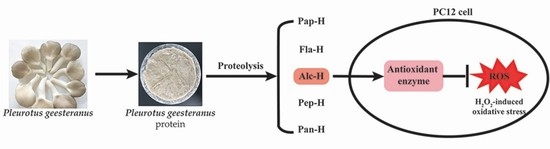
Preparation of Antioxidant Protein Hydrolysates from Pleurotus geesteranus and Their Protective Effects on H2O2 Oxidative Damaged PC12 Cells
Abstract
1. Introduction
2. Results and Discussion
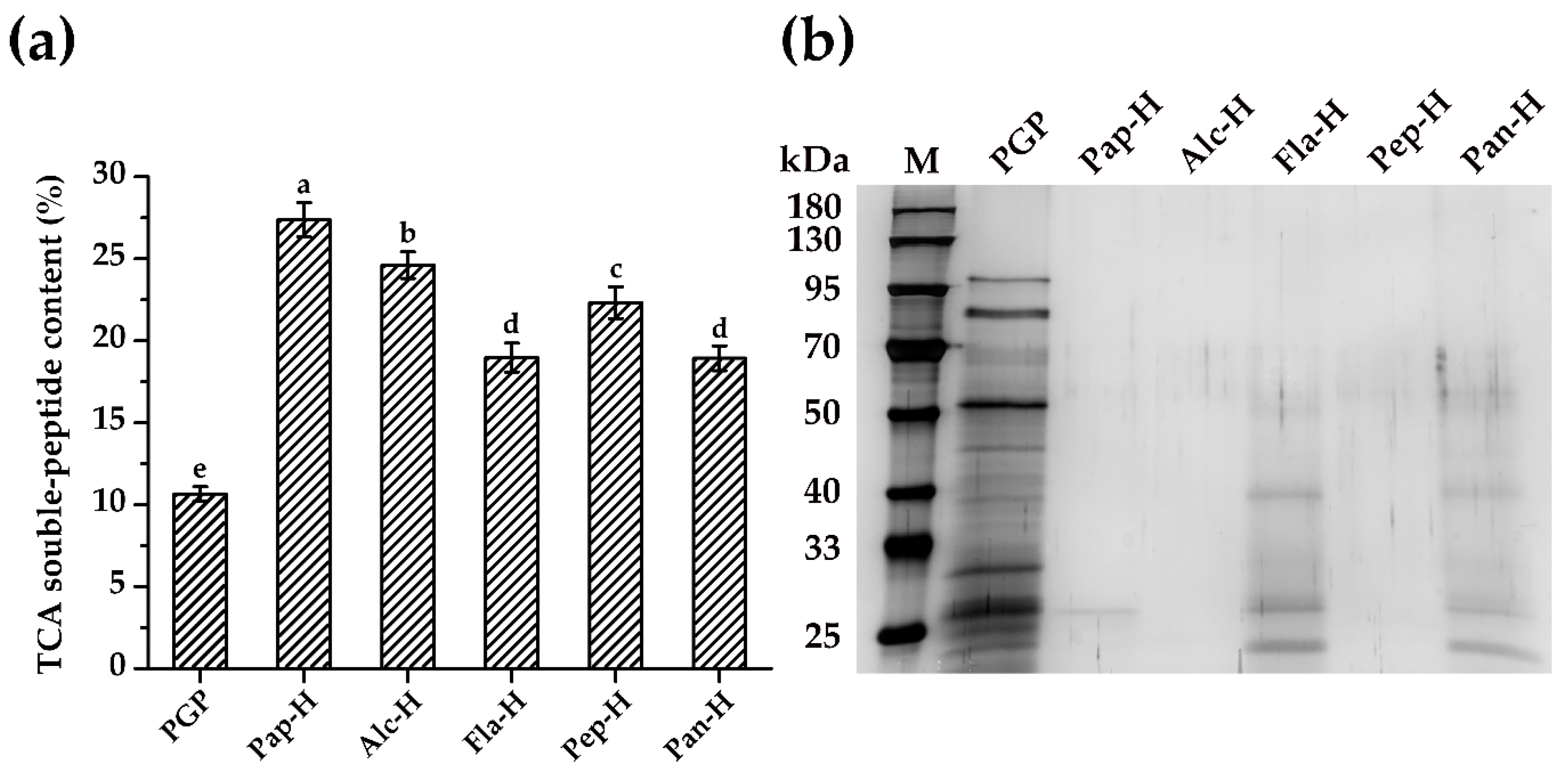
2.1. Enzymatic Hydrolysis
2.2. Antioxidant Activity of PGP and PGPHs
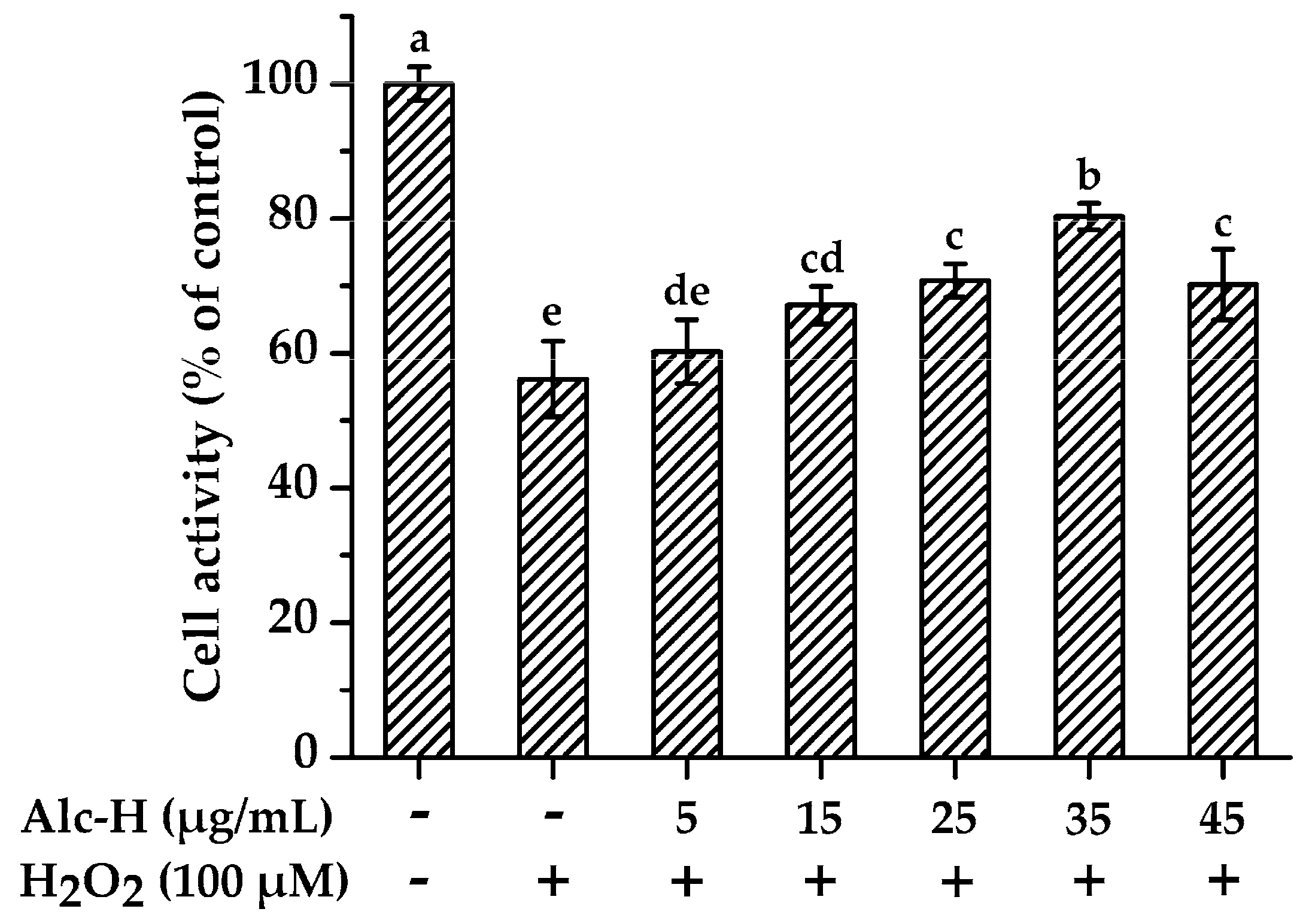
2.3. Effects of Alcalase Hydrolysate on Hydrogen Peroxide (H2O2)-Induced Oxidative Damage
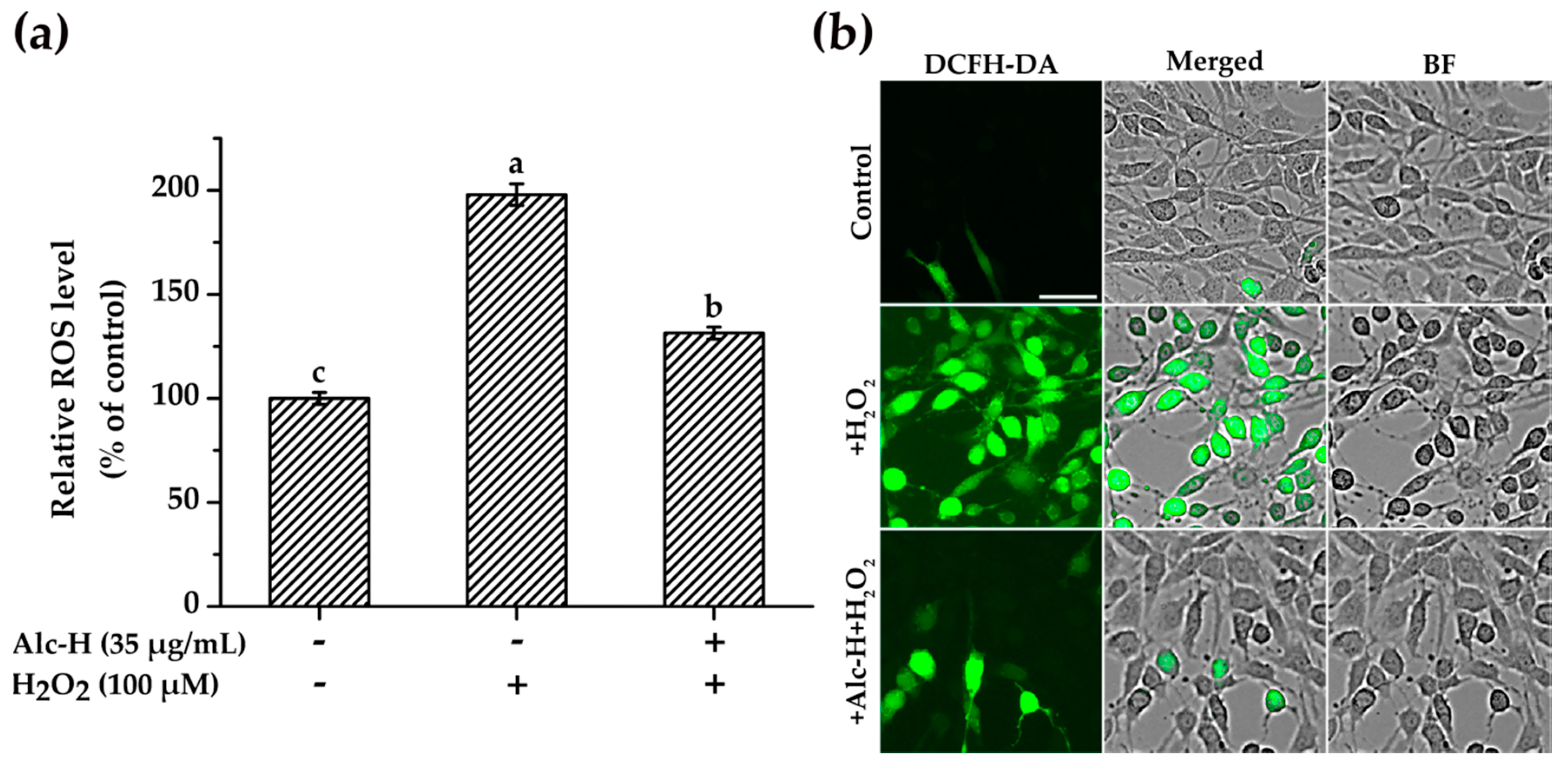
2.4. Effects of Alcalase Hydrolysate on H2O2-Induced ROS Accumulation
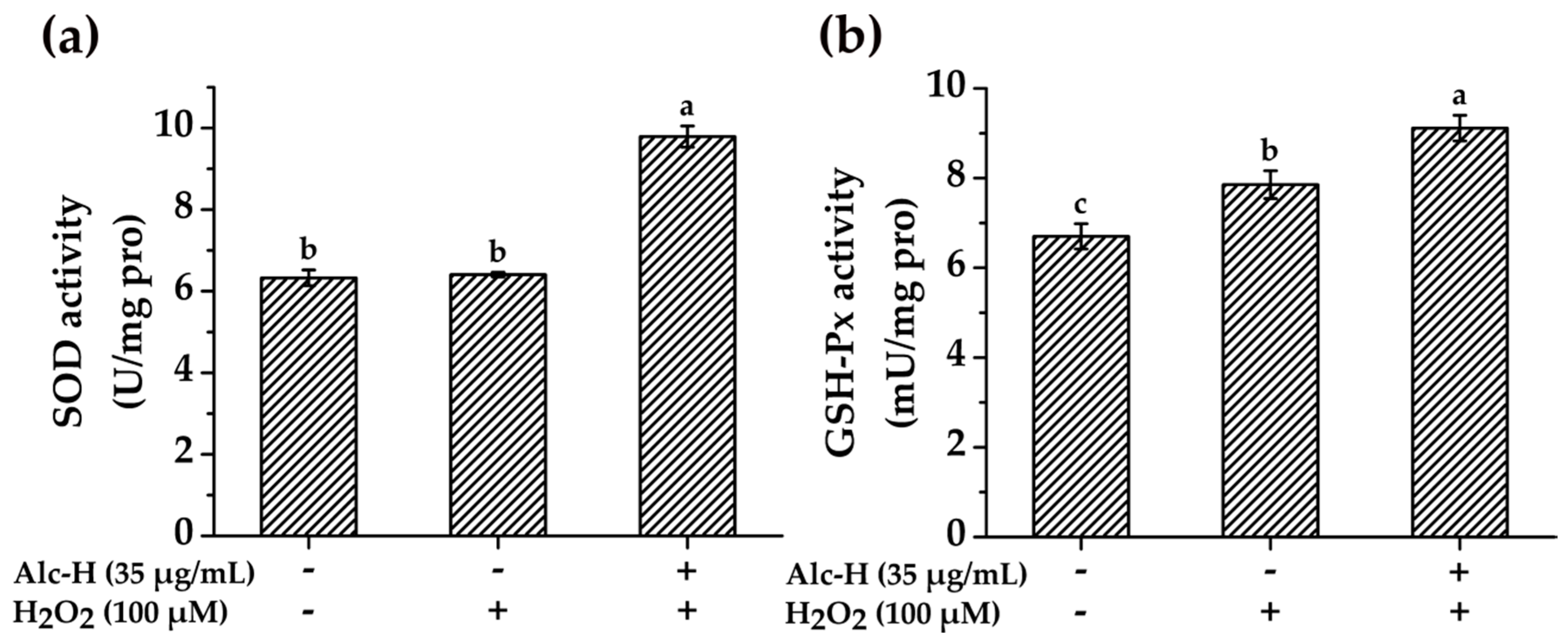
2.5. Effects of Alcalase Hydrolysate on Cellular Antioxidant Enzyme Activities
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Sample
3.2.1. Extraction of PGP
3.2.2. Preparation of PGPHs
3.3. TCA-Soluble Peptide Content
3.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
3.5. Amino acid (AA) Composition
3.6. Determination of Antioxidant Activities
3.6.1. DPPH Radical Scavenging Activity
3.6.2. ABTS Radical Scavenging Activity
3.6.3. Ferric Reducing Antioxidant Power (FRAP)
3.6.4. Oxygen Radical Absorbance Capacity (ORAC)
3.6.5. Ferrous Ion Chelating Activity
3.6.6. Inhibition of Linoleic Acid Peroxidation
3.7. Effects of Alcalase Hydrolysate on PC12 Cells Proliferation
3.8. Inducing PC12 Cells with H2O2
3.9. Cytoprotective Effects of Alcalase Hydrolysate on H2O2-Damaged PC12 Cells
3.10. Determination of Intracellular ROS Accumulation
3.11. Measurement of SOD and GSH-Px Levels
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liang, L.L.; Cai, S.Y.; Gao, M.; Chu, X.M.; Pan, X.Y.; Gong, K.K.; Xiao, C.W.; Chen, Y.; Zhao, Y.Q.; Wang, B. Purification of antioxidant peptides of Moringa oleifera seeds and their protective effects on H2O2 oxidative damaged Chang liver cells. J. Funct. Foods 2020, 64, 103698. [Google Scholar] [CrossRef]
- Fão, L.; Mota, S.I.; Rego, A.C. Shaping the Nrf2-ARE-related pathways in Alzheimer’s and Parkinson’s diseases. Ageing Res. Rev. 2019, 54, 100942. [Google Scholar] [CrossRef] [PubMed]
- Poljsak, B.; Šuput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Koo, N.S.; Min, D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004, 3, 21–33. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals, antioxidants, and human disease: Curiosity, cause, or consequence? Lancet 1994, 344, 721–724. [Google Scholar] [CrossRef]
