Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment
Abstract
1. Introduction
2. Targeting Approaches in the Context of Cancer
2.1. Targeting Different Populations of the Tumor Microenvironment
2.1.1. Targeting Cancer Cells and Cancer Stem Cells (CSCs)
2.1.2. Targeting Endothelial Cells from Tumor Angiogenic Blood Vessels
2.1.3. Simultaneous Targeting of Different Cell Populations of the Tumor Microenvironment
| Peptide | Receptor | Target Cells of TME | Ref. |
|---|---|---|---|
| Lyp-1 | p32, NRP | Cancer cells, tumor lymphatic endothelial cells and tumor associated macrophages | [65,79] |
| F3 | Nucleolin | Cancer cells, CSCs and tumor endothelial cells | [71,72,73,80,81] |
| iRGD | αvβ3, αvβ5 NRP | Cancer cells and tumor endothelial cells | [77,82] |
| T1 | p32, NRP | Cancer cells, tumor lymphatic endothelial cells and tumor associated macrophages | [70,83] |
| F56 | VEGFR1 | Cancer cells, tumor endothelial cells, fibroblasts and tumor associated macrophages | [84] |
2.2. Ligands for Active Targeting
2.3. Strategies to Identify New Ligands
3. Ligand-Targeted Photosensitizers
3.1. Folate and Transferrin-Targeted PS
3.2. Antibody and Nanobody-Targeted PSs
3.3. Peptides-Targeted PS
3.4. Other Targeting Strategies
| Strategy | PS | Ligand | Target | Application | Ref. |
|---|---|---|---|---|---|
| Endogenous ligand | Chlorin derivatives | Biotin | Biotin receptor | In vitro: CT26 cells | [168] |
| Endogenous ligand | (Phthalocyaninato)zinc(II) | Biotin | Biotin receptor | In vitro: HeLa and HuH-7 cells | [169] |
| Endogenous ligand | Ruthenium (II) polypyridyl complex | Biotin | Biotin receptor | In vitro: A549R cells | [170] |
| Endogenous ligand | Silicon (IV) phthalocyanine | Biotin | Biotin receptor | In vivo: mice bearing HeLa tumors | [171] |
| Endogenous ligand | Pyropheophorbide a | 17-substituted testosterone and epitestosterone | Androgen receptor | In vitro: LNCaP and PC-3 cells | [174] |
| Carbohydrate | H2TFPC (chlorin) | d-glucose | Glucose transporter | In vitro: MKN28, MKN45, HT29 and HCT116 cells; In vivo: mice bearing HT29 or HCT116 tumors | [175] |
| Carbohydrate | H2TFPC (chlorin) | d-mannose | CD206 (mannose receptor) | In vitro: MKN28, MKN45, HT29, HCT116 and M1- and M2-polarized THP-1 macrophages; In vivo: mice bearing CT26 tumors | [179] |
| Aptamer | Chlorin e6 free acid | AIR-3A (RNA aptamer) | Interleukin-6 receptor | In vitro: BaF3/gp130/IL6R/TNF cells expressing interleukin-6 receptor | [177] |
| Aptamer | Chlorin e6 free acid | AS1411 (DNA aptamer) | Nucleolin | In vitro: MCF-7, HCT 116 and SKOV-3 cells; Ex vivo: MCF-7 and HCT 116 tumours | [114] |
4. Ligand-Targeted Nanocarriers for the Delivery of Photosensitizers
4.1. Ligand-Targeted Lipid-Based NPs
| Nanocomposition | PS | Ligand | Target | Extra Features | Application | Ref. |
|---|---|---|---|---|---|---|
| Liposomes | Erythrosine-decyl ester | Biotin | Biotin receptor | _ | In vitro: ATCC® CCL1.3™ cells | [192] |
| Liposomes | ICG | FA | FR | DOX, Gadolinium (III) | In vitro: HeLa, NIH-3T3 cells; In vivo: mice bearing HeLa tumors | [198] |
| Liposomes | Pyropheophorbide a-lipid | FA | FR | _ | In vitro: A549, H647, H460, SBC5 and DFC1024 cell lines; In vivo: mice bearing A549 tumors | [196] |
| Liposomes | (5,10,15,20-Tetraporphyrinato)zinc(II) | FA | FR | _ | In vitro: HeLa cells | [202] |
| Liposomes | Temoporfin | FA | FR | PEG | In vitro: A549, KB and HeLa cells | [193] |
| Liposomes | Verteporfin | Anti-EGFR antibody (Cetuximab) | EGFR | _ | In vitro: Ovcar-5, CAMA-1 and A431 cells | [189] |
| Liposomes | Verteporfin | Anti-EGFR antibody (Cetuximab) | EGFR | Irinotecan | In vitro: OVCAR-5, U87 and J774 cells | [203] |
| Liposomes | Pheophorbide a derivative | Anti-EGFR antibody (Cetuximab) | EGFR | DOX | In vitro: A-431 SK-BR-3 cells; In vivo: A-431 tumors | [204] |
| Liposomes | Hydrophobically modified ICG with octadecylamine (ODA) | Anti-Her2 antibodies | Her2 | DOX | In vitro: MCF7, SKOV3, A549 and S180 cells; In vivo: mice bearing SKOV3, A549 and MCF7 tumors | [190] |
| Liposomes | Chlorin e6 free acid | cRGD | αvβ3 integrin receptor | TPZ, Gadolinium (III), ICG | In vitro: A549 cells In vivo: mice bearing A549 tumors | [93] |
| Liposomes | Verteporfin | Factor VII (fVII) protein | VEGFR | _ | In vitro: CHO-K1, EMT6, HEK 293, MDA-MB-231 and HUVEC cells; In vivo: mice bearing EMT6 tumors | [205] |
| Liposomes | ICG | HA | CD44 | PEG | In vitro: U-87MG; In vivo: mice bearing U87MG tumors | [199] |
| Liposomes | Porphyrin derivatives: 5,10,15,20-tetrakis(4-aminophenyl) porphyrin, 5, 10,15,20-tetrakis(4-hydroxyphenyl) porphyrin, 5, 10,15,20-tetraphenyl porphyrin, 5,10,15,20-tetra(4-pyridyl) porphyrin | HA | CD44 | Rhodamine | In vitro: MDA-MB-231 cells | [194] |
| NLC | ICG | FA | FR | Paclitaxel, PEG | In vitro: HepG2 and NIH3T3 cells; In vivo: mice bearing HepG2 tumors | [201] |
| NLC | 1,2,3,4,8,9,10,11,15,16,17,18,22,23,24,25-hexadecafluoro-29H,31H-phthalocyanine | FA | FR | _ | In vitro: MCF-7 cells | [206] |
4.2. Ligand-Targeted Polymer-Based NPs and Hydrogels
| Nanocomposition | PS | Ligand | Target | Extra Features | Application | Ref. |
|---|---|---|---|---|---|---|
| Methoxy-PEG-PLGA-based PNP | Chlorin e6 free acid | FA | FR | PEG, RBC membranes, DOX | In vitro: HepG2 cells; In vivo: mice bearing HepG2 tumors | [211] |
| PEGylated PLG-co-hydroxymethyl GA-based PNP | meso-tetraphenylchlorine disulphonic acid disodium (TPCS2a) | anti-HER2 nanobody (11A4) | HER2 | PEG, Saporin | In vitro: SkBr3 (HER2+), MDA-MB-231 (HER2-) cells | [223] |
| PLGA-based PNP | Pheophorbide a | FA | FR | PEG | In vitro: MKN28 cells; In vivo: mice bearing MKN28 tumors | [224] |
| PLGA-based PNP | Verteporfin | FA | FR | _ | In vitro: HCT116 cells | [225] |
| HA-b-PLGA-based PNP | Pp IX | HA | CD44 | _ | In vitro: A549 cells | [226] |
| (PLGA) and carboxymethyl chitosan (CMC)- based PNP | Hypocrellin A | Tf | Tf receptor | _ | In vitro: A549, NIH-3T3 cells; In vivo: Mice bearing A549 tumors | [227] |
| PLGA-based PNP | meso-tetraphenylchlorine disulphonic acid disodium (TPCS2a) | HA | CD44 | Docetaxel | In vitro: MCF-7 and MDA-MB-231 cells | [208] |
| PLGA-based PNP | meso-tetraphenylchlorine disulphonic acid disodium (TPCS2a) | HA | CD44 | Docetaxel | In vitro: MDA-MB-231 and HeLa cells | [228] |
| PEG-based PNP | Coumarin chromophore | Biotin | Biotin receptor | PEG | In vitro: HeLa cells | [208] |
| 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(PEG-2000)-based PNP | benzo[1,2-b:4,5-b′]dithiophene 1,1,5,5-tetraoxide | RGD-4R peptide | αvβ3 integrin receptor | 4,4′-(2,2-diphenylethene-1,1-diyl)bis(N,N-diphenylaniline) | In vitro: SKOV-3, HeLa, PC3 and MCF7 cells; In vivo: mice bearing SKOV-3 tumors | [229] |
| PLGA- PNP | MB | c(RGDfK) peptide | αvβ3 integrin receptor | Catalase in the aqueous core, Black hole quencher-3 | In vitro: U87-MG, MCF-7, SKOV-3 and HaCaT cells; In vivo: mice bearing U87-MG tumors | [212] |
| PLGA-PEG-based PNP | Verteporfin | hTf peptide | Tf receptor | _ | In vitro: MDA-MB-231 cells | [139] |
| PEG-PCL-based Polymeric micelles | HOSiPcOSi(CH3)2-(CH2)3N(CH3)2, (Pc 4) | GE-11 peptide | EGFR | _ | In vitro: SCC-15 cells; In vivo: mice bearing SCC-15 tumors | [213] |
| Chitosan-based hydrogel | Tetrakis(4-aminophenyl)porphyrin | FA | FR | _ | In vitro: The MCF-7 (FR+) and HepG2 (FR−) cells | [219] |
| Chitosan/alginate-based hydrogel | meso-Tetra(N-methyl-4-pyridyl) porphine tetra tosylate (TMPyP) | Anti-DR5 antibody | Death receptor 5 | _ | In vitro: HCT116 cells | [218] |
| HA-based hydrogel | ICG | MMP-2 | MMP-2 receptor | DOX | In vitro: SCC-15 cancer cells; In vivo: SCC-15 tumor bearing mice | [222] |
| Polyacrylamide-based hydrogel | MB | F3 peptide | Nucleolin | PEG | In vitro: MDA-MB-435 and F98 cells | [220] |
| Polyacrylamide-based hydrogel | HPPH | F3 peptide | Nucleolin | PEG | In vitro: MDA-MB-435 and 9 L cells | [221] |
4.3. Cyclodextrin (CDs)
| Nanocomposition | PS | Ligand | Target | Extra Features | Application | Ref. |
|---|---|---|---|---|---|---|
| β-cyclodextrin | Adamantane-modified 5,10,15,20-tetrakis(4-hydroxyphenyl)-21H,23H-porphine (THPP) | HA | CD44 receptor | Adamantane-modified camptothecin prodrug | In vitro: MDA-MB-231 cells; In vivo: mice bearing MDA-MB-231 tumors | [233] |
| γ-cyclodextrin | Fullerene C60 | FA | FR | GO | In vitro: HeLa cells | [236] |
| β-cyclodextrin | Chlorin e6 free acid | Adamantine-CGKRK-GFLG-EE-HAIYPRH (T7) peptide | Tf receptor | _ | In vitro: MCF-7 cells | [237] |
| β-cyclodextrin | 1,8-dihydroxy-3-methylanthraquinone (DHMA) | Lactobionic acid (LA) | Asialoglycoprotein receptors | PEG, camptothecin prodrug (NBCCPT), | In vitro: HepG2 cells; In vivo: mice bearing HepG2 tumors | [234] |
| β-cyclodextrin | Pheophorbide a | FA | FR | Adamantane | In vitro: MCF-7 and PC3 cells | [238] |
| β-cyclodextrin | Adamantane-modified BODIPY (BTA) | Mannose | Mannose receptor | Adamantane | In vitro: MDA-MB-231 and MCF-10A cells; In vivo: mice bearing MDA-MB-231 tumors | [235] |
| β-cyclodextrin | Phenanthroline modified CD-Ruthenium complex | Tf | Tf receptor | Adamantane | In vitro: A549 cells 293T cells | [239] |
| β-cyclodextrin | GO | HA | CD44 | DOX, Fe3O4 | In vitro: BEL-7402 cells | [240] |
| β-cyclodextrin | (Phthalocyaninato)zinc(II) | FA | FR | Camptothecin | In vitro: HEP2 cells; In vivo: mice bearing HEP2 tumors | [241] |
| β-cyclodextrin | 5,10,15,20-Tetrakis(m-hydroxyphenyl)-21,23H-porphyrin (mTHPP) | Tamoxifen | Estrogen receptor | _ | In vitro: MCF7 and MDA-MB-231 cells | [242] |
| β-cyclodextrin | Adamantane-modified 5-(4-carboxyphenyl)-10,15,20-triphenylporphyrin | FA | FR | DOX, GO | In vitro: HeLa and OCT-1 cells; In vivo: mice bearing HeLa tumors | [243] |
4.4. Carbon Nanomaterials (CNMs)
| Nanocomposition | PS | Ligand | Target | Extra Features | Application | Ref. |
|---|---|---|---|---|---|---|
| CNT | ICG | FA | FR | PTT | In vitro: HeLa cells; In vivo: mice bearing HeLa tumors | [251] |
| CD | ICG | FA | FR | Polydopamine | In vitro: HeLa cells | [252] |
| CNT | Organoselenium compound (PSeD) | AE105 polypeptide (uPAR) | Urokinase-type plasminogen activator receptor (uPAR) | pH-responsive triblock polymer composed of PEG-COOH, polyethyleneimine (PEI) and 3,4,5,6-tetrahydrophthalic anhydride (TA) (PPTA) | In vitro: MDA-MB-231 and L02 cells | [253] |
| CNT | (2-amino-phthalocyaninato)zinc(II) | FA | FR | _ | In vitro: A375 cells | [254] |
| CNT | HMME | HA | CD44 | _ | In vitro: B16F10 cells; In vivo: Mice bearing B16F10 tumors | [248] |
| CNT | ICG | HA | CD44 | _ | In vitro: SCC7; In vivo: mice bearing SCC7 tumors | [255] |
| GO | ICG | Anti-epithelial cell adhesion molecule (EpCAM) antibody and A9-aptamer | PSMA | _ | In vitro: LNCaP cells | [256] |
| GO | Chlorin e6 free acid | HA | CD44 | _ | In vitro: A549 cells | [257] |
| GO | Chlorin e6 free acid | RGD4C peptide | αvβ3 integrin receptor | Polyvinylpyrrolidone (PVP) | In vitro: MGC803 cells | [258] |
| GO | Chlorin e6 free acid | FA | FR | _ | In vitro: MGC803 cell line | [259] |
| GO | Chlorin e6 free acid | HA | CD44 | _ | In vitro: HeLa and NIH3T3 cells | [260] |
| GO | MB | FA | FR | DOX | In vitro: HeLa and MCF-7 cells | [261] |
| GO | Verteporfin | c(RGDfK) peptide | αvβ3 integrin receptor | Banoxantrone dihydrochloride (AQ4N), and HIF-1α siRNA (siHIF-1α) | In vitro: Human PC-3 prostate cancer cell line; In vivo: mice bearing PC-3 tumor | [262] |
| GO | 3-[1-hydroxyethyl]-3-devinyl-131-β,β-dicyanomethylene-131-deoxopyropheophorbide a | FA | FR | DOX | In vitro: Hep-G2 cells | [263] |
| GO | Chlorin e6 free acid | FA | FR | 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine-PEG2000 | In vitro: KB, A549, HeLa, HaCaT cells; In vivo: mice bearing HeLa tumors | [264] |
| GO | Pyropheophorbide a | Anti-integrin αvβ3 antibody | αvβ3 integrin receptor | _ | In vitro: MCF-7, U87-MG cells | [265] |
| GO | Tetrakis(4-carboxyphenyl)porphyrin (TCPP) | FA | FR | _ | In vitro: HeLa cells | [266] |
| GO | HPPH | HK peptide | αvβ3 integrin receptor | PEG | In vitro: 4T1 cells; In vivo: mice bearing 4T1 tumors | [267] |
| Fullerene | Fullerene (C60) | FA | FR | DOX | In vitro: HeLa (FR+) and A549 and L929 (FR-) cells | [268] |
| Fullerene | Fullerene (C60) | FA | FR | _ | In vitro: HeLa cells | [269] |
| Fullerene | Fullerene (C60) | HA | CD44 | In vitro: HCT-116 cells; In vivo: mice bearing HCT-116 tumors | [270] | |
| Fullerene | Fullerene (C60) | Pullulan | Asialoglycoprotein receptors (ASGPR) | _ | In vitro: HepG2 cell lines; In vivo: mice bearing tumors | [271,272] |
| Fullerene | Fullerene (C70) | R13 Aptamer | EGFR | _ | In vitro: A549 cells | [273] |
| Fullerene | Fullerene (C60) | D-glucosamine | GLUT-1 receptor | _ | In vitro: PANC1 and PSC cells | [274] |
| Fullerene | Fullerene (C60) | NGR peptide | CD13/aminopeptidase N receptor | DOX, 1, 2-Distearoyl-sn-glycero-3-phosphoethanolamine -PEG | In vitro: 4T1 cells; In vivo: mice-bearing 4T1 tumors | [250] |
| Fullerene | Diadduct malonic acid-fullerene (C60) | NGR peptide | CD13/aminopeptidase N receptor | 2-methoxyestradiol (2ME) | In vitro: MCF-7 cells | [275] |
| Fullerene | Fullerene (C60) | Tf | Tf receptor | HA, Artesunate | In vitro: MCF-7 cells; In vivo: mice bearing S180 tumors | [276] |
| CD | Pp IX | FA | FR | In vitro: HeLa and HT-29 cells | [277] | |
