Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies
Abstract
:1. Introduction
2. PDT and Urological Cancer
3. VTP Mechanism of Action
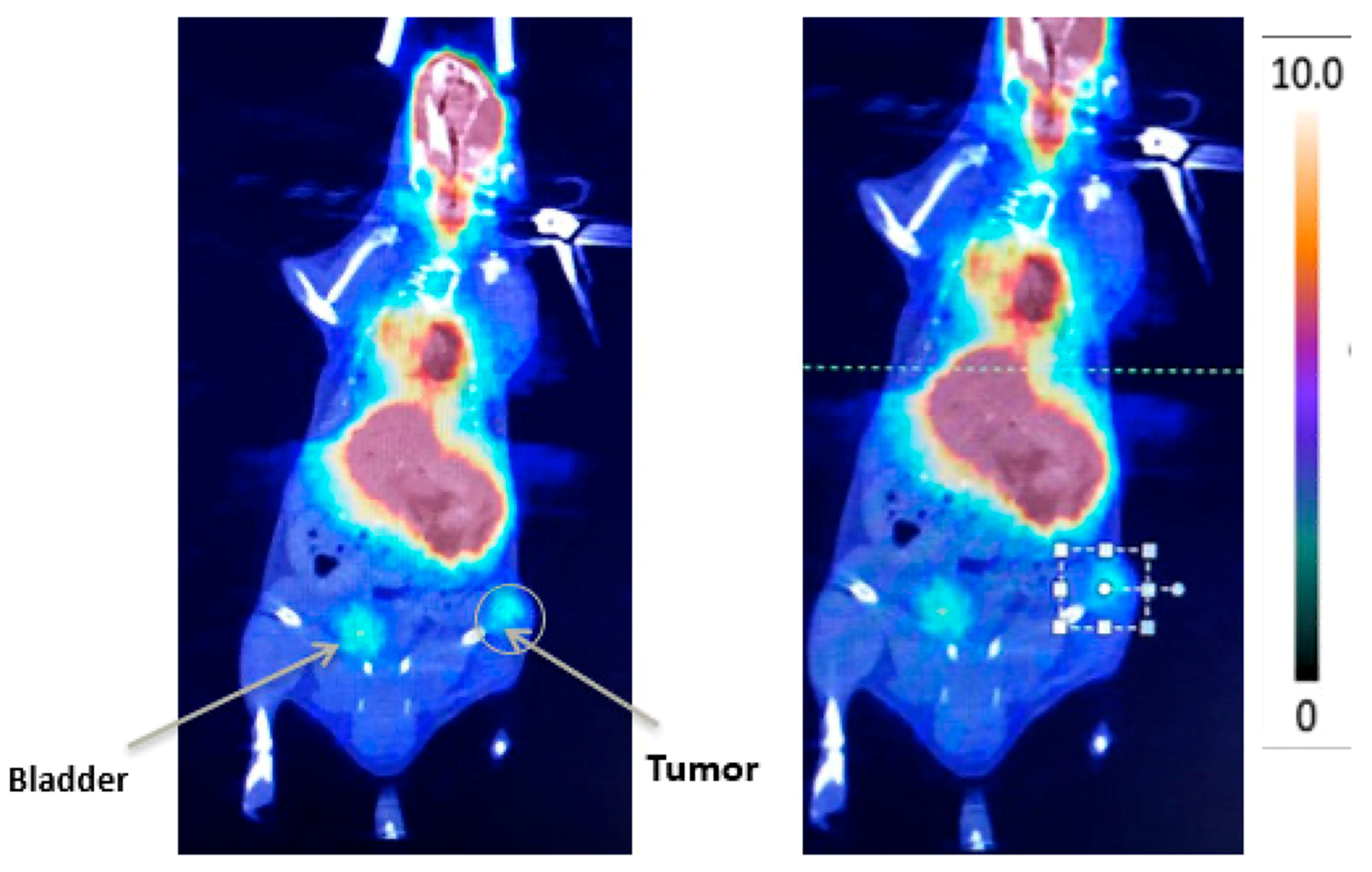
4. New Imaging Methods to Evaluate a VTP Response in Urologic Cancers
5. Immune Modulation by VTP and Adjunct Immunotherapy
6. VTP and Phosphatidylinositol 3-Kinase (Pi3k) Signaling Pathway
7. Clinical Trials in Urologic Cancers
7.1. Phase I
7.2. Phase I/II
7.3. Phase II
7.4. Phase III
8. Current Studies in Prostate and Urothelial Upper Tract Cancers
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Koob, T.J.; Lim, J.J.; Massee, M.; Zabek, N.; Denozière, G. Properties of dehydrated human amnion/chorion composite grafts: Implications for wound repair and soft tissue regeneration. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1353–1362. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, A.F.; De Almeida DR, Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic therapy in cancer treatment—An update review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Von Tappeiner, H. Therapeutische Versuche mit fluoreszierenden Stoffen. Munch. Med. Wochenschr. 1903, 1, 2042–2044. [Google Scholar]
- Hamblin, M.R. Photodynamic Therapy for Cancer: What’s Past is Prologue. Photochem. Photobiol. 2020, 96, 506–516. [Google Scholar] [CrossRef] [Green Version]
- Casas, A. Clinical uses of 5-aminolaevulinic acid in photodynamic treatment and photodetection of cancer: A review. Cancer Lett. 2020, 490, 165–173. [Google Scholar] [CrossRef]
- Tampa, M.; Sarbu, M.-I.; Matei, C.; Mitran, C.-I.; Mitran, M.-I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.-R. Photodynamic therapy: A hot topic in dermato-oncology. Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, F.S.; Bentley, M.V.L.B. Photodynamic Therapy of Skin Cancers: Sensitizers, Clinical Studies and Future Directives. Pharm. Res. 2000, 17, 1447–1455. [Google Scholar] [CrossRef]
- Azzouzi, A.-R.; Emberton, M. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer—Authors’ reply. Lancet Oncol. 2017, 18, e188. [Google Scholar] [CrossRef] [Green Version]
- Tracey, A.; Noguiera, L.; Alvim, R.; Wong, N.; Demac, Q.; McGill, M.; Sjoberg, D.; Estes, C.; ODea, C.; Benfante, N.; et al. LBA02-04 Interim Results: A Phase 2B Trial of Padeliporfin (WST11) Vascular-Targeted Photodynamic Therapy as Partial-Gland Ablation for Men with Intermediate-Risk Prostate Cancer. J. Urol. 2020, 203 (Suppl. S4), e1116. [Google Scholar] [CrossRef] [Green Version]
- Yanovsky, R.L.; Bartenstein, D.W.; Rogers, G.S.; Isakoff, S.J.; Chen, S.T. Photodynamic therapy for solid tumors: A review of the literature. Photodermatol. Photoimmunol. Photomed. 2019, 35, 295–303. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Morgan, J.; Pandey, S.K.; Chen, Y.; Tracy, E.; Baumann, H.; Missert, R.G.; Batt, C.; Jackson, J.; Bellnier, D.A.; et al. Conjugation of 2-(1′-hexyloxyethyl)-2-devinylpyropheophorbide-a (HPPH) to carbohydrates changes its subcellular distribution and enhances photodynamic activity in vivo. J. Med. Chem. 2009, 52, 4306–4318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.-J.; Lei, Q.; Zhang, X.-Z. Recent advances in photonanomedicines for enhanced cancer photodynamic therapy. Prog. Mater. Sci. 2020, 114, 100685. [Google Scholar] [CrossRef]
