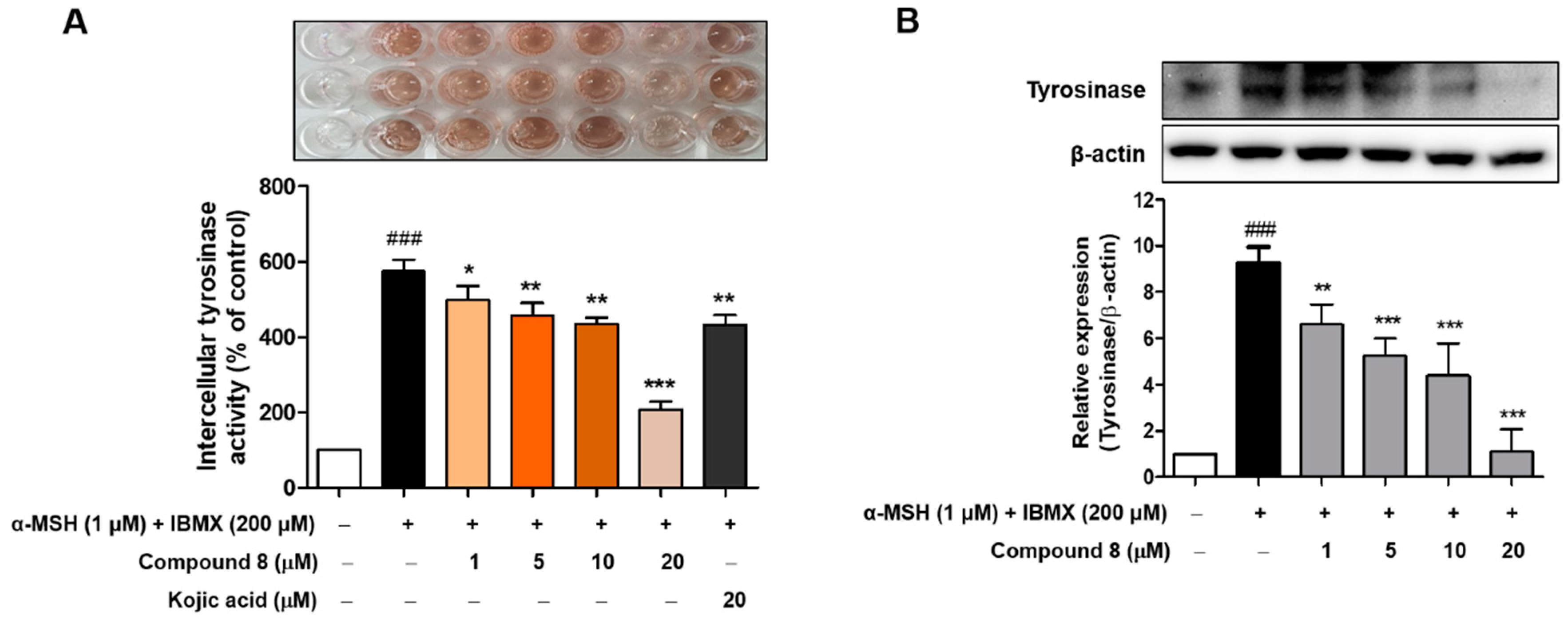
2.7. Melanin Contents Measurement of Compound 8
2.8. Cellular Tyrosinase Activities and Tyrosinase Protein Levels of Compound 8
3. Material and Methods
3.1. Chemicals and Reagents
3.2. General Experimental Procedures
3.2.1. General Procedure for the Preparation of (E)-1-(Furan-2-yl)-3-(substituted phenyl)prop-2-en-1-one Derivatives 1–6
(E)-1-(Furan-2-yl)-3-(4-hydroxyphenyl)prop-2-en-1-one (1)
(E)-3-(3,4-Dihydroxyphenyl)-1-(furan-2-yl)prop-2-en-1-one (2)
(E)-1-(Furan-2-yl)-3-(4-hydroxy-3-methoxyphenyl)prop-2-en-1-one (3)
(E)-1-(Furan-2-yl)-3-(3-hydroxy-4-methoxyphenyl)prop-2-en-1-one (4)
(E)-1-(Furan-2-yl)-3-(4-hydroxy-3,5-dimethoxyphenyl)prop-2-en-1-one (5)
(E)-3-(3-Bromo-4-hydroxyphenyl)-1-(furan-2-yl)prop-2-en-1-one (6)
3.2.2. Synthesis of Compound 7
(E)-3-(3,5-Dibromo-4-hydroxyphenyl)-1-(furan-2-yl)prop-2-en-1-one (7)
3.2.3. Synthesis of Compound 8
(E)-3-(2,4-Dihydroxyphenyl)-1-(furan-2-yl)prop-2-en-1-one (8)
3.2.4. Synthesis of Compound 9
(E)-3-(2,4-Dimethoxyphenyl)-1-(furan-2-yl)prop-2-en-1-one (9)
3.3. Mushroom Tyrosinase Inhibition Assay
3.4. Enzyme Kinetics Analysis of the Inhibition of Tyrosinase
3.5. Computational Study
3.5.1. Docking on the Mushroom Tyrosinase
3.5.2. Molecular Dynamics (MD) Analyses
3.6. Bioactivity
3.6.1. Cell Culture and Cell Viability
3.6.2. Extracellular and Intracellular Melanin Contents Assay
3.6.3. Cellular Tyrosinase Activity
3.6.4. Western Blot Analysis
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Videira, I.F.; Moura, D.F.; Magina, S. Mechanisms regulating melanogenesis. An. Bras. Dermatol. 2013, 88, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Riley, P.A. Mechanistic Aspects of the Control of Tyrosinase Activity. Pigment. Cell Res. 1993, 6, 182–185. [Google Scholar] [CrossRef] [PubMed]
- García-Borrón, J.C.; Solano, F. Molecular anatomy of tyrosinase and its related proteins: Beyond the histidine-bound metal catalytic center. Pigment. Cell Res. 2002, 15, 162–173. [Google Scholar]
- Hu, S.; Zheng, Z.; Zhang, X.; Chen, F.; Wang, M. Oxyresveratrol and trans-dihydromorin from the twigs of Cudrania tricuspidata as hypopigmenting agents against melanogenesis. J. Funct. Foods 2015, 13, 375–383. [Google Scholar] [CrossRef]