| CD | CD | Heavy-chain ferritin | Tf receptor | DOX | In vitro: MCF-7 cells; In vivo: mice bearing S180 tumors | [278] |
| CD | Pp IX | RGD peptide | αvβ3 integrin receptor | Carbon nitride | In vitro: MCF-7 and 4T1 cells; In vivo: mice bearing 4T1 tumors | [249] |
4.5. Inorganic NPs
4.6. Metal Organic Frameworks (MOFs)
| Nanocomposition | PS | Ligand | Target | Extra Features | Application | Ref. |
|---|---|---|---|---|---|---|
| SiNPs | MB | Nuclear localization signal peptide (KKKRK) | Nuclear receptor | DOX | In vitro: U87MG cancer cells, In vivo: U87MG tumor bearing mice | [285] |
| SiNPs | (Phthalocyaninato)zinc(II) | FA | FR | _ | In vitro: A431, SCC12, CAL27 and NHEKs cells | [291] |
| SiNPs | (5-{p-[3-(2′,5′-dioxo-2′,5′-dihydro-1H-pyrrol-1′-yl)-N-3-phenoxypropyl)propanamide]-phenyl}-10,15,20-tri-p-pyridyl-porphyrine derivative | Dimannoside-carboxylate | Mannose 6-phosphate receptor | - | In vitro: LNCaP cells | [292] |
| SiNPs | Chlorin e6 free acid | FA | FR | FA polyethylene glycol-b-poly(asparaginyl-chidamide), DOX | In vitro: MCF-7/ADR cells | [293] |
| SiNPs | Chlorin e6 free acid | HA | CD44 | DOX | In vitro: SCC7 cells | [294] |
| SiNPs | 5,10,15,20--Tetrakis(N-methyl-4-pyridyl)porphyrin tetra tosylate (TMPyP4) | FA | FR | G-quadruplex DNA, DOX | In vitro: HepG2 and 3T3 cells | [295] |
| SiNPs | N-[3-(triethoxysilyl)propyl]-O-[4-(10,15,20-tri(3-hydroxyphenyl)-(2,3-dihydro)porphyrin-5-yl) phenyl]-carbamate | FA and Biotin; RGD and RAD; Cetuximab and Bovine Serum Albumin-conjugated nanoparticles | PEG | In vitro: A549, CCD-34Lu, KB cells, HeLa, A431 and HUVEC cells | [296] | |
| SiNPs | 5-ALA | FA | FR | _ | In vitro: B16F10 cells | [297] |
| SiNPs | 5-(4-carboxyphenyl)-10,15,20-triphenylchlorin (TPC) | Neuropilin-1 (NRP-1) | VEGFR | Gadolinium | In vitro: MDA-MB-23 cells; In vivo: mice bearing U87 tumors | [298] |
| SiNPs | 5,10,15-Trisulphonatophenyl-20-(N-phenyl-N’-propyltriethoxysilanecarbamide)porphyrin | HA | CD44 | _ | In vitro: HCT-116 cells | [299] |
| SiNPs | 5,10,15-Trisulphonatophenyl-20-(N-phenyl-N’-propyltriethoxysilanecarbamide)porphyrin | Mannose, galactose | Mannose, galactose receptors | Camptothecin, fluorescein isothiocyanate | In vitro: Y-79 cells | [300] |
| SiNPs | (5,10,15,20-Tetraphenylporphyrinato)palladium(II) | cRGDyK peptides | αvβ3 integrin receptor | fluorescent contrast agent, ATTO647N | In vitro: MCF-7 and U87-MG cells | [301] |
| SiNPs | 5,10,15-Trisulphonatophenyl-20-(N-phenyl-N’-propyltriethoxysilanecarbamide)porphyrin | Galactose | Galactose receptor | Camptothecin | In vitro: HCT-116, Capan-1 and MDA-MB-231 cells | [302] |
| AuNPs | (5,10,15,20-Tetraphenylporphyrinato)zinc(II) | FA | FR | Thioglucose | In vitro: HeLa and MCF-7 cells | [303] |
| AuNPs | ICG | RGD peptide | αvβ3 integrin receptor | Doxycycline, Combretastatin A4 phosphate, PEG | In vitro: HUVEC and HT-1080 cells | [304] |
| AuNPs | Chlorin e6 (Ce6-labeled aptamer sequence) | Nucleolin-targeting aptamer AS1411 | Nucleolin | DNA-programmed polymeric SNA, DOX | In vitro: HeLa cells | [305] |
| AuNPs | 5-ALA | Anti-HER2 antibody, HA | HER2, CD44 | PEG, Cy7.5 | In vitro: MCF-7 cells; In vivo: mice bearing MCF-7 tumors | [286] |
| AuNPs | Chlorin e6 free acid | Anti-CD3 antibody | CIK-cells | _ | In vitro: MGC-803 and GES-1 cells; In vivo: mice bearing MGC-803 tumors | [306] |
| AuNPs | HOSiPcOSi(CH3)2-(CH2)3N(CH3)2, (Pc 4) | PSMA | PSMA receptor | PEG | In vitro: PC3pip (PSMA+ ) and PC3flu (PSMA−) cells; In vivo: mice bearing PC3pip or PC3flu tumors | [307] |
| AuNPs | Chlorin e6 free acid | α-lipoic acid-EGF | EGFR | _ | In vitro: MDA-MB-468 cells | [308] |
| AuNPs | (Phthalocyaninato)zinc(II) | Lactose-containing thiol derivative | Galectin-1 receptor | In vitro: SK-BR-3 and MDA-MB-231 cells | [309] | |
| AuNPs | 5-ALA | U11 peptide | Urokinase-type plasminogen activator receptor (uPAR) | CTSE-sensitive imaging agent, PEG | In vitro: PANC1-CSTE cells; In vivo: mice bearing PANC1-CSTE tumors | [310] |
| AuNPs | (5-[4-(11-mercaptoundecyloxy)phenyl]-10,15,20-triphenylporphyrin | Anti-erbB2 ICR55 antibody | ErbB2 | Thiolated carboxyl terminated PEG | In vitro: SK-BR-3 cells | [311] |
| AuNPs | 5-ALA | R8-PLGLAG-EK10 peptide | MMP-2 | _ | In vitro: SCC-7cells; In vivo: mice bearing SCC-7 tumors | [312] |
| AuNPs | Verteporfin | FA | FR | PEG-P(Asp-Hyd)-DHLA block copolymer | In vitro: HeLA cells | [313] |
| AuNPs | HOSiPcOSi(CH3)2-(CH2)3N(CH3)2, Pc 4 | EGF, Tf | EGFR, Tf receptor | _ | In vitro: U87-MG and LN229 cells; In vivo: mice bearing U87-MG tumors | [314] |
| Au nanoclusters | Pp IX | FA | FR | Lipoic acid | In vitro: L929 and C6 cells; In vivo: mice bearing C6 tumors | [315] |
| AuNPs | HOSiPcOSi(CH3)2-(CH2)3N(CH3)2, (Pc 4) | EGF | EGFR | _ | In vitro: 9L.E29 cells; In vivo: mice bearing 9L.E29 tumors | [37] |
| AuNPs | HOSiPcOSi(CH3)2-(CH2)3N(CH3)2, (Pc 4) | Tf | Tf receptor | _ | In vitro: LN229 and U87 cells; In vivo: mice bearing U87 tumors | [140] |
| AuNPs | (Phthalocyaninato)zinc(II) | Jacalin (lectin) | T antigen | Thiol-functionalized PEG | In vitro: HT-29 cells | [316] |
| IONPs | ICG | HA | CD44 | amino PEG | In vitro: A2780 and HCT-116 cells; In vivo: mice bearing HCT-116 tumors | [287] |
| IONPs | 5, 10, 15, 20-tetra(phenyl-4-N-met32hyl-4-pyridyl)porphyrin | AS1411 aptamer | Nucleolin | Daunomycin | In vitro: A549 and C26 cells | [317] |
| IONPs | Chlorin e6 free acid | HA | CD44 | _ | In vitro: B16F1 cells | [318] |
| IONPs | Hypericin | Lactose | Asialoglycoprotein receptors (ASGP-R) | Polydopamine | In vitro: HepG2 and MCF-7 cells | [319] |
| IONPs | Pheophorbide a | FA | FR32 | PEG, Caffeic Acid | In vitro: MDA-MB-231 NIH3T3 and MCF-7 cells | [320] |
| IONPs | HOSiPcOSi(CH3)2-(CH2)3N(CH3)2, (Pc 4) | Fibronectin-mimetic peptide (Fmp) | Integrin β1 | _ | In vitro: HNSCC, M4E, 686LN and TU212 cells; In vivo: mice bearing M4E tumors | [321] |
| MOF | Tetrakis(4-carboxyphenyl)porphyrin (TCCP) | HA | CD44 | CHC | In vitro: CT26, 4T1, HeLa, COS7, MCF-7 and HepG2 cells; In vivo: mice bearing CT26 tumors | [291] |
| MOF | TCPP | HA | CD44 | HIF signaling inhibitor (ACF), Zirconium ions | In vitro: H22 and NIH3T3 cells; In vivo: mice bearing H22 tumors | [322] |
| MOF | (Phthalocyaninato)zinc(II) | FA | FR | DOX | In vitro: HeLa cells | [323] |
| MOF | Al(III) phthalocyanine chloride tetrasulfonic acid (AlPcS4) | Catalase (CAT) protein molecules | Cancer cell membrane antigens | Cancer cell membrane | In vitro: HeLa, COS7; In vivo: mice bearing HeLa tumors | [324] |
| MOF | TCPP | FA | FR | TPP | In vitro: SMMC-7721 cells | [325] |
| MOF | MB | cRGD | αvβ3 integrin receptor | _ | In vitro: A549 and HeLa cells | [326] |
| MOF | TCPP | Bovine Serum Albumin-sulfonamides (SAs) complexes | Carbonic anhydrase IX | _ | In vitro: 4T1 cells; In vivo: mice bearing 4T1 tumors | [327] |
| MOF | TCPP | Sulfadiazines | Carbonic anhydrase IX | Bovine serum albumin, MnO2 | In vitro: 4T1 cells; In vivo: mice bearing 4T1 tumors | [328] |
| MOF | TCPP | Aptamer of A549 lung cancer cells | A549 lung cancer cells | DOX | In vitro: A549, MCF-7 and LO2 cells | [329] |
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Nichols, J.W.; Bae, Y.H. EPR: Evidence and fallacy. J. Control. Release 2014, 190, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Elkhodiry, M.A.; Momah, C.C.; Suwaidi, S.R.; Gadalla, D.; Martins, A.M.; Vitor, R.F.; Husseini, G.A. Synergistic Nanomedicine: Passive, Active, and Ultrasound-Triggered Drug Delivery in Cancer Treatment. J. Nanosci. Nanotechnol. 2016, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Vasir, J.K.; Labhasetwar, V. Targeted Drug Delivery in Cancer Therapy. Technol. Cancer Res. Treat. 2005, 4, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Z.; Chen, H.; Gao, Y. Nanoparticle-based drug delivery systems for controllable photodynamic cancer therapy. Eur. J. Pharm. Sci. 2020, 144, 105213. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A New Concept for Macromolecular Therapeutics in Cancer Chemotherapy: Mechanism of Tumoritropic Accumulation of Proteins and the Antitumor Agent Smancs. Cancer Res. 1986, 46, 6387. [Google Scholar]
- Lin, L.; Xiong, L.; Wen, Y.; Lei, S.; Deng, X.; Liu, Z.; Chen, W.; Miao, X. Active Targeting of Nano-Photosensitizer Delivery Systems for Photodynamic Therapy of Cancer Stem Cells. J. Biomed. Nanotechnol. 2015, 11, 531–554. [Google Scholar] [CrossRef]
- Zhu, G.; Niu, G.; Chen, X. Aptamer–Drug Conjugates. Bioconjug. Chem. 2015, 26, 2186–2197. [Google Scholar] [CrossRef]
- Trapani, G.; Denora, N.; Trapani, A.; Laquintana, V. Recent advances in ligand targeted therapy. J. Drug Target. 2012, 20, 1–22. [Google Scholar] [CrossRef]
- Bartlett, D.W.; Su, H.; Hildebrandt, I.J.; Weber, W.A.; Davis, M.E. Impact of tumor-specific targeting on the biodistribution and efficacy of siRNA nanoparticles measured by multimodality in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 15549. [Google Scholar] [CrossRef]
- Kirpotin, D.B.; Drummond, D.C.; Shao, Y.; Shalaby, M.R.; Hong, K.; Nielsen, U.B.; Marks, J.D.; Benz, C.C.; Park, J.W. Antibody Targeting of Long-Circulating Lipidic Nanoparticles Does Not Increase Tumor Localization but Does Increase Internalization in Animal Models. Cancer Res. 2006, 66, 6732. [Google Scholar] [CrossRef] [PubMed]
- David, A. Peptide ligand-modified nanomedicines for targeting cells at the tumor microenvironment. Adv. Drug Deliv. Rev. 2017, 119, 120–142. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Venkatraman, G.; Batra, S.K. Optimization of Radioimmunotherapy of Solid Tumors: Biological Impediments and Their Modulation. Clin. Cancer Res. 2007, 13, 1374. [Google Scholar] [CrossRef] [PubMed]
- Gosk, S.; Moos, T.; Gottstein, C.; Bendas, G. VCAM-1 directed immunoliposomes selectively target tumor vasculature in vivo. Biochim. Biophys. Acta BBA Biomembr. 2008, 1778, 854–863. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef]
- Callaghan, S.; Senge, M.O. The good, the bad, and the ugly – controlling singlet oxygen through design of photosensitizers and delivery systems for photodynamic therapy. Photochem. Photobiol. Sci. 2018, 17, 1490–1514. [Google Scholar] [CrossRef]
- Weishaupt, K.R.; Gomer, C.J.; Dougherty, T.J. Identification of Singlet Oxygen as the Cytotoxic Agent in Photo-inactivation of a Murine Tumor. Cancer Res. 1976, 36, 2326. [Google Scholar]
- Zhang, J.; Jiang, C.; Figueiró Longo, J.P.; Azevedo, R.B.; Zhang, H.; Muehlmann, L.A. An updated overview on the development of new photosensitizers for anticancer photodynamic therapy. Acta Pharm. Sin. B 2018, 8, 137–146. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef]
- Lucky, S.S.; Soo, K.C.; Zhang, Y. Nanoparticles in Photodynamic Therapy. Chem. Rev. 2015, 115, 1990–2042. [Google Scholar] [CrossRef]
- Moserova, I.; Kralova, J. Role of ER Stress Response in Photodynamic Therapy: ROS Generated in Different Subcellular Compartments Trigger Diverse Cell Death Pathways. PLoS ONE 2012, 7, e32972. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, C.; Senge, M.O.; Arnaut, L.G.; Gomes-da-Silva, L.C. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim. Biophys. Acta BBA Rev. Cancer 2019, 1872, 188308. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Photodynamic Therapy: A Brief History. J. Clin. Med. 2019, 8, 1581. [Google Scholar] [CrossRef] [PubMed]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.; Yoon, J. Supramolecular photosensitizers rejuvenate photodynamic therapy. Chem. Soc. Rev. 2018, 47, 1174–1188. [Google Scholar] [CrossRef]
- Polo, L.; Valduga, G.; Jori, G.; Reddi, E. Low-density lipoprotein receptors in the uptake of tumour photosensitizers by human and rat transformed fibroblasts. Int. J. Biochem. Cell Biol. 2002, 34, 10–23. [Google Scholar] [CrossRef]
- Chitgupi, U.; Qin, Y.; Lovell, J.F. Targeted Nanomaterials for Phototherapy. Nanotheranostics 2017, 1, 38–58. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Das, M.; Mohanty, C.; Sahoo, S.K. Ligand-based targeted therapy for cancer tissue. Expert Opin. Drug Deliv. 2009, 6, 285–304. [Google Scholar] [CrossRef]
- Cheung, A.; Bax, H.J.; Josephs, D.H.; Ilieva, K.M.; Pellizzari, G.; Opzoomer, J.; Bloomfield, J.; Fittall, M.; Grigoriadis, A.; Figini, M.; et al. Targeting folate receptor alpha for cancer treatment. Oncotarget 2016, 7, 52553–52574. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Li, H.; Sun, H.; Ho, K. Targeted Drug Delivery via the Transferrin Receptor-Mediated Endocytosis Pathway. Pharmacol. Rev. 2002, 54, 561–587. [Google Scholar] [CrossRef] [PubMed]
- Daniels, T.R.; Bernabeu, E.; Rodríguez, J.A.; Patel, S.; Kozman, M.; Chiappetta, D.A.; Holler, E.; Ljubimova, J.Y.; Helguera, G.; Penichet, M.L. The transferrin receptor and the targeted delivery of therapeutic agents against cancer. Biochim. Biophys. Acta 2012, 1820, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Alexander-Bryant, A.A.; Vanden Berg-Foels, W.S.; Wen, X. Chapter One—Bioengineering Strategies for Designing Targeted Cancer Therapies. In Advances in Cancer Research; Tew, K.D., Fisher, P.B., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 118, pp. 1–59. ISBN 0065-230X. [Google Scholar]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Danhier, F.; Feron, O.; Préat, V. To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control. Release 2010, 148, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Cheng, Y.; Broome, A.-M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-Targeted Gold Nanoparticles for Photodynamic Therapy of Brain Cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef]
