Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption
Abstract
1. Introduction
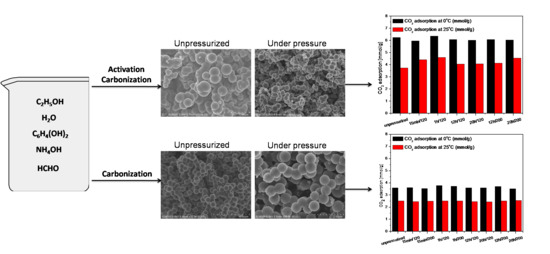
2. Results and Discussions
3. Materials Preparation
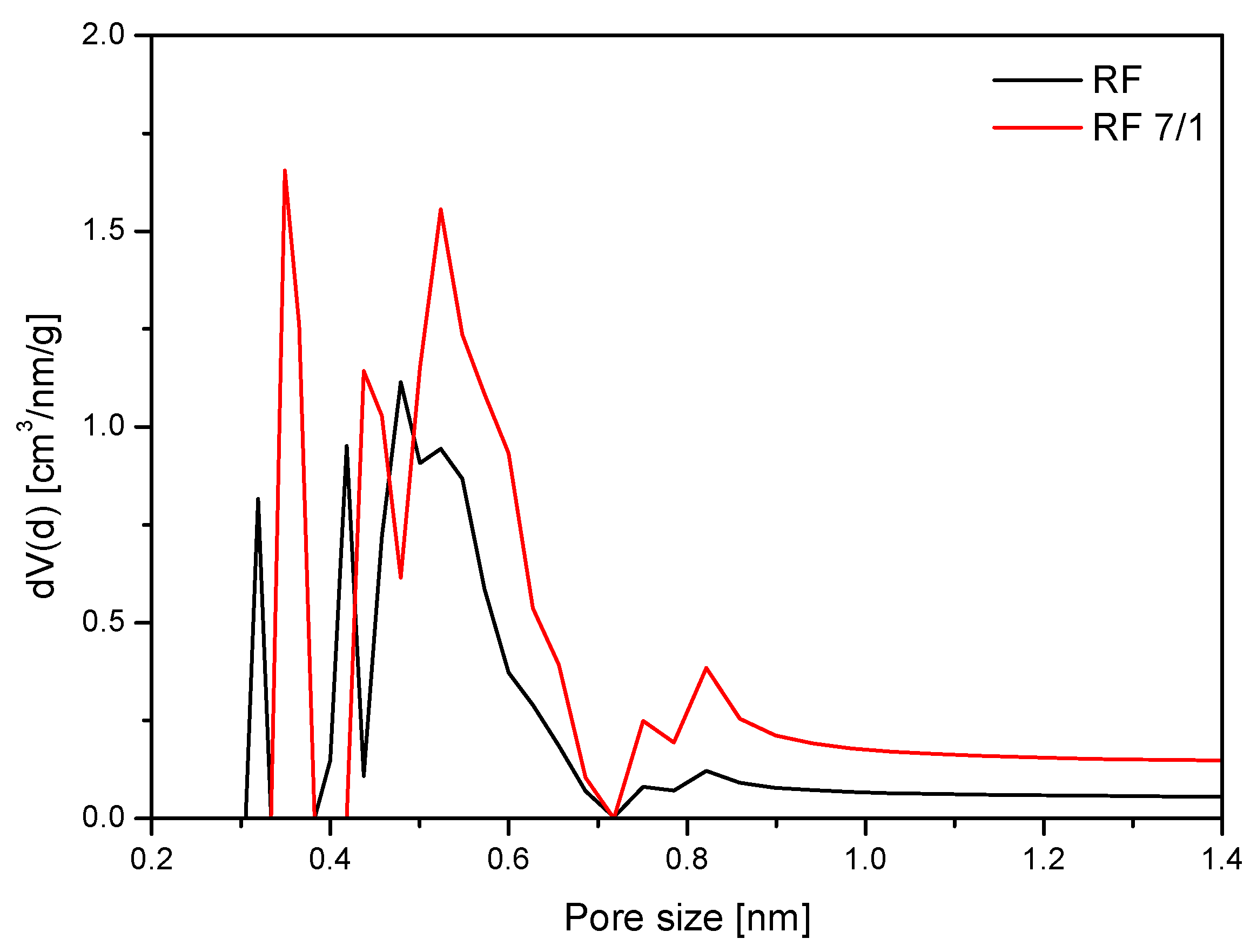
4. Materials Characterization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission, Brussels, 11.12.2019 COM(2019) 640 Final. Available online: https://ec.europa.eu/info/sites/info/files/european-green-deal-communication_en.pdf (accessed on 2 September 2020).
