A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020)
Abstract
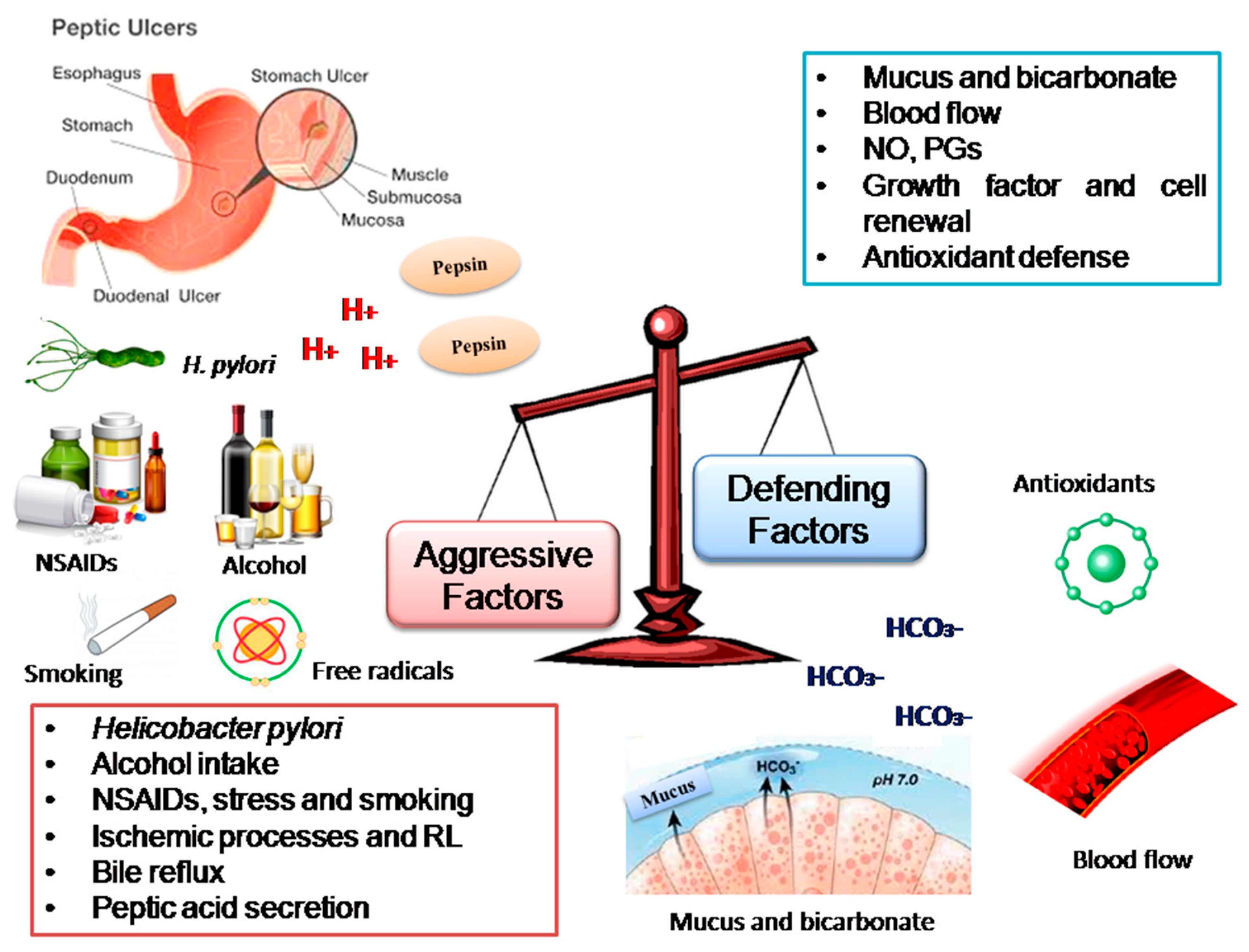
1. Introduction
2. Results
2.1. Flavonols
2.1.1. Kaempferol
2.1.2. Kaempferide
2.1.3. Quercetin
2.1.4. Morin
2.1.5. Rutin
2.1.6. Quercitrin and Afzelin
2.2. Flavanols
Catechin, Epicatechin, Epigallocatechin Gallate and Derivates
2.3. Flavones
2.3.1. Baicalein
2.3.2. Baicalin
2.3.3. Chrysin
2.3.4. Diosmin
2.3.5. Isoorientin
2.3.6. Nobiletin
2.4. Flavanones
2.4.1. Naringin
2.4.2. Pinostrobin
2.4.3. Hesperidin and Neohesperidine
2.5. Isoflavonoids
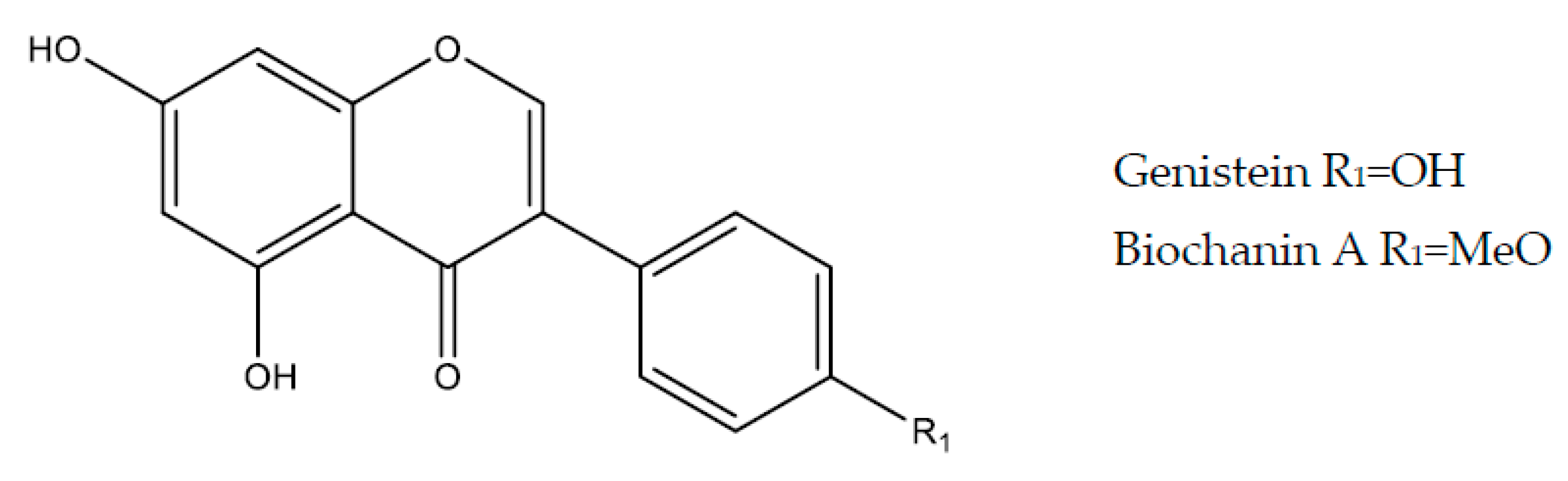
2.5.1. Genistein
2.5.2. Biochanin A
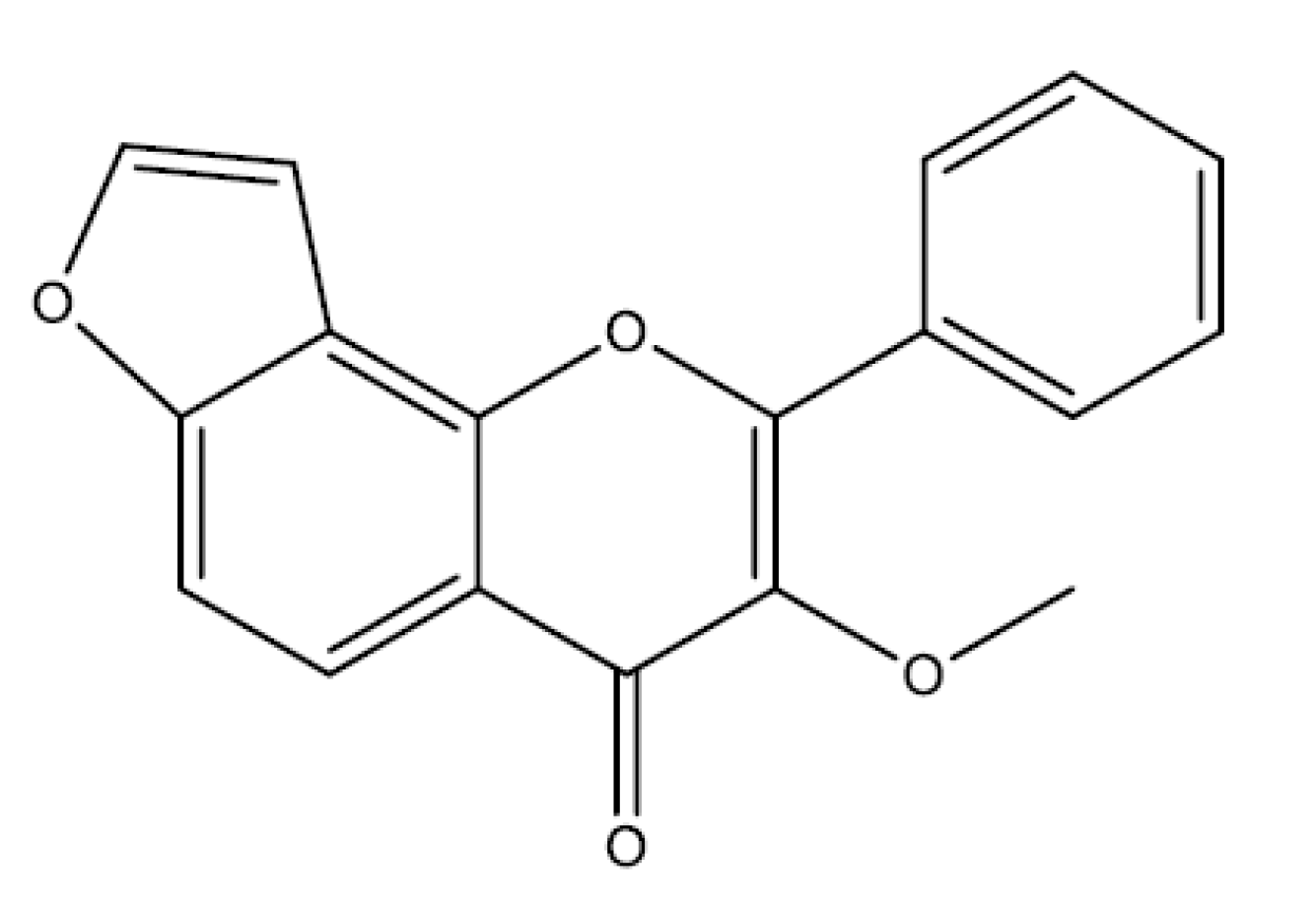
2.6. Furanoflavonoids
Karanjin
2.7. Biflavonoids
Kolaviron
2.8. Other Flavonoids
Silymarin
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ach | Acetylcholine |
| Bcl-2 | B-cell lymphoma-2 |
| Cag A | Cytotoxin associated gene A |
| CAT | Catalase |
| COX | Cyclooxygenase |
| Cit C | Cytochrome C |
| eNOS | Endothelial nitric oxide synthase |
| FGF | Fibroblast growth factor |
| GCs | Soluble guanylyl cyclase |
| GPx | Glutathione peroxidase |
| GR | Glutathione reductase |
| GSH | Glutathione |
| GST | Glutathione transferase |
| HCl | Hydrochloric acid |
| HSP | Heat shock protein |
| HCO3− | Bicarbonate |
| HO-1 | Heme oxygenase-1 |
| H2O2 | Hydrogen peroxide |
| HSF-1 | Thermal shock factor 1 |
| IL | Interleukin |
| iNOS | Inducible nitric oxide synthase |
| MDA | Malondialdehyde |
| MIC | Minimum inhibitory concentration |
| MMP | Matrix metalloproteinase |
| MPO | Myeloperoxidase |
| NF-kB | Nuclear factor-kappa B |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| Nrf2 | Nuclear factor related to erythroid 2 |
| NSAIDs | Non-steroidal anti-inflammatory |
| nNOS | Neuronal nitric oxide synthase |
| PGs | Prostaglandin |
| PGE2 | Prostaglandin E2 |
| PPIs | Proton pump inhibitors |
| ROS | Reactive oxygen species |
| SH | Sulfhydryl compounds |
| SOD | Superoxide dismutase |
| TAC | Total antioxidant capacity |
| TNF-α | Tumor necroses factor-α |
| VEGF | Vascular endothelial growth factor |
| VacA | Vacuolating cytotoxin |
References
- Del Valle, J. Peptic ulcer disease and related disorders. Harrison’s Principles of Internal Medicine, 19th ed.; Kasper, D.L., Fauci, A.S., Hauser, S.L., Longo, D.L., Jameson, J.L., Loscalzo, J., Eds.; McGraw Hill Education: New York, NY, USA, 2015; pp. 1911–1932. [Google Scholar]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Dimaline, R.; Varro, A. Attack and defence in the gastric epithelium—A delicate balance. Exp. Physiol. 2007, 92, 591–601. [Google Scholar] [CrossRef]
- Yandrapu, H.; Sarosiek, J. Protective Factors of the Gastric and Duodenal Mucosa: An Overview. Curr. Gastroenterol. Rep. 2015, 17, 24. [Google Scholar] [CrossRef] [PubMed]
- Galura, G.M.; Chavez, L.O.; Robles, A.; McCallum, R. Gastroduodenal Injury: Role of Protective Factors. Curr. Gastroenterol. Rep. 2019, 21, 34. [Google Scholar] [CrossRef] [PubMed]
- Min, C.; Hesheng, L.; Jihong, C.; Qiaoyun, T.; Xianzhen, L.; Chireyeth, S. Effects and Mechanism of Changes of Local Neurotransmitters in Rats’ Pylorus and Bile Reflux to the Stomach with Stress Ulcer. Dig. Dis. Sci. 2005, 50, 1898–1903. [Google Scholar] [CrossRef] [PubMed]
- Kanaizumi, T.; Nakano, H.; Matsui, T.; Tatsumi, H.; Ishikawa, H.; Kuramoto, H.; Shimizu, R.; Shiratori, T. Gastric emptying in patients with gastric and duodenal ulcer. Tohoku J. Exp. Med. 1989, 158, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tytgat, G.N. Etiopathogenetic principles and peptic ulcer disease classification. Dig. Dis. 2011, 29, 454–458. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, R.; Sharma, R.; Kumar, S. Peptic Ulcer: A Review on Etiology and Pathogenesis. Int. Res. J. Pharm. 2012, 3, 34–38. [Google Scholar]
- Narayanan, M.; Reddy, K.M.; Marsicano, E. Peptic Ulcer Disease and Helicobacter pylori infection. Mo. Med. 2018, 115, 219–224. [Google Scholar]
- Yegen, B.C. Lifestyle and Peptic Ulcer Disease. Curr. Pharm. Des. 2018, 24, 2034–2040. [Google Scholar] [CrossRef]
- Vomero, N.D.; Colpo, E. Nutritional care in peptic ulcer. Arq. Bras. Cir. Dig. 2014, 27, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Yoshida, M.; Filakovszky, J.; Juhasz, G. “Stress” is 80 Years Old: From Hans Selye Original Paper in 1936 to Recent Advances in GI Ulceration. Curr. Pharm. Des. 2017, 23, 4029–4041. [Google Scholar] [CrossRef]
- Amaral, G.P.; de Carvalho, N.R.; Barcelos, R.P.; Dobrachinski, F.; de Lima Portella, R.; da Silva, M.H.; Lugokenski, T.H.; Dias, G.R.M.; da Luz, S.C.A.; Boligon, A.A.; et al. Protective action of ethanolic extract of Rosmarinus officinalis L. in gastric ulcer prevention induced by ethanol in rats. Food Chem. Toxicol. 2013, 55, 48–55. [Google Scholar] [CrossRef]
- Zhang, L.; Ren, J.W.; Wong, C.C.; Wu, W.K.; Ren, S.X.; Shen, J.; Chan, R.L.Y.; Cho, C.H. Effects of cigarette smoke and its active components on ulcer formation and healing in the gastrointestinal mucosa. Curr. Med. Chem. 2012, 19, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I.; Scarpignato, C.; Holmgren, E.; Olszewski, M.; Rainsford, K.D.; Lanas, A. Mechanisms of Damage to the Gastrointestinal Tract From Nonsteroidal Anti-Inflammatory Drugs. Gastroenterology 2018, 154, 500–514. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Sougioultzis, S.; Archimandritis, A.J. Effects of Helicobacter pylori and nonsteroidal anti-inflammatory drugs on peptic ulcer disease: A systematic review. Clin. Gastroenterol. Hepatol. 2006, 4, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Sarri, G.L.; Grigg, S.E.; Yeomans, N.D. Helicobacter pylori and low-dose aspirin ulcer risk: A meta-analysis. J. Gastroenterol. Hepatol. 2019, 34, 517–525. [Google Scholar] [CrossRef]
- Sverdén, E.; Brusselaers, N.; Wahlin, K.; Lagergren, J. Time latencies of Helicobacter pylori eradication after peptic ulcer and risk of recurrent ulcer, ulcer adverse events, and gastric cancer: A population-based cohort study. Gastrointest. Endosc. 2018, 88, 242–250. [Google Scholar] [CrossRef]
- Lanas, A. We Are Using Too Many PPIs, and We Need to Stop: A European Perspective. Am. J. Gastroenterol. 2016, 111, 1085–1086. [Google Scholar] [CrossRef]
- Scally, B.; Emberson, J.R.; Spata, E.; Reith, C.; Davies, K.; Halls, H.; Holland, L.; Wilson, K.; Bhala, N.; Hawkey, C.; et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: A meta-analysis of randomised trials. Lancet Gastroenterol. Hepatol. 2018, 3, 231–241. [Google Scholar] [CrossRef]