- Schneider, M.R.; Wolf, E. The epidermal growth factor receptor ligands at a glance. J. Cell. Physiol. 2009, 218, 460–466. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, R.; Wu, X.; Sun, Y.; Yao, M.; Li, J.; Xu, Y.; Gu, J. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J. 2005, 19, 1978–1985. [Google Scholar] [CrossRef]
- Masoud, V.; Pagès, G. Targeted therapies in breast cancer: New challenges to fight against resistance. World J. Clin. Oncol. 2017, 8, 120–134. [Google Scholar] [CrossRef]
- Spector, N.L.; Blackwell, K.L. Understanding the Mechanisms Behind Trastuzumab Therapy for Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. J. Clin. Oncol. 2009, 27, 5838–5847. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983. [Google Scholar] [CrossRef] [PubMed]
- Boiko, A.D.; Razorenova, O.V.; van de Rijn, M.; Swetter, S.M.; Johnson, D.L.; Ly, D.P.; Butler, P.D.; Yang, G.P.; Joshua, B.; Kaplan, M.J.; et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nature 2010, 466, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Pestell, T.G.; Lisanti, M.P.; Pestell, R.G. Cancer stem cells. Int. J. Biochem. Cell Biol. 2012, 44, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z. Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases. Antibodies 2018, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.C.N.; Fecteau, J.-F.; Cui, B.; Chen, L.; Zhang, L.; Wu, R.; Rassenti, L.; Lao, F.; Weigand, S.; et al. Targeting chronic lymphocytic leukemia cells with a humanized monoclonal antibody specific for CD44. Proc. Natl. Acad. Sci. USA 2013, 110, 6127–6132. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 8. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Papetti, M.; Herman, I.M. Mechanisms of normal and tumor-derived angiogenesis. Am. J. Physiol. Cell Physiol. 2002, 282, C947–C970. [Google Scholar] [CrossRef]
- Senge, M.O.; Radomski, M.W. Platelets, photosensitizers, and PDT. Photodiagn. Photodyn. Ther. 2013, 10, 1–16. [Google Scholar] [CrossRef]
- Seebacher, N.A.; Stacy, A.E.; Porter, G.M.; Merlot, A.M. Clinical development of targeted and immune based anti-cancer therapies. J. Exp. Clin. Cancer Res. 2019, 38, 156. [Google Scholar] [CrossRef] [PubMed]
- Hamidi, H.; Pietilä, M.; Ivaska, J. The complexity of integrins in cancer and new scopes for therapeutic targeting. Br. J. Cancer 2016, 115, 1017–1023. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dai, J.; Yao, Z.; Shelley, G.; Keller, E.T. Abituzumab Targeting of αV-Class Integrins Inhibits Prostate Cancer Progression. Mol. Cancer Res. 2017, 15, 875. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.; Rothweiler, F.; Anhorn, M.G.; Sauer, D.; Riemann, I.; Weiss, E.C.; Katsen-Globa, A.; Michaelis, M.; Cinatl, J.; Schwartz, D.; et al. Enhanced drug targeting by attachment of an anti αv integrin antibody to doxorubicin loaded human serum albumin nanoparticles. Biomaterials 2010, 31, 2388–2398. [Google Scholar] [CrossRef] [PubMed]
- Élez, E.; Kocáková, I.; Höhler, T.; Martens, U.M.; Bokemeyer, C.; Van Cutsem, E.; Melichar, B.; Smakal, M.; Cso˝szi, T.; Topuzov, E.; et al. 507PD—Poseidon Phase I/II Trial: Abituzumab Combined with Cetuximab Plus Irinotecan As Second-Line Treatment for Patients with Kras Wild-Type Metastatic Colorectal Cancer. Ann. Oncol. 2014, 25, iv171. [Google Scholar] [CrossRef][Green Version]
- Raab-Westphal, S.; Marshall, J.F.; Goodman, S.L. Integrins as Therapeutic Targets: Successes and Cancers. Cancers 2017, 9, 110. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, R.; Khaket, T.P.; Dutta, C.; Chakraborty, B.; Mukherjee, T.K. Breast cancer metastasis: Putative therapeutic role of vascular cell adhesion molecule-1. Cell. Oncol. 2017, 40, 199–208. [Google Scholar] [CrossRef]
- Byrne, J.D.; Betancourt, T.; Brannon-Peppas, L. Active targeting schemes for nanoparticle systems in cancer therapeutics. Adv. Drug Deliv. Rev. 2008, 60, 1615–1626. [Google Scholar] [CrossRef]
- Schlesinger, M.; Bendas, G. Vascular cell adhesion molecule-1 (VCAM-1)—An increasing insight into its role in tumorigenicity and metastasis. Int. J. Cancer 2015, 136, 2504–2514. [Google Scholar] [CrossRef]
- Chang, A.-C.; Chen, P.-C.; Lin, Y.-F.; Su, C.-M.; Liu, J.-F.; Lin, T.-H.; Chuang, S.-M.; Tang, C.-H. Osteoblast-secreted WISP-1 promotes adherence of prostate cancer cells to bone via the VCAM-1/integrin α4β1 system. Cancer Lett. 2018, 426, 47–56. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; Chen, L.; Lin, Y.-L.; Li, F. A Unified Mechanism for Aminopeptidase N-based Tumor Cell Motility and Tumor-homing Therapy. J. Biol. Chem. 2014, 289, 34520–34529. [Google Scholar] [CrossRef] [PubMed]
- Saiki, I.; Yoneda, J.; Azuma, I.; Fujii, H.; Abe, F.; Nakajima, M.; Tsuruo, T. Role of aminopeptidase N (CD13) in tumor-cell invasion and extracellular matrix degradation. Int. J. Cancer 1993, 54, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, R.; Koivunen, E.; Kain, R.; Lahdenranta, J.; Sakamoto, M.; Stryhn, A.; Ashmun, R.A.; Shapiro, L.H.; Arap, W.; Ruoslahti, E. Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res. 2000, 60, 722–727. [Google Scholar] [PubMed]
- Laakkonen, P.; Porkka, K.; Hoffman, J.A.; Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 2002, 8, 751–755. [Google Scholar] [CrossRef]
- Fogal, V.; Zhang, L.; Krajewski, S.; Ruoslahti, E. Mitochondrial/Cell-Surface Protein p32/gC1qR as a Molecular Target in Tumor Cells and Tumor Stroma. Cancer Res. 2008, 68, 7210. [Google Scholar] [CrossRef]
- Fidler, I.J. The pathogenesis of cancer metastasis: The “seed and soil” hypothesis revisited. Nat. Rev. Cancer 2003, 3, 453–458. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncogen. 2013, 18, 43–73. [Google Scholar] [CrossRef]
- Laakkonen, P.; Akerman, M.E.; Biliran, H.; Yang, M.; Ferrer, F.; Karpanen, T.; Hoffman, R.M.; Ruoslahti, E. Antitumor activity of a homing peptide that targets tumor lymphatics and tumor cells. Proc. Nat. Acad. Sci. USA 2004, 101, 9381–9386. [Google Scholar] [CrossRef]
- Paasonen, L.; Sharma, S.; Braun, G.B.; Kotamraju, V.R.; Chung, T.D.Y.; She, Z.-G.; Sugahara, K.N.; Yliperttula, M.; Wu, B.; Pellecchia, M.; et al. New p32/gC1qR Ligands for Targeted Tumor Drug Delivery. ChemBioChem 2016, 17, 570–575. [Google Scholar] [CrossRef]
- Lam, P.Y.H.; Hillyar, C.R.T.; Able, S.; Vallis, K.A. Synthesis and evaluation of an 18F-labeled derivative of F3 for targeting surface-expressed nucleolin in cancer and tumor endothelial cells. J. Label. Comp. Radiopharm. 2016, 59, 492–499. [Google Scholar] [CrossRef]
- Fonseca, N.A.; Rodrigues, A.S.; Rodrigues-Santos, P.; Alves, V.; Gregório, A.C.; Valério-Fernandes, Â.; Gomes-da-Silva, L.C.; Rosa, M.S.; Moura, V.; Ramalho-Santos, J.; et al. Nucleolin overexpression in breast cancer cell sub-populations with different stem-like phenotype enables targeted intracellular delivery of synergistic drug combination. Biomaterials 2015, 69, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Porkka, K.; Laakkonen, P.; Hoffman, J.A.; Bernasconi, M.; Ruoslahti, E. A fragment of the HMGN2 protein homes to the nuclei of tumor cells and tumor endothelial cells in vivo. Proc. Natl. Acad. Sci. USA 2002, 99, 7444–7449. [Google Scholar] [CrossRef] [PubMed]
- Gomes-da-Silva, L.C.; Santos, A.O.; Bimbo, L.M.; Moura, V.; Ramalho, J.S.; Pedroso de Lima, M.C.; Simões, S.; Moreira, J.N. Toward a siRNA-containing nanoparticle targeted to breast cancer cells and the tumor microenvironment. Int. J. Pharm. 2012, 434, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Gomes-da-Silva, L.C.; Fernández, Y.; Abasolo, I.; Schwartz, S.; Ramalho, J.S.; Pedroso de Lima, M.C.; Simões, S.; Moreira, J.N. Efficient intracellular delivery of siRNA with a safe multitargeted lipid-based nanoplatform. Nanomedicine 2013, 8, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Gomes-da-Silva, L.C.; Ramalho, J.S.; Pedroso de Lima, M.C.; Simões, S.; Moreira, J.N. Impact of anti-PLK1 siRNA-containing F3-targeted liposomes on the viability of both cancer and endothelial cells. Eur. J. Pharm. Biopharm. 2013, 85, 356–364. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-Penetrating Delivery of Compounds and Nanoparticles into Tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Park, J.-H.; von Maltzahn, G.; Xu, M.J.; Fogal, V.; Kotamraju, V.R.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Cooperative nanomaterial system to sensitize, target, and treat tumors. Proc. Natl. Acad. Sci. USA 2010, 107, 981–986. [Google Scholar] [CrossRef]
- Christian, S.; Pilch, J.; Akerman, M.E.; Porkka, K.; Laakkonen, P.; Ruoslahti, E. Nucleolin expressed at the cell surface is a marker of endothelial cells in angiogenic blood vessels. J. Cell. Biol. 2003, 163, 871–878. [Google Scholar] [CrossRef]
- Winer, I.; Wang, S.; Lee, Y.-E.K.; Fan, W.; Gong, Y.; Burgos-Ojeda, D.; Spahlinger, G.; Kopelman, R.; Buckanovich, R.J. F3-Targeted Cisplatin-Hydrogel Nanoparticles as an Effective Therapeutic That Targets Both Murine and Human Ovarian Tumor Endothelial Cells In vivo. Cancer Res. 2010, 70, 8674. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, J.; Ji, Y.; Lu, J.; Chan, R.; Meng, H. Targeted drug delivery using iRGD peptide for solid cancer treatment. Mol. Syst. Des. Eng. 2017, 2, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kotamraju, V.R.; Mölder, T.; Tobi, A.; Teesalu, T.; Ruoslahti, E. Tumor-Penetrating Nanosystem Strongly Suppresses Breast Tumor Growth. Nano Lett. 2017, 17, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Lei, H.; Zhang, J.; Song, S.; He, L.; Jin, G.; Liu, X.; Wu, J.; Meng, L.; Liu, M.; et al. Suppression of tumor growth and metastasis by a VEGFR-1 antagonizing peptide identified from a phage display library. Int. J. Cancer 2004, 111, 165–173. [Google Scholar] [CrossRef] [PubMed]
- O’Keefe, D.O.; Draper, R.K. Characterization of a transferrin-diphtheria toxin conjugate. J. Biol. Chem. 1985, 260, 932–937. [Google Scholar] [PubMed]
- Cavanaugh, P.G. Synthesis of Chlorin e6-Transferrin and Demonstration of Its Light-Dependent in vitro Breast Cancer Cell Killing Ability. Breast Cancer Res. Treat. 2002, 72, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Dai, L.; Li, C.; Liu, J.; Wang, L.; Lei, J. Self-assembled targeted nanoparticles based on transferrin-modified eight-arm-polyethylene glycol–dihydroartemisinin conjugate. Sci. Rep. 2016, 6, 29461. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Sharma, G.; Sonali; Agrawal, P.; Pandey, B.L.; Koch, B.; Muthu, M.S. Transferrin receptor targeted PLA-TPGS micelles improved efficacy and safety in docetaxel delivery. Int. J. Biol. Macromol. 2016, 83, 335–344. [Google Scholar] [CrossRef]
- Zhong, Y.; Meng, F.; Deng, C.; Zhong, Z. Ligand-Directed Active Tumor-Targeting Polymeric Nanoparticles for Cancer Chemotherapy. Biomacromolecules 2014, 15, 1955–1969. [Google Scholar] [CrossRef]
- Li, Z.J.; Cho, C.H. Peptides as targeting probes against tumor vasculature for diagnosis and drug delivery. J. Transl. Med. 2012, 10 (Suppl. 1), S1. [Google Scholar] [CrossRef]
- Brown, K.C. Peptidic tumor targeting agents: The road from phage display peptide selections to clinical applications. Curr. Pharm. Des. 2010, 16, 1040–1054. [Google Scholar] [CrossRef]
- Dubey, P.K.; Mishra, V.; Jain, S.; Mahor, S.; Vyas, S.P. Liposomes Modified with Cyclic RGD Peptide for Tumor Targeting. J. Drug Target. 2004, 12, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Wang, B.; Sun, Z.; Cheng, J.; Zhao, H.; Wu, K.; Sun, P.; Shen, Q.; Li, M.; Fan, Q. Multifunctional Theranostic Liposomes Loaded with a Hypoxia-Activated Prodrug for Cascade-Activated Tumor Selective Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 39410–39423. [Google Scholar] [CrossRef] [PubMed]
- Mansur, A.A.P.; Carvalho, S.M.; Lobato, Z.I.P.; de FáTIMA Leite, M.; da Silva Cunha, A.; Mansur, H.S. Design and Development of Polysaccharide-Doxorubicin-Peptide Bioconjugates for Dual Synergistic Effects of Integrin-Targeted and Cell-Penetrating Peptides for Cancer Chemotherapy. Bioconjug. Chem. 2018, 29, 1973–2000. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Diao, Y.; Li, W.; Yang, Z.; Zhang, L.; Chen, Z.; Wu, Y. RGD peptide conjugation results in enhanced antitumor activity of PD0325901 against glioblastoma by both tumor-targeting delivery and combination therapy. Int. J. Pharm. 2016, 505, 329–340. [Google Scholar] [CrossRef]
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef]
- Lewis Phillips, G.D.; Li, G.; Dugger, D.L.; Crocker, L.M.; Parsons, K.L.; Mai, E.; Blättler, W.A.; Lambert, J.M.; Chari, R.V.J.; Lutz, R.J.; et al. Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 2008, 68, 9280. [Google Scholar] [CrossRef]
- Sandland, J.; Boyle, R.W. Photosensitizer Antibody–Drug Conjugates: Past, Present, and Future. Bioconjug. Chem. 2019, 30, 975–993. [Google Scholar] [CrossRef]
- Chen, F.; Hong, H.; Zhang, Y.; Valdovinos, H.F.; Shi, S.; Kwon, G.S.; Theuer, C.P.; Barnhart, T.E.; Cai, W. In Vivo Tumor Targeting and Image-Guided Drug Delivery with Antibody-Conjugated, Radiolabeled Mesoporous Silica Nanoparticles. ACS Nano 2013, 7, 9027–9039. [Google Scholar] [CrossRef]