- Lai, X.; Wichers, H.J.; Soler-Lopez, M.; Dijkstra, B.W. Structure and Function of Human Tyrosinase and Tyrosinase-Related Proteins. Chem.– Eur. J. 2018, 24, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Ferrer, Á.; Neptuno Rodríguez-López, J.; García-Cánovas, F.; García-Carmona, F. Tyrosinase: A comprehensive review of its mechanism. Biochim. Biophys. Acta 1995, 1247, 1–11. [Google Scholar] [CrossRef]
- Nerya, O.; Vaya, J.; Musa, R.; Izrael, S.; Ben-Arie, R.; Tamir, S. Glabrene and Isoliquiritigenin as Tyrosinase Inhibitors from Licorice Roots. J. Agric. Food Chem. 2003, 51, 1201–1207. [Google Scholar] [CrossRef]
- Slominski, A.; Tobin, D.J.; Shibahara, S.; Wortsman, J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004, 84, 1155–1228. [Google Scholar] [CrossRef]
- Andersen, F.A.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; Snyder, P.W. Final Amended Safety Assessment of Hydroquinone as Used in Cosmetics. Int. J. Toxicol. 2010, 29, 274S–287S. [Google Scholar] [CrossRef]
- Saito, Y.; Kishimoto, M.; Yoshizawa, Y.; Kawaii, S. Synthesis and structure-activity relationship studies of furan-ring fused chalcones as antiproliferative agents. Anticancer Res. 2015, 35, 811–817. [Google Scholar]
- Liew, C.Y.; Lam, K.W.; Kim, M.K.; Harith, H.H.; Tham, C.L.; Cheah, Y.K.; Sulaiman, M.R.; Lajis, N.H.; Israf, D.A. Effects of 3-(2-Hydroxyphenyl)-1-(5-methyl-furan-2-y-l) propenone (HMP) upon signalling pathways of lipopolysaccharide-induced iNOS synthesis in RAW 264.7 cells. Int. Immunopharmacol. 2011, 11, 85–95. [Google Scholar] [CrossRef]
- Robinson, S.J.; Petzer, J.P.; Petzer, A.; Bergh, J.J.; Lourens, A.C.U. Selected furanochalcones as inhibitors of monoamine oxidase. Bioorg. Med. Chem. Lett. 2013, 23, 4985–4989. [Google Scholar] [CrossRef]
- Minders, C.; Petzer, J.P.; Petzer, A.; Lourens, A.C.U. Monoamine oxidase inhibitory activities of heterocyclic chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 5270–5276. [Google Scholar] [CrossRef]
