Approach to the Dynamic of Carbamazepine and its Main Metabolites in Soil Contamination through the Reuse of Wastewater and Sewage Sludge
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Experiments
2.2. Adsorption of Studied Compounds
2.2.1. Single-Solute Systems
2.2.2. Four-Solute Systems
2.3. Adsorption Isotherms
2.3.1. Single-Solute Systems
2.3.2. Four-Solute Systems
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sampling and Soil Preparation
3.3. Batch Experiments
3.3.1. Preliminary Experiments
3.3.2. Influence of pH Experiments on the Adsorption of Studied Compounds
3.3.3. Adsorption Isotherm Experiments
3.4. Chromatographic Determination
3.5. Method Performance and Quality Control
3.6. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Boix, C.; Ibáñez, M.; Zamora, T.; Sancho, J.; Niessen, W.; Hernández, F. Identification of new omeprazole metabolites in wastewaters and surface waters. Sci. Total Environ. 2014, 468–469, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Ferrando-Climent, L.; Collado, N.; Buttiglieri, G.; Gros, M.; Layret, I.R.-R.; Rodríguez-Mozaz, S.; Barceló, J. Comprehensive study of ibuprofen and its metabolites in activated sludge batch experiments and aquatic environment. Sci. Total Environ. 2012, 438, 404–413. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Yonetani, T.; Asada, Y.; Echigo, S.; Itoh, S. Simultaneous determination of carbamazepine-N-glucuronide and carbamazepine phase I metabolites in the wastewater by liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 145, 1191–1198. [Google Scholar] [CrossRef]
- Cao, S.-S.; Duan, Y.; Tu, Y.-J.; Tang, Y.; Liu, J.; Zhi, W.-D.; Dai, C. Pharmaceuticals and personal care products in a drinking water resource of Yangtze River Delta Ecology and Greenery Integration Development Demonstration Zone in China: Occurrence and human health risk assessment. Sci. Total Environ. 2020, 721, 137624. [Google Scholar] [CrossRef] [PubMed]
- García-Galán, M.J.; Díaz-Cruz, M.S.; Barceló, J. Multiresidue trace analysis of sulfonamide antibiotics and their metabolites in soils and sewage sludge by pressurized liquid extraction followed by liquid chromatography–electrospray-quadrupole linear ion trap mass spectrometry. J. Chromatogr. A 2013, 1275, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Peysson, W.; Vulliet, E. Determination of 136 pharmaceuticals and hormones in sewage sludge using quick, easy, cheap, effective, rugged and safe extraction followed by analysis with liquid chromatography–time-of-flight-mass spectrometry. J. Chromatogr. A 2013, 1290, 46–61. [Google Scholar] [CrossRef]
- Rossini, D.; Ciofi, L.; Ancillotti, C.; Checchini, L.; Bruzzoniti, M.; Rivoira, L.; Fibbi, D.; Orlandini, S.; Del Bubba, M. Innovative combination of QuEChERS extraction with on-line solid-phase extract purification and pre-concentration, followed by liquid chromatography-tandem mass spectrometry for the determination of non-steroidal anti-inflammatory drugs and their metabolites in sewage sludge. Anal. Chim. Acta 2016, 935, 269–281. [Google Scholar]
- Wu, C.; Pongberg, A.L.S.; Itter, J.D.W.; Ang, M.F.; Zajkowski, K.P.C. Uptake of Pharmaceutical and Personal Care Products by Soybean Plants from Soils Applied with Biosolids and Irrigated with Contaminated Water. Environ. Sci. Technol. 2010, 44, 6157–6161. [Google Scholar] [CrossRef]
- Milieu Ltd.; WRc; RPA. Environmental, Economic and Social Impacts of the Use of Sewage Sludge on Land. Final Report Part I: Overview Report 2008. Available online: http://ec.europa.eu/environment/archives/waste/sludge/pdf/part_i_report.pdf (accessed on 12 November 2020).