- Marques, A.C.; Costa, P.J.; Velho, S.; Amaral, M.H. Functionalizing nanoparticles with cancer-targeting antibodies: A comparison of strategies. J. Control. Release 2020, 320, 180–200. [Google Scholar] [CrossRef]
- Wu, A.M.; Senter, P.D. Arming antibodies: Prospects and challenges for immunoconjugates. Nat. Biotechnol. 2005, 23, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Sadeqzadeh, E.; Rahbarizadeh, F.; Ahmadvand, D.; Rasaee, M.J.; Parhamifar, L.; Moghimi, S.M. Combined MUC1-specific nanobody-tagged PEG-polyethylenimine polyplex targeting and transcriptional targeting of tBid transgene for directed killing of MUC1 over-expressing tumour cells. J. Control. Release 2011, 156, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Deken, M.M.; Kijanka, M.M.; Beltrán Hernández, I.; Slooter, M.D.; de Bruijn, H.S.; van Diest, P.J.; van Bergen en Henegouwen, P.M.P.; Lowik, C.W.G.M.; Robinson, D.J.; Vahrmeijer, A.L.; et al. Nanobody-targeted photodynamic therapy induces significant tumor regression of trastuzumab-resistant HER2-positive breast cancer, after a single treatment session. J. Control. Release 2020, 323, 269–281. [Google Scholar] [CrossRef] [PubMed]
- van Driel, P.B.A.A.; Boonstra, M.C.; Slooter, M.D.; Heukers, R.; Stammes, M.A.; Snoeks, T.J.A.; de Bruijn, H.S.; van Diest, P.J.; Vahrmeijer, A.L.; van Bergen en Henegouwen, P.M.P.; et al. EGFR targeted nanobody–photosensitizer conjugates for photodynamic therapy in a pre-clinical model of head and neck cancer. J. Control. Release 2016, 229, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Low, P.S.; Antony, A.C. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv. Drug Deliv. Rev. 2004, 56, 1055–1058. [Google Scholar] [CrossRef]
- Leamon, C.; Low, P. Selective Targeting of Malignant Cells with Cytotoxin-Folate Conjugates. J. Drug Target. 1994, 2, 101–112. [Google Scholar] [CrossRef]
- Yang, M.; Deng, J.; Guo, D.; Zhang, J.; Yang, L.; Wu, F. A folate-conjugated platinum porphyrin complex as a new cancer-targeting photosensitizer for photodynamic therapy. Org. Biomol. Chem. 2019, 17, 5367–5374. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.; Xu, R.; Li, S.; Hu, H.; Xiao, C.; Wu, H.; Zhu, L.; Ming, J.; Chu, Z.; et al. Self-assembly of folic acid dextran conjugates for cancer chemotherapy. Nanoscale 2018, 10, 17265–17274. [Google Scholar] [CrossRef]
- Yoo, J.; Park, C.; Yi, G.; Lee, D.; Koo, H. Active Targeting Strategies Using Biological Ligands for Nanoparticle Drug Delivery Systems. Cancers 2019, 11, 640. [Google Scholar] [CrossRef]
- Rogers, L.; Majer, F.; Sergeeva, N.N.; Paszko, E.; Gilmer, J.F.; Senge, M.O. Synthesis and biological evaluation of Foscan® bile acid conjugates to target esophageal cancer cells. Bioorg. Med. Chem. Lett. 2013, 23, 2495–2499. [Google Scholar] [CrossRef]
- Morita, Y.; Leslie, M.; Kameyama, H.; Volk, D.E.; Tanaka, T. Aptamer Therapeutics in Cancer: Current and Future. Cancers 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.-N.T.; LaVan, D.A.; Langer, R. Nanoparticle-Aptamer Bioconjugates. Cancer Res. 2004, 64, 7668. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, W.; Kim, D.; Lee, E.S.; Lee, D.H.; Jeong, S.; Park, J.M.; Na, K. Tumor-Specific Aptamer-Conjugated Polymeric Photosensitizer for Effective Endo-Laparoscopic Photodynamic Therapy. Adv. Funct. Mater. 2019, 29, 1900084. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, W.; Cheng, L.; Cai, R.; Yi, X.; He, J.; Pan, X.; Yang, L.; Yang, K.; Liu, Z.; et al. Tumor microenvironment (TME)-activatable circular aptamer-PEG as an effective hierarchical-targeting molecular medicine for photodynamic therapy. Biomaterials 2020, 246, 119971. [Google Scholar] [CrossRef]
- Smith, G. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Nobel Media AB 2020 The Nobel Prize in Chemistry. 2018. Available online: https://www.nobelprize.org/prizes/chemistry/2018/prize-announcement/ (accessed on 11 April 2020).
- Haeri, A.; Zalba, S.; ten Hagen, T.L.M.; Dadashzadeh, S.; Koning, G.A. EGFR targeted thermosensitive liposomes: A novel multifunctional platform for simultaneous tumor targeted and stimulus responsive drug delivery. Colloid. Surf. B 2016, 146, 657–669. [Google Scholar] [CrossRef]
- Chen, J.; Ouyang, J.; Chen, Q.; Deng, C.; Meng, F.; Zhang, J.; Cheng, R.; Lan, Q.; Zhong, Z. EGFR and CD44 Dual-Targeted Multifunctional Hyaluronic Acid Nanogels Boost Protein Delivery to Ovarian and Breast Cancers In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2017, 9, 24140–24147. [Google Scholar] [CrossRef]
- Colzani, B.; Speranza, G.; Dorati, R.; Conti, B.; Modena, T.; Bruni, G.; Zagato, E.; Vermeulen, L.; Dakwar, G.R.; Braeckmans, K.; et al. Design of smart GE11-PLGA/PEG-PLGA blend nanoparticulate platforms for parenteral administration of hydrophilic macromolecular drugs: Synthesis, preparation and in vitro/ex vivo characterization. Int. J. Pharm. 2016, 511, 1112–1123. [Google Scholar] [CrossRef]
- Wang, A.; Cui, M.; Qu, H.; Di, J.; Wang, Z.; Xing, J.; Wu, F.; Wu, W.; Wang, X.; Shen, L.; et al. Induction of anti-EGFR immune response with mimotopes identified from a phage display peptide library by panitumumab. Oncotarget 2016, 7. [Google Scholar] [CrossRef][Green Version]
- Sun, J.; Zhang, C.; Liu, G.; Liu, H.; Zhou, C.; Lu, Y.; Zhou, C.; Yuan, L.; Li, X. A novel mouse CD133 binding-peptide screened by phage display inhibits cancer cell motility in vitro. Clin. Exp. Metastasis 2012, 29, 185–196. [Google Scholar] [CrossRef]
- De Groof, T.W.M.; Mashayekhi, V.; Fan, T.S.; Bergkamp, N.D.; Sastre Toraño, J.; van Senten, J.R.; Heukers, R.; Smit, M.J.; Oliveira, S. Nanobody-Targeted Photodynamic Therapy Selectively Kills Viral GPCR-Expressing Glioblastoma Cells. Mol. Pharm. 2019, 16, 3145–3156. [Google Scholar] [CrossRef] [PubMed]
- Gallo, E.; Kelil, A.; Bayliss, P.E.; Jeganathan, A.; Egorova, O.; Ploder, L.; Adams, J.J.; Giblin, P.; Sidhu, S.S. In situ antibody phage display yields optimal inhibitors of integrin α11/β1. mAbs 2020, 12, 1717265. [Google Scholar] [CrossRef] [PubMed]
- Nikfarjam, S.; Tohidkia, M.R.; Mehdipour, T.; Soleimani, R.; Rahimi, A.A.R.; Nouri, M. Successful Application of Whole Cell Panning for Isolation of PhageAntibody Fragments Specific to Differentiated Gastric Cancer Cells. Adv. Pharm. Bull. 2019, 9, 624–631. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.; Silva, A.P.; Nobrega, F.L.; Martins, I.M.; Barbosa-Matos, C.; Granja, S.; Martins, S.F.; Baltazar, F.; Rodrigues, L.R. Rational Identification of a Colorectal Cancer Targeting Peptide through Phage Display. Sci. Rep. 2019, 9, 3958. [Google Scholar] [CrossRef]
- Pasqualini, R.; Ruoslahti, E. Organ targeting In vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef]
- Liu, X.; Peng, J.; He, J.; Li, Q.; Zhou, J.; Liang, X.; Tang, S. Selection and identification of novel peptides specifically targeting human cervical cancer. Amino Acids 2018, 50, 577–592. [Google Scholar] [CrossRef]
- Hassan Baig, M.; Ahmad, K.; Roy, S.; Mohammad Ashraf, J.; Adil, M.; Haris Siddiqui, M.; Khan, S.; Amjad Kamal, M.; Provazník, I.; Choi, I. Computer Aided Drug Design: Success and Limitations. Curr. Pharm. Des. 2016, 22, 572–581. [Google Scholar] [CrossRef]
- Hidayat, A.T.; Yusuf, M.; Bachti, H.H.; Diantini, A.; Zainuddin, A. Computational Model of Doxorubicin Conjugate with Docosahexaenoic Acid and Integrin avß3 Ligand for Anticancer. J. Appl. Pharm. Sci. 2018, 1–6. [Google Scholar] [CrossRef][Green Version]
- Hudson, R.; Boyle, R.W. Strategies for selective delivery of photodynamic sensitisers to biological targets. J. Porphyrins Phthalocyanines 2004, 08, 954–975. [Google Scholar] [CrossRef]
- Muro, S. Challenges in design and characterization of ligand-targeted drug delivery systems. J. Control. Release 2012, 164, 125–137. [Google Scholar] [CrossRef]
- Stallivieri, A.; Colombeau, L.; Jetpisbayeva, G.; Moussaron, A.; Myrzakhmetov, B.; Arnoux, P.; Acherar, S.; Vanderesse, R.; Frochot, C. Folic acid conjugates with photosensitizers for cancer targeting in photodynamic therapy: Synthesis and photophysical properties. Bioorg. Med. Chem. 2017, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Suvorov, N.V.; Mironov, A.F.; Grin, M.A. Folic acid and its derivatives for targeted photodynamic therapy of cancer. Russ. Chem. Bull. 2017, 66, 1982–2008. [Google Scholar] [CrossRef]
- Jenni, S.; Sour, A.; Bolze, F.; Ventura, B.; Heitz, V. Tumour-targeting photosensitisers for one- and two-photon activated photodynamic therapy. Org. Biomol. Chem. 2019, 17, 6585–6594. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, J.; Li, S.; Li, G.; Chen, Q.; Hong, Z. Folate-Targeted Polyethylene Glycol–Modified Photosensitizers for Photodynamic Therapy. J Pharm. Sci. 2019, 108, 2102–2111. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Newman, E.L. Photosensitizer targeting in photodynamic therapy I. Conjugates of haematoporphyrin with albumin and transferrin. J. Photochem. Photobiol. B Biol. 1994, 26, 45–56. [Google Scholar] [CrossRef]
- Kaspler, P.; Lazic, S.; Forward, S.; Arenas, Y.; Mandel, A.; Lilge, L. A ruthenium( ii ) based photosensitizer and transferrin complexes enhance photo-physical properties, cell uptake, and photodynamic therapy safety and efficacy. Photochem. Photobiol. Sci. 2016, 15, 481–495. [Google Scholar] [CrossRef]
- Jadia, R.; Kydd, J.; Rai, P. Remotely Phototriggered, Transferrin-Targeted Polymeric Nanoparticles for the Treatment of Breast Cancer. Photochem. Photobiol. 2018, 94, 765–774. [Google Scholar] [CrossRef]
- Dixit, S.; Novak, T.; Miller, K.; Zhu, Y.; Kenney, M.E.; Broome, A.-M. Transferrin receptor-targeted theranostic gold nanoparticles for photosensitizer delivery in brain tumors. Nanoscale 2015, 7, 1782–1790. [Google Scholar] [CrossRef]
- Zhu, X.; Zhou, H.; Liu, Y.; Wen, Y.; Wei, C.; Yu, Q.; Liu, J. Transferrin/aptamer conjugated mesoporous ruthenium nanosystem for redox-controlled and targeted chemo-photodynamic therapy of glioma. Acta Biomater. 2018, 82, 143–157. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Ogawa, M.; Kosaka, N.; Rosenblum, L.T.; Choyke, P.L.; Kobayashi, H. Cancer cell-selective in vivo near infrared photoimmunotherapy targeting specific membrane molecules. Nat. Med. 2011, 17, 1685–1691. [Google Scholar] [CrossRef]
- Railkar, R.; Krane, L.S.; Li, Q.Q.; Sanford, T.; Siddiqui, M.R.; Haines, D.; Vourganti, S.; Brancato, S.J.; Choyke, P.L.; Kobayashi, H.; et al. Epidermal Growth Factor Receptor (EGFR)-targeted Photoimmunotherapy (PIT) for the Treatment of EGFR-expressing Bladder Cancer. Mol. Cancer Ther. 2017, 16, 2201–2214. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.R.; Railkar, R.; Sanford, T.; Crooks, D.R.; Eckhaus, M.A.; Haines, D.; Choyke, P.L.; Kobayashi, H.; Agarwal, P.K. Targeting Epidermal Growth Factor Receptor (EGFR) and Human Epidermal Growth Factor Receptor 2 (HER2) Expressing Bladder Cancer Using Combination Photoimmunotherapy (PIT). Sci. Rep. 2019, 9, 2084. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Kobayashi, H. Near-Infrared Photoimmunotherapy Targeting Prostate Cancer with Prostate-Specific Membrane Antigen (PSMA) Antibody. Mol. Cancer Res. 2017, 15, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, R.; Hanaoka, H.; Sato, K.; Nagaya, T.; Harada, T.; Mitsunaga, M.; Kim, I.; Paik, C.H.; Wu, A.M.; Choyke, P.L.; et al. Photoimmunotherapy targeting prostate-specific membrane antigen: Are antibody fragments as effective as antibodies? J. Nucl. Med. 2015, 56, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, T.; Nakamura, Y.; Okuyama, S.; Ogata, F.; Maruoka, Y.; Choyke, P.L.; Allen, C.; Kobayashi, H. Syngeneic Mouse Models of Oral Cancer Are Effectively Targeted by Anti-CD44-Based NIR-PIT. Mol. Cancer Res. 2017, 15, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Isobe, Y.; Sato, K.; Nishinaga, Y.; Takahashi, K.; Taki, S.; Yasui, H.; Shimizu, M.; Endo, R.; Koike, C.; Kuramoto, N.; et al. Near infrared photoimmunotherapy targeting DLL3 for small cell lung cancer. EBioMedicine 2020, 52, 102632. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Weidensteiner, C.; Reichardt, W.; Gaedicke, S.; Zhu, X.; Grosu, A.-L.; Kobayashi, H.; Niedermann, G. Imaging and Selective Elimination of Glioblastoma Stem Cells with Theranostic Near-Infrared-Labeled CD133-Specific Antibodies. Theranostics 2016, 6, 862–874. [Google Scholar] [CrossRef]
- Wei, W.; Jiang, D.; Ehlerding, E.B.; Barnhart, T.E.; Yang, Y.; Engle, J.W.; Luo, Q.; Huang, P.; Cai, W. CD146-Targeted Multimodal Image-Guided Photoimmunotherapy of Melanoma. Adv. Sci. 2019, 6, 1801237. [Google Scholar] [CrossRef]
- Sato, K.; Ando, K.; Okuyama, S.; Moriguchi, S.; Ogura, T.; Totoki, S.; Hanaoka, H.; Nagaya, T.; Kokawa, R.; Takakura, H.; et al. Photoinduced Ligand Release from a Silicon Phthalocyanine Dye Conjugated with Monoclonal Antibodies: A Mechanism of Cancer Cell Cytotoxicity after Near-Infrared Photoimmunotherapy. ACS Cent. Sci. 2018, 4, 1559–1569. [Google Scholar] [CrossRef]
- Kobayashi, H. Near infrared photoimmunotherapy: A new type of immune theranostic technology for cancer. Proc. SPIE 2020, 11362, 113620S. [Google Scholar]
- Kobayashi, H.; Choyke, P.L. Near-Infrared Photoimmunotherapy of Cancer. Acc. Chem. Res. 2019, 52, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.gov. Study of RM-1929 and Photoimmunotherapy in Patients with Recurrent Head and Neck Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02422979 (accessed on 21 September 2020).