- Suresh, J.; Baek, S.C.; Ramakrishnan, S.P.; Kim, H.; Mathew, B. Discovery of potent and reversible MAO-B inhibitors as furanochalcones. Int. J. Biol. Macromol. 2018, 108, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Solomon, V.R.; Lee, H. Anti-breast cancer activity of heteroaryl chalcone derivatives. Biomed. Pharmacother 2012, 66, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.A.N.; Tintino, S.R.; Teixeira, A.M.R.; Bandeira, P.N.; Santos, H.S.; Cruz, B.G.; Nogueira, C.E.S.; Moura, T.F.; Pereira, R.L.S.; Sena, D.M., Jr.; et al. Potentiation of antibiotic activity by chalcone (E)-1-(4’-aminophenyl)-3-(furan-2-yl)-prop-2-en-1-one against gram-positive and gram-negative MDR strains. Microb. Pathog. 2020, 148, 104453. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Jiang, S.M.; Chen, Z.H.; Ye, B.J.; Piao, H.R. Synthesis and anti-bacterial activity of some heterocyclic chalcone derivatives bearing thiofuran, furan, and quinoline moieties. Arch. Pharm. 2011, 344, 689–695. [Google Scholar] [CrossRef]
- El-Sayed, Y.S.; Gaber, M. Studies on chalcone derivatives: Complex formation, thermal behavior, stability constant and antioxidant activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 137, 423–431. [Google Scholar] [CrossRef]
- Ismail, N.I.; Ming-Tatt, L.; Lajis, N.; Akhtar, M.N.; Akira, A.; Perimal, E.K.; Israf, D.A.; Sulaiman, M.R. Antinociceptive Effect of 3-(2,3-Dimethoxyphenyl)-1-(5-methylfuran-2-yl)prop-2-en-1-one in Mice Models of Induced Nociception. Molecules 2016, 21, 1077. [Google Scholar] [CrossRef]
- Liu, J.; Chen, C.; Wu, F.; Zhao, L. Microwave-Assisted Synthesis and Tyrosinase Inhibitory Activity of Chalcone Derivatives. Chem. Biol. Drug Des. 2013, 82, 39–47. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Shimmon, R.; Conn, C.; Baker, A. Design, synthesis and biological evaluation of hydroxy substituted amino chalcone compounds for antityrosinase activity in B16 cells. Bioorg. Chem. 2015, 62, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, S.K.; Shimmon, R.G.; Conn, C.; Baker, A.T. Evaluation of Novel Chalcone Oximes as Inhibitors of Tyrosinase and Melanin Formation in B16 Cells. Arch. Pharm. 2016, 349, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Barros, M.R.; Menezes, T.M.; da Silva, L.P.; Pires, D.S.; Princival, J.L.; Seabra, G.; Neves, J.L. Furan inhibitory activity against tyrosinase and impact on B16F10 cell toxicity. Int. J. Biolog. Macromol. 