- Kočárek, M.; Kodešová, R.; Vondráčková, L.; Golovko, O.; Fér, M.; Klement, A.; Nikodem, A.; Jakšík, O.; Grabic, R. Simultaneous sorption of four ionizable pharmaceuticals in different horizons of three soil types. Environ. Pollut. 2016, 218, 563–573. [Google Scholar] [CrossRef]
- Biel-Maeso, M.; González-González, C.; Lara-Martín, P.A.; Corada-Fernández, C. Sorption and degradation of contaminants of emerging concern in soils under aerobic and anaerobic conditions. Sci. Total Environ. 2019, 666, 662–671. [Google Scholar] [CrossRef]
- Yu, C.; Bi, E. Adsorption site-dependent transport of diclofenac in water saturated minerals and reference soils. Chemosphere 2019, 236, 124256. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.; Boateng, L.K.; Flora, J.R.; Oh, J.; Braswell, M.C.; Son, A.; Yoon, Y. Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: Experimental and molecular modeling study. Chem. Eng. J. 2015, 264, 1–9. [Google Scholar] [CrossRef]
- Lerman, I.; Chen, Y.; Xing, B.; Chefetz, B. Adsorption of carbamazepine by carbon nanotubes: Effects of DOM introduction and competition with phenanthrene and bisphenol A. Environ. Pollut. 2013, 182, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kaserzon, S.L.; Vermeirssen, E.L.M.; Hawker, D.W.; Kennedy, K.; Bentley, C.; Thompson, J.; Booij, K.; Mueller, J. Passive sampling of perfluorinated chemicals in water: Flow rate effects on chemical uptake. Environ. Pollut. 2013, 177, 58–63. [Google Scholar] [CrossRef]
- Orta, M.D.M.; Martín, J.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Adsorption of propranolol onto montmorillonite: Kinetic, isotherm and pH studies. Appl. Clay Sci. 2019, 173, 107–114. [Google Scholar] [CrossRef]
- Fenet, H.; Mathieu, O.; Mahjoub, O.; Li, Z.; Hillaire-Buys, D.; Casellas, C.; Gomez, E. Carbamazepine, carbamazepine epoxide and dihydroxycarbamazepine sorption to soil and occurrence in a wastewater reuse site in Tunisia. Chemosphere 2012, 88, 49–54. [Google Scholar] [CrossRef]
- Leclercq, M.; Mathieu, O.; Gomez, E.; Casellas, C.; Fenet, H.; Hillaire-Buys, D. Presence and Fate of Carbamazepine, Oxcarbazepine, and Seven of Their Metabolites at Wastewater Treatment Plants. Arch. Environ. Contam. Toxicol. 2009, 56, 408–415. [Google Scholar] [CrossRef]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Routine analytical method for monitoring the main metabolites for a recurrent group of parabens and pharmaceuticals in wastewater and tap water. Anal. Bioanal. Chem. 2019, 411, 6625–6635. [Google Scholar] [CrossRef]
- Wojsławski, J.; Białk-Bielińska, A.; Stepnowski, P.; Dołżonek, J. Leaching behavior of pharmaceuticals and their metabolites in the soil environment. Chemosphere 2019, 231, 269–275. [Google Scholar] [CrossRef]