- ClinicalTrials.gov. ASP-1929 Photoimmunotherapy (PIT) Study in Recurrent Head/Neck Cancer for Patients Who Have Failed at Least Two Lines of Therapy. Available online: https://clinicaltrials.gov/ct2/show/NCT03769506 (accessed on 21 September 2020).
- Aung, W.; Tsuji, A.B.; Sugyo, A.; Takashima, H.; Yasunaga, M.; Matsumura, Y.; Higashi, T. Near-infrared photoimmunotherapy of pancreatic cancer using an indocyanine green-labeled anti-tissue factor antibody. World. J. Gastroenterol. 2018, 24, 5491–5504. [Google Scholar] [CrossRef] [PubMed]
- Darwish, W.M.; Bayoumi, N.A.; El-Shershaby, H.M.; Allahloubi, N.M. Targeted photoimmunotherapy based on photosensitizer-antibody conjugates for multiple myeloma treatment. J. Photochem. Photobiol. B Biol. 2020, 203, 111777. [Google Scholar] [CrossRef] [PubMed]
- Heukers, R.; Mashayekhi, V.; Ramirez-Escudero, M.; de Haard, H.; Verrips, T.; van Bergen en Henegouwen, P.; Oliveira, S. VHH-Photosensitizer Conjugates for Targeted Photodynamic Therapy of Met-Overexpressing Tumor Cells. Antibodies 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Heukers, R.; Fan, T.S.; de Wit, R.H.; van Senten, J.R.; De Groof, T.W.M.; Bebelman, M.P.; Lagerweij, T.; Vieira, J.; de Munnik, S.M.; Smits-de Vries, L.; et al. The constitutive activity of the virally encoded chemokine receptor US28 accelerates glioblastoma growth. Oncogene 2018, 37, 4110–4121. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Pantarat, N.; Suzuki, T.; Evdokiou, A. Near-Infrared Photoimmunotherapy Using a Small Protein Mimetic for HER2-Overexpressing Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5835. [Google Scholar] [CrossRef]
- Yu, L.; Wang, Q.; Wong, R.C.-H.; Zhao, S.; Ng, D.K.P.; Lo, P.-C. Synthesis and biological evaluation of phthalocyanine-peptide conjugate for EGFR-targeted photodynamic therapy and bioimaging. Dyes Pigments 2019, 163, 197–203. [Google Scholar] [CrossRef]
- Williams, T.M.; Sibrian-Vazquez, M.; Vicente, M.G.H. Design and Synthesis of Photosensitizer-Peptide Conjugates for PDT. In Handbook of Photodynamic Therapy; Pandey, R.K., Kessel, D., Dougherty, T.J., Eds.; World Scientific: Hackensack, NJ, USA, 2016; pp. 45–93. ISBN 978-981-4719-64-3. [Google Scholar]
- Kim, J.; Chae, J.; Kim, J.S.; Goh, S.-H.; Choi, Y. Photosensitizer-conjugated tryptophan-containing peptide ligands as new dual-targeted theranostics for cancers. Int. J. Pharm. 2016, 513, 584–590. [Google Scholar] [CrossRef]
- Kim, J.; Won, Y.; Goh, S.-H.; Choi, Y. A redox-responsive theranostic agent for target-specific fluorescence imaging and photodynamic therapy of EGFR-overexpressing triple-negative breast cancers. J. Mater. Chem. B 2016, 4, 6787–6790. [Google Scholar] [CrossRef]
- Xu, P.; Jia, Y.; Yang, Y.; Chen, J.; Hu, P.; Chen, Z.; Huang, M. Photodynamic Oncotherapy Mediated by Gonadotropin-Releasing Hormone Receptors. J. Med. Chem. 2017, 60, 8667–8672. [Google Scholar] [CrossRef]
- Pethő, L.; Murányi, J.; Pénzes, K.; Gurbi, B.; Brauswetter, D.; Halmos, G.; Csík, G.; Mező, G. Suitability of GnRH Receptors for Targeted Photodynamic Therapy in Head and Neck Cancers. Int. J. Mol. Sci. 2019, 20, 5027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gao, F.; Wu, W.; Qiu, W.-X.; Zhang, L.; Li, R.; Zhuang, Z.-N.; Yu, W.; Cheng, H.; Zhang, X.-Z. Enzyme-Driven Membrane-Targeted Chimeric Peptide for Enhanced Tumor Photodynamic Immunotherapy. ACS Nano 2019, 13, 11249–11262. [Google Scholar] [CrossRef] [PubMed]
- Isaac-Lam, M.; Hammonds, D. Biotinylated Chlorin and Its Zinc and Indium Complexes: Synthesis and In Vitro Biological Evaluation for Photodynamic Therapy. Pharmaceuticals 2017, 10, 41. [Google Scholar] [CrossRef] [PubMed]
- Balçik-Erçin, P.; Çetin, M.; Göksel, M.; Durmuş, M. Improved targeting for photodynamic therapy via a biotin–phthalocyanine conjugate: Synthesis, photophysical and photochemical measurements, and in vitro cytotoxicity assay. New J. Chem. 2020, 44, 3392–3401. [Google Scholar] [CrossRef]
- Li, J.; Zeng, L.; Xiong, K.; Rees, T.W.; Jin, C.; Wu, W.; Chen, Y.; Ji, L.; Chao, H. A biotinylated ruthenium( ii ) photosensitizer for tumor-targeted two-photon photodynamic therapy. Chem. Commun. 2019, 55, 10972–10975. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Dong, W.; Liu, Q.; Lv, G.; Xie, M.; Sun, X.; Qiu, L.; Lin, J. A biotin receptor-targeted silicon(IV) phthalocyanine for in vivo tumor imaging and photodynamic therapy. J. Photochem. Photobiol. B Biol. 2019, 190, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Osati, S.; Ali, H.; Guérin, B.; van Lier, J.E. Steroid-photosensitizer conjugates: Syntheses and applications. J. Porphyrins Phthalocyanines 2017, 21, 701–730. [Google Scholar] [CrossRef]
- El-Akra, N.; Noirot, A.; Faye, J.-C.; Souchard, J.-P. Synthesis of estradiol–pheophorbide a conjugates: Evidence of nuclear targeting, DNA damage and improved photodynamic activity in human breast cancer and vascular endothelial cells. Photochem. Photobiol. Sci. 2006, 5, 996–999. [Google Scholar] [CrossRef]
- Zolottsev, V.A.; Ponomarev, G.V.; Taratynova, M.O.; Morozevich, G.E.; Novikov, R.A.; Timofeev, V.P.; Solyev, P.N.; Zavialova, M.G.; Zazulina, O.V.; Tkachev, Y.V.; et al. Conjugates of 17-substituted testosterone and epitestosterone with pyropheophorbide a differing in the length of linkers. Steroids 2018, 138, 82–90. [Google Scholar] [CrossRef]
- Tanaka, M.; Kataoka, H.; Mabuchi, M.; Sakuma, S.; Takahashi, S.; Tujii, R.; Akashi, H.; Ohi, H.; Yano, S.; Morita, A.; et al. Anticancer Effects of Novel Photodynamic Therapy with Glycoconjugated Chlorin for Gastric and Colon Cancer. Anticancer Res. 2011, 31, 763–767. [Google Scholar]
- Kataoka, H.; Nishie, H.; Hayashi, N.; Tanaka, M.; Nomoto, A.; Yano, S.; Joh, T. New photodynamic therapy with next-generation photosensitizers. Ann. Transl. Med. 2017, 5, 183. [Google Scholar] [CrossRef] [PubMed]
- Kruspe, S.; Meyer, C.; Hahn, U. Chlorin e6 Conjugated Interleukin-6 Receptor Aptamers Selectively Kill Target Cells Upon Irradiation. Mol. Ther. Nucl Acids 2014, 3, e143. [Google Scholar] [CrossRef] [PubMed]
- Mallikaratchy, P.; Tang, Z.; Tan, W. Cell Specific Aptamer–Photosensitizer Conjugates as a Molecular Tool in Photodynamic Therapy. ChemMedChem 2008, 3, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, N.; Kataoka, H.; Yano, S.; Tanaka, M.; Moriwaki, K.; Akashi, H.; Suzuki, S.; Mori, Y.; Kubota, E.; Tanida, S.; et al. A Novel Photodynamic Therapy Targeting Cancer Cells and Tumor-Associated Macrophages. Mol. Cancer Ther. 2015, 14, 452–460. [Google Scholar] [CrossRef]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagn. Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Thanos, C.G. The pinpoint promise of nanoparticle-based drug delivery and molecular diagnosis. Biomol. Eng. 2006, 23, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.M.; Wittrup, K.D. A modeling analysis of the effects of molecular size and binding affinity on tumor targeting. Mol. Cancer Ther. 2009, 8, 2861–2871. [Google Scholar] [CrossRef]
- Puri, A.; Loomis, K.; Smith, B.; Lee, J.-H.; Yavlovich, A.; Heldman, E.; Blumenthal, R. Lipid-Based Nanoparticles as Pharmaceutical Drug Carriers: From Concepts to Clinic. Crit. Rev. Ther. Drug Carrier Syst. 2009, 26, 523–580. [Google Scholar] [CrossRef]
- Düzgüneş, N.; Piskorz, J.; Skupin-Mrugalska, P.; Goslinski, T.; Mielcarek, J.; Konopka, K. Photodynamic therapy of cancer with liposomal photosensitizers. Ther. Deliv. 2018, 9, 823–832. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Hasan, T. Mechanisms of Action of Photodynamic Therapy with Verteporfin for the Treatment of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 195–214. [Google Scholar] [CrossRef]
- Bangham, A.D.; Standish, M.M.; Watkins, J.C. Diffusion of univalent ions across the lamellae of swollen phospholipids. J. Mol. Biol. 1965, 13, 238–252. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef] [PubMed]
- Mir, Y.; Elrington, S.A.; Hasan, T. A new nanoconstruct for epidermal growth factor receptor-targeted photo-immunotherapy of ovarian cancer. Nanomed. Nanotechn. Biol. Med. 2013, 9, 1114–1122. [Google Scholar] [CrossRef]
- Li, Q.; Li, W.; Di, H.; Luo, L.; Zhu, C.; Yang, J.; Yin, X.; Yin, H.; Gao, J.; Du, Y.; et al. A photosensitive liposome with NIR light triggered doxorubicin release as a combined photodynamic-chemo therapy system. J. Control. Release 2018, 277, 114–125. [Google Scholar] [CrossRef]
- Ichikawa, K.; Hikita, T.; Maeda, N.; Yonezawa, S.; Takeuchi, Y.; Asai, T.; Namba, Y.; Oku, N. Antiangiogenic photodynamic therapy (PDT) by using long-circulating liposomes modified with peptide specific to angiogenic vessels. Biochim. Biophys. Acta BBA Biomembranes 2005, 1669, 69–74. [Google Scholar] [CrossRef]
- de Freitas, C.F.; Montanha, M.C.; Pellosi, D.S.; Kimura, E.; Caetano, W.; Hioka, N. Biotin-targeted mixed liposomes: A smart strategy for selective release of a photosensitizer agent in cancer cells. Mater. Sci. Eng. C 2019, 104, 109923. [Google Scholar] [CrossRef]
- Moret, F.; Scheglmann, D.; Reddi, E. Folate-targeted PEGylated liposomes improve the selectivity of PDT with meta-tetra(hydroxyphenyl)chlorin (m-THPC). Photochem. Photobiol. Sci. 2013, 12, 823–834. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, J.; Song, H.; Zhuo, L.; Wang, G.; Liao, W.; Feng, Y.; Wei, H.; Chen, Y.; Yang, Y.; et al. Uptake and light-induced cytotoxicity of hyaluronic acid-grafted liposomes containing porphyrin in tumor cells. J. Drug Deliv. Sci. Technol. 2018, 47, 137–143. [Google Scholar] [CrossRef]
- Lovell, J.F.; Jin, C.S.; Huynh, E.; Jin, H.; Kim, C.; Rubinstein, J.L.; Chan, W.C.W.; Cao, W.; Wang, L.V.; Zheng, G. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 2011, 10, 324–332. [Google Scholar] [CrossRef]
- Kato, T.; Jin, C.S.; Ujiie, H.; Lee, D.; Fujino, K.; Wada, H.; Hu, H.; Weersink, R.A.; Chen, J.; Kaji, M.; et al. Nanoparticle targeted folate receptor 1-enhanced photodynamic therapy for lung cancer. Lung Cancer 2017, 113, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Mehnert, W.; Mäder, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug Deliv. Rev. 2012, 64, 83–101. [Google Scholar] [CrossRef]
- Dai, Y.; Su, J.; Wu, K.; Ma, W.; Wang, B.; Li, M.; Sun, P.; Shen, Q.; Wang, Q.; Fan, Q. Multifunctional Thermosensitive Liposomes Based on Natural Phase-Change Material: Near-Infrared Light-Triggered Drug Release and Multimodal Imaging-Guided Cancer Combination Therapy. ACS Appl. Mater. Interfaces 2019, 11, 10540–10553. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, T.S.; Lu, Y.-J.; Chen, H.-A.; Hsu, H.-L.; Jose, G.; Chen, J.-P. Dual targeted magnetic photosensitive liposomes for photothermal/photodynamic tumor therapy. J. Magn. Magn. Mater. 2019, 473, 241–252. [Google Scholar] [CrossRef]
- Pardeike, J.; Hommoss, A.; Müller, R.H. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int. J. Pharm. 2009, 366, 170–184. [Google Scholar] [CrossRef]
- Ding, X.; Xu, X.; Zhao, Y.; Zhang, L.; Yu, Y.; Huang, F.; Yin, D.; Huang, H. Tumor targeted nanostructured lipid carrier co-delivering paclitaxel and indocyanine green for laser triggered synergetic therapy of cancer. RSC Adv. 2017, 7, 35086–35095. [Google Scholar] [CrossRef]
- García-Díaz, M.; Nonell, S.; Villanueva, Á.; Stockert, J.C.; Cañete, M.; Casadó, A.; Mora, M.; Sagristá, M.L. Do folate-receptor targeted liposomal photosensitizers enhance photodynamic therapy selectivity? Biochim. Biophys. Acta BBA Biomembr. 2011, 1808, 1063–1071. [Google Scholar] [CrossRef]
- Liang, B.J.; Pigula, M.; Baglo, Y.; Najafali, D.; Hasan, T.; Huang, H.-C. Breaking the selectivity-uptake trade-off of photoimmunoconjugates with nanoliposomal irinotecan for synergistic multi-tier cancer targeting. J. Nanobiotechnol. 2020, 18, 1. [Google Scholar] [CrossRef]