- Yu, L.-Y.; Sun, L.N.; Zhang, X.-H.; Li, Y.-Q.; Yu, L.; Yuan, Z.-Q.-Y.; Meng, L.; Zhang, H.-W. A review of the new application and the possible adverse effects of proton pump inhibitors. Adv. Ther. 2017, 34, 1070–1086. [Google Scholar]
- Varcus, F.; Paun, I.; Duta, C.; Dobrescu, A.; Frandes, M.; Tarta, C. Laparoscopic repair of perforated peptic ulcer. Minerva Chir. 2018, 73, 188–193. [Google Scholar] [PubMed]
- Kuna, L.; Jakab, J.; Smolic, R.; Raguz-Lucic, N.; Vcev, A.; Smolic, M. Peptic Ulcer Disease: A Brief Review of Conventional Therapy and Herbal Treatment Options. J. Clin. Med. 2019, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, J.E. What is hidden in biodiversity? The role of natural products and medicinal chemistry in the drug discovery process. An. Acad. Bras. Sci. 2019, 91. [Google Scholar] [CrossRef] [PubMed]
- Calixto, J.B. The role of natural products in modern drug discovery. An. Acad. Bras. Sci. 2019, 91. [Google Scholar] [CrossRef] [PubMed]
- Mathesius, U. Flavonoid Functions in Plants and Their Interactions with Other Organisms. Plants 2018, 7, 30. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr Sci. 2016, 5. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Science 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef]
- Cheng, Y.; Tan, J.; Li, H.; Kong, X.; Liu, Y.; Guo, R.; Li, G.; Yang, B.; Pei, M. Cardioprotective effects of total flavonoids from Jinhe Yangxin prescription by activating the PI3K/Akt signaling pathway in myocardial ischemia injury. Biomed. Pharmacother. 2018, 98, 308–317. [Google Scholar] [CrossRef]
- Calis, Z.; Mogulkoc, R.; Baltaci, A.K. The roles of Flavonoles/Flavonoids in Neurodegeneration and Neuroinflammation. Mini Rev. Med. Chem. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef] [PubMed]
- Oteiza, P.I.; Fraga, C.G.; Mills, D.A.; Taft, D.H. Flavonoids and the gastrointestinal tract: Local and systemic effects. Mol. Aspects Med. 2018, 61, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Santos-Fagundes, D.; Grasa, L.; Gonzalo, S.; Valero, M.S.; Castro, M.; Arruebo, M.P.; Plaza, M.A.; Murillo, M.D. Different mechanisms of actions of genistein, quercetin on spontaneous contractions of rabbit duodenum. Rev. Esp. Enferm. Dig. 2015, 107, 413–416. [Google Scholar]
- Yao, W.R.; Wang, H.Y.; Wang, S.T.; Sun, S.L.; Zhou, J.; Luan, Y.Y. Assessment of the antibacterial activity and the antidiarrheal function of flavonoids from bayberry fruit. J. Agric. Food Chem. 2011, 59, 5312–5317. [Google Scholar] [CrossRef]
- Mota, K.S.; Dias, G.E.; Pinto, M.E.; Luiz-Ferreira, A.; Souza-Brito, A.R.M.; Hiruma-Lima, C.A.; Barbosa-Filho, J.M.; Batista, L.M. Flavonoids with gastroprotective activity. Molecules 2009, 14, 979–1012. [Google Scholar] [CrossRef]
- Musumeci, L.; Maugeri, A.; Cirmi, S.; Lombardo, G.E.; Russo, C.; Gangemi, S.; Calapai, G.; Navarra, M. Citrus fruits and their flavonoids in inflammatory bowel disease: An overview. Nat. Prod. Res. 2020, 34, 122–136. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Muschietti, L.V.; Martino, V.S. Atividades biológicas dos flavonóides naturais. Química de Produtos Naturais, Novos Fármacos e a Moderna Farmacognosia, 1st ed.; Yunes, R.A., Cechinel Filho, V., Eds.; Univali: Itajaí, Brasil, 2007; pp. 183–207. [Google Scholar]
- Simões, C.M.O.; Schenkel, E.P.; Palazzo de Mello, J.C.; Mentz, L.A.; RosPetrovick, P. Farmacognosia: Do Produto Natural ao Medicamento, 7th ed.; Artmed Editora: Porto Alegre, Brasil, 2017. [Google Scholar]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Du, W.; An, Y.; He, X.; Zhang, D.; He, W. Protection of Kaempferol on Oxidative Stress-Induced Retinal Pigment Epithelial Cell Damage. Oxid. Med. Cell. Longev. 2018, 2018, 1610751. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Marra, M.V. Dietary Quercetin and Kaempferol: Bioavailability and Potential Cardiovascular-Related Bioactivity in Humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Salehi, B.; Sharifi-Rad, J.; Gondal, T.A.; Saeed, F.; Imran, A.; Shahbaz, M.; Fokou, P.V.T.; Arshad, M.; et al.; Khan, H. Kaempferol: A Key Emphasis to Its Anticancer Potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Hu, X.; Xuan, Y.; Ying, J.; Fei, Y.; Rong, J.; Zhang, Y.; Zhang, J.; Liu, C.; Liu, Z. Kaempferol protects ethanol-induced gastric ulcers in mice via pro-inflammatory cytokines and NO. Acta Biochim. Biophys Sin. 2018, 50, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Yeon, M.J.; Lee, M.H.; Kim, D.H.; Yang, J.Y.; Woo, H.J.; Kwon, H.J.; Moon, C.; Kim, S.H.; Kim, J.B. Anti-inflammatory effects of Kaempferol on inflammation induced by Helicobacter pylori. Biosci. Biotech. Bioch. 2019, 83, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Almeida, M.O.; Lemos, M.; Arruda, C.; Casoti, R.; Somensi, L.B.; Boeing, T.; Mariott, M.; da Silva, R.C.M.V.A.F.; Stein, B.P.; et al. Artepillin C, drupanin, aromadendrin-4′-O-methyl-ether and kaempferide from Brazilian green propolis promote gastroprotective action by diversified mode of action. J. Ethnopharmacol. 2018, 226, 82–89. [Google Scholar] [CrossRef]
- Moustafa, Y.M.; El-Azab, M.F.; Fouda, A. 15-PGDH inhibitors: The antiulcer effects of carbenoxolone, pioglitazone and verapamil in indomethacin induced peptic ulcer rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2000–2009. [Google Scholar]
- David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar]
- Suzuki, Y.; Ishihara, M.; Segami, T.M. Anti-ulcer effects of antioxidants, quercetin, alpha-tocopherol, nifedipine and tetracycline in rats. Jpn. J. Pharmacol. 1998, 78, 435–441. [Google Scholar] [CrossRef]
- Coskun, O.; Kanter, M.; Armutçu, F.; Çetin, K.; Kaybolmaz, B.; Yazgan, Ö. Protective effects of quercetin, a flavonoid antioxidant, in absolute ethanol-induced acut gastric ulcer. Eur. J. Intern. Med. 2004, 1, 37–42. [Google Scholar]
- Chakraborty, S.; Stalin, S.N.; Choudhury, S.T.; Ghosh, S.; Swarnakar, S. The use of nano-quercetin to arrest mitochondrial damage and MMP-9 upregulation during prevention of gastric inflammation induced by ethanol in rat. Biomaterial 2012, 33, 2991–3001. [Google Scholar] [CrossRef]
- Alkushi, A.G.R.; Elsawy, N.A.M. Quercetin attenuates indomethacin-induced acute gastric ulcer in rats. Folia Morphol. 2017, 76, 252–261. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Castillo, R.L.; Beltrán, C.; Miranda, A.; Fuentes, J.; Gotteland, M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: Role of NF-κB and Nrf2. J. Nutr. Biochem. 2016, 27, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Wang, J.; Kasman, L.; Jiang, X.; Haley-Zitlin, V. Activities of muscadine grape skin and quercetin against Helicobacter pylori infection in mice. J. Appl. Microbiol. 2011, 110, 139–146. [Google Scholar] [CrossRef]