- Yang, Q.; Cai, X.; Yan, A.; Tian, Y.; Du, M.; Wang, S. A specific antioxidant peptide: Its properties in controlling oxidation and possible action mechanism. Food Chem. 2020, 327, 126984. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, C.; Fan, G.; Li, T.; Sun, Y. Improvement of antioxidant activity of Morchella esculenta protein hydrolysate by optimized glycosylation reaction. CYTA J. Food 2018, 16, 238–246. [Google Scholar] [CrossRef]
- Chen, C.; Sun-Waterhouse, D.; Zhang, Y.; Zhao, M.; Sun, W. The chemistry behind the antioxidant actions of soy protein isolate hydrolysates in a liposomal system: Their performance in aqueous solutions and liposomes. Food Chem. 2020, 323, 126789. [Google Scholar] [CrossRef]
- Zanutto-Elgui, M.R.; Vieira, J.C.S.; do Prado, D.Z.; Buzalaf, M.A.R.; de Magalhães Padilha, P.; de Oliveira, D.E.; Fleuri, L.F. Production of milk peptides with antimicrobial and antioxidant properties through fungal proteases. Food Chem. 2019, 278, 823–831. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Borawska-Dziadkiewicz, J.; Kulawik, P.; Duda, I.; Morawska, M.; Mickowska, B. The effects of hydrolysis condition on the antioxidant activity of protein hydrolysate from Cyprinus carpio skin gelatin. LWT Food Sci. Technol. 2020, 117, 108616. [Google Scholar] [CrossRef]
- Li, T.; Shi, C.; Zhou, C.; Sun, X.; Ang, Y.; Dong, X.; Huang, M.; Zhou, G. Purification and characterization of novel antioxidant peptides from duck breast protein hydrolysates. LWT Food Sci. Technol. 2020, 125, 109215. [Google Scholar] [CrossRef]
- Farzaneh, P.; Khanahamadi, M.; Ehsani, M.R.; Sharifan, A. Bioactive properties of Agaricus bisporus and Terfezia claveryi proteins hydrolyzed by gastrointestinal proteases. LWT Food Sci. Technol. 2018, 91, 322–329. [Google Scholar] [CrossRef]
- Zhou, J.; Chen, M.; Wu, S.; Liao, X.; Wang, J.; Wu, Q.; Zhuang, M.; Ding, Y. A review on mushroom-derived bioactive peptides: Preparation and biological activities. Food Res. Int. 2020, 134, 109230. [Google Scholar] [CrossRef] [PubMed]