2019, 136, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Jung, H.J.; Moon, H.R. A Potent Tyrosinase Inhibitor, (E)-3-(2,4-Dihydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one, with Anti-Melanogenesis Properties in α-MSH and IBMX-Induced B16F10 Melanoma Cells. Molecules 2018, 23, 2725. [Google Scholar] [CrossRef]
- Ashraf, Z.; Rafiq, M.; Nadeem, H.; Hassan, M.; Afzal, S.; Waseem, M.; Afzal, K.; Latip, J. Carvacrol derivatives as mushroom tyrosinase inhibitors; synthesis, kinetics mechanism and molecular docking studies. PLoS ONE 2017, 12, e0178069. [Google Scholar] [CrossRef]
- Şöhretoğlu, D.; Sari, S.; Barut, B.; Özel, A. Tyrosinase inhibition by some flavonoids: Inhibitory activity, mechanism by in vitro and in silico studies. Bioorg. Chem. 2018, 81, 168–174. [Google Scholar]
- Hassani, S.; Haghbeen, K.; Fazli, M. Non-specific binding sites help to explain mixed inhibition in mushroom tyrosinase activities. Eur. J. Med. Chem. 2016, 122, 138–148. [Google Scholar] [CrossRef]
- Khatib, S.; Nerya, O.; Musa, R.; Shmuel, M.; Tamir, S.; Vaya, J. Chalcones as potent tyrosinase inhibitors: The importance of a 2,4-substituted resorcinol moiety. Bioorg. Med. Chem. 2005, 13, 433–441. [Google Scholar] [CrossRef]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors. Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef]
- Ito, Y.; Mitani, T.; Harada, N.; Isayama, A.; Tanimori, S.; Takenaka, S.; Nakano, Y.; Inui, H.; Yamaji, R. Identification of carbonyl reductase 1 as a resveratrol-binding protein by affinity chromatography using 4’-amino-3,5-dihydroxy-trans-stilbene. J. Nutr. Sci. Vitaminol. 2013, 59, 358–364. [Google Scholar] [CrossRef][Green Version]
- Yin, S.-J.; Si, Y.-X.; Qian, G.-Y. Inhibitory effect of phthalic Acid on tyrosinase: The mixed-type inhibition and docking simulations. Enzyme Res. 2011, 2011, 294724. [Google Scholar] [CrossRef] [PubMed]