- Bahlmann, A.; Brack, W.; Schneider, R.J.; Krauss, M. Carbamazepine and its metabolites in wastewater: Analytical pitfalls and occurrence in Germany and Portugal. Water Res. 2014, 57, 104–114. [Google Scholar] [CrossRef]
- Kodešová, R.; Klement, A.; Golovko, O.; Fér, M.; Kočárek, M.; Nikodem, A.; Grabic, R. Soil influences on uptake and transfer of pharmaceuticals from sewage sludge amended soils to spinach. J. Environ. Manag. 2019, 250, 109407. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Tadmor, G.; Malchi, T.; Blotevogel, J.; Borch, T.; Polubesova, T.; Chefetz, B. Fate of carbamazepine, its metabolites, and lamotrigine in soils irrigated with reclaimed wastewater: Sorption, leaching and plant uptake. Chemosphere 2016, 160, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Mas-Pla, J.; Boy-Roura, M.; Geli, I.; Domingo, F.; Petrović, M. Veterinary pharmaceuticals and antibiotics in manure and slurry and their fate in amended agricultural soils: Findings from an experimental field site (Baix Empordà, NE Catalonia). Sci. Total Environ. 2019, 654, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Conde-Cid, M.; Nóvoa-Muñoz, J.; Fernández-Sanjurjo, M.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Arias-Estévez, M. Pedotransfer functions to estimate the adsorption and desorption of sulfadiazine in agricultural soils. Sci. Total Environ. 2019, 691, 933–942. [Google Scholar] [CrossRef] [PubMed]
- Giles, C.H.; MacEwan, T.H.; Nakhwa, S.N.; Smith, D. 786. Studies in adsorption. Part XI. A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc. 1960, 4, 3973–3993. [Google Scholar] [CrossRef]
- Pavlović, D.M.; Glavač, A.; Gluhak, M.; Runje, M. Sorption of albendazole in sediments and soils: Isotherms and kinetics. Chemosphere 2018, 193, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Evers, D.C.; Keane, S.E.; Basu, N.; Buck, D. Evaluating the effectiveness of the Minamata Convention on Mercury: Principles and recommendations for next steps. Sci. Total Environ. 2016, 323, 888–903. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, I.; Gómez, M.J.; Molina-Díaz, A.; Huijbregts, M.A.; Fernández-Alba, A.R.; García-Calvo, E. Ranking potential impacts of priority and emerging pollutants in urban wastewater through life cycle impact assessment. Chemosphere 2008, 74, 37–44. [Google Scholar] [CrossRef]
- Lee, S.; Kang, S.-I.; Lim, J.-L.; Huh, Y.J.; Kim, K.-S.; Cho, J. Evaluating controllability of pharmaceuticals and metabolites in biologically engineered processes, using corresponding octanol–water distribution coefficient. Ecol. Eng. 2011, 37, 1595–1600. [Google Scholar] [CrossRef]