- Kozarski, M.; Klaus, A.; Jakovljevic, D.; Todorovic, N.; Vunduk, J.; Petrović, P.; Niksic, M.; Vrvic, M.M.; Van Griensven, L. Antioxidants of edible mushrooms. Molecules 2015, 20, 19489–19525. [Google Scholar] [CrossRef] [PubMed]
- Reis, F.S.; Martins, A.; Vasconcelos, M.H.; Morales, P.; Ferreira, I.C. Functional foods based on extracts or compounds derived from mushrooms. Trends Food Sci. Technol. 2017, 66, 48–62. [Google Scholar] [CrossRef]
- Sánchez, C. Reactive oxygen species and antioxidant properties from mushrooms. Synth. Syst. Biotechnol. 2017, 2, 13–22. [Google Scholar] [CrossRef]
- Chandra, P.; Sharma, R.K.; Arora, D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020, 129, 108849. [Google Scholar] [CrossRef]
- Girjal, V.U.; Neelagund, S.; Krishnappa, M. Antioxidant properties of the peptides isolated from Ganoderma lucidum fruiting body. Int. J. Pept. Res. Ther. 2012, 18, 319–325. [Google Scholar] [CrossRef]
- Ren, D.; Jiao, Y.; Yang, X.; Yuan, L.; Guo, J.; Zhao, Y. Antioxidant and antitumor effects of polysaccharides from the fungus Pleurotus abalonus. Chem. Biol. Interact. 2015, 237, 166–174. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, L.; Cui, S.W.; Wang, Q.; Zhou, T.; Shen, H. Fractionation, partial characterization and bioactivity of water-soluble polysaccharides and polysaccharide-protein complexes from Pleurotus geesteranus. Int. J. Biol. Macromol. 2011, 48, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Q.; Xu, M.; Fu, L.; Sun, P.L. Structural elucidation of a novel mannogalactan isolated from the fruiting bodies of Pleurotus geesteranus. Carbohydr. Polym. 2013, 92, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Shen, Q.; Liu, M.; Zhang, C.; Zhang, L.; Ren, Z.; Wang, W.; Dong, Y.; Wang, X.; Zhang, J. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus against alcoholic liver diseases. Int. J. Biol. Macromol. 2018, 114, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhou, C.; Huang, S.; Jiang, C. Selenium polysaccharide SPMP-2a from Pleurotus geesteranus alleviates H2O2-induced oxidative damage in HaCaT cells. Biomed. Res. Int. 2017, 2017, 4940384. [Google Scholar] [CrossRef] [PubMed]
- Ketnawa, S.; Benjakul, S.; Martínez-Alvarez, O.; Rawdkuen, S. Fish skin gelatin hydrolysates produced by visceral peptidase and bovine trypsin: Bioactivity and stability. Food Chem. 2017, 215, 383–390. [Google Scholar] [CrossRef]