- National Academies of Sciences, Engineering, and Medicine. In Negative Emissions Technologies and Reliable Sequestration: A Research Agenda; The National Academies Press: Washington, DC, USA, 2019.
- European Academies’ Science Advisory Council. In Negative Emission Technologies: What Role in Meeting Paris Agreement Targets? German National Academy of Sciences Leopoldina: Harley, Germany, 2018.
- Rifka, T.; Morosuk, T.; Tsatsaronis, G. Carbon capture and storage using low-temperature post-combustion technologies. Energy Sources Part A 2019. [Google Scholar] [CrossRef]
- Gómez-Díaz, D.; Muñiz-Mouro, A.; Navaza, J.M.; Rumbo, A. Diamine versus amines blend for CO2 chemical absorption. AIChE J. 2020. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Hashim, A.S.; Othman, M.R. Carbon dioxide removal through physical adsorption using carbonaceous and non-carbonaceous adsorbents: A review. J. Environ. Chem. Eng. 2020, 8, 104142. [Google Scholar] [CrossRef]
- Rosli, A.; Ahmad, A.L.; Low, S.C. Enhancing membrane hydrophobicity using silica end-capped with organosilicon for CO2 absorption in membrane contactor. Sep. Purif. Technol. 2020, 251, 117429. [Google Scholar] [CrossRef]
- Gadipelli, S.; Howard, C.A.; Guo, J.; Skipper, N.T.; Zhang, H.; Shearing, P.R.; Brett, D.J.L. Superior multifunctional activity of nanoporous carbons with widely tunable porosity: Enhanced storage capacities for carbon-dioxide, hydrogen, water, and electric charge. Adv. Energy Mater. 2020, 10, 1903649. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Staciwa, P.; Narkiewicz, U.; Sibera, D.; Moszyński, D.; Wróbel, R.J.; Cormia, R.D. Carbon spheres carbon spheres as CO2 sorbents. Appl. Sci. 2019, 9, 3349. [Google Scholar] [CrossRef]
- Khandaker, T.; Hossain, M.S.; Dhar, P.K.; Rahman, S.; Hossain, A.; Ahmed, M.B. Efficacies of carbon-based adsorbents for carbon dioxide capture. Processes 2020, 8, 654. [Google Scholar] [CrossRef]
- Qiao, W.; Song, Y.; Lim, S.; Hong, S.; Yoon, S.; Mochida, I.; Imaoka, T. Carbon nanospheres produced in an arc-discharge process. Carbon 2006, 44, 187–190. [Google Scholar] [CrossRef]
- Yang, S.; Zeng, H.; Zhao, H.; Zhang, H.; Cai, W. Luminescent hollow carbon shells and fullerene-like carbon spheres produced by laser ablation with toluene. J. Mater. Chem. 2011, 21, 4432–4436. [Google Scholar] [CrossRef]
- Choma, J.; Jamioła, D.; Augustynek, K.; Marszewski, M.; Gao, M.; Jaroniec, M. New opportunities in Stöber synthesis: Preparation of microporous and mesoporous carbon spheres. J. Mater. Chem. 2012, 22, 12636–12642. [Google Scholar] [CrossRef]
- Mi, Y.; Hu, W.; Dan, Y.; Liu, Y. Synthesis of carbon micro-spheres by a glucose hydrothermal method. Mater. Lett. 2008, 62, 1194–1196. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Y.; Chu, W. Comparative study of textural characteristics on methane adsorption for carbon spheres produced by CO2 Activation. Int. J. Chem. Eng. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Rey-Raap, N.; Villanueva, S.F.; Menéndez, J.; Arenillas, A. Microporous carbon spheres derived from resorcinol-formaldehyde solutions. A new approach to coat supports. Microporous Mesoporous Mater. 2017, 252, 154–160. [Google Scholar] [CrossRef]