- Chen, Z.; Woodburn, K.W.; Shi, C.; Adelman, D.C.; Rogers, C.; Simon, D.I. Photodynamic therapy with motexafin lutetium induces redox-sensitive apoptosis of vascular cells. Arter. Thromb. Vasc. Biol. 2001, 21, 759–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, S.R.H.; Weersink, R.; Haider, M.A.; Gertner, M.R.; Bogaards, A.; Giewercer, D.; Scherz, A.; Sherar, M.D.; Elhilali, M.; Chin, J.L.; et al. Treatment planning and dose analysis for interstitial photodynamic therapy of prostate cancer. Phys. Med. Biol. 2009, 54, 2293–2313. [Google Scholar] [CrossRef]
- Bugaj, A.M. Vascular targeted photochemotherapy using padoporfin and padeliporfin as a method of the focal treatment of localised prostate cancer—Clinician’s insight. World J. Methodol. 2016, 6, 65–76. [Google Scholar] [CrossRef]
- Nogueira, L.; Wang, L.; Fine, S.W.; Pinochet, R.; Kurta, J.M.; Katz, D.; Savage, C.J.; Cronin, A.M.; Hricak, H.; Scardino, P.T.; et al. Focal treatment or observation of prostate cancer: Pretreatment accuracy of transrectal ultrasound biopsy and T2-weighted MRI. Urology 2010, 75, 472–477. [Google Scholar] [CrossRef] [Green Version]
- Bedi, N.; Reddy, D.; Ahmed, H.U. Targeting the cancer lesion, not the whole prostate. Transl. Androl. Urol. 2020, 9, 1518–1525. [Google Scholar] [CrossRef]
- Ahmad, S.; Aboumarzouk, O.; Somani, B.; Nabi, G.; Kata, S.G. Oral 5-aminolevulinic acid in simultaneous photodynamic diagnosis of upper and lower urinary tract transitional cell carcinoma—A prospective audit. BJU Int. 2012, 110, E596–E600. [Google Scholar] [CrossRef]
- Fukuhara, H.; Kurabayashi, A.; Furihata, M.; Setuda, S.; Takahashi, K.; Murakami, K.; Tanakac, T.; Inouea, K. 5-aminolevulinic acid-mediated photodynamic diagnosis using fluorescence ureterorenoscopy for urinary upper tract urothelial carcinoma approximately Preliminary prospective single centre trial approximately. Photodiagnosis Photodyn. Ther. 2020, 29, 101617. [Google Scholar] [CrossRef]
- Fukuhara, H.; Yamamoto, S.; Karashima, T.; Inoue, K. Photodynamic diagnosis and therapy for urothelial carcinoma and prostate cancer: New imaging technology and therapy. Int. J. Clin. Oncol. 2020, 1–8. [Google Scholar] [CrossRef]
- Eymerit-Morin, C.; Zidane, M.; Lebdai, S.; Triau, S.; Azzouzi, A.R.; Rousselet, M.-C. Histopathology of prostate tissue after vascular-targeted photodynamic therapy for localized prostate cancer. Virchows Archiv. 2013, 463, 547–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madar-Balakirski, N.; Tempel-Brami, C.; Kalchenko, V.; Brenner, O.; Varon, D.; Scherz, A.; Salomon, Y. Permanent occlusion of feeding arteries and draining veins in solid mouse tumors by vascular targeted photodynamic therapy (VTP) with Tookad. PLoS ONE 2010, 5, e10282. [Google Scholar] [CrossRef] [PubMed]