- Kristinsson, H.G.; Rasco, B.A. Hydrolysis of salmon muscle proteins by an enzyme mixture extracted from atlantic salmon (Salmo salar) pyloric caeca. J. Food Biochem. 2000, 24, 177–187. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef]
- Cotabarren, J.; Rosso, A.M.; Tellechea, M.; García-Pardo, J.; Rivera, J.L.; Obregón, W.D.; Parisi, M.G. Adding value to the chia (Salvia hispanica L.) expeller: Production of bioactive peptides with antioxidant properties by enzymatic hydrolysis with papain. Food Chem. 2019, 274, 848–856. [Google Scholar] [CrossRef]
- Jemil, I.; Mora, L.; Nasri, R.; Abdelhedi, O.; Aristoy, M.C.; Hajji, M.; Nasri, M.; Toldrá, F. A peptidomic approach for the identification of antioxidant and ACE-inhibitory peptides in sardinelle protein hydrolysates fermented by Bacillus subtilis A26 and Bacillus amyloliquefaciens An6. Food Res. Int. 2016, 89, 347–358. [Google Scholar] [CrossRef]
- Sarbon, N.M.; Badii, F.; Howell, N.K. Purification and characterization of antioxidative peptides derived from chicken skin gelatin hydrolysate. Food. Hydrocoll. 2018, 85, 311–320. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- Girgih, A.T.; He, R.; Hasan, F.M.; Udenigwe, C.C.; Gill, T.A.; Aluko, R.E. Evaluation of the in vitro antioxidant properties of a cod (Gadus morhua) protein hydrolysate and peptide fractions. Food Chem. 2015, 173, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.S.; Winkler-Moser, J.K. Antioxidant activity of amino acids in soybean oil at frying temperature: Structural effects and synergism with tocopherols. Food Chem. 2017, 221, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.C.; Xiao, J.; Wang, S.; Ee, K.Y.; Chai, T.T. Advances on the antioxidant peptides from edible plant sources. Trends Food Sci. Technol. 2020, 99, 44–57. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Samaranayaka, A.G.P.; Li-Chan, E.C.Y. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar] [CrossRef]
- Sangtitanu, T.; Sangtanoo, P.; Srimongkol, P.; Saisavoey, T.; Reamtong, O.; Karnchanatat, A. Peptides obtained from edible mushrooms: Hericium erinaceus offers the ability to scavenge free radicals and induce apoptosis in lung cancer cells in humans. Food Funct. 2020, 11, 4927–4939. [Google Scholar] [CrossRef]