- Sibera, D.; Narkiewicz, U.; Kapica, J.; Serafin, J.; Michalkiewicz, B.; Wróbel, R.J.; Morawski, A.W. Preparation and characterisation of carbon spheres for carbon dioxide capture. J. Porous Mater. 2018, 26, 19–27. [Google Scholar] [CrossRef]
- Tian, H.; Liu, J.; O’Donnell, K.; Liu, T.; Liu, X.; Yan, Z.; Liu, S.; Jaroniec, M. Revisiting the Stöber method: Design of nitrogen-doped porous carbon spheres from molecular precursors of different chemical structures. J. Colloid Interface Sci. 2016, 476, 55–61. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, W.; Zhang, L.; Cai, H.; Han, M.; Yuan, Y.; Majeed, S.; Anjum, S.; Xu, G. A Template-free and surfactant-free method for high-yield synthesis of highly monodisperse 3-aminophenol–formaldehyde resin and carbon nano/microspheres. Macromolecules 2012, 46, 140–145. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated carbon spheres for CO2 adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.; Liu, H.; Chen, J.; Orpe, A.; Zhao, D.; Lu, G.Q. (Max) Extension of the stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres. Angew. Chem. Int. Ed. 2011, 50, 5947–5951. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.; Wang, M.; Zhu, L.; Dai, L.; Jaroniec, M. Nitrogen enriched porous carbon spheres: Attractive materials for supercapacitor electrodes and CO2 adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Ludwinowicz, J.; Jaroniec, M. Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption. Carbon 2015, 82, 297–303. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Xing, W.; Liu, B.; Zhang, J.; Lin, H.; Cui, H.; Zhuo, S. Resorcinol–formaldehyde resin-based porous carbon spheres with high CO2 capture capacities. J. Energy Chem. 2017, 26, 1007–1013. [Google Scholar] [CrossRef]
- Pari, G.; Darmawan, S.; Prihandoko, B. Porous carbon spheres from hydrothermal carbonization and KOH activation on Cassava and tapioca flour raw material. Procedia Environ. Sci. 2014, 20, 342–351. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Okunev, A.; Sharonov, V.; Aristov, Y.; Parmon, V. Sorption of carbon dioxide from wet gases by K2CO3-in-porous matrix: Influence of the matrix nature. React. Kinet. Catal. Lett. 2000, 71, 355–362. [Google Scholar] [CrossRef]
- Tripathi, N.K. Porous carbon spheres: Recent developments and applications. AIMS Mater. Sci. 2018, 5, 1016–1052. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, B.; Guan, D.; Cheng, F. Mesoporous activated carbon spheres derived from resorcinol-formaldehyde resin with high performance for supercapacitors. J. Solid State Electrochem. 2015, 19, 1783–1791. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Park, S.-J. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Colloid Interface Sci. 2013, 389, 230–235. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef]
- Chen, L.; Watanabe, T.; Kanoh, H.; Hata, K.; Ohba, T. Cooperative CO2 adsorption promotes high CO2 adsorption density over wide optimal nanopore range. Adsorpt. Sci. Technol. 2017, 36, 625–639. [Google Scholar] [CrossRef]
- Staciwa, P.; Sibera, D.; Pełech, I.; Narkiewicz, U.; Łojkowski, W.; Dąbrowska, S.; Cormia, R. Effect of microwave assisted solvothermal process parameters on carbon dioxide adsorption properties of microporous carbon materials. Micropor. Mesopor. Mater. Unpublished work.