- Abourehab, M.; Khaled, K.; Sarhan, H.A.; Ahmed, O.A.A. Evaluation of famotidine combined with quercetin for the treatment of peptic ulcer: Study in animals in vivo. Drug Des. Devel. Ther. 2015, 9, 2159–2169. [Google Scholar] [PubMed]
- Singh, D.P.; Borse, S.P.; Nivsarkar, M. Co-administration of quercetin with pantoprazole sodium prevents NSAID-induced severe gastroenteropathic damage efficiently: Evidence from a preclinical study in rats. Exp. Toxicol. Pathol. 2017, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Caselli, A.; Cirri, P.; Santi, A.; Paoli, P. Morin: A Promising Natural Drug. Curr. Med. Chem. 2016, 23, 774–791. [Google Scholar] [CrossRef]
- Sinha, K.; Sadhukhan, P.; Saha, S.; Pal, P.B.; Sil, P.C. Morin protects gastric mucosa from nonsteroidal anti-inflammatory drug, indomethacin induced inflammatory damage and apoptosis by modulating NF-κB pathway. Biochim. Biophys. Acta 2015, 1850, 769–783. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of Rutin. Saudi. Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef]
- Enogieru, A.B.; Haylett, W.; Hiss, D.C.; Bardien, S.; Ekpo, O. Rutin as a potent antioxidant: Implications for neurodegenerative disorders. Oxid. Med. Cell. Longev. 2018, 2018, 6241017. [Google Scholar] [CrossRef]
- Abdel-Raheem, I.T. Gastroprotective effect of rutin against indomethacin-induced ulcers in rats. Basic Clin. Pharmacol. Toxicol. 2010, 107, 742–750. [Google Scholar] [CrossRef]
- Liu, Y.; Gou, L.; Fu, X.; Li, S.; Lan, N.; Yin, X. Protective effect of rutin against acute gastric mucosal lesions induced by ischemia-reperfusion. Pharm. Biol. 2013, 51, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Olaleye, M.T.; Akinmoladun, A.C. Comparative gastroprotective effect of post-treatment with low doses of rutin and cimetidine in rats. Fundam. Clin. Pharmacol. 2013, 27, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Dubey, S.; Ganeshpurkar, A.; Shrivastava, A.; Bansal, D.; Dubey, N. Rutin exerts antiulcer effect by inhibiting the gastric proton pump. Indian J. Pharmacol. 2013, 45, 415–417. [Google Scholar] [PubMed]
- Teves, M.R.; Rotelli, A.E.; Wendel, G.H.; Paredes, J.D.; Giraudo, E.; Petenatti, M.E.; Pelzer, L.E. Records of medicinal plants utilized as gastroprotective and for treatment of gastrointestinal ulcers, gastritis, and heartburn in Argentina: A survey of the literature. J. Herbs Spices Med. Plants 2015, 21, 333–371. [Google Scholar] [CrossRef]
- De Barros, M.; Mota da Silva, L.; Boeing, T.; Somensi, L.B.; Cury, B.J.; de Moura Burci, L.; Santin, J.R.; De Andrade, S.F.; Monache, F.D.; Cechinel-Filho, V. Pharmacological reports about gastroprotective effects of methanolic extract from leaves of Solidago chilensis (Brazilian arnica) and its components quercitrin and afzelin in rodents. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 403–417. [Google Scholar] [CrossRef]
- Peluso, I.; Serafini, M. Antioxidants from black and green tea: From dietary modulation of oxidative stress to pharmacological mechanisms. Br. J. Pharmacol. 2017, 174, 1195–1208. [Google Scholar] [CrossRef]
- Adhikary, B.; Yadav, S.K.; Bandyopadhyay, S.K.; Chattopadhyay, S. Role of COX independent pathways in the healing action of epigallocatechin gallate ulcer. Food Funct. 2011, 2, 338–347. [Google Scholar] [CrossRef]
- Adhikary, B.; Yadav, S.K.; Bandyopadhyay, S.K.; Chattopadhyay, S. Epigallocatechin gallate accelerates the healing of stomach ulcers induced by indomethacin in mice. Pharmacol. Rep. 2011, 63, 527–536. [Google Scholar] [CrossRef]
- Rozza, A.L.; Hiruma-Lima, C.A.; Tanimoto, A.; Pellizzon, C.H. Morphological and pharmacological investigations on the gastroprotective effect of epicatechin. Complement. Based Evid. Alternat. Med. 2012, 2012, 708156. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Wu, C.H.; Ho, C.Y.; Yen, G.C. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J. Nutr. Biochem. 2013, 24, 475–483. [Google Scholar] [CrossRef]
- Ankolekar, C.; Johnson, D.; Pinto, M.S.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory potential of tea polyphenols and influence of extraction time against Helicobacter pylori and lack of inhibition of beneficial lactic acid bacteria. J. Med. Food. 2011, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Pastene, E.; Parada, V.; Avello, H.; Ruiz, A.; Garcia, U. Procyanidins based on the catechin of Peumusboldus Mol. Aqueous extract inhibits Helicobacter pylori urease and adenocarcinoma gastric cells adherence. Phytother. Res. 2014, 28, 1637–1645. [Google Scholar] [CrossRef]
- Boyanova, L.; Ilieva, J.; Gergova, G.; Vladimirov, B.; Nikolov, R.; Mitov, I. The consumption of honey and green/black tea can reduce the risk of Helicobacter pylori infection. Diagn. Microbiol. Infect. Dis. 2015, 82, 85–86. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Gohil, N.; Ramírez-García, R. New insight into the control of peptic ulcer by targeting the histamine H2 receptor. J. Cell. Biochem. 2018, 119, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Choi, E.O.; Kim, G.Y.; Hwang, H.J.; Kim, B.W.; Yoo, Y.H.; Park, H.T.; Choi, Y.H. Protective effect of Baicalein on DNA damage induced by oxidative stress and apoptosis in Schwann RT4-D6P2T cells. Int. J. Med. Sci. 2019, 16, 8–16. [Google Scholar] [CrossRef]
- Yu, X.; Liu, Y.; Wang, Y.; Mao, X.; Zhang, Y.; Xia, J. Baicalein induces cervical cancer apoptosis through the NF-κB signaling pathway. Mol. Med. Rep. 2018, 17, 5088–5094. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, T.; Wang, K.; Hou, C.; Cai, S.; Huang, Y.; Du, Z.; Huang, H.; Kong, J.; Chen, Y. Baicalein Inhibits Staphylococcus aureus Biofilm Formation and the Quorum Sensing System In Vitro. PLoS ONE 2016, 11, e0153468. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; do Nascimento Valença, J.D.; da Silva Santos, J.; Boeing, T.; da Silva, L.M.; de Andrade, S.F.; Albuquerque-Júnior, R.L.; Thomazzi, S.M. The effects of baicalein on gastric mucosal ulcerations in mice: Protective pathways and anti-secretory mechanisms. Chem. Biol. Interact. 2016, 260, 33–41. [Google Scholar] [CrossRef]
- Chen, M.E.; Su, C.H.; Yang, J.S.; Lu, C.C.; Hou, Y.C.; Wu, J.B.; Hsu, Y.M. Baicalin, Baicalein, and Lactobacillus Rhamnosus JB3 Alleviated Helicobacter pylori Infections in Vitro and in Vivo. J. Food Sci. 2018, 83, 3118–3125. [Google Scholar] [CrossRef]
- Zhang, T.; Yang, D.; Meng, X. Baicalin protects against gastroduodenal ulcers through the modulation of Nrf2 expression: Experimental, biochemical and histological analyzes. Pharmacol. Rep. 2017, 69, 1154–1158. [Google Scholar] [CrossRef]