- Song, K.K.; Chen, Q.X.; Wang, Q.; Qiu, L.; Huang, H. Inhibitory effects of 4-vinylbenzaldehyde and 4-vinylbenzoic acid on the activity of mushroom tyrosinase. J. Enzyme Inhib. Med. Chem. 2005, 20, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Battaini, G.; Monzani, E.; Casella, L.; Santagostini, L.; Pagliarin, R. Inhibition of the catecholase activity of biomimetic dinuclear copper complexes by kojic acid. J. Biol. Inorg. Chem. 2000, 5, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Picot-Allain, M.C.N.; Zengin, G.; Llorent-Martínez, E.J.; Abdullah, H.H.; Ak, G.; Senkardes, I.; Chiavaroli, A.; Menghini, L.; Recinella, L.; et al. Phytochemical Analysis, Network Pharmacology and in silico Investigations on Anacamptis pyramidalis Tuber Extracts. Molecules 2020, 25, 2422. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kosmadaki, M.; Yaar, M.; Gilchrest, B.A. Cellular mechanisms regulating human melanogenesis. Cell Mol. Life Sci. 2009, 66, 1493–1506. [Google Scholar] [CrossRef]
- Di Petrillo, A.; González-Paramás, A.M.; Era, B.; Medda, R.; Pintus, F.; Santos-Buelga, C.; Fais, A. Tyrosinase inhibition and antioxidant properties of Asphodelus microcarpus extracts. BMC Complement. Altern. Med. 2016, 16, 453. [Google Scholar] [CrossRef]
- Jung, H.J.; Noh, S.G.; Park, Y.; Kang, D.; Chun, P.; Chung, H.Y.; Moon, H.R. In vitro and in silico insights into tyrosinase inhibitors with (E)-benzylidene-1-indanone derivatives. Comput. Struct. Biotechnol. J. 2019, 17, 1255–1264. [Google Scholar] [CrossRef]
- Lineweaver, H.; Burk, D. The Determination of Enzyme Dissociation Constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Ismaya, W.T.; Rozeboom, H.J.; Weijn, A.; Mes, J.J.; Fusetti, F.; Wichers, H.J.; Dijkstra, B.W. Crystal Structure of Agaricus bisporus Mushroom Tyrosinase: Identity of the Tetramer Subunits and Interaction with Tropolone. Biochemistry 2011, 50, 5477–5486. [Google Scholar] [CrossRef]
- Tsuboi, T.; Kondoh, H.; Hiratsuka, J.; Mishima, Y. Enhanced melanogenesis induced by tyrosinase gene-transfer increases boron-uptake and killing effect of boron neutron capture therapy for amelanotic melanoma. Pigment. Cell Res. 1998, 11, 275–282. [Google Scholar] [CrossRef]
Sample Availability: Available from the authors. |
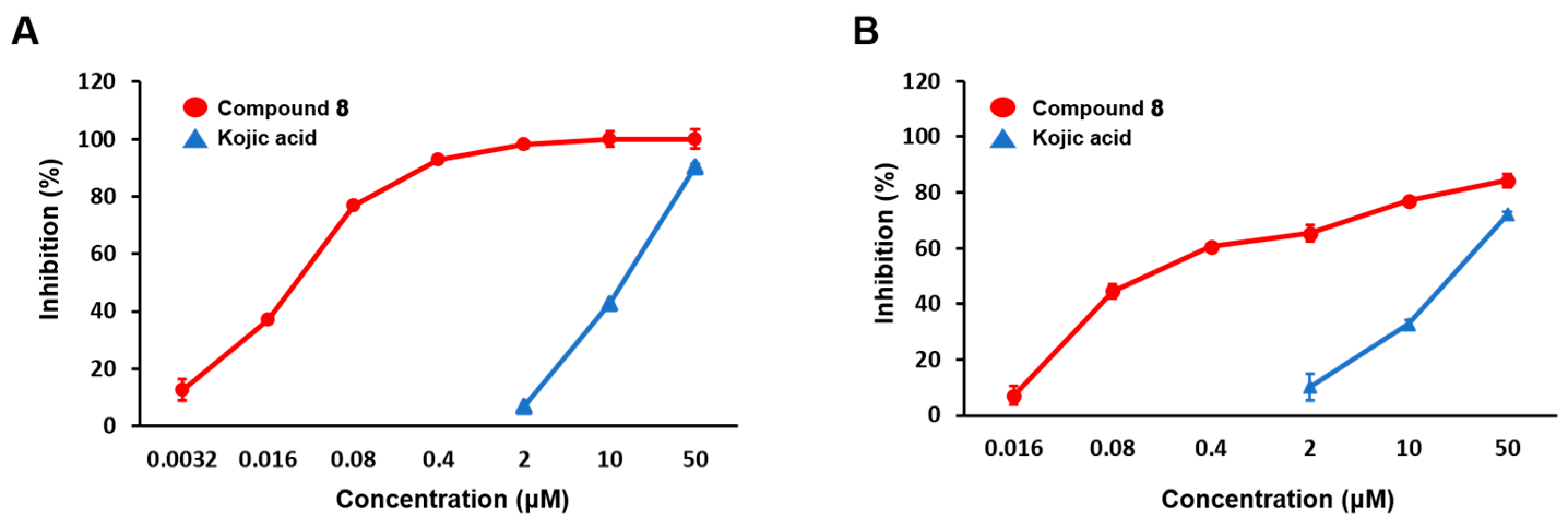



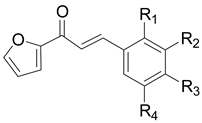
| Compounds | R1 | R2 | R3 | R4 | IC50 (μM) a | IC50 (μM) b |
|---|---|---|---|---|---|---|
| 1 | H | H | OH | H | 28.85 ± 1.21 | >200 |
| 2 | H | OH | OH | H | 84.90 ± 3.79 | 24.86 ± 3.25 |