- Kimm, S.Y.; Tarin, T.V.; Monette, S.; Srimathveeravalli, G.; Gerber, D.; Durack, J.C.; Solomon, S.B.; Scardino, P.T.; Scherz, A.; Coleman, J. Nonthermal Ablation by Using Intravascular Oxygen Radical Generation with WST11: Dynamic Tissue Effects and Implications for Focal Therapy. Radiology 2016, 281, 109–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, K.S.; Winter, A.G.; Corradi, R.B.; LaRosa, S.; Jebiwott, S.; Somma, A.; Takaki, H.; Srimathveeravalli, G.; Lepherd, M.; Monette, S.; et al. Treatment Effects of WST11 Vascular Targeted Photodynamic Therapy for Urothelial Cell Carcinoma in Swine. J. Urol. 2016, 196, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Muller, B.G.; Fütterer, J.J.; Gupta, R.T.; Katz, A.; Kirkham, A.; Kurhanewicz, J.; Moul, J.W.; Pinto, P.A.; Rastinehad, A.R.; Robertson, C.; et al. The role of magnetic resonance imaging (MRI) in focal therapy for prostate cancer: Recommendations from a consensus panel. BJU Int. 2014, 113, 218–227. [Google Scholar] [CrossRef] [Green Version]
- Haider, M.A.; Davidson, S.R.H.; Kale, A.V.; Weersink, R.; Evans, A.J.; Toi, A.; Gertner, M.R.; Bogaards, A.; Wilson, B.; Chin, J.L.; et al. Prostate gland: MR imaging appearance after vascular targeted photodynamic therapy with palladium-bacteriopheophorbide. Radiology 2007, 244, 196–204. [Google Scholar] [CrossRef]
- Vargas, H.A.; Wassberg, C.; Akin, O.; Hricak, H. MR imaging of treated prostate cancer. Radiology 2012, 262, 26–42. [Google Scholar] [CrossRef]
- Cornelis, F.H.; Kim, K.; Durack, J.; Jebiwott, S.; Scherz, A.; Srimathveeravalli, G.; Coleman, J. Contrast enhanced ultrasound imaging can predict vascular-targeted photodynamic therapy induced tumor necrosis in small animals. Photodiagnosis Photodyn. Ther. 2017, 20, 165–168. [Google Scholar] [CrossRef]
- Neuschmelting, V.; Kim, K.; Malekzadeh-Najafabadi, J.; Jebiwott, S.; Prakash, J.; Scherz, A.; Coleman, J.A.; Kircher, M.F.; Ntziachristos, V. WST11 Vascular Targeted Photodynamic Therapy Effect Monitoring by Multispectral Optoacoustic Tomography (MSOT) in Mice. Theranostics 2018, 8, 723–734. [Google Scholar] [CrossRef]
- Haedicke, K.; Agemy, L.; Omar, M.; Berezhnoi, A.; Roberts, S.; Longo-Machado, C.; Skubal, M.; Nagar, K.; Hsu, H.-T.; Kim, K.; et al. High-resolution optoacoustic imaging of tissue responses to vascular-targeted therapies. Nat. Biomed. Eng. 2020, 4, 286–297. [Google Scholar] [CrossRef]
- Alvim, R.; Nagar, K.; Das, S.; Lebdai, S.; Wong, N.; Somma, A.; Hughes, C.; Thomas, J.; Monette, S.; Scherz, A.; et al. Positron Emission Tomography/Computed Tomography with Gallium-68-labeled Prostate-specific Membrane Antigen Detects Relapse After Vascular-targeted Photodynamic Therapy in a Prostate Cancer Model. Eur. Urol. Focus 2019. [Google Scholar] [CrossRef] [PubMed]