- Yan-Qiang, H.; Gan-Rong, H.; Ming-Hui, W.; Hua-Ying, T.; Zan-Song, H.; Xi-Han, Z.; Wen-Qiang, Y.; Jian-Wei, S.; Xiao-Qiang, M.; Bing-Pu, C.; et al. Inhibitory effects of emodin, baicalin, schizandrine and berberine on the hefA gene: Treatment of multiple drug resistance induced by Helicobacter pylori. World J. Gastroenterol. 2015, 21, 4225–4231. [Google Scholar]
- Yang, H.; Lu, Y.; Zeng, X.F.; Li, L.; Zhang, R.P.; Ren, Z.K.; Liu, X. Antichronic Gastric Ulcer Effect of Zinc-Baicalin Complex on the Acetic Acid-Induced Chronic Gastric Ulcer Rat Model. Gastroenterol. Res. Pract. 2018, 2018, 1275486. [Google Scholar] [CrossRef] [PubMed]
- Celińska-Janowicz, K.; Zaręba, I.; Lazarek, U.; Teul, J.; Tomczyk, M.; Pałka, J.; Miltyk, W. Constituents of Propolis: Chrysin, Caffeic Acid, p-Coumaric Acid, and Ferulic Acid Induce PRODH/POX-Dependent Apoptosis in Human Tongue Squamous Cell Carcinoma Cell (CAL-27). Front. Pharmacol. 2018, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Mani, R.; Natesan, V. Chrysin: Sources, beneficial pharmacological activities, and molecular mechanism of action. Phytochemistry 2018, 145, 187–196. [Google Scholar] [CrossRef]
- Naz, S.; Imran, M.; Rauf, A.; Orhan, I.E.; Shariati, M.A.; Ul-Haq, I.; Yasmin, I.; Shahbaz, M.; Qaisrani, T.B.; Shah, Z.A.; et al. Chrysin: Pharmacological and therapeutic properties. Life Sci. 2019, 235. [Google Scholar] [CrossRef]
- George, M.Y.; Esmat, A.; Tadros, M.G.; El-Demerdash, E. In vivo cellular and molecular gastroprotective mechanisms of chrysin; Emphasis on oxidative stress, inflammation and angiogenesis. Eur. J. Pharmacol. 2018, 818, 486–498. [Google Scholar] [CrossRef] [PubMed]
- Fagundes, F.L.; de Morais Piffer, G.; Périco, L.L.; Rodrigues, V.P.; Hiruma-Lima, C.A.; Dos Santos, R.C. Chrysin Modulates Genes Related to Inflammation, Tissue Remodeling, and Cell Proliferation in the Gastric Ulcer Healing. Int. J. Mol. Sci. 2020, 21, 760. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Khalifa, H.A.; Abushouk, A.I.; Dkhil, M.A.; Al-Quraishy, S.A. Diosmin Attenuates Methotrexate-Induced Hepatic, Renal, and Cardiac Injury: A biochemical and histopathological study in mice. Oxid. Med. Cell. Longev. 2017, 2017. [Google Scholar] [CrossRef]
- Arab, H.H.; Salama, S.A.; Omar, H.A.; El-Shaimaa, A.A.; Maghrabi, I.A. Diosmin protects against gastric injury induced by ethanol in rats: New anti-ulcer actions. PLoS ONE 2015, 10, e0122417. [Google Scholar] [CrossRef]
- Kataria, R.; Khatkar, A. In-silico Designing, ADMET Analysis, Synthesis and Biological Evaluation of Novel Derivatives of Diosmin Against Urease Protein and Helicobacter pylori Bacterium. Curr. Top. Med. Chem. 2019, 19, 2658–2675. [Google Scholar] [CrossRef]
- Abd El Hady, W.E.; Mohamed, E.A.; Soliman, O.A.E.; El-Sabbagh, H.M. In vitro and in vivo evaluation of chitosan-PLGA nanoparticles for potentiated gastric retention and anti-ulcer activity of diosmin. Int. J. Nanomed. 2019, 14, 7191–7213. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, N.; Zhu, C.; Ma, L.; Yang, J.; Du, J.; Zhang, W.; Sun, T.; Niu, J.; Yu, J. Antinociceptive effect of isoorientin against neuropathic pain induced by the chronic constriction injury of the sciatic nerve in mice. Int. Immunopharmacol. 2019, 75, 105753. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Kwon, S.H.; Lee, S.Y.; Jang, C.G. Isoorientin improves scopolamine-induced cognitive impairments by restoring the cholinergic system, antioxidant defense, and p-CREB/BDNF signaling in the hippocampus and frontal cortex. Arch. Pharm. Res. 2019, 42, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Lv, H.; Wang, L.; Deng, X.; Ci, X. Isoorientin Ameliorates APAP-Induced Hepatotoxicity via Activation Nrf2 Antioxidative Pathway: The Involvement of AMPK/Akt/GSK3β. Front. Pharmacol. 2018, 9, 1334. [Google Scholar] [CrossRef]
- Karaoğlan, E.S.; Albayrak, A.; Kutlu, Z.; Bayır, Y. Gastroprotective and antioxidant effects of EremurusspectabilisBieb.methanol extract and its isolated component isoorientin on indomethacin induced gastric ulcers in rats. Acta Cir. Bras. 2018, 33, 609–618. [Google Scholar] [CrossRef]
- Lin, Z.; Wu, D.; Huang, L.; Jiang, C.; Pan, T.; Kang, X.; Pan, J. Nobiletin Inhibits IL-1β-Induced Inflammation in Chondrocytes via Suppression of NF-κB Signaling and Attenuates Osteoarthritis in Mice. Front. Pharmacol. 2019, 10, 570. [Google Scholar] [CrossRef]
- Dusabimana, T.; Kim, S.R.; Kim, H.J.; Park, S.W.; Kim, H. Nobbiletine improves liver ischemia and reperfusion injury by activating SIRT-1/FOXO3a-mediated autophagy and mitochondrial biogenesis. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef]
- Goh, J.X.H.; Tan, L.T.; Goh, J.K.; Goh, J.K.; Chan, K.G.; Pusparajah, P.; Lee, L.H.; Goh, B.H. Nobiletin and Derivatives: Functional Compounds from Citrus Fruit Peel for Colon Cancer Chemoprevention. Cancers 2019, 11, 867. [Google Scholar] [CrossRef]
- Li, W.; Wang, X.; Zhi, W.; Chan, K.G.; Pusparajah, P.; Lee, L.H.; Goh, B.H. The gastroprotective effect of nobiletin against ethanol-induced acute gastric lesions in mice: Impact on oxidative stress and inflammation. Immunopharmacol. Immunotoxicol. 2017, 39, 354–363. [Google Scholar] [CrossRef]
- Chen, R.; Qi, Q.L.; Wang, M.T.; Li, Q.Y. Therapeutic potential of naringin: An overview. Pharm. Biol. 2016, 54, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.J.; Marhuenda, E.; Pérez-Guerrero, C.; Franco, J.M. Antiulcerous effect of naringin on gastric lesions induced by ethanol in rats. Pharmacology 1994, 49, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Galati, E.M.; Monforte, M.T.; D’Aquino, A.; Miceli, N.; Di Mauro, D.; Sanogo, R. Effects of naringin on experimental ulcer in rats. Phytomedicine 1998, 5, 361–366. [Google Scholar] [CrossRef]
- Mohamed, E.A.; Abu Hashim, I.I.; Yusif, R.M.; Shaaban, A.A.A.; El-Sheakh, A.R.; Hamed, M.F.; Badria, F.A.E. Polymeric micelles for potentiated antiulcer and anticancer activities of naringin. Int. J. Nanomed. 2018, 13, 1009–1027. [Google Scholar] [CrossRef]
- Patel, N.K.; Jaiswal, G.; Bhutani, K.K. A review on biological sources, chemistry and pharmacological activities of pinostrobin. Nat. Prod. Res. 2016, 30, 2017–2027. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, S.I.; Mohan, S.; Abdulla, M.A.; Sukari, M.A.; Abdul, A.B.; Taha, M.M.E.; Syam, S.; Ahmad, S.; Lee, K.H. The methanolic extract of Boesenbergia rotunda (L.)Mansf.and its major compound pinostrobin induces anti-ulcerogenic property in vivo: Possible involvement of indirect antioxidant action. J. Ethnopharmacol. 2011, 137, 963–970. [Google Scholar] [CrossRef]