- Savellano, M.D.; Owusu-Brackett, N.; Son, J.; Ganga, T.; Leung, N.L.; Savellano, D.H. Photodynamic Tumor Eradication With a Novel Targetable Photosensitizer: Strong Vascular Effects and Dependence on Treatment Repetition Versus Potentiation. Photochem. Photobiol. 2013, 89, 687–697. [Google Scholar] [CrossRef]
- Hu, Z.; Rao, B.; Chen, S.; Duanmu, J. Targeting tissue factor on tumour cells and angiogenic vascular endothelial cells by factor VII-targeted verteporfin photodynamic therapy for breast cancer in vitro and in vivo in mice. BMC Cancer 2010, 10, 235. [Google Scholar] [CrossRef]
- Oshiro-Junior, J.A.; Sato, M.R.; Boni, F.I.; Santos, K.L.M.; de Oliveira, K.T.; de Freitas, L.M.; Fontana, C.R.; Nicholas, D.; McHale, A.; Callan, J.F.; et al. Phthalocyanine-loaded nanostructured lipid carriers functionalized with folic acid for photodynamic therapy. Mater. Sci. Eng. C 2020, 108, 110462. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xue, Y.; Tian, J.; Liu, Z.; Zhuang, A.; Gu, P.; Zhou, H.; Zhang, W.; Fan, X. Fluorinated-functionalized hyaluronic acid nanoparticles for enhanced photodynamic therapy of ocular choroidal melanoma by ameliorating hypoxia. Carbohydr. Polym. 2020, 237, 116119. [Google Scholar] [CrossRef] [PubMed]
- Gaio, E.; Conte, C.; Esposito, D.; Reddi, E.; Quaglia, F.; Moret, F. CD44 Targeting Mediated by Polymeric Nanoparticles and Combination of Chlorine TPCS2a-PDT and Docetaxel-Chemotherapy for Efficient Killing of Breast Differentiated and Stem Cancer Cells In Vitro. Cancers 2020, 12, 278. [Google Scholar] [CrossRef] [PubMed]
- Gangopadhyay, M.; Singh, T.; Behara, K.K.; Karwa, S.; Ghosh, S.K.; Singh, N.D.P. Coumarin-containing-star-shaped 4-arm-polyethylene glycol: Targeted fluorescent organic nanoparticles for dual treatment of photodynamic therapy and chemotherapy. Photochem. Photobiol. Sci. 2015, 14, 1329–1336. [Google Scholar] [CrossRef]
- Rezvantalab, S.; Drude, N.I.; Moraveji, M.K.; Güvener, N.; Koons, E.K.; Shi, Y.; Lammers, T.; Kiessling, F. PLGA-Based Nanoparticles in Cancer Treatment. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, N.; Luo, C.; Zhu, J.; Bao, C. Photosensitizer-loaded cell membrane biomimetic nanoparticles for enhanced tumor synergetic targeted therapy. RSC Adv. 2020, 10, 9378–9386. [Google Scholar] [CrossRef]
- Chen, H.; Tian, J.; He, W.; Guo, Z. H2O2-Activatable and O2-Evolving Nanoparticles for Highly Efficient and Selective Photodynamic Therapy against Hypoxic Tumor Cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef]
- Master, A.; Malamas, A.; Solanki, R.; Clausen, D.M.; Eiseman, J.L.; Sen Gupta, A. A Cell-Targeted Photodynamic Nanomedicine Strategy for Head and Neck Cancers. Mol. Pharm. 2013, 10, 1988–1997. [Google Scholar] [CrossRef]
- Buwalda, S.J.; Boere, K.W.M.; Dijkstra, P.J.; Feijen, J.; Vermonden, T.; Hennink, W.E. Hydrogels in a historical perspective: From simple networks to smart materials. J. Control. Release 2014, 190, 254–273. [Google Scholar] [CrossRef]
- Khurana, B.; Gierlich, P.; Meindl, A.; Gomes-da-Silva, L.C.; Senge, M.O. Hydrogels: Soft matters in photomedicine. Photochem. Photobiol. Sci. 2019, 18, 2613–2656. [Google Scholar] [CrossRef]
- Belali, S.; Savoie, H.; O’Brien, J.M.; Cafolla, A.A.; O’Connell, B.; Karimi, A.R.; Boyle, R.W.; Senge, M.O. Synthesis and Characterization of Temperature-Sensitive and Chemically Cross-Linked Poly(N-isopropylacrylamide)/Photosensitizer Hydrogels for Applications in Photodynamic Therapy. Biomacromolecules 2018, 19, 1592–1601. [Google Scholar] [CrossRef] [PubMed]
- Bae, B.; Na, K. Self-quenching polysaccharide-based nanogels of pullulan/folate-photosensitizer conjugates for photodynamic therapy. Biomaterials 2010, 31, 6325–6335. [Google Scholar] [CrossRef] [PubMed]
- Abdelghany, S.M.; Schmid, D.; Deacon, J.; Jaworski, J.; Fay, F.; McLaughlin, K.M.; Gormley, J.A.; Burrows, J.F.; Longley, D.B.; Donnelly, R.F.; et al. Enhanced Antitumor Activity of the Photosensitizer meso-Tetra(N-methyl-4-pyridyl) Porphine Tetra Tosylate through Encapsulation in Antibody-Targeted Chitosan/Alginate Nanoparticles. Biomacromolecules 2013, 14, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Belali, S.; Karimi, A.R.; Hadizadeh, M. Cell-specific and pH-sensitive nanostructure hydrogel based on chitosan as a photosensitizer carrier for selective photodynamic therapy. Int. J. Biol. Macromol. 2018, 110, 437–448. [Google Scholar] [CrossRef]
- Hah, H.J.; Kim, G.; Lee, Y.-E.K.; Orringer, D.A.; Sagher, O.; Philbert, M.A.; Kopelman, R. Methylene Blue-Conjugated Hydrogel Nanoparticles and Tumor-Cell Targeted Photodynamic Therapy. Macromol. Biosci. 2011, 11, 90–99. [Google Scholar] [CrossRef]
- Wang, S.; Kim, G.; Lee, Y.-E.K.; Hah, H.J.; Ethirajan, M.; Pandey, R.K.; Kopelman, R. Multifunctional Biodegradable Polyacrylamide Nanocarriers for Cancer Theranostics—A “See and Treat” Strategy. ACS Nano 2012, 6, 6843–6851. [Google Scholar] [CrossRef]
- Wang, H.; Fu, Z.; Li, W.; Li, Y.; Zhao, L.; Wen, L.; Zhang, J.; Wen, N. The synthesis and application of nano doxorubicin-indocyanine green matrix metalloproteinase-responsive hydrogel in chemophototherapy for head and neck squamous cell carcinoma. Int. J. Nanomed. 2019, 14, 623–638. [Google Scholar] [CrossRef]
- Martínez-Jothar, L.; Beztsinna, N.; van Nostrum, C.F.; Hennink, W.E.; Oliveira, S. Selective Cytotoxicity to HER2 Positive Breast Cancer Cells by Saporin-Loaded Nanobody-Targeted Polymeric Nanoparticles in Combination with Photochemical Internalization. Mol. Pharm. 2019, 16, 1633–1647. [Google Scholar] [CrossRef]
- Son, J.; Yang, S.M.; Yi, G.; Roh, Y.J.; Park, H.; Park, J.M.; Choi, M.-G.; Koo, H. Folate-modified PLGA nanoparticles for tumor-targeted delivery of pheophorbide a in vivo. Biochem. Biophys. Res. Commun. 2018, 498, 523–528. [Google Scholar] [CrossRef]
- Clement, S.; Chen, W.; Deng, W.; Goldys, E.M. X-ray radiation-induced and targeted photodynamic therapy with folic acid-conjugated biodegradable nanoconstructs. Int. J. Nanomed. 2018, 13, 3553–3570. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Li, J.; Huang, H.; Sun, X.; Lv, Y. Development and evaluation of hyaluronic acid-based polymeric micelles for targeted delivery of photosensitizer for photodynamic therapy in vitro. J. Drug Deliv. Sci. Technol. 2018, 48, 414–421. [Google Scholar] [CrossRef]
- Lin, X.; Yan, S.-Z.; Qi, S.-S.; Xu, Q.; Han, S.-S.; Guo, L.-Y.; Zhao, N.; Chen, S.-L.; Yu, S.-Q. Transferrin-Modified Nanoparticles for Photodynamic Therapy Enhance the Antitumor Efficacy of Hypocrellin A. Front. Pharmacol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Gaio, E.; Conte, C.; Esposito, D.; Miotto, G.; Quaglia, F.; Moret, F.; Reddi, E. Co-delivery of Docetaxel and Disulfonate Tetraphenyl Chlorin in One Nanoparticle Produces Strong Synergism between Chemo- and Photodynamic Therapy in Drug-Sensitive and -Resistant Cancer Cells. Mol. Pharm. 2018, 15, 4599–4611. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, Y.; Long, Z.; Jiang, R.; Zhuang, Z.; Wang, Z.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Efficient Near-Infrared Photosensitizer with Aggregation-Induced Emission for Imaging-Guided Photodynamic Therapy in Multiple Xenograft Tumor Models. ACS Nano 2020, 14, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Hua, S.; Liu, J. Cyclodextrin-based delivery systems for chemotherapeutic anticancer drugs: A review. Carbohydr. Polym. 2020, 232, 115805. [Google Scholar] [CrossRef] [PubMed]
- Ben Mihoub, A.; Larue, L.; Moussaron, A.; Youssef, Z.; Colombeau, L.; Baros, F.; Frochot, C.; Vanderesse, R.; Acherar, S. Use of Cyclodextrins in Anticancer Photodynamic Therapy Treatment. Molecules 2018, 23, 1936. [Google Scholar] [CrossRef]
- Laza-Knoerr, A.L.; Gref, R.; Couvreur, P. Cyclodextrins for drug delivery. J. Drug Target. 2010, 18, 645–656. [Google Scholar] [CrossRef]
- Phua, S.Z.F.; Xue, C.; Lim, W.Q.; Yang, G.; Chen, H.; Zhang, Y.; Wijaya, C.F.; Luo, Z.; Zhao, Y. Light-Responsive Prodrug-Based Supramolecular Nanosystems for Site-Specific Combination Therapy of Cancer. Chem. Mater. 2019, 31, 3349–3358. [Google Scholar] [CrossRef]
- Yao, X.; Li, M.; Li, B.; Xue, C.; Cai, K.; Zhao, Y.; Luo, Z. Tumor-targeted upconverting nanoplatform constructed by host-guest interaction for near-infrared-light-actuated synergistic photodynamic-/chemotherapy. Chem. Eng. 2020, 390, 124516. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, Y.; Wang, X.-J.; Xu, J.-L.; Ye, Z.; Wang, S.; Seeberger, P.H.; Yin, J. Targeted Photodynamic Killing of Breast Cancer Cells Employing Heptamannosylated β-Cyclodextrin-Mediated Nanoparticle Formation of an Adamantane-Functionalized BODIPY Photosensitizer. ACS Appl. Mater. Interfaces 2016, 8, 33405–33411. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, C.; Zhao, F.; Xu, X.; Wang, S.; Yu, L.; Zhang, D.; Huang, Y. Fabrication of a graphene/C60 nanohybrid via γ-cyclodextrin host–guest chemistry for photodynamic and photothermal therapy. Nanoscale 2017, 9, 8825–8833. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.; Du, J.; Li, H.; Jin, Q.; Wang, Y.; Ji, J. Programmed photosensitizer conjugated supramolecular nanocarriers with dual targeting ability for enhanced photodynamic therapy. Chem. Commun. 2016, 52, 11935–11938. [Google Scholar] [CrossRef] [PubMed]
- Zagami, R.; Rapozzi, V.; Piperno, A.; Scala, A.; Triolo, C.; Trapani, M.; Xodo, L.E.; Monsù Scolaro, L.; Mazzaglia, A. Folate-Decorated Amphiphilic Cyclodextrins as Cell-Targeted Nanophototherapeutics. Biomacromolecules 2019, 20, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.-G.; Chen, Y.; Yu, Q.; Liu, Y. A tumor-targeting Ru/polysaccharide/protein supramolecular assembly with high photodynamic therapy ability. Chem. Commun. 2019, 55, 3148–3151. [Google Scholar] [CrossRef]
- Liang, W.; Huang, Y.; Lu, D.; Ma, X.; Gong, T.; Cui, X.; Yu, B.; Yang, C.; Dong, C.; Shuang, S. β-Cyclodextrin–Hyaluronic Acid Polymer Functionalized Magnetic Graphene Oxide Nanocomposites for Targeted Photo-Chemotherapy of Tumor Cells. Polymers 2019, 11, 133. [Google Scholar] [CrossRef]
- Hu, X.; Gao, Z.; Tan, H.; Zhang, L. A pH-Responsive Multifunctional Nanocarrier in the Application of Chemo-Photodynamic Therapy. J. Nanomater. 2019, 1–12. [Google Scholar] [CrossRef]
- Theodossiou, T.A.; Gonçalves, A.R.; Yannakopoulou, K.; Skarpen, E.; Berg, K. Photochemical Internalization of Tamoxifens Transported by a “Trojan-Horse” Nanoconjugate into Breast-Cancer Cell Lines. Angew. Chem. Int. Ed. 2015, 54, 4885–4889. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.-M.; Chen, Y.; Zhao, D.; Chen, J.-T.; Liu, Y. Construction of a Graphene Oxide Based Noncovalent Multiple Nanosupramolecular Assembly as a Scaffold for Drug Delivery. Chem. Eur. J. 2012, 18, 4208–4215. [Google Scholar] [CrossRef]
- Jiang, B.-P.; Zhou, B.; Lin, Z.; Liang, H.; Shen, X.-C. Recent Advances in Carbon Nanomaterials for Cancer Phototherapy. Chem. Eur. J. 2019, 25, 3993–4004. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. J. Cell. Physiol. 2019, 234, 298–319. [Google Scholar] [CrossRef]
- Bartelmess, J.; Quinn, S.J.; Giordani, S. Carbon nanomaterials: Multi-functional agents for biomedical fluorescence and Raman imaging. Chem. Soc. Rev. 2015, 44, 4672–4698. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.J.; Choi, D.G.; Shim, M.S. Targeted and effective photodynamic therapy for cancer using functionalized nanomaterials. Acta Pharm. Sin. B 2016, 6, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Ma, R.; Wang, L.; Zhang, J.; Liu, R.; Li, L.; Liu, Y.; Hou, L.; Yu, X.; Gao, J.; et al. The application of hyaluronic acid-derivatized carbon nanotubes in hematoporphyrin monomethyl ether-based photodynamic therapy for in vivo and in vitro cancer treatment. Int. J. Nanomed. 2013, 8, 2361–2373. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.-W.; Li, B.; Li, C.-X.; Fan, J.-X.; Lei, Q.; Li, C.; Xu, Z.; Zhang, X.-Z. Carbon-Dot-Decorated Carbon Nitride Nanoparticles for Enhanced Photodynamic Therapy against Hypoxic Tumor via Water Splitting. ACS Nano 2016, 10, 8715–8722. [Google Scholar] [CrossRef]