- He, R.; Alashi, A.; Malomo, S.A.; Girgih, A.T.; Chao, D.; Ju, X.; Aluko, R.E. Antihypertensive and free radical scavenging properties of enzymatic rapeseed protein hydrolysates. Food Chem. 2013, 141, 153–159. [Google Scholar] [CrossRef]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.N.T. The berry constituents quercetin, kaempferol, and pterostilbene synergistically attenuate reactive oxygen species: Involvement of the Nrf2-ARE signaling pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef]
- Zou, B.; Xiao, G.; Xu, Y.; Wu, J.; Yu, Y.; Fu, M. Persimmon vinegar polyphenols protect against hydrogen peroxide-induced cellular oxidative stress via Nrf2 signalling pathway. Food Chem. 2018, 255, 23–30. [Google Scholar] [CrossRef]
- Pozzolini, M.; Millo, E.; Oliveri, C.; Mirata, S.; Salis, A.; Damonte, G.; Arkel, M.; Scarfì, S. Elicited ROS scavenging activity, photoprotective, and wound-healing properties of collagen-derived peptides from the marine sponge Chondrosia reniformis. Mar. Drugs 2018, 16, 465. [Google Scholar] [CrossRef] [PubMed]
- Yarnpakdee, S.; Benjakul, S.; Kristinsson, H.G.; Bakken, H.E. Preventive effect of Nile tilapia hydrolysate against oxidative damage of HepG2 cells and DNA mediated by H2O2 and AAPH. J. Food Sci. Technol. 2015, 52, 6194–6205. [Google Scholar] [CrossRef] [PubMed]
- Meng, D.; Zhang, P.; Zhang, L.; Wang, H.; Ho, C.T.; Li, S.; Shahidi, F.; Zhao, H. Detection of cellular redox reactions and antioxidant activity assays. J. Funct. Foods 2017, 37, 467–479. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.S.; Gómez, B.; Barba, F.J.; Mora, L.; Pérez-Santaescolástica, C.; Toldrá, F. Bioactive peptides as natural antioxidants in food products—A review. Trends Food Sci. Technol. 2018, 79, 136–147. [Google Scholar] [CrossRef]
- Weydert, C.J.; Cullen, J.J. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nat. Protoc. 2009, 5, 51–66. [Google Scholar] [CrossRef]
- Chai, H.J.; Wu, C.J.; Yang, S.H.; Li, T.L.; Pan, B.S. Peptides from hydrolysate of lantern fish (Benthosema pterotum) proved neuroprotective in vitro and in vivo. J. Funct. Foods 2016, 24, 438–449. [Google Scholar] [CrossRef]