- Huntscha, S.; Singer, H.P.; McArdell, C.S.; Frank, C.E.; Hollender, J. Multiresidue analysis of 88 polar organic micropollutants in ground, surface and wastewater using online mixed-bed multilayer solid-phase extraction coupled to high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2012, 1268, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Miao, X.-S.; Yang, J.-J.; Metcalfe, C.D. Carbamazepine and Its Metabolites in Wastewater and in Biosolids in a Municipal Wastewater Treatment Plant. Environ. Sci. Technol. 2005, 39, 7469–7475. [Google Scholar] [CrossRef] [PubMed]
- Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Simultaneous pressurized liquid extraction and clean-up for the determination of metabolites in complex environmental solid matrices. Microchem. J. 2020, 152, 104370. [Google Scholar] [CrossRef]
- OECD. Adsorption-Desorption Using a Batch Equilibrium Method. OECD Guideline for the Testing of Chemicals; Organization for Economic Cooperation and Development: Paris, France, 2000; Volume 106. [Google Scholar]
- Kiecak, A.; Sassine, L.; Boy-Roura, M.; Elsner, M.; Mas-Pla, J.; Salle, C.L.G.L.; Stumpp, C. Sorption properties and behaviour at laboratory scale of selected pharmaceuticals using batch experiments. J. Contam. Hydrol. 2019, 225, 103500. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, W.; Stefanska, J. Adsorption and biodegradation of antidiabetic pharmaceuticals in soils. Chemosphere 2014, 95, 281–288. [Google Scholar] [CrossRef] [PubMed]


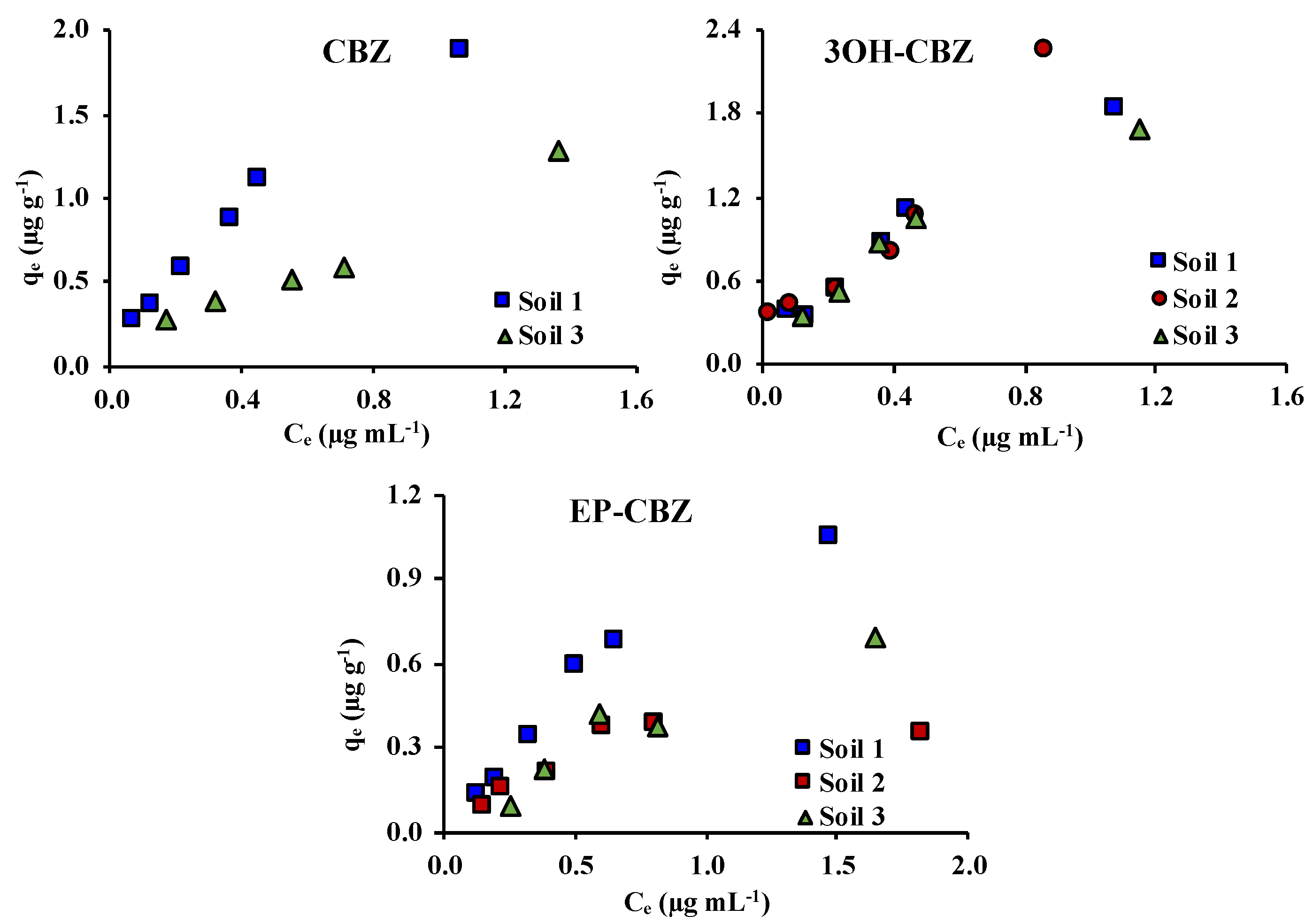
| Henry | Freundlich | Langmuir | ||||||
|---|---|---|---|---|---|---|---|---|
| Kd (mL g−1) | R2 | KF ((µg g−1)) (mL µg−1)1/n) | 1/n | R2 | KL (mL g−1) | qmax (µg g−1) | R2 | |
| Single-Solute System | ||||||||
| CBZ | ||||||||
| Soil 1 | 1.65 | 0.9783 | 1.69 | 0.848 | 0.9698 | 0.45 | 5.30 | 0.9801 |
| Soil 2 | - | - | - | - | - | - | - | - |
| Soil 3 | 2.12 | 0.9975 | 2.19 | 0.842 | 0.9990 | 1.05 | 3.38 | 0.9914 |
| 3OH-CBZ | ||||||||
| Soil 1 | 9.89 | 0.9974 | 7.12 | 0.762 | 0.9835 | 8.27 | 2.71 | 0.9473 |
| Soil 2 | 1.58 | 0.9906 | 1.52 | 0.803 | 0.9692 | 2.04 | 1.54 | 0.9176 |
| Soil 3 | 4.29 | 0.9895 | 3.31 | 0.709 | 0.9713 | 5.73 | 1.97 | 0.9525 |
| 10OH-CBZ | ||||||||
| Soil 1 | 0.14 | 0.4142 | 0.49 | 0.432 | 0.6911 | 1.27 | 0.98 | 0.9055 |
| Soil 2 | - | - | - | - | - | - | - | - |
| Soil 3 | 0.28 | 0.9225 | 0.41 | 0.723 | 0.9501 | 0.56 | 1.21 | 0.9830 |
| EP-CBZ | ||||||||
| Soil 1 | 0.68 | 0.8432 | 0.94 | 0.953 | 0.9052 | -0.21 | -4.35 | 0.9599 |
| Soil 2 | 0.32 | 0.8879 | 0.37 | 1.005 | 0.8923 | -0.46 | -0.65 | 0.9249 |
| Soil 3 | 0.93 | 0.9353 | 1.16 | 0.934 | 0.9711 | 0.04 | 35.06 | 0.9926 |
| Four-Solute System | ||||||||
| CBZ | ||||||||
| Soil 1 | 1.60 | 0.9732 | 1.82 | 0.713 | 0.9911 | 2.57 | 1.83 | 0.9584 |