- Xu, S.; Liu, C.; Ye, F.; Guo, Y.; Wiezorek, J.M. Alkali-assisted hydrothermal route to control submicron-sized nanoporous carbon spheres with uniform distribution. Colloids Surf. A Physicochem. Eng. Asp. 2017, 515, 1–11. [Google Scholar] [CrossRef]
- Liu, X.; Song, P.; Hou, J.; Wang, B.; Xu, F.; Zhang, X. Revealing the dynamic formation process and mechanism of hollow carbon spheres: From bowl to sphere. ACS Sustain. Chem. Eng. 2018, 6, 2797–2805. [Google Scholar] [CrossRef]
- Kukulka, W.; Wenelska, K.; Baca, M.; Chen, X.; Mijowska, E. From hollow to solid carbon spheres: Time-dependent facile synthesis. Nanomaterials 2018, 8, 861. [Google Scholar] [CrossRef]
- Juhl, A.C.; Schneider, A.; Ufer, B.; Brezesinski, T.; Janek, J.; Fröba, M. Mesoporous hollow carbon spheres for lithium–sulfur batteries: Distribution of sulfur and electrochemical performance. Beilstein J. Nanotechnol. 2016, 7, 1229–1240. [Google Scholar] [CrossRef]
- Krishnamurthy, G.; Namitha, R. Synthesis of structurally novel carbon micro/nanospheres by low temperature-hydrothermal process. J. Chil. Chem. Soc. 2013, 58, 1930–1933. [Google Scholar] [CrossRef]
- Lima, A.M.F.; Musumeci, A.W.; Liu, H.-W.; Waclawik, E.R.; Silva, G.G. Purity evaluation and influence of carbon nanotube on carbon nanotube/graphite thermal stability. J. Therm. Anal. Calorim. 2009, 97, 257–263. [Google Scholar] [CrossRef]