- Li, C.; Schluesener, H. Health-promoting effects of the citrus flavanone hesperidin. Crit. Rev. Food Sci. Nutr. 2017, 57, 613–631. [Google Scholar] [CrossRef]
- Rossi, G.V.; Packman, E.W.; Goldberg, M.E. Studies on the effects of ascorbic acid and hesperidin upon histamine-induced gastric ulcers in guinea pigs. Am. J. Pharm. Sci. Support. Public Health 1957, 129, 89–94. [Google Scholar]
- Suárez, J.; Herrera, M.D.; Marhuenda, E. Hesperidin and neohesperidin dihydrochalcone in different experimental models of Phytother-induced gastric ulcer. Phytother. Res. 1996, 10, 616–618. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.H.; Kim, Y.S.; Jeong, C.S. Protective effects of neohesperidine and poncirin isolated from the fruits of Poncirus trifoliata in the potential gastric disease. Phytother. Res. 2009, 23, 1748–1753. [Google Scholar] [CrossRef]
- Cheng, T.; Wen-Li, L.; Liu, H.Y.; Kun-Teng, W.; Chun-Chao, C.; Ching-Chiung, W. Gastroprotective effects of Ping-Wei San on indomethacin-induced gastric ulcer. Food Drug Anal. 2011, 19, 509–516. [Google Scholar]
- Yao, J. Tiao He Yi Wei Granule, a Traditional Chinese Medicine, against Ethanol-Induced Gastric Ulcer in Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 647283. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hamdan, D.I. Effect of hesperidin and neohesperidin from bittersweet orange (Citrus aurantium var. bigaradia) peel on indomethacin-induced peptic ulcers in rats. Environ. Toxicol. Pharmacol. 2014, 37, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Bigoniya, P.; Singh, K. Ulcer protective potential of standardized hesperidin, a citrus flavonoid isolated from Citrus sinensis. Rev. Bras. Farmacogn. 2014, 24, 330–340. [Google Scholar] [CrossRef]
- Jain, D.; Katti, N. Combination treatment of lycopene and hesperidin protect experimentally induced ulcer in laboratory rats. J. Intercult. Ethnopharmacol. 2015, 4, 143–146. [Google Scholar] [CrossRef]
- Selmi, S.; Rtibi, K.; Grami, D.; Sebai, H.; Marzouki, L. Protective effects of orange (Citrus sinensis L.) peel aqueous extract and hesperidin on oxidative stress and peptic ulcer induced by alcohol in rat. Lipids Health Dis. 2017, 16, 152. [Google Scholar] [CrossRef]
- Elshazly, S.M.; Abd El Motteleb, D.M.; Ibrahim, I.A.A.E. Hesperidin protects against stress induced gastric ulcer through regulation of peroxisome proliferator activator receptor gamma in diabetic rats. Chem. Biol. Interact. 2018, 291, 153–161. [Google Scholar] [CrossRef]
- Da Silva, L.M.; Pezzini, B.C.; Somensi, L.B.; Bolda Mariano, L.N.; Mariott, M.; Boeing, T.; Ana Carolina Dos Santos, A.C.; Longo, B.; Cechinel-Filho, V.; De Souza, P.; et al. Hesperidin, a citrus flavanone glycoside, accelerates the gastric healing process of acetic acid-induced ulcer in rats. Chem. Biol. Interact. 2019, 308, 45–50. [Google Scholar] [CrossRef]
- Lee, Y.S.; Huh, J.Y.; Nam, S.H.; Lua, S.K.; Lee, S.B. Enzymatic bioconversion of citrus hesperidin by Aspergillusoyaenaringinase: Increased solubility of hesperetin-7-O-glucoside with in vitro inhibition of human intestinal maltase, HMG-CoA reductase and Helicobacter pylori growth. Food Chem. 2012, 135, 2253–2259. [Google Scholar] [CrossRef]
- Jaiswal, N.; Akhtar, J.; Singh, S.P.; Badruddeen, F.A. An Overview on Genistein and its Various Formulations. Drug Res. 2019, 69, 305–313. [Google Scholar] [CrossRef]
- Kavoosi, F.; Dastjerdi, M.N.; Valiani, A.; Esfandiari, E.; Sanaei, M.; Hakemi, M.G. Genistein potentiates the effect of 17-beta estradiol on the human hepatocellular carcinoma cell line. Adv. Biomed. Res. 2016, 5, 133. [Google Scholar] [PubMed]
- Vivatvakin, S.; Werawatganon, D.; Somanawat, K.; Klaikeaw, N.; Siriviriyakul, P. Genistein-attenuated Gastric Injury on Indomethacin-induced Gastropathy in Rats. Pharmacogn. Mag. 2017, 13, S306–S310. [Google Scholar] [PubMed]
- Abdel-Raheem, I.; Bamagous, G.; Omran, G. Anti-ulcerogenic effect of genistein against indomethacin-induced gastric ulcer in rats. Asian J. Pharm Clin. Res. 2016, 9, 58–63. [Google Scholar]
- Hegab, I.I.; Abd-Ellatif, R.N.; Sadek, M.T. The gastroprotective effect of N-acetylcysteine and genistein in indomethacin-induced gastric injury in rats. Can. J. Physiol. Pharmacol. 2018, 96, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, P.; Lou, L.; Wang, Y. Perspectives Regarding the Role of Biochanin A in Humans. Front. Pharmacol. 2019, 10, 793. [Google Scholar] [CrossRef]
- Hajrezaie, M.; Salehen, N.; Karimian, H.; Zahedifard, M.; Shams, K.; Batran, R.A.; Majid, N.A.; Khalifa, S.A.M.; Ali, H.M.; El-Seedi, H.; et al. Biochanin a gastroprotective effects in ethanol-induced gastric mucosal ulceration in rats. PLoS ONE 2015, 10, e0121529. [Google Scholar] [CrossRef]
- Maurya, R.; Yadav, P. Furanoflavonoids: An overview. Nat. Prod. Rep. 2005, 22, 400–424. [Google Scholar] [CrossRef]
- Saini, P.; Lakshmayya, L.; Bisht, V.S. Anti-Alzheimer activity of isolated karanjin from Pongamia pinnata (L.) pierre and embelin from Embeliaribes Burm.f. AYU 2017, 38, 76–81. [Google Scholar]
- Roy, R.; Pal, D.; Sur, S.; Mandal, S.; Saha, P.; Panda, C.K. Pongapin and Karanjin, furanoflavanoids of Pongamiapinnata, induce G2/M arrest and apoptosis in cervical cancer cells by differential reactive oxygen species modulation, DNA damage, and nuclear factor kappa-light-chain-enhancer of activated B cell signaling. Phytother. Res. 2019, 33, 1084–1094. [Google Scholar]
- Vismaya Belagihally, S.M.; Rajashekhar, S.; Jayaram, V.B.; Dharmesh, S.M.; Thirumakudalu, S.K. Gastroprotective Properties of Karanjin from Karanja (Pongamia pinnata) Seeds; Role as Antioxidant and H, K-ATPase Inhibitor. Evid. Based Complement. Altern. Med. 2011, 2011, 747246. [Google Scholar]
- Geiger, H.; Quinn, C. Biflavonoids. In The Flavonoides, 1st ed.; Harborne, J.B., Mabry, T.J., Mabry, H., Eds.; Springer: Boston, MA, USA, 1975. [Google Scholar]
- Omotoso, G.O.; Olajide, O.J.; Gbadamosi, I.T.; Rasheed, M.A.; Izuogu, C.T. Kolaviron protects the prefrontal cortex and hippocampus against histomorphological and neurobehavioral changes in the cuprizone model of multiple sclerosis. Malaio J. Med. Sci. 2018, 25, 50–63. [Google Scholar]
- Oyagbemi, A.A. Cardiorespiral dysfunction induced by Kolaviron arsenic acid via regulation of ROS, C-reactive proteins (CRP), cardiac troponin I (CTnI) and BCL2. J. Tradit Complement. Med. 2018, 8, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Alabi, Q.K.; Akomolafe, R.O. Kolaviron diminishes diclofenac-induced liver and kidney toxicity in wistar rats via suppressing inflammatory events, upregulating antioxidant defenses, and improving hematological indices. Dose Response 2020, 18, 1559325819899256. [Google Scholar] [CrossRef] [PubMed]