- Shi, J.; Wang, B.; Wang, L.; Lu, T.; Fu, Y.; Zhang, H.; Zhang, Z. Fullerene (C60)-based tumor-targeting nanoparticles with “off-on” state for enhanced treatment of cancer. J. Control. Release 2016, 235, 245–258. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, R.; Zhou, Y.; Yu, N.; Chen, Z.; Gao, D.; Shi, X.; Shen, M. Targeted dual-mode imaging and phototherapy of tumors using ICG-loaded multifunctional MWCNTs as a versatile platform. J. Mater. Chem. B 2018, 6, 6122–6132. [Google Scholar] [CrossRef]
- Li, N.; Li, T.; Hu, C.; Lei, X.; Zuo, Y.; Han, H. Targeted Near-Infrared Fluorescent Turn-on Nanoprobe for Activatable Imaging and Effective Phototherapy of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 15013–15023. [Google Scholar] [CrossRef]
- Mei, C.; Wang, N.; Zhu, X.; Wong, K.-H.; Chen, T. Photothermal-Controlled Nanotubes with Surface Charge Flipping Ability for Precise Synergistic Therapy of Triple-Negative Breast Cancer. Adv. Funct. Mater. 2018, 28, 1805225. [Google Scholar] [CrossRef]
- Ogbodu, R.O.; Ndhundhuma, I.; Karsten, A.; Nyokong, T. Photodynamic therapy effect of zinc monoamino phthalocyanine–folic acid conjugate adsorbed on single walled carbon nanotubes on melanoma cells. Spectrochim. Acta A 2015, 137, 1120–1125. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, F.; Tian, R.; Zhang, L.; Fu, G.; Yang, L.; Zhu, L. Nanotubes-Embedded Indocyanine Green–Hyaluronic Acid Nanoparticles for Photoacoustic-Imaging-Guided Phototherapy. ACS Appl. Mater. Interfaces 2016, 8, 5608–5617. [Google Scholar] [CrossRef]
- Chavva, S.R.; Pramanik, A.; Nellore, B.P.V.; Sinha, S.S.; Yust, B.; Kanchanapally, R.; Fan, Z.; Crouch, R.A.; Singh, A.K.; Neyland, B.; et al. Theranostic Graphene Oxide for Prostate Cancer Detection and Treatment. Part. Part. Syst. Charact. 2014, 31, 1252–1259. [Google Scholar] [CrossRef]
- Cho, Y.; Kim, H.; Choi, Y. A graphene oxide–photosensitizer complex as an enzyme-activatable theranostic agent. Chem. Commun. 2013, 49, 1202–1204. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, S.; Wang, X.; Shen, G.; Lin, J.; Wang, Z.; Guo, S.; Cui, D.; Yang, M.; Chen, X. Surface Functionalization of Chemically Reduced Graphene Oxide for Targeted Photodynamic Therapy. J. Biomed. Nanotechnol. 2015, 11, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Xu, C.; Lin, J.; Wang, C.; Wang, X.; Zhang, C.; Zhou, X.; Guo, S.; Cui, D. Folic Acid-conjugated Graphene Oxide loaded with Photosensitizers for Targeting Photodynamic Therapy. Theranostics 2011, 1, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Park, S.-J.; Ling, D.; Park, W.; Han, J.Y.; Na, K.; Char, K. Hyaluronic acid-conjugated graphene oxide/photosensitizer nanohybrids for cancer targeted photodynamic therapy. J. Mater. Chem. B 2013, 1, 1678–1686. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Chen, B.; Hu, J.; Huang, Q.; Zhang, D.; Wan, J.; Hu, Z.; Wang, B. pH and Thermal Dual-Responsive Graphene Oxide Nanocomplexes for Targeted Drug Delivery and Photothermal-Chemo/Photodynamic Synergetic Therapy. ACS Appl. Bio Mater. 2019, 2, 5859–5871. [Google Scholar] [CrossRef]
- Luan, X.; Guan, Y.-Y.; Liu, H.-J.; Lu, Q.; Zhao, M.; Sun, D.; Lovell, J.F.; Sun, P.; Chen, H.-Z.; Fang, C. A Tumor Vascular-Targeted Interlocking Trimodal Nanosystem That Induces and Exploits Hypoxia. Adv. Sci. 2018, 5, 1800034. [Google Scholar] [CrossRef]
- Qin, X.; Wang, Z.; Guo, C.; Jin, Y. Multi-responsive drug delivery nanoplatform for tumor-targeted synergistic photothermal/dynamic therapy and chemotherapy. New J. Chem. 2020, 44, 3593–3603. [Google Scholar] [CrossRef]
- Tian, J.; Ding, L.; Wang, Q.; Hu, Y.; Jia, L.; Yu, J.-S.; Ju, H. Folate Receptor-Targeted and Cathepsin B-Activatable Nanoprobe for In Situ Therapeutic Monitoring of Photosensitive Cell Death. Anal. Chem. 2015, 87, 3841–3848. [Google Scholar] [CrossRef]
- Wei, Y.; Zhou, F.; Zhang, D.; Chen, Q.; Xing, D. A graphene oxide based smart drug delivery system for tumor mitochondria-targeting photodynamic therapy. Nanoscale 2016, 8, 3530–3538. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Wang, Z.; Wu, X.; Jin, Y. Mitochondria-targeted tri-triphenylphosphonium substituted meso-tetra(4-carboxyphenyl)porphyrin(TCPP) by conjugation with folic acid and graphene oxide for improved photodynamic therapy. J. Porphyrins Phthalocyanines 2019, 23, 1028–1040. [Google Scholar] [CrossRef]
- Yu, X.; Gao, D.; Gao, L.; Lai, J.; Zhang, C.; Zhao, Y.; Zhong, L.; Jia, B.; Wang, F.; Chen, X.; et al. Inhibiting Metastasis and Preventing Tumor Relapse by Triggering Host Immunity with Tumor-Targeted Photodynamic Therapy Using Photosensitizer-Loaded Functional Nanographenes. ACS Nano 2017, 11, 10147–10158. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Fang, G.; Zeng, F.; Wang, X.; Wu, S. Water-Dispersible Fullerene Aggregates as a Targeted Anticancer Prodrug with both Chemo- and Photodynamic Therapeutic Actions. Small 2013, 9, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, C.; Huang, Y.; Sun, S.; Guan, W.; Yao, Y. Photodynamic anticancer activities of water-soluble C60 derivatives and their biological consequences in a HeLa cell line. Chem. Biol. Interact. 2012, 195, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Park, J.; Youn, Y.S.; Oh, K.T.; Bae, J.H.; Lee, E.S. Hoechst 33258–conjugated hyaluronated fullerene for efficient photodynamic tumor therapy and necrotic tumor targeting. J. Bioact. Compat. Polym. 2015. [Google Scholar] [CrossRef]
- Liu, J.; Tabata, Y. Photodynamic therapy of fullerene modified with pullulan on hepatoma cells. J. Drug Target. 2010, 18, 602–610. [Google Scholar] [CrossRef]
- Liu, J.; Tabata, Y. Effect of Modification Manner on the Photodynamic Antitumor Activity of C60 Modified with Pullulan. J. Biomater. Sci. Polym. Ed. 2011, 22, 2147–2163. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, L.; Zhang, X.; Li, N.; Zheng, J.; Guan, M.; Fang, X.; Wang, C.; Shu, C. Enhanced Photodynamic Efficiency of an Aptamer-Guided Fullerene Photosensitizer toward Tumor Cells. Chem. Asian J. 2013, 8, 2370–2376. [Google Scholar] [CrossRef]
- Serda, M.; Ware, M.J.; Newton, J.M.; Sachdeva, S.; Krzykawska-Serda, M.; Nguyen, L.; Law, J.; Anderson, A.O.; Curley, S.A.; Wilson, L.J.; et al. Development of photoactive Sweet-C60 for pancreatic cancer stellate cell therapy. Nanomedicine 2018, 13, 2981–2993. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Z.; Wang, L.; Wang, H.; Li, L.; Yu, X.; Zhang, J.; Ma, R.; Zhang, Z. Photodynamic therapy of a 2-methoxyestradiol tumor-targeting drug delivery system mediated by Asn-Gly-Arg in breast cancer. Int. J. Nanomed. 2013, 8, 1551–1562. [Google Scholar] [CrossRef]
- Zhang, H.; Hou, L.; Jiao, X.; Ji, Y.; Zhu, X.; Zhang, Z. Transferrin-mediated fullerenes nanoparticles as Fe2+-dependent drug vehicles for synergistic anti-tumor efficacy. Biomaterials 2015, 37, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, D.; Fowley, C.; McHale, A.P.; Kamila, S.; Sheng, J.; Atchison, J.; Callan, J.F. A folic acid labelled carbon quantum dot-protoporphryin IX conjugate for use in folate receptor targeted two-photon excited photodynamic therapy. Proc. SPIE 2015, 9338, 933813. [Google Scholar]
- Yao, H.; Zhao, W.; Zhang, S.; Guo, X.; Li, Y.; Du, B. Dual-functional carbon dot-labeled heavy-chain ferritin for self-targeting bio-imaging and chemo-photodynamic therapy. J. Mater. Chem. B 2018, 6, 3107–3115. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef]
- Bhattacharya, R.; Mukherjee, P. Biological properties of “naked” metal nanoparticles. Adv. Drug Deliv. Rev. 2008, 60, 1289–1306. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and Beyond: Molecular Mechanisms of Action and Drug Resistance Development in Cancer Chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. APL Bioeng. 2019, 3, 011502. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Lo, L.-W. Inorganic Nanoparticles for Enhanced Photodynamic Cancer Therapy. Curr. Drug Discov. Technol. 2011, 8, 250–268. [Google Scholar] [CrossRef]
- Erathodiyil, N.; Ying, J.Y. Functionalization of Inorganic Nanoparticles for Bioimaging Applications. Acc. Chem. Res. 2011, 44, 925–935. [Google Scholar] [CrossRef]
- Wang, J.; Xu, D.; Deng, T.; Li, Y.; Xue, L.; Yan, T.; Huang, D.; Deng, D. Self-Decomposable Mesoporous Doxorubicin@Silica Nanocomposites for Nuclear Targeted Chemo-Photodynamic Combination Therapy. ACS Appl. Nano Mater. 2018, 1, 1976–1984. [Google Scholar] [CrossRef]
- Xu, W.; Qian, J.; Hou, G.; Wang, Y.; Wang, J.; Sun, T.; Ji, L.; Suo, A.; Yao, Y. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019, 83, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Shi, Y.; Zhang, S.; Huang, X.; Zhang, J.; Zhang, Y.; Si, W.; Dong, X. Hydrogen Peroxide Responsive Iron–Based Nanoplatform for Multimodal Imaging–Guided Cancer Therapy. Small 2019, 15, 1803791. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-X.; Yang, Y.-W. Metal–Organic Framework (MOF)-Based Drug/Cargo Delivery and Cancer Therapy. Adv. Mater. 2017, 29, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Lan, G.; Ni, K.; Lin, W. Nanoscale metal–organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-X.; Liu, M.-D.; Zhang, M.-K.; Wang, S.-B.; Xu, L.; Li, C.-X.; Gao, F.; Xie, B.-R.; Zhong, Z.-L.; Zhang, X.-Z. Interfering with Lactate-Fueled Respiration for Enhanced Photodynamic Tumor Therapy by a Porphyrinic MOF Nanoplatform. Adv. Funct. Mater. 2018, 28, 1803498. [Google Scholar] [CrossRef]
- Dam, D.H.M.; Zhao, L.; Jelsma, S.A.; Zhao, Y.; Paller, A.S. Folic acid functionalized hollow nanoparticles for selective photodynamic therapy of cutaneous squamous cell carcinoma. Mater. Chem. Front. 2019, 3, 1113–1122. [Google Scholar] [CrossRef]
- Bouffard, E.; Mauriello Jimenez, C.; El Cheikh, K.; Maynadier, M.; Basile, I.; Raehm, L.; Nguyen, C.; Gary-Bobo, M.; Garcia, M.; Durand, J.-O.; et al. Efficient Photodynamic Therapy of Prostate Cancer Cells through an Improved Targeting of the Cation-Independent Mannose 6-Phosphate Receptor. Int. J. Mol. Sci. 2019, 20, 2809. [Google Scholar] [CrossRef]
- Wang, D.; Li, X.; Li, X.; Kang, A.; Sun, L.; Sun, M.; Yang, F.; Xu, C. Magnetic and pH dual-responsive nanoparticles for synergistic drug-resistant breast cancer chemo/photodynamic therapy. Int. J. Nanomed. 2019, 14, 7665–7679. [Google Scholar] [CrossRef]
- Park, S.; Park, H.; Jeong, S.; Yi, B.G.; Park, K.; Key, J. Hyaluronic Acid-Conjugated Mesoporous Silica Nanoparticles Loaded with Dual Anticancer Agents for Chemophotodynamic Cancer Therapy. J. Nanomater. 2019, 2019, 3481397. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, L.; Geng, J.; Ren, J.; Qu, X. Photosensitizer-Incorporated Quadruplex DNA-Gated Nanovechicles for Light-Triggered, Targeted Dual Drug Delivery to Cancer Cells. Small 2013, 9, 2793–2800. [Google Scholar] [CrossRef]
- Selvestrel, F.; Moret, F.; Segat, D.; Woodhams, J.H.; Fracasso, G.; Echevarria, I.M.R.; Baù, L.; Rastrelli, F.; Compagnin, C.; Reddi, E.; et al. Targeted delivery of photosensitizers: Efficacy and selectivity issues revealed by multifunctional ORMOSIL nanovectors in cellular systems. Nanoscale 2013, 5, 6106–6116. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Qu, Q.; Zhao, Y. Targeted Delivery of 5-Aminolevulinic Acid by Multifunctional Hollow Mesoporous Silica Nanoparticles for Photodynamic Skin Cancer Therapy. ACS Appl. Mater. Interfaces 2015, 7, 10671–10676. [Google Scholar] [CrossRef] [PubMed]