- Qian, L.; Zhang, Y.; Liu, F. Purification and characterization of a ~43 kDa antioxidant protein with antitumor activity from Pholiota nameko. J. Sci. Food Agric. 2016, 96, 1044–1052. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, F.; Gao, P.; Yu, D.; Yu, P.; Jiang, Q.; Xu, Y.; Xia, W. Comparative study on quality characteristics of pickled and fermented sturgeon (Acipenser sinensis) meat in retort cooking. Int. J. Food Sci. Technol. 2019, 54, 2553–2562. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.; Mallia, A.; Gartner, F.; Provenzano, M.; Fujimoto, E.; Goeke, N.; Olson, B.; Klenk, D. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Prieto, D.A.; Whitely, G.; Johann, D.J.; Blonder, J. Protocol for the analysis of laser capture microdissected fresh-frozen tissue homogenates by silver-stained 1D SDS-PAGE. In Laser Capture Microdissection: Methods and Protocols; Murray, G.I., Ed.; Springer: New York, NY, USA, 2018; pp. 95–110. [Google Scholar]
- Liu, P.; Yuan, J.; Jiang, Z.; Wang, Y.; Weng, B.; Li, G. A lower cadmium accumulating strain of Agaricus brasiliensis produced by 60Co-γ-irradiation. LWT Food Sci. Technol. 2019, 114, 108370. [Google Scholar] [CrossRef]
- Kimatu, B.M.; Zhao, L.; Biao, Y.; Ma, G.; Yang, W.; Pei, F.; Hu, Q. Antioxidant potential of edible mushroom (Agaricus bisporus) protein hydrolysates and their ultrafiltration fractions. Food Chem. 2017, 230, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, F.B.; Cermeño, M.; Kleekayai, T.; Harnedy, P.A.; FitzGerald, R.J.; Alves, R.C.; Oliveira, M.B.P. Effect of in vitro simulated gastrointestinal digestion on the antioxidant activity of the red seaweed Porphyra dioica. Food Res. Int. 2020, 136, 109309. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.R.A.; Tuenter, E.; Patria, G.D.; Foubert, K.; Pieters, L.; Dewettinck, K. Phytochemical composition and antioxidant activity of Cinnamomum burmannii blume extracts and their potential application in white chocolate. Food Chem. 2020, 340, 127983. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Yu, Q.; Liao, W.; Liu, S.; He, Z.; Hu, X.; Chen, Y.; Xie, J.; Nie, S.; Xie, M. Composition of bound polyphenols from carrot dietary fiber and its in vivo and in vitro antioxidant activity. Food Chem. 2020, 339, 127879. [Google Scholar] [CrossRef]