- Lohrmann, C.; Zhang, H.; Thorek, D.L.; Desai, P.; Zanzonico, P.B.; O’Donoghue, J.A.; Irwin, C.P.; Reiner, T.; Grimm, J.; Weber, W.A. Cerenkov Luminescence Imaging for Radiation Dose Calculation of a ⁹⁰Y-Labeled Gastrin-Releasing Peptide Receptor Antagonist. J. Nucl. Med. 2015, 56, 805–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.; Zhang, H.; La Rosa, S.; Jebiwott, S.; Desai, P.; Kimm, S.; Scherz, A.; O’Donoghue, J.A.; Weber, W.A.; Coleman, J. Bombesin Antagonist-Based Radiotherapy of Prostate Cancer Combined with WST-11 Vascular Targeted Photodynamic Therapy. Clin. Cancer Res. 2017, 23, 3343–3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preise, D.; Oren, R.; Glinert, I.; Kalchenko, V.; Jung, S.; Scherz, A.; Salomon, Y. Systemic antitumor protection by vascular-targeted photodynamic therapy involves cellular and humoral immunity. Cancer Immunol. Immunother. 2008, 58, 71–84. [Google Scholar] [CrossRef] [PubMed]
- O’Shaughnessy, M.J.; Murray, K.S.; La Rosa, S.P.; Budhu, S.; Merghoub, T.; Somma, A.; Monette, S.; Kim, K.; Corradi, R.B.; Scherz, A.; et al. Systemic Antitumor Immunity by PD-1/PD-L1 Inhibition Is Potentiated by Vascular-Targeted Photodynamic Therapy of Primary Tumors. Clin. Cancer Res. 2018, 24, 592–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corradi, R.B.; LaRosa, S.; Jebiwott, S.; Murray, K.S.; Rosenzweig, B.; Somma, A.J.; Gomez, R.S.; Scherz, A.; Kim, K.; Coleman, J.A. Effectiveness of the combination of vascular targeted photodynamic therapy and anti-cytotoxic T-lymphocyte-associated antigen 4 in a preclinical mouse model of urothelial carcinoma. Int. J. Urol. 2019, 26, 414–422. [Google Scholar] [CrossRef]
- Lebdai, S.; Gigoux, M.; Alvim, R.; Somma, A.; Nagar, K.; Azzouzi, A.R.; Cussenot, O.; Merghoub, T.; Wolchok, J.D.; Scherz, A.; et al. Potentiating vascular-targeted photodynamic therapy through CSF-1R modulation of myeloid cells in a preclinical model of prostate cancer. OncoImmunology 2019, 8, e1581528. [Google Scholar] [CrossRef] [Green Version]
- Kraus, D.; Palasuberniam, P.; Chen, B. Targeting Phosphatidylinositol 3-Kinase Signaling Pathway for Therapeutic Enhancement of Vascular-Targeted Photodynamic Therapy. Mol. Cancer Ther. 2017, 16, 2422–2431. [Google Scholar] [CrossRef] [Green Version]
- Weersink, R.A.; Forbes, J.; Bisland, S.; Trachtenberg, J.; Elhilali, M.; Brún, P.H.; Wilson, B.C. Assessment of cutaneous photosensitivity of TOOKAD (WST09) in preclinical animal models and in patients. Photochem. Photobiol. 2005, 81, 106. [Google Scholar] [CrossRef]
- Weersink, R.; Wilson, B.C.; Patterson, M.S. Determination of the peak absorption wavelength and disaggregation kinetics of TOOKAD in vivo using dynamic, spatially resolved diffuse reflectance spectroscopy in a rabbit model. Int. Symp. Biomed. Opt. 2002, 4613, 135–143. [Google Scholar] [CrossRef]