- Onasanwo, S.A.; Singh, N.; Olaleye, S.B.; Palit, L. Inhibitory activity of the anti-ulcerogenic pump and the proton pump (H +, K + ATPase) of Garcinia kola Heckel Kolaviron in rodents. Indian J. Exp. Biol. 2011, 49, 461–468. [Google Scholar]
- Odukanmi, O.A.; Salami, A.T.; Ashaolu, O.P.; Adegoke, A.G.; Olaleye, S.B. Kolaviron attenuates ischemia/reperfusion injury in the stomach of rats. Appl. Physiol. Nutr. Metab. 2018, 43, 30–37. [Google Scholar] [CrossRef]
- Jee, S.C.; Kim, M.; Sung, J.S. Modulating effects of silymarin on benzo [a] pyrene-induced hepatotoxicity. Int. J. Mol. Sci. 2020, 21, 2369. [Google Scholar] [CrossRef]
- Karabag, H.; Koçarslan, S. Comparison of the effects of thymoquinone and silymarin in the brain of rats with ischemia-reperfusion in the lower extremities. Ann. Ital. Chir. 2020, 91, 131–136. [Google Scholar]
- Sheta, N.M.; Elfeky, Y.A.; Boshra, S.A. Cardioprotector Efficacy of silymarin Liquisolid in repeated myocardial isoproterenol in rats. AAPS PharmSciTech 2020, 21, 81. [Google Scholar] [CrossRef]
- Tan, J.; Hu, J.; He, Y.; Cui, F. Protective role of silymarin in a mouse model with renal ischemia and reperfusion injury. Diagn Pathol. 2015, 198. [Google Scholar] [CrossRef]
- Akbari-Kordkheyli, V.; Abbaszadeh-Goudarzi, K.; Nejati-Laskokalayeh, M.; Zarpou, S.; Khonakdar-Tarsi, A. The protective effects of silymarin on ischemia-reperfusion injuries: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 968–976. [Google Scholar]
- Bijak, M. Silybin, a Major Bioactive Component of Milk Thistle (Silybummarianum L. Gaernt.)-Chemistry, Bioavailability, and Metabolism. Molecules 2017, 22, 1942. [Google Scholar] [CrossRef] [PubMed]
- Alarcon de la Lastra, C.; Martin, M.J.; Marhuenda, E. Anti-gastric ulcer activity of silymarin, a lipoxygenase inhibitor, in rats. J. Pharm. Pharmacol. 1992, 44, 929–931. [Google Scholar] [CrossRef] [PubMed]
- Alarcon de Lastra, C.; Martin, M.J.; Motilva, V.; Jimenez, M.; La Casa, C.; Lopez, A. Silymarin-induced gastroprotection, the hepatoprotective principle of Silybummarianum in mucosal injury due to ischemia reperfusion: The role of neuterophils. Plant. Med. 1995, 61, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Huilgol, S.V.; Jamadar, M.G. Silymarin, an antioxidant bioflavonoid, inhibits experimentally induced peptic ulcers in rats by dual mechanisms. Int. J. Appl. Basic Med. Res. 2012, 2, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Lee, C.W.; Oh, S.J.; Yun, J.; Lee, K.; Park, S.K.; Kim, H.M.; Han, S.B.; Kim, Y.; Kim, H.C.; et al. Protective effect of silymarin against ethanol-induced gastritis in rats: Role of sulfhydryls, nitric oxide and gastric sensory afferents. Food Chem. Toxicol. 2013, 55, 353–357. [Google Scholar] [CrossRef]
- Arafa Keshk, W.; Zahran, S.M.; Katary, M.A.; Abd-Elaziz Ali, D. Modulatory effect of silymarin on redox status regulated by factor 2 related to nuclear factor eryroid-2, inflammation mediated by nuclear factor κB and apoptosis in experimental gastric ulcer. Chem. Biol. Interact. 2017, 273, 266–272. [Google Scholar] [CrossRef]
- Liju, V.B.; Jeena, K.; Kuttan, R. Gastroprotective activity of essential oils from turmeric and ginger. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 95–103. [Google Scholar] [CrossRef]
- Ribeiro, A.R.S.; Diniz, P.B.F.; Pinheiro, M.S.; Albuquerque-Júnior, R.L.C.; Thomazzi, S.M. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, ATP-sensitive K+ channels, and gastric mucus secretion. Chem. Biol. Interact. 2016, 25, 121–128. [Google Scholar] [CrossRef]
- Ateufack, G.; Domgnim Mokam, E.C.; Mbiantcha, M.; Dongmo Feudjio, R.B.; David, N.; Kamanyi, A. Gastroprotective and ulcer healing effects of Piptadeniastrum africanum on experimentally induced gastric ulcers in rats. BMC Complement. Altern. Med. 2015, 15, 214. [Google Scholar] [CrossRef]
- Chauhan, A.K.; Kang, S.C. Therapeutic potential and mechanism of thymol action against ethanol induced gastric mucosal injury in rat model. Alcohol 2015, 49, 739–745. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Me, N.; Ma, C.; Lou, Z.; Lv, W.; He, G. Protective effects of polysaccharide from Dendrobiumnobile against ethanol-induced gastric damage in rats. Int. J. Biol. Macromol. 2018, 107, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Kazmi, I.; Saleem, S.; Ahmad, T.; Afzal, M.; Al-Abbasi, F.A.; Kumar, V.; Anwar, F. Protective effect of oleane-12-en-3b-ol-28-oic acid 3b-Dglucopyranoside in ethanol induced gastric ulcer by enhancing the prostaglandin E2 level. J. Ethnopharmacol. 2018, 211, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Almasaudi, S.B.; Abbas, A.T.; Al-Hindi, R.R.; El-Shitany, N.A.; Abdel-Dayem, U.A.; Ali, S.S.; Saleh, R.M.; Soad, K.; Jaouni, A.; Kamal, M.A.; et al. Manuka honey exerts antioxidant and antiInflammatory activities that promote healing of acetic acid-induced gastric ulcer in rats. Evid. Based Complement. Altern. Med. 2017, 2017, 5413917. [Google Scholar] [CrossRef] [PubMed]
- Saxena, B.; Singh, S. Comparison of three acute stress models for simulating the pathophysiology of stress-related mucosal disease. Drug Discov. Ther. 2017, 11, 98–103. [Google Scholar] [CrossRef]
- Dostal, C.R.; Sulzer, M.C.; Keith, W.K.; Freund, G.G.; Cusker, R.H.M. Glial and tissue-specific regulation of Kynurenine Pathwaydioxygenases by acute stress of mice. Neurobiology 2017, 7, 1–15. [Google Scholar]
- Tanyeli, A.; Eraslan, E.; Polat, E.; Bal, T. The biochemical and histopathological effects of salusinalphaandsalusin beta on cold restricted stress induced gastric injury. Medicine 2017, 6, 236–241. [Google Scholar]
- Boeing, T.; da Silva, L.M.; Somensi, L.B.; Cury, B.J.; Costa, A.P.M.; Petreanu, M.; Niero, R.; de Andrade, S.B. Antiulcer mechanisms of Vernonia condensata Baker: Amedicinal plant used in the treatment of gastritis and gastric ulcer. J. Ethnopharmacol. 2016, 184, 196–207. [Google Scholar] [CrossRef]
- Scheiman, J.M. NSAID-induced gastrointestinal injury: A focused update forclinicians. J. Clin. Gastroenterol. 2016, 50, 5–10. [Google Scholar] [CrossRef]
- Bastaki, S.M.A.; Padol, I.T.; Amir, N.; Hunt, R.H. Effect of Aspirin and ibuprofen either alone or incombination on gastric mucosa and bleeding time and on serum prostaglandin E2 and thromboxane A2 levels in the anaesthetized rats in vivo. Mol. Cell Biochem. 2017, 438, 25–34. [Google Scholar] [CrossRef]
- Utzeri, E.; Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3954–3963. [Google Scholar] [CrossRef]