- Zhang, Q.; Tong, X.; Sui, X.; Wang, Z.; Qi, B.; Li, Y.; Jiang, L. Antioxidant activity and protective effects of alcalase-hydrolyzed soybean hydrolysate in human intestinal epithelial Caco-2 cells. Food Res. Int. 2018, 111, 256–264. [Google Scholar] [CrossRef]
- Zhao, W.H.; Luo, Q.B.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Preparation, identification, and activity evaluation of ten antioxidant peptides from protein hydrolysate of swim bladders of miiuy croaker (Miichthys miiuy). J. Funct. Foods 2018, 47, 503–511. [Google Scholar] [CrossRef]
- Wang, L.; Ding, L.; Yu, Z.; Zhang, T.; Ma, S.; Liu, J. Intracellular ROS scavenging and antioxidant enzyme regulating capacities of corn gluten meal-derived antioxidant peptides in HepG2 cells. Food Res. Int. 2016, 90, 33–41. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Amino Acids | PGP 1 | Pap-H 2 | Alc-H 3 | Fla-H 4 | Pep-H 5 | Pan-H 6 |
|---|---|---|---|---|---|---|
| Asp | 35.53 ± 0.13 | 71.57 ± 0.43 | 109.26 ± 0.89 | 86.25 ± 0.24 | 63.28 ± 0.25 | 87.73 ± 0.92 |
| Thr | 20.34 ± 0.11 | 40.25 ± 0.25 | 62.17 ± 0.26 | 48.67 ± 0.09 | 35.11 ± 0.16 | 48.50 ± 0.57 |
| Ser | 16.51 ± 0.05 | 33.30 ± 0.21 | 51.97 ± 0.16 | 40.30 ± 0.07 | 29.17 ± 0.11 | 40.90 ± 0.42 |
| Glu | 29.80 ± 0.17 | 54.87 ± 0.25 | 86.45 ± 0.53 | 65.75 ± 0.12 | 49.95 ± 0.17 | 68.68 ± 0.56 |
| Gly | 13.67 ± 0.04 | 27.95 ± 0.10 | 45.55 ± 0.20 | 34.29 ± 0.04 | 25.02 ± 0.05 | 35.09 ± 0.25 |
| Ala | 16.45 ± 0.10 | 31.48 ± 0.06 | 58.22 ± 0.19 | 38.52 ± 0.04 | 28.17 ± 0.06 | 39.05 ± 0.30 |
| Cys | 4.38 ± 0.09 f | 7.06 ± 0.16 d | 9.92 ± 0.34 a | 8.17 ± 0.10 c | 5.22 ± 0.08 e | 8.75 ± 0.39 b |
| Val | 16.50 ± 0.02 | 34.75 ± 0.19 | 58.94 ± 0.30 | 41.82 ± 0.05 | 29.68 ± 0.14 | 42.86 ± 0.38 |
| Met | 3.07 ± 0.12 f | 8.78 ± 0.09 d | 14.32 ± 0.09 a | 10.67 ± 0.05 c | 3.78 ± 0.17 e | 11.21 ± 0.24 b |
| Ile | 12.21 ± 0.04 | 27.68 ± 0.09 | 46.56 ± 0.21 | 33.54 ± 0.08 | 22.98 ± 0.15 | 34.48 ± 0.42 |
| Leu | 16.80 ± 0.10 | 37.99 ± 0.04 | 63.83 ± 0.33 | 46.27 ± 0.16 | 33.40 ± 0.17 | 47.88 ± 0.28 |
| Tyr | 16.05 ± 0.17 e | 34.77 ± 0.25 c | 44.88 ± 0.15 a | 40.94 ± 0.09 b | 27.74 ± 0.28 d | 41.07 ± 0.76 b |
| Phe | 17.15 ± 0.21 | 34.91 ± 0.11 | 53.67 ± 0.56 | 41.23 ± 0.14 | 29.89 ± 0.18 | 42.08 ± 0.35 |
| Lys | 19.95 ± 0.21 | 30.39 ± 0.15 | 47.28 ± 0.66 | 35.16 ± 0.10 | 26.62 ± 0.30 | 38.47 ± 0.24 |