- Trachtenberg, J.; Bogaards, A.; Weersink, R.; Haider, M.; Evans, A.; McCluskey, S.; Scherz, A.; Gertner, M.; Yue, C.; Appu, S.; et al. Vascular Targeted Photodynamic Therapy With Palladium-Bacteriopheophorbide Photosensitizer for Recurrent Prostate Cancer Following Definitive Radiation Therapy: Assessment of Safety and Treatment Response. J. Urol. 2007, 178, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Gertner, M.R.; Bogaards, A.; Weersink, R.A.; McCluskey, S.A.; Haider, M.A.; Yue, C.K.K.; Savard, J.; Simpson, S.; Brun, P.H.; Cohen, P. 839 Initial results of a phase I II trial of WST09-mediated photodynamic therapy (WST09-PDT) for recurrent prostate cancer following failed external beam radiation therapy (EBRT). Eur. Urol. Suppl. 2004, 3, 212. [Google Scholar] [CrossRef]
- Steba Biotech, S. Safety and Tolerability Study Using WST11 in Patients with Localized Prostate Cancer; ClinicalTrials; National Library of Medicine (US): Bethesda, MD, USA, 2009.
- Azzouzi, A.R.; Lebdai, S.; Benzaghou, F.; Stief, C. Vascular-targeted photodynamic therapy with TOOKAD(R) Soluble in localized prostate cancer: Standardization of the procedure. World J. Urol. 2015, 33, 937–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzouzi, A.R.; Barret, E.; Bennet, J.; Moore, C.M.; Taneja, S.S.; Muir, G.H.; Villers, A.; Coleman, J.; Allen, C.; Scherz, A.; et al. TOOKAD® Soluble focal therapy: Pooled analysis of three phase II studies assessing the minimally invasive ablation of localized prostate cancer. World J. Urol. 2015, 33, 945–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noweski, A.; Roosen, A.; Lebdai, S.; Barret, E.; Emberton, M.; Benzaghou, F.; Apfelbeck, M.; Gaillac, B.; Gratzke, C.; Stief, C.; et al. Medium-term Follow-up of Vascular-targeted Photodynamic Therapy of Localized Prostate Cancer Using TOOKAD Soluble WST-11 (Phase II Trials). Eur. Urol. Focus 2019, 5, 1022–1028. [Google Scholar] [CrossRef] [Green Version]
- Trachtenberg, J.; Weersink, R.; Davidson, S.R.; Haider, M.A.; Bogaards, A.; Gertner, M.R.; Evans, A.; Scherz, A.; Savard, J.; Chin, J.L.; et al. Vascular-targeted photodynamic therapy (padoporfin, WST09) for recurrent prostate cancer after failure of external beam radiotherapy: A study of escalating light doses. BJU Int. 2008, 102, 556–562. [Google Scholar] [CrossRef]
- Azzouzi, A.R.; Barret, E.; Moore, C.M.; Villers, A.; Allen, C.; Scherz, A.; Muir, G.H.; De Wildt, M.J.; Barber, N.J.; Lebdai, S.; et al. TOOKAD®Soluble vascular-targeted photodynamic (VTP) therapy: Determination of optimal treatment conditions and assessment of effects in patients with localised prostate cancer. BJU Int. 2013, 112, 766–774. [Google Scholar] [CrossRef]
- Moore, C.M.; Azzouzi, A.-R.; Barret, E.; Villers, A.; Muir, G.H.; Barber, N.J.; Bott, S.; Trachtenberg, J.; Arumainayagam, N.; Gaillac, B.; et al. Determination of optimal drug dose and light dose index to achieve minimally invasive focal ablation of localised prostate cancer using WST11-vascular-targeted photodynamic (VTP) therapy. BJU Int. 2015, 116, 888–896. [Google Scholar] [CrossRef]