- El-Ashmawy, N.E.; Khedr, E.G.; El-Bahrawy, H.A.; Selim, H.M. Nebivolol prevents indomethacin-induced gastric ulcer in rats. J. Immunotoxicol. 2016, 13, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moawad, H.; Awdan, S.A.; Sallam, N.A.; Eraky, W.I.; Alkhawlani, M.A. Gastroprotective effect of cilostazol against ethanol- and pylorus ligation-induced gastric lesions in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2019, 392, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sahoo, H.B.; Priyadarshini, D.; Soundarya, G.; Kishore, C.K.; Ran, K.U. Antiulcer Activity of Ethanolic Extract of Salvadoraindica (W.) Leaves on Albino Rats. J. Clin. Diagn. Res. 2016, 10, 7–10. [Google Scholar]
- Schubert, M. Gastric acid secretion. Curr. Opin. Gastroenterol. 2016, 32, 452–460. [Google Scholar] [CrossRef]
- Marosti, A.R.; Silva, V.M.; Palombit, K.; Mendes, C.E.; Tavares De Lima, W.; Catelucci, P. Differential effects of intestinal ischemia and reperfusion in rat enteric neurouns and glial cells expressing P2×2 receptors. Histol. Histopathol. 2015, 30, 489–501. [Google Scholar]
- Viana, A.F.S.C.; Lopes, M.T.P.; Oliveira, F.T.B.; Nunes, P.I.G.; Santos, V.G.; Braga, A.D.; Silva, A.C.A.; Sousa, D.P.; Viana, D.A.; Rao, V.S.; et al. Myrtenol accelerates the healing of gastric ulcers induced by acetic acid in rats and in human gastric adenocarcinoma cells. Eur. J. Pharmacol. 2019, 854, 139–148. [Google Scholar] [CrossRef]
- Kolgazi, M.; Ozdemir-Kumral, Z.N.; Cantali-Ozturk, C.; Demirci, E.K.; Yuksel, M.; Sirvanci, S.; Yegen, B.C. Anti-inflammatory effects of nesfatin-1 on acetic acid-induced gastric ulcers in rats: Involvement of the cyclooxygenase pathway. J. Physiol. Pharmacol. 2017, 68, 765–777. [Google Scholar]
- D’Amelia, V.; Aversano, R.; Chiaiese, P.; Carputo, D. The antioxidant properties of plant flavonoids: Their exploitation by molecular plant breeding. Phytochem Rev. 2018, 17, 611–625. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Yanaka, A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell Mol. Life Sci. 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.S.; Yoon, J.J.; Han, B.H.; Jeong, D.H.; Lee, Y.J.; Kang, D.G.; Lee, H.S. Ligustilide attenuates vascular inflammation and activates Nrf2/HO-1 induction and, NO synthesis in HUVECs. Phytomedicine 2018, 38, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.R.; Kim, I.S.; Ahn, D.; Tae, H.J.; Nam, H.H.; Choo, B.K.; Kim, K.; Parque, B.Y. Anti-Inflammatory and Gastroprotective Roles of Rabdosiainflexa through Downregulation of Pro-Inflammatory Cytokines and MAPK/NF-κB Signaling Pathways. Int. J. Mol. Sci. 2018, 19, 584. [Google Scholar] [CrossRef]
- Wu, J.Z.; Liu, Y.H.; Liang, J.L.; Huang, Q.H.; Dou, Y.X.; Nie, J.; Zhuo, J.Y.; Wu, X.; Chen, J.N.; Su, Z.R.; et al. Protective role of β-patchoulene from Pogostemoncablin against indomethacin-induced gastric ulcer in rats: Involvement of anti-inflammation and angiogenesis. Phytomedicine 2018, 39, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Adinortey, M.B.; Ansah, C.; Adinortey, C.A.; McGiboney, J.; Nyarko, A. In vitro H+/K+-ATPase Inhibition, Antiradical Effects of a Flavonoid-rich Fraction of Dissotisrotundifolia, and In silico PASS Prediction of its Isolated Compounds. J. Nat. Sci. Biol. Med. 2018, 9, 47–53. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Ichimura, A.; Sato, S.; Fujii, T. The natural flavonoid myricetin inhibits gastric H+, K+-ATPase. Eur. J. Pharmacol. 2018, 820, 217–221. [Google Scholar] [CrossRef]
- Røn, T.; Patil, N.J.; Ajalloueian, F.; Sankaranarayanan, R.; Zappone, B.; Chronakis, I.S.; Lee, S. Gastric mucus and mucuslike hydrogels: Thin film lubricating properties at soft interfaces. Biointerphases 2017, 12, 051001. [Google Scholar] [CrossRef]
- Wallace, J.L. Nitric oxide in the gastrointestinal tract: Opportunities for drug development. Br. J. Pharmacol. 2019, 176, 147–154. [Google Scholar] [CrossRef]
- Sanders, K.M.; Ward, S.M. Nitric oxide and its role as a non-adrenergic, non-cholinergic inhibitory neurotransmitter in the gastrointestinal tract. Br. J. Pharmacol. 2019, 176, 212–227. [Google Scholar] [CrossRef]
- Da Silva Monteiro, C.E.; Franco, Á.X.; Sousa, J.A.O.; Matos, V.E.A.; de Souza, E.P.; Fraga, C.A.M.; Barreiro, E.J.; de Souza, M.H.L.P.; Soares, P.M.G.; Barbosa, A.L.R. Gastroprotective effects of N-acylarylhydrazone derivatives on ethanol-induced gastric lesions in mice are dependent on the NO/cGMP/KATP pathway. Biochem. Pharmacol. 2019, 169, 113629. [Google Scholar] [CrossRef]
- Takeuchi, K.; Amagase, K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef] [PubMed]
- Kocak, B.T.; Saribas, S.; Demiryas, S.; Yilmaz, E.; Uysal, O.; Kepil, N.; Demirci, M.; Dinc, H.O.; Akkus, S.; Gulergun, R.; et al. Association between polymorphisms in the HLA-A, HLA-B, HLA-DR and DQ genes of patients with gastric cancer and duodenal ulcer and cagL among Helicobacter pylori strains cagA-positive: The first study in a Turkish population. Infect. Genet. Evol. 2020, 13, 104288. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Dou, Y.; Wu, X.; Li, H.; Wu, J.; Huang, Q.; Luo, D.; Yi, T.; Liu, Y.; Su, Z.; et al. Prophylactic efficacy of patchoulene epoxide against ethanol-induced gastric ulcer in rats: Influence on oxidative stress, inflammation and apoptosis. Chem. Biol. Interact. 2018, 283, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Khotib, J.; Rahmadi, M.; Ardianto, C.; Nisak, K.; Oktavia, R.; Ratnasari, A.; Dinintia, Y.; Shinta, D.W.; Aryani, T. Selective serotonin reuptake inhibitor fluvoxamine ameliorates stress- and NSAID-induced peptic ulcer possibly by involving Hsp70. J. Basic Clin. Physiol. Pharmacol. 2019, 30, 195–203. [Google Scholar] [CrossRef]
- Park, C.H.; Son, H.U.; Yoo, C.Y.; Lee, S.H. Low molecular-weight gel fraction of Aloe vera exhibits gastroprotection by inducing matrix metalloproteinase-9 inhibitory activity in alcohol-induced acute gastric lesion tissues. Pharm. Biol. 2017, 55, 2110–2115. [Google Scholar] [CrossRef]







| Compound | Natural and/or Food Source | Experimental Protocol/Dose | Route Administration/Organism Test | Effect or Mechanism | Reference |
|---|---|---|---|---|---|
| Flavonols | |||||
| Quercetin | Food source: onions, broccoli, apple, cherry and grape | in vivo Ethanol—2.5 mg/kg | (p.o.)/Rat | ↓ MMP-9, iNOS, MPO ↑GSH | [53] |
| in vivo NSAIDs (indomethacin)—50 and 100 mg/kg | (p.o.)/Rat | ↑ Nrf2, SOD, GPx ↓ ICAM-1, MPO, P-selectin | [55] | ||
| in vitro NSAIDs (indomethacin)—0.0331 mMol/L | Caco-2 cell | ↓ NF-κB, IL-8 | [55] | ||