| His | 8.51 ± 0.24 f | 15.13 ± 0.04 d | 23.28 ± 0.45 a | 17.42 ± 0.06 c | 13.13 ± 0.16 e | 18.72 ± 0.08 b |
| Arg | 9.85 ± 0.19 | 4.93 ± 0.10 | 20.57 ± 0.11 | 6.09 ± 0.06 | 10.96 ± 0.13 | 8.03 ± 0.06 |
| Pro | 12.29 ± 0.11 | 29.54 ± 1.28 | 43.13 ± 0.34 | 34.94 ± 0.13 | 25.69 ± 0.10 | 35.71 ± 1.60 |
| Trp | 4.16 ± 0.11 f | 14.85 ± 0.15 b | 13.20 ± 0.13 d | 13.49 ± 0.12 c | 9.36 ± 0.08 e | 16.69 ± 0.16 a |
| HAA 7 | 119.05 ± 1.05 f | 261.81 ± 2.42 d | 406.68 ± 2.65 a | 309.60 ± 0.95 c | 215.91 ± 1.38 e | 319.80 ± 4.87 b |
| NCAA 8 | 65.34 ± 0.30 f | 126.44 ± 0.68 d | 195.71 ± 1.43 a | 151.99 ± 0.36 c | 113.24 ± 0.36 e | 156.41 ± 1.47 b |
| AAA 9 | 37.36 ± 0.49 f | 84.54 ± 0.51 d | 111.76 ± 0.84 a | 95.66 ± 0.35 c | 66.99 ± 0.53 e | 99.85 ± 1.27 b |
| Papain | Alcalase | Flavourzyme | Pepsin | Pancreatin | |
|---|---|---|---|---|---|
| Temperature (°C) | 55 | 50 | 50 | 37 | 37 |
| pH | 7 | 9 | 7 | 2 | 7.5 |
| Time (h) | 2 | 2 | 2 | 2 | 2 |
| E 1/S 2 ration (w/w) | 4/100 | 4/100 | 4/100 | 4/100 | 4/100 |
| Substrate concentration (w/v) | 2/100 | 2/100 | 2/100 | 2/100 | 2/100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Zhu, Z.; Wu, S.; Chen, M.; Huang, R.; Wang, J.; Wu, Q.; Ding, Y. Preparation of Antioxidant Protein Hydrolysates from Pleurotus geesteranus and Their Protective Effects on H2O2 Oxidative Damaged PC12 Cells. Molecules 2020, 25, 5408. https://doi.org/10.3390/molecules25225408
Liao X, Zhu Z, Wu S, Chen M, Huang R, Wang J, Wu Q, Ding Y. Preparation of Antioxidant Protein Hydrolysates from Pleurotus geesteranus and Their Protective Effects on H2O2 Oxidative Damaged PC12 Cells. Molecules. 2020; 25(22):5408. https://doi.org/10.3390/molecules25225408
Chicago/Turabian StyleLiao, Xiyu, Zhenjun Zhu, Shujian Wu, Mengfei Chen, Rui Huang, Juan Wang, Qingping Wu, and Yu Ding. 2020. "Preparation of Antioxidant Protein Hydrolysates from Pleurotus geesteranus and Their Protective Effects on H2O2 Oxidative Damaged PC12 Cells" Molecules 25, no. 22: 5408. https://doi.org/10.3390/molecules25225408
APA StyleLiao, X., Zhu, Z., Wu, S., Chen, M., Huang, R., Wang, J., Wu, Q., & Ding, Y. (2020). Preparation of Antioxidant Protein Hydrolysates from Pleurotus geesteranus and Their Protective Effects on H2O2 Oxidative Damaged PC12 Cells. Molecules, 25(22), 5408. https://doi.org/10.3390/molecules25225408