| 3 | H | OMe | OH | H | >200 | >200 |
| 4 | H | OH | OMe | H | 13.61 ± 3.74 | 37.36 ± 4.89 |
| 5 | H | OMe | OH | OMe | >200 | >200 |
| 6 | H | Br | OH | H | 51.25 ± 3.73 | >200 |
| 7 | H | Br | OH | Br | >200 | 27.17 ± 0.38 |
| 8 | OH | H | OH | H | 0.0433 ± 0.0016 | 0.28 ± 0.01 |
| Kojic acid c | 19.97 ± 0.36 | 33.47 ± 0.05 | ||||
| Phthalic acid d | >200 | >200 | ||||
| Cinnamic acid d | >200 | >200 |
| Compounds | Binding Energy (kcal/mol) a | Binding Residues b | |
|---|---|---|---|
| Catalytic site | 8 | −5.69 | VAL248, MET257, ASN260, PHE264, MET280, VAL283, ALA286 |
| Kojic acid c | −4.21 | HIS263, MET280 | |
| Allosteric site 1 | 8 | −4.52 | LEU24, TYR140, PHE147, ILE217, ALA221 |
| Phthalic acid d | −3.19 | TRP136, ILE217, ALA221, PHE224, LEU265 | |
| Allosteric site 2 | 8 | −5.72 | THR308, TYR311, ASP312, GLU356 |
| Cinnamic acid d | −4.08 | LYS379 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, H.J.; Noh, S.G.; Ryu, I.Y.; Park, C.; Lee, J.Y.; Chun, P.; Moon, H.R.; Chung, H.Y. (E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives as Tyrosinase Inhibitors and Melanogenesis Inhibition: An In Vitro and In Silico Study. Molecules 2020, 25, 5460. https://doi.org/10.3390/molecules25225460
Jung HJ, Noh SG, Ryu IY, Park C, Lee JY, Chun P, Moon HR, Chung HY. (E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives as Tyrosinase Inhibitors and Melanogenesis Inhibition: An In Vitro and In Silico Study. Molecules. 2020; 25(22):5460. https://doi.org/10.3390/molecules25225460
Chicago/Turabian StyleJung, Hee Jin, Sang Gyun Noh, Il Young Ryu, Chaeun Park, Ji Young Lee, Pusoon Chun, Hyung Ryong Moon, and Hae Young Chung. 2020. "(E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives as Tyrosinase Inhibitors and Melanogenesis Inhibition: An In Vitro and In Silico Study" Molecules 25, no. 22: 5460. https://doi.org/10.3390/molecules25225460
APA StyleJung, H. J., Noh, S. G., Ryu, I. Y., Park, C., Lee, J. Y., Chun, P., Moon, H. R., & Chung, H. Y. (2020). (E)-1-(Furan-2-yl)-(substituted phenyl)prop-2-en-1-one Derivatives as Tyrosinase Inhibitors and Melanogenesis Inhibition: An In Vitro and In Silico Study. Molecules, 25(22), 5460. https://doi.org/10.3390/molecules25225460