- Long, J.W.; Laskoski, M.; Keller, T.M.; Pettigrew, K.A.; Zimmerman, T.N.; Qadri, S.B.; Peterson, G.W. Selective-combustion purification of bulk carbonaceous solids to produce graphitic nanostructures. Carbon 2010, 48, 501–508. [Google Scholar] [CrossRef]
- Datsyuk, V.; Kalyva, M.; Papagelis, K.; Parthenios, J.; Tasis, D.; Siokou, A.; Kallitsis, I.; Galiotis, C. Chemical oxidation of multiwalled carbon nanotubes. Carbon 2008, 46, 833–840. [Google Scholar] [CrossRef]
- Sing, K.S.W. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Provisional). Pure Appl. Chem. 1982, 54, 2201–2218. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Musa, M.S.; Sanagi, M.M.; Nur, H.; Ibrahim, W.A.W. Understanding pore formation and structural deformation in carbon spheres during KOH activation. Sains Malays. 2015, 44, 613–618. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for preparation and activation of activated carbon: A review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Sun, Y.; Sun, C.; Liu, H.; Stevens, L.A.; Li, K.; Snape, C.P. High density and super ultra-microporous-activated carbon macrospheres with high volumetric capacity for CO2 capture. Adv. Sustain. Syst. 2018, 2, 1700115–1700123. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Czekajło, Ł.; Sibera, D.; Moszyński, D.; Sreńscek-Nazzal, J.; Morawski, A.; Wrobel, R.J.; Michalkiewicz, B.; Arabczyk, W.; Narkiewicz, U. Surface characteristics of KOH-treated commercial carbons applied for CO2 adsorption. Adsorpt. Sci. Technol. 2017, 36, 478–492. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Rao, Y.; Zhao, X.; Wu, M. Superior CO2, CH4, and H2 uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass-derived char by CO2 activation. Carbon 2016, 105, 454–462. [Google Scholar] [CrossRef]