| Soil 2 | - | - | - | - | - | - | - | - |
| Soil 3 | 0.83 | 0.9747 | 0.86 | 0.702 | 0.9445 | 0.83 | 1.17 | 0.9287 |
| 3OH-CBZ | ||||||||
| Soil 1 | 1.53 | 0.9679 | 1.71 | 0.646 | 0.9285 | 4.43 | 1.37 | 0.7284 |
| Soil 2 | 2.23 | 0.9405 | 1.45 | 0.387 | 0.7464 | 55.84 | 0.84 | 0.5626 |
| Soil 3 | 1.25 | 0.9461 | 1.64 | 0.695 | 0.9736 | 17.29 | 2.45 | 0.9636 |
| 10OH-CBZ | ||||||||
| Soil 1 | - | - | - | - | - | - | - | - |
| Soil 2 | - | - | - | - | - | - | - | - |
| Soil 3 | - | - | - | - | - | - | - | - |
| EP-CBZ | ||||||||
| Soil 1 | 0.68 | 0.9271 | 0.90 | 0.891 | 0.9680 | 0.08 | 12.57 | 0.9912 |
| Soil 2 | 0.14 | 0.4900 | 0.36 | 0.559 | 0.8074 | 0.84 | 0.80 | 0.9601 |
| Soil 3 | 0.39 | 0.9100 | 0.50 | 0.966 | 0.8947 | −0.52 | −1.18 | 0.9147 |
| Compound | Molecular Weight | pKa | Log Kow | Water Solubility (mg/L) | Structure |
|---|---|---|---|---|---|
| Carbamazepine (CBZ) | 236.27 | 13.9 a | 2.5 b | 0.15 f |  |
| 3-Hydroxycarbamazepine (3OH-CBZ) | 252.27 | 9.19 c | 2.41d | n.a. |  |
| 10,11-Dihydro-10-hydroxy carbamazepine (10OH-CBZ) | 254.28 | 12.8 d | 0.93 e | n.a. |  |
| Carbamazepine-10,11-epoxide (EP-CBZ) | 252.27 | 16.0 d | 1.0 b | 1.34 f |  |
| Soil 1 | Soil 2 | Soil 3 | |
|---|---|---|---|
| Fine sand, wt.% | 16.4 | 4.70 | 14.0 |
| Coarse sand, wt.% | 8.20 | 69.5 | 51.6 |
| Silt, wt.% | 44.5 | 5.80 | 18.4 |
| Clay, wt.% | 30.8 | 19.9 | 16.0 |
| pH | 8.27 | 8.21 | 8.06 |
| EC, µS·cm−1 | 129 | 75 | 126 |
| OM, wt.% | 0.91 | 0.58 | 2.01 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malvar, J.L.; Santos, J.L.; Martín, J.; Aparicio, I.; Alonso, E. Approach to the Dynamic of Carbamazepine and its Main Metabolites in Soil Contamination through the Reuse of Wastewater and Sewage Sludge. Molecules 2020, 25, 5306. https://doi.org/10.3390/molecules25225306
Malvar JL, Santos JL, Martín J, Aparicio I, Alonso E. Approach to the Dynamic of Carbamazepine and its Main Metabolites in Soil Contamination through the Reuse of Wastewater and Sewage Sludge. Molecules. 2020; 25(22):5306. https://doi.org/10.3390/molecules25225306
Chicago/Turabian StyleMalvar, José Luis, Juan Luis Santos, Julia Martín, Irene Aparicio, and Esteban Alonso. 2020. "Approach to the Dynamic of Carbamazepine and its Main Metabolites in Soil Contamination through the Reuse of Wastewater and Sewage Sludge" Molecules 25, no. 22: 5306. https://doi.org/10.3390/molecules25225306
APA StyleMalvar, J. L., Santos, J. L., Martín, J., Aparicio, I., & Alonso, E. (2020). Approach to the Dynamic of Carbamazepine and its Main Metabolites in Soil Contamination through the Reuse of Wastewater and Sewage Sludge. Molecules, 25(22), 5306. https://doi.org/10.3390/molecules25225306