- Benachour, H.; Sève, A.; Bastogne, T.; Frochot, C.; Vanderesse, R.; Jasniewski, J.; Miladi, I.; Billotey, C.; Tillement, O.; Lux, F.; et al. Multifunctional Peptide-Conjugated Hybrid Silica Nanoparticles for Photodynamic Therapy and MRI. Theranostics 2012, 2, 889–904. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Brevet, D.; Benkirane-Jessel, N.; Raehm, L.; Maillard, P.; Garcia, M.; Durand, J.-O. Hyaluronic acid-functionalized mesoporous silica nanoparticles for efficient photodynamic therapy of cancer cells. Photodiagn. Photodyn. Ther. 2012, 9, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Hocine, O.; Maynadier, M.; Gallud, A.; Da Silva, A.; Mongin, O.; Blanchard-Desce, M.; et al. Multifunctionalized mesoporous silica nanoparticles for the in vitro treatment of retinoblastoma: Drug delivery, one and two-photon photodynamic therapy. Int. J. Pharm. 2012, 432, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-H.; Lee, C.-H.; Chen, M.-C.; Souris, J.S.; Tseng, F.-G.; Yang, C.-S.; Mou, C.-Y.; Chen, C.-T.; Lo, L.-W. Tri-functionalization of mesoporous silica nanoparticles for comprehensive cancer theranostics—the trio of imaging, targeting and therapy. J. Mater. Chem. 2010, 20, 6149–6157. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Hocine, O.; Brevet, D.; Maynadier, M.; Raehm, L.; Richeter, S.; Charasson, V.; Loock, B.; Morère, A.; Maillard, P.; et al. Cancer therapy improvement with mesoporous silica nanoparticles combining targeting, drug delivery and PDT. Int. J. Pharm. 2012, 423, 509–515. [Google Scholar] [CrossRef]
- Chabloz, N.G.; Perry, H.L.; Yoon, I.-C.; Coulson, A.J.; White, A.J.P.; Stasiuk, G.J.; Botnar, R.M.; Wilton-Ely, J.D.E.T. Combined Magnetic Resonance Imaging and Photodynamic Therapy Using Polyfunctionalised Nanoparticles Bearing Robust Gadolinium Surface Units. Chem. Eur. J. 2020, 26, 4552–4566. [Google Scholar] [CrossRef]
- Paris, J.L.; Villaverde, G.; Gómez-Graña, S.; Vallet-Regí, M. Nanoparticles for multimodal antivascular therapeutics: Dual drug release, photothermal and photodynamic therapy. Acta Biomater. 2020, 101, 459–468. [Google Scholar] [CrossRef]
- Li, N.; Xiang, M.-H.; Liu, J.-W.; Tang, H.; Jiang, J.-H. DNA Polymer Nanoparticles Programmed via Supersandwich Hybridization for Imaging and Therapy of Cancer Cells. Anal. Chem. 2018, 90, 12951–12958. [Google Scholar] [CrossRef]
- Xia, F.; Hou, W.; Liu, Y.; Wang, W.; Han, Y.; Yang, M.; Zhi, X.; Li, C.; Qi, D.; Li, T.; et al. Cytokine induced killer cells-assisted delivery of chlorin e6 mediated self-assembled gold nanoclusters to tumors for imaging and immuno-photodynamic therapy. Biomaterials 2018, 170, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, T.T.; Castilho, M.L.; de Oliveira, I.R.; Jesus, V.P.S.; Hewitt, K.C.; Raniero, L. FTIR study of secondary structure changes in Epidermal Growth Factor by gold nanoparticle conjugation. Biochim. Biophys. Acta BBA Gen. Subj. 2018, 1862, 495–500. [Google Scholar] [CrossRef] [PubMed]
- García Calavia, P.; Chambrier, I.; Cook, M.J.; Haines, A.H.; Field, R.A.; Russell, D.A. Targeted photodynamic therapy of breast cancer cells using lactose-phthalocyanine functionalized gold nanoparticles. J. Colloid Interface Sci. 2018, 512, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, P.; Deng, Y.; Zeng, M.; Tang, Y.; Zhu, W.-H.; Cheng, Y. Combination of active targeting, enzyme-triggered release and fluorescent dye into gold nanoclusters for endomicroscopy-guided photothermal/photodynamic therapy to pancreatic ductal adenocarcinoma. Biomaterials 2017, 139, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Penon, O.; Marín, M.J.; Russell, D.A.; Pérez-García, L. Water soluble, multifunctional antibody-porphyrin gold nanoparticles for targeted photodynamic therapy. J. Colloid Interface Sci. 2017, 496, 100–110. [Google Scholar] [CrossRef]
- Wu, J.; Han, H.; Jin, Q.; Li, Z.; Li, H.; Ji, J. Design and Proof of Programmed 5-Aminolevulinic Acid Prodrug Nanocarriers for Targeted Photodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 14596–14605. [Google Scholar] [CrossRef]
- Zhao, L.; Kim, T.-H.; Kim, H.-W.; Ahn, J.-C.; Kim, S.Y. Enhanced cellular uptake and phototoxicity of Verteporfin-conjugated gold nanoparticles as theranostic nanocarriers for targeted photodynamic therapy and imaging of cancers. Mater. Sci. Eng. C 2016, 67, 611–622. [Google Scholar] [CrossRef]
- Dixit, S.; Miller, K.; Zhu, Y.; McKinnon, E.; Novak, T.; Kenney, M.E.; Broome, A.-M. Dual Receptor-Targeted Theranostic Nanoparticles for Localized Delivery and Activation of Photodynamic Therapy Drug in Glioblastomas. Mol. Pharm. 2015, 12, 3250–3260. [Google Scholar] [CrossRef]
- Nair, L.V.; Nazeer, S.S.; Jayasree, R.S.; Ajayaghosh, A. Fluorescence Imaging Assisted Photodynamic Therapy Using Photosensitizer-Linked Gold Quantum Clusters. ACS Nano 2015, 9, 5825–5832. [Google Scholar] [CrossRef]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Targeting the Oncofetal Thomsen–Friedenreich Disaccharide Using Jacalin-PEG Phthalocyanine Gold Nanoparticles for Photodynamic Cancer Therapy. Angew. Chem. Int. Ed. 2012, 51, 6158–6162. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Liu, B.; Chen, X.; Lin, H.; Peng, Y.; Li, Y.; Zheng, H.; Xu, Y.; Ou, X.; Yan, S.; et al. Aptamer-assisted superparamagnetic iron oxide nanoparticles as multifunctional drug delivery platform for chemo-photodynamic combination therapy. J. Mater. Sci. Mater. Med. 2019, 30, 76. [Google Scholar] [CrossRef] [PubMed]
- Dehvari, K.; Lin, P.-T.; Chang, J.-Y. Fluorescence-guided magnetic nanocarriers for enhanced tumor targeting photodynamic therapy. J. Mater. Chem. B 2018, 6, 4676–4686. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.; Shang, K.; Xu, H.; Zhang, Y.; Pei, Z.; Pei, Y. Facile fabrication of hypericin-entrapped glyconanoparticles for targeted photodynamic therapy. Int. J. Nanomed. 2018, 13, 4319–4331. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, K.S.; Na, K. Caffeic acid-coated multifunctional magnetic nanoparticles for the treatment and bimodal imaging of tumours. J. Photochem. Photobiol. B Biol. 2016, 160, 210–216. [Google Scholar] [CrossRef]
- Wang, D.; Fei, B.; Halig, L.V.; Qin, X.; Hu, Z.; Xu, H.; Wang, Y.A.; Chen, Z.; Kim, S.; Shin, D.M.; et al. Targeted Iron-Oxide Nanoparticle for Photodynamic Therapy and Imaging of Head and Neck Cancer. ACS Nano 2014, 8, 6620–6632. [Google Scholar] [CrossRef]
- Cai, Z.; Xin, F.; Wei, Z.; Wu, M.; Lin, X.; Du, X.; Chen, G.; Zhang, D.; Zhang, Z.; Liu, X.; et al. Photodynamic Therapy Combined with Antihypoxic Signaling and CpG Adjuvant as an In Situ Tumor Vaccine Based on Metal–Organic Framework Nanoparticles to Boost Cancer Immunotherapy. Adv. Healthc. Mater. 2020, 9, 1900996. [Google Scholar] [CrossRef]
- Chen, P.; Huang, Y.-F.; Xu, G.-Y.; Xue, J.-P.; Chen, J.-J. Functionalized Eu(III)-based nanoscale metal-organic framework for enhanced targeted anticancer therapy. J. Porphyrins Phthalocyanines 2019, 23, 619–627. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, J.-Y.; Li, S.-Y.; Zeng, J.-Y.; Lei, Q.; Chen, K.-W.; Zhang, C.; Zhang, X.-Z. An O2 Self-Sufficient Biomimetic Nanoplatform for Highly Specific and Efficient Photodynamic Therapy. Adv. Funct. Mater. 2016, 26, 7847–7860. [Google Scholar] [CrossRef]
- Gong, M.; Yang, J.; Zhuang, Q.; Li, Y.; Gu, J. Mitochondria-Targeted Nanoscale MOFs for Improved Photodynamic Therapy. ChemNanoMat 2020, 6, 89–98. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Zheng, M.; Shan, C.; Yan, H.; Wu, W.; Gao, X.; Cheng, B.; Liu, W.; Tang, Y. Functionalized Eu(III)-Based Nanoscale Metal–Organic Framework To Achieve Near-IR-Triggered and -Targeted Two-Photon Absorption Photodynamic Therapy. Inorg. Chem. 2018, 57, 300–310. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Yang, Z.; Zhang, L.; Xiao, L.; Liu, P.; Wang, J.; Yi, C.; Xu, Z.; Ren, J. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: Targeting tumor hypoxia by carbonic anhydrase IX inhibition and T1–T2 dual mode MRI guided photodynamic/photothermal therapy. J. Mater. Chem. B 2018, 6, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhang, L.; Yang, Z.; Liu, P.; Wang, J.; Cao, J.; Shen, A.; Xu, Z.; Wang, J. An efficient tumor-inducible nanotheranostics for magnetic resonance imaging and enhanced photodynamic therapy. Chem. Eng. J. 2019, 358, 969–979. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Chen, G.; Shi, P. DNA-Functionalized Metal–Organic Framework: Cell Imaging, Targeting Drug Delivery and Photodynamic Therapy. Inorg. Chem. 2019, 58, 6593–6596. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.; Qu, P.; Miley, M.J.; Zhao, Y.; Li, Z.; Ming, X. P-glycoprotein targeted photodynamic therapy of chemoresistant tumors using recombinant Fab fragment conjugates. Biomater. Sci. 2018, 6, 3063–3074. [Google Scholar] [CrossRef] [PubMed]
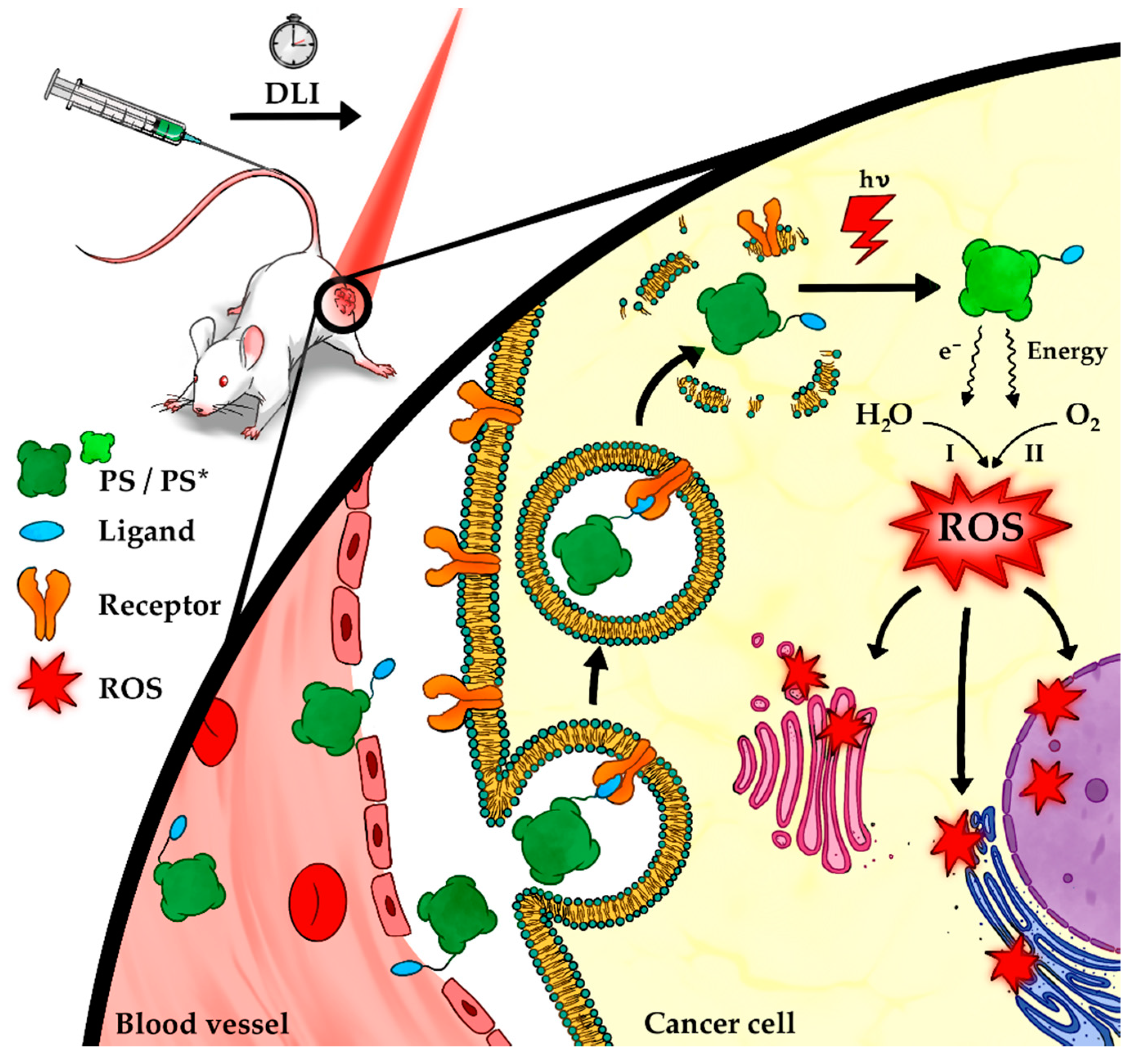
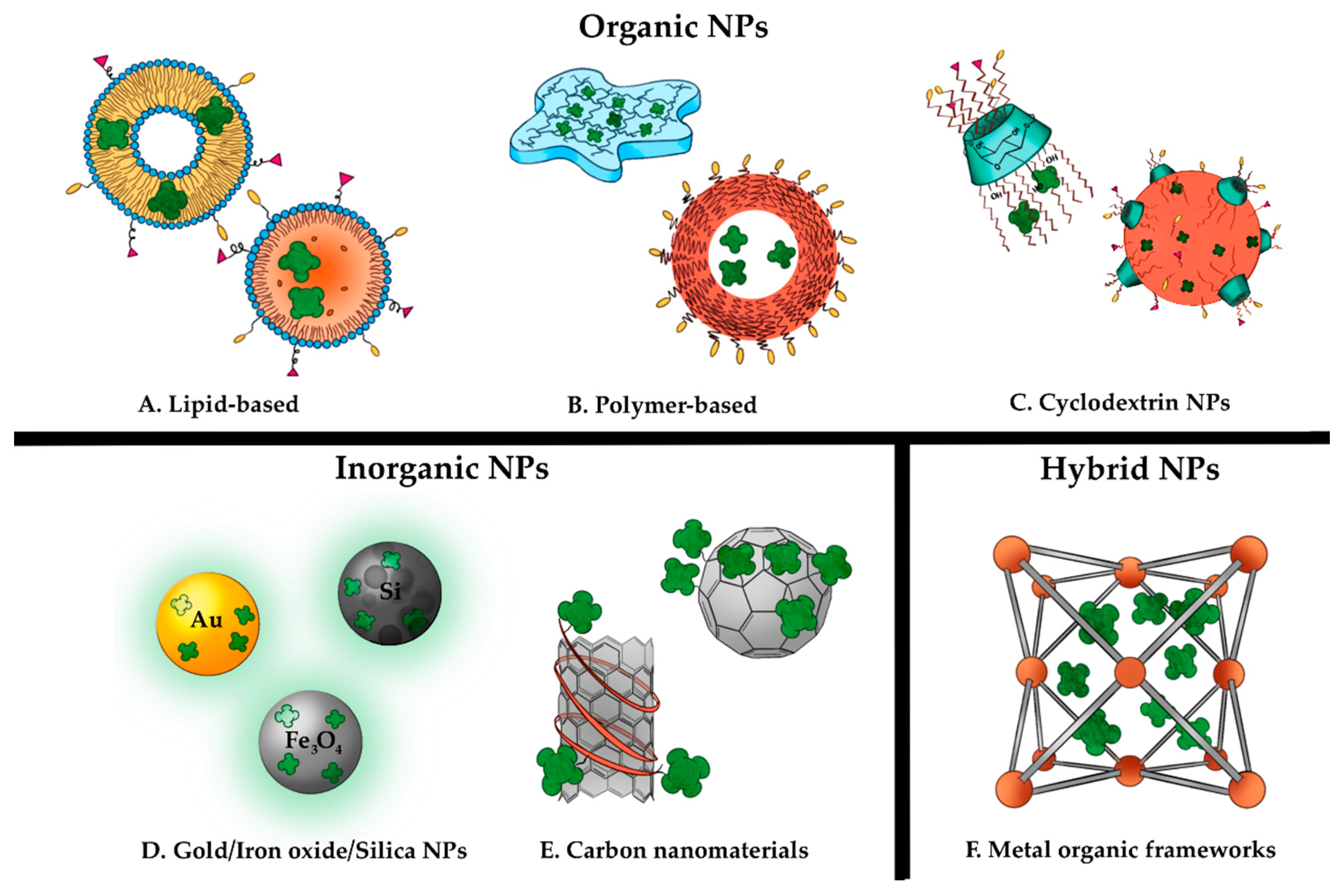
| Ligand Type | Examples | Characteristics | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Proteins | Transferrin | Glycoprotein Aids iron transport via TfR1 | High affinity/specificity of TfR1 interaction | Potential off-target toxicity with high doses Potential competitive binding to malignant cell receptors | [33,34,85,86,87,88] |
| Peptides | RGD, Lyp-1, GE11, F3 | Low molecular weight Typically <50 aas | High target receptor affinity/specificity Enhanced tumor diffusion Biocompatibility Low manufacture costs Ease of conjugation | Slow receptor identification Low stability in vivo which may be improved by chemical modifications. | [27,89,90,91,92,93,94,95] |
| Antibodies | Trastuzumab, Cetuximab | Y shaped macromolecules | High receptor target affinity/specificity Stability in vivo | Potential immunogenicity Heterogenous tumor antigen expression High cost/resource intensive production Large size limits tumor penetration | [7,30,96,97,98,99,100] |
| Nanobodies | 7D12, 7D12-9G8 | Small/fully functional antibody fragment | High receptor target affinity/specificity High tissue penetration High thermal and chemical stability Reduced immunogenicity relative to mAbs | Small size can lead to unfavorably high blood clearance rate which may be avoided by chemical modification | [97,101,102,103,104,105] |
| Non-protein | Folate, Polysaccharides–Hyaluronic acid (HA) Bile acids (BAs) | Folate is used for purine and pyrimidine biosynthesis HA is a component of the extracellular matrix BAs facilitate targeting of apical sodium dependent bile acid transporter (ASB) | High affinity Minimal immunogenicity | Folate conjugates may undergo slow release HA may cause off-target effects | [35,106,107,108,109,110,111] |
| Aptamers | A10 PSMA AS1411 | ss-DNA/RNA Fold into distinct secondary/tertiary structures. | High target receptor affinity Minimal immunogenicity Low manufacturing cost Suitable for large scale production High thermal and chemical stability | Off-target effects may result in toxicity Susceptible to nuclease degradation in vivo if unmodified | [112,113,114,115] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gierlich, P.; Mata, A.I.; Donohoe, C.; Brito, R.M.M.; Senge, M.O.; Gomes-da-Silva, L.C. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules 2020, 25, 5317. https://doi.org/10.3390/molecules25225317
Gierlich P, Mata AI, Donohoe C, Brito RMM, Senge MO, Gomes-da-Silva LC. Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules. 2020; 25(22):5317. https://doi.org/10.3390/molecules25225317
Chicago/Turabian StyleGierlich, Piotr, Ana I. Mata, Claire Donohoe, Rui M. M. Brito, Mathias O. Senge, and Lígia C. Gomes-da-Silva. 2020. "Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment" Molecules 25, no. 22: 5317. https://doi.org/10.3390/molecules25225317
APA StyleGierlich, P., Mata, A. I., Donohoe, C., Brito, R. M. M., Senge, M. O., & Gomes-da-Silva, L. C. (2020). Ligand-Targeted Delivery of Photosensitizers for Cancer Treatment. Molecules, 25(22), 5317. https://doi.org/10.3390/molecules25225317