- Rodriguez-Rivera, J.; Rodriguez-Lay, R.; Zegarra-Montes, L.; Benzaghou, F.; Gaillac, B.; Azzouzi, A.; Reis, L.O.; Palma, P. Expanding indication of padeliporfin (WST11) vascular-targeted photodynamic therapy: Results of prostate cancer Latin-American multicenter study. Actas Urol. Esp. 2018, 42, 632–638. [Google Scholar] [CrossRef]
- Azzouzi, A.-R.; Vincendeau, S.; Barret, E.; Cicco, A.; Kleinclauss, F.; Van Der Poel, H.G.; Stief, C.G.; Rassweiler, J.; Salomon, G.; Solsona, E.; et al. Padeliporfin vascular-targeted photodynamic therapy versus active surveillance in men with low-risk prostate cancer (CLIN1001 PCM301): An open-label, phase 3, randomised controlled trial. Lancet Oncol. 2017, 18, 181–191. [Google Scholar] [CrossRef]
| Agent | Cancer Types | Administration |
|---|---|---|
| Porfimer sodium [4] | Lung, esophagus, bile duct, bladder, brain, ovarian, breast, skin metastases | Intravenous injection |
| 5-aminolevulinic acid (5-ALA) [5] | Skin, bladder, brain, esophagus | Topical, oral, or intravenous injection |
| Methyl-aminolevulinate (MAL) [6] | Skin | Topical |
| Hexyl aminolevulinate (h-ALA) [7] | Skin | Topical |
| Verteporfin/ benzoporphyrin derivative (BDP) [4] | Pancreas, breast | Intravenous injection |
| Padeliporfin/ WST-11 [8,9] | Prostate, esophagus, pancreas, urothelial | Intravenous injection |
| Temoporfin [10] | Head and neck, lung, brain, bileduct, pancreas skin, breast | Intravenous injection |
| Talaporfin [4] | Liver, colon, brain, lung, breast skin metastases | Intravenous injection |
| HPPH [11] | Head and neck, esophagus, lung | Intravenous injection |
| Rostaporfin [4] | Skin, breast | Intravenous injection |
| Fimaporfin [12] | Skin, bile duct | Intra-tumoral or intravenous injection |
| Motexafin lutetium [13] | Breast | Intravenous injection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogueira, L.; Tracey, A.T.; Alvim, R.; Reisz, P.; Scherz, A.; Coleman, J.A.; Kim, K. Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies. Molecules 2020, 25, 5417. https://doi.org/10.3390/molecules25225417
Nogueira L, Tracey AT, Alvim R, Reisz P, Scherz A, Coleman JA, Kim K. Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies. Molecules. 2020; 25(22):5417. https://doi.org/10.3390/molecules25225417
Chicago/Turabian StyleNogueira, Lucas, Andrew T. Tracey, Ricardo Alvim, Peter Reisz, Avigdor Scherz, Jonathan A. Coleman, and Kwanghee Kim. 2020. "Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies" Molecules 25, no. 22: 5417. https://doi.org/10.3390/molecules25225417
APA StyleNogueira, L., Tracey, A. T., Alvim, R., Reisz, P., Scherz, A., Coleman, J. A., & Kim, K. (2020). Developments in Vascular-Targeted Photodynamic Therapy for Urologic Malignancies. Molecules, 25(22), 5417. https://doi.org/10.3390/molecules25225417