- Travis, W.; Gadipelli, S.; Guo, Z. Superior CO2 adsorption from waste coffee ground derived carbons. RSC Adv. 2015, 5, 29558–29562. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.K.; Yeon, S.-H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds used in the experiments are available from the authors. |

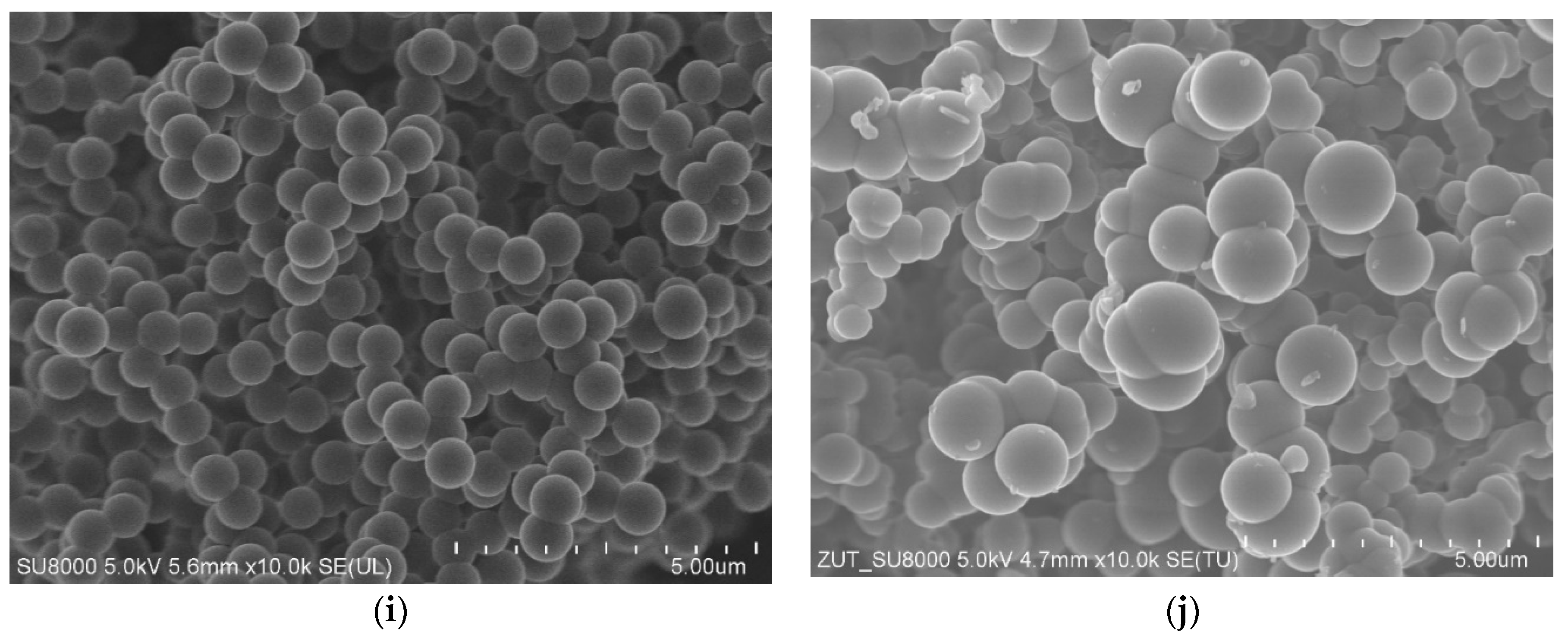
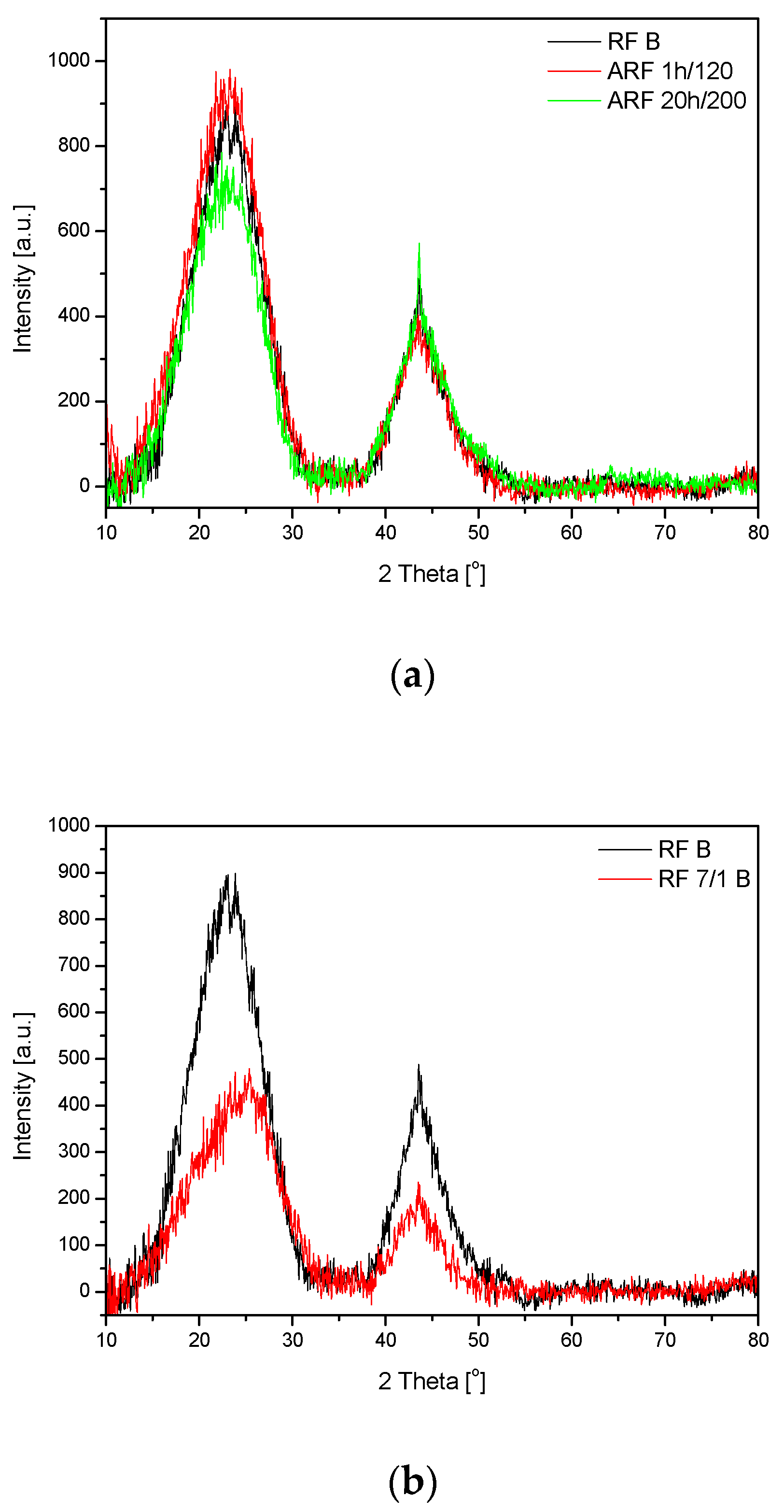

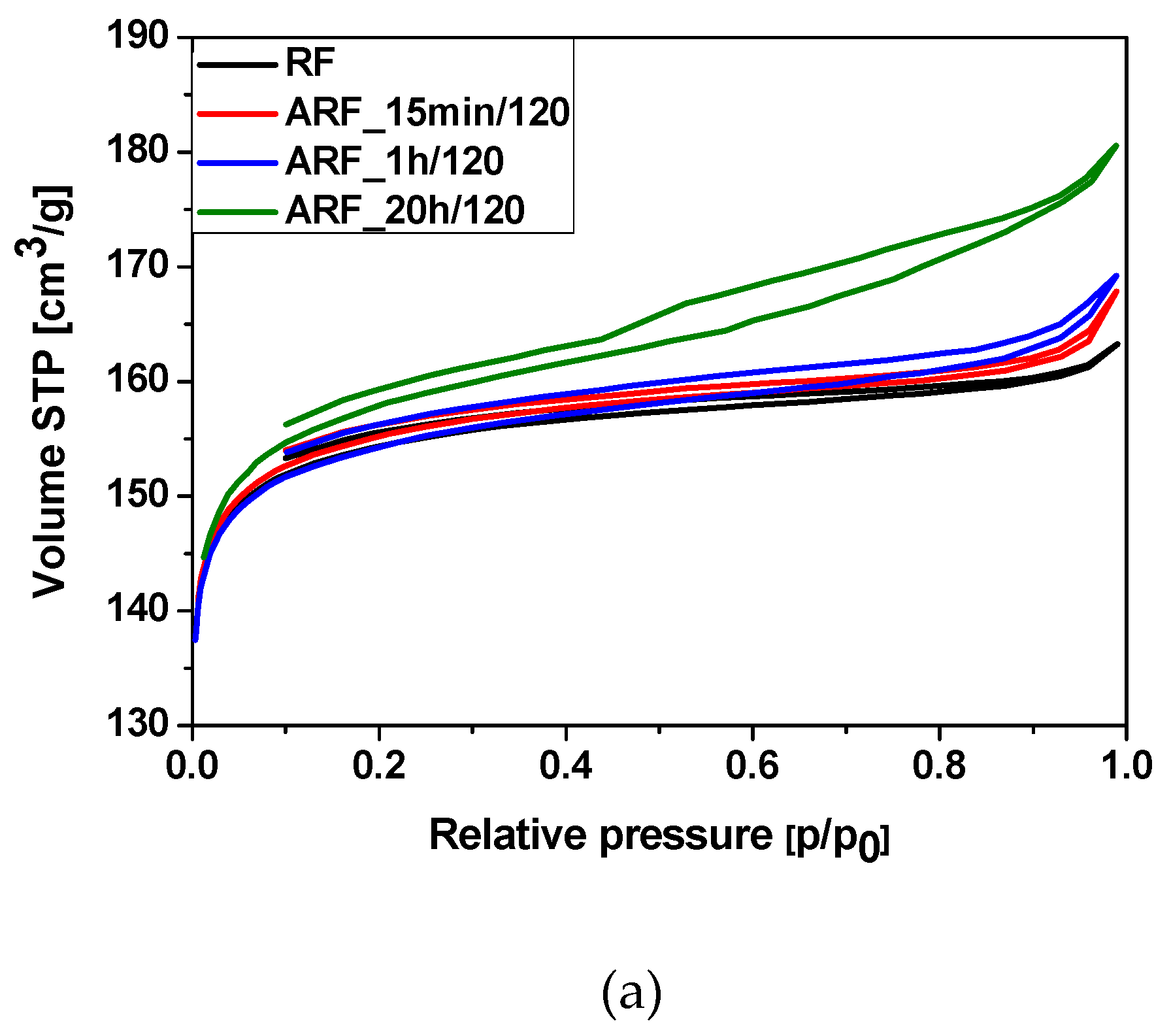




| Designation of the Sample | SBET | Total Pore Volume | CO2 Adsorption at 0 °C | CO2 Adsorption at 25 °C |
|---|---|---|---|---|
| (m2/g) | (cm3/g) | (mmol/g) | (mmol/g) | |
| The Samples without Addition of Potassium Oxalate | ||||
| RF | 472 | 0.25 | 3.59 | 2.52 |
| ARF_15 min/120 | 476 | 0.25 | 3.61 | 2.46 |
| ARF_15 min/200 | 478 | 0.26 | 3.53 | 2.49 |
| ARF_1 h/120 | 474 | 0.26 | 3.78 | 2.52 |
| ARF_1 h/200 | 483 | 0.27 | 3.73 | 2.52 |
| ARF_12 h/120 | 470 | 0.26 | 3.60 | 2.46 |
| ARF_20 h/120 | 486 | 0.28 | 3.59 | 2.44 |
| ARF_12 h/200 | 462 | 0.25 | 3.70 | 2.52 |
| ARF_20 h/200 | 473 | 0.29 | 3.51 | 2.55 |
| The Samples with Addition of Potassium Oxalate | ||||
| RF_7/1 | 904 | 0.49 | 6.25 | 3.74 |
| ARF_7/1_15 min/120 | 903 | 0.49 | 5.96 | 4.41 |
| ARF_7/1_1 h/120 | 969 | 0.51 | 6.35 | 4.60 |