| in vitro anti-H. pylori—0.000212–0.423 mMol/L | Cell culture | Inhibition | [56] | ||
| in vivo anti-H. pylori—25 mg/kg | (p.o.)/Mice | ↓ TNF-α, IL-1β | [56] | ||
| Kaempferol | Food source: broccoli, cabbage, beans, leeks, tomatoes, strawberries, grapes and propolis | in vivo Ethanol—40, 80 and 160 mg/kg | (p.o.)/Mice | ↓ MPO, TNF-α, IL-1β, IL-6 ↑ NO | [46] |
| in vitro anti-H. pylori—0.0015625 to 0.2 mMol/L | AGS cells | ↓ CagA, VacA, TNF-α, IL-1β, IL-8 | [47] | ||
| Kaempferide | Propolis | in vivo Pylorus ligature—3 mg/kg | (i.g)/Mice | ↓ pH, H+ concentration, secretion of volume, pepsin activity | [48] |
| in vivo HCl/Ethanol—3 mg/kg | (i.p)/Mice | ↑ SOD, CAT, GST, mucus ↓ MPO | [48] | ||
| Morin | Species of Moraceae family | in vivo NSAIDs (indomethacin)—50 mg/kg | (p.o.)/Rat | ↓ MPO, NF-κB, TNF-α, iNOS, ICAM-1, IL-6, caspase-3 ↑ PGE2, SOD | [60] |
| Quercitrin | Solidago chilensis | in vivo HCl/Ethanol—1, 38 mg/kg | (p.o.)/Mice | ↑ GSH ↓ MPO | [68] |
| in vitro Proton pump activity—0.00223 to 0.223 mMol/L | Cell culture | Inhibition | [68] | ||
| Afzelin | Solidago chilensis | in vivo HCl/Ethanol—0.026 and 0.078 mg/kg | (p.o.)/Mice | ↓ MPO | [68] |
| in vitro Proton pump activity—0.00231 to 0.231 mMol/L | Cell culture | Inhibition | [68] | ||
| Rutin | Food source: tomatoes, orange, carrots, sweet potatoes, black tea, and apple peels | in vivo NSAIDs (indomethacin)—200 mg/kg | (p.o)/Rat | ↑ GSH, SOD ↓ MPO | [63] |
| in vivo-Ischemia and reperfusion—50, 100, and 200 mg/kg | (p.o)/Rat | ↓ iNOS, MPO, MDA | [64] | ||
| in vivo Ethanol—20, 40 and 80 mg/kg | (p.o)/Rat | ↓ MDA ↑ GPx | [65] | ||
| in vivo Acetic Acid—20, 40 and 80 mg/kg | (p.o)/Rat | ↓ MDA ↑ GPx | [65] | ||
| in vitro Proton pump activity—IC50 of 0.0590 mMol/L | Cell culture | Inhibition | [66] | ||
| Flavanols | |||||
| Catechin | Green tea | in vivo NSAIDs (ketoprofen)—25 mg/kg | (p.o)/Rat | ↑ GPx, GR, Nrf2 | [73] |
| Epicatechin | Green tea | in vivo Pylorus ligature—25 and 50 mg/kg | (p.o)/Rat | ↓ H+ secretion | [72] |
| in vivo Ethanol—25 and 50 mg/kg | (p.o)/Rat | ↑ Mucus, SHs, NO, SOD, HSP-7 | [72] | ||
| Epigallocatechin gallate | Green tea | in vivo NSAIDs (indomethacin)—0,5, 1, 2, 3 and 5 mg/kg | (p.o)/Rat | ↓ iNOS, MPO ↑ Mucin, PGE2 | [70,71] |
| Flavones | |||||
| Isoorientin | Gentiana triflora and Eremurus spectabilis | in vivo NSAIDs (indomethacin)—25, 50, 100 mg/kg | (p.o)/Rat | ↓ MDA ↑ GSH, SOD | [98] |
| Chrysin | Passiflora incarnate, Oroxylum indicum, Matricaria chamomilla, propolis | in vivo NSAIDs (indomethacin)—50 and 100 mg/kg | (p.o)/Rat | ↑ Mucus, GSH, CAT, VEGF ↓ MDA | [89] |
| in vivo Acetic Acid—10 mg/kg | (p.o)/Mice | ↓ COX-2, MMP-9, caspase-3 ↑ EGF, COX-1 | [90] | ||
| Nobiletin | Citrus fruits | in vivo Ethanol—5, 10 or 20 mg/kg | (p.o)/Mice | ↑ PGE2, SOD ↓ TNF-α, IL-6 | [103] |
| Baicalein | Scutellaria baicalensis | in vivo HCl/Ethanol—10, 30 and 100 mg/kg | (p.o)/Mice | ↑ SHs, NO, GSH, mucus ↓ MPO | [81] |
| in vitro Proton pump activity—0.0370 and 0.111 mMol/L | Culture cell | Inhibition | [81] | ||
| in vitro anti-H. pylori—IC50 0.331 mMol/L | AGS cells | ↓ IL-8, Vac A, capacity of adhesion | [82] | ||
| Baicalin | Scutellaria baicalensis | in vivo Pylorus ligature—5, 10 and 15 mg/kg | (p.o)/Rat | ↓ H+ secretion | [83] |
| in vivo Acetic Acid—6.5 and 13 mg/kg | (p.o)/Rat | ↑ SOD, GSH, GPx ↓ MDA, IL-8, TNF-α | [85] | ||
| in vitro anti-H. pylori—IC50 0.431 mMol/L | GES-1 cells | ↓ IL-8, IL-1β, Vac A, urease, adhesion, hefA | [82,84] | ||
| Diosmin | Citrus fruits (lemon) | in vivo Ethanol—100 mg/kg | (p.o)/Mice | ↓ TNF-α, NF-kB, MPO, caspase-3, Cit C, Bcl2 ↑ IL-10, GSH, GPx, PGE2, NO | [92] |
| in vitro anti-H. pylori—CIM 0.822 mMol/L | Culture cell | Inhibition | [93] | ||
| Flavanones | |||||
| Naringin | Citrus fruits (orange, lemon) | in vivo Ethanol—100 and 200 mg/kg | (p.o)/Rat | ↓ MDA, IL-6, TNF-α, caspase-3 ↑ GSH, SOD | [107] |
| Pinostrobin | Boesenbergia rotunda | in vivo Ethanol—20 and 40 mg/kg | (p.o)/Rat | ↓ COX-2 | [109] |
| Flavanones | |||||
| Hesperidin | Citrus fruits (orange, lemon) | in vivo NSAIDs (indomethacin)—150, 300 and 450 mg/kg | (p.o)/Rat | ↑ GSH, SOD, CAT | [117] |
| in vivo Ethanol—50 mg/kg | (p.o)/Rat | ↓ COX-2, TNF-α, MDA ↑ SOD, CAT, GPx | [119] | ||
| in vivo Stress—100 mg/kg | (p.o)/Rat | ↑ GSH, SOD, CAT, mucin ↓ MDA | [120] | ||
| in vivo Acetic Acid—3 and 10 mg/kg | (p.o)/Rat | ↑ Mucin, GSH, SOD, CAT ↓ MPO | [121] | ||
| in vitro anti-H. pylori—0.05–0.5 mMol/L | Culture cell | Inhibition | [122] | ||
| Neohesperidine | Citrus fruits (orange, lemon) | in vivo NSAIDs (indomethacin)—100 mg/kg | (p.o)/Rat | ↓ COX-2, TNF-α, MDA ↑ GSH | [116] |
| Isoflavonoids | |||||
| Genistein | Soy-based foods (Glycine max or Soy hispida) | in vivo NSAIDs (indomethacin)—10 mg/kg | (p.o)/Rat | ↓ TNF-α, MDA, iNOS ↑ SOD, PGE2 | [125] |
| in vivo NSAIDs (indomethacin)—10 mg/kg for 7 days | (p.o)/Rat | ↑ NO, PGE2, SOD ↓ MDA, TNF-α, MPO | [126] | ||
| Biochanin A | Soy-based foods | in vivo Ethanol—25 and 50 mg/kg | (p.o)/Rat | ↑ NO, SOD ↓ MDA | [129] |
| Furanoflavonoids | |||||
| Karanjin | Pongamia pinnata | in vivo Ethanol—10 and 20 mg/kg | (p.o)/Rat | ↑ CAT, GPx, SOD ↓ MDA | [133] |
| in vitro Proton pump activity-0.0273–0.192 mMol/L | Culture cell | Inhibition | [133] | ||
| Biflavonoids | |||||
| Kolaviron | Garcinia kola | in vivo Ethanol—100 and 200 mg/kg | (p.o)/Rat | ↑ NO, mucus ↓ MDA | [138] |
| in vitro Proton pump activity—IC50 of 0.0744 mMol/L | Culture cell | Inhibition | [139] | ||
| Other flavonoids | |||||
| Silymarin | Silybum marianum | in vivo Ethanol—50 mg/kg | (p.o)/Rat | ↓ MPO, TNF-α, IL-6, NF-kB ↑ GPx, SOD, Nfr2 | [149,150] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serafim, C.; Araruna, M.E.; Júnior, E.A.; Diniz, M.; Hiruma-Lima, C.; Batista, L. A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules 2020, 25, 5431. https://doi.org/10.3390/molecules25225431
Serafim C, Araruna ME, Júnior EA, Diniz M, Hiruma-Lima C, Batista L. A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules. 2020; 25(22):5431. https://doi.org/10.3390/molecules25225431
Chicago/Turabian StyleSerafim, Catarina, Maria Elaine Araruna, Edvaldo Alves Júnior, Margareth Diniz, Clélia Hiruma-Lima, and Leônia Batista. 2020. "A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020)" Molecules 25, no. 22: 5431. https://doi.org/10.3390/molecules25225431
APA StyleSerafim, C., Araruna, M. E., Júnior, E. A., Diniz, M., Hiruma-Lima, C., & Batista, L. (2020). A Review of the Role of Flavonoids in Peptic Ulcer (2010–2020). Molecules, 25(22), 5431. https://doi.org/10.3390/molecules25225431