| ARF_7/1_12 h/120 | 847 | 0.46 | 6.08 | 4.06 |
| ARF_7/1_20 h/120 | 831 | 0.45 | 6.02 | 4.08 |
| ARF_7/1_12 h/200 | 923 | 0.49 | 6.07 | 4.14 |
| ARF_7/1_20 h/200 | 986 | 0.54 | 6.03 | 4.55 |
| Carbon Precursor | CO2 Adsorption at 0 °C | CO2 Adsorption at 25 °C | Reference |
|---|---|---|---|
| Resorcinol-formaldehyde resin | 6.30 | 4.70 | [24] |
| Coal tar pitch | 6.00 | 4.03 | [47] |
| Fern leaves | 4.52 | 4.12 | [49] |
| Carrot peels | 5.64 | 4.18 | [49] |
| Starch | 4.40 | 3.40 | [50] |
| Pinecone biochar | 7.90 | [8] | |
| Waste coffee grounds | 7.50 | 4.21 | [51] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pełech, I.; Sibera, D.; Staciwa, P.; Narkiewicz, U.; Cormia, R. Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption. Molecules 2020, 25, 5328. https://doi.org/10.3390/molecules25225328
Pełech I, Sibera D, Staciwa P, Narkiewicz U, Cormia R. Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption. Molecules. 2020; 25(22):5328. https://doi.org/10.3390/molecules25225328
Chicago/Turabian StylePełech, Iwona, Daniel Sibera, Piotr Staciwa, Urszula Narkiewicz, and Robert Cormia. 2020. "Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption" Molecules 25, no. 22: 5328. https://doi.org/10.3390/molecules25225328
APA StylePełech, I., Sibera, D., Staciwa, P., Narkiewicz, U., & Cormia, R. (2020). Pressureless and Low-Pressure Synthesis of Microporous Carbon Spheres Applied to CO2 Adsorption. Molecules, 25(22), 5328. https://doi.org/10.3390/molecules25225328