Sugar Profiling of Honeys for Authentication and Detection of Adulterants Using High-Performance Thin Layer Chromatography
Abstract
1. Introduction
2. Results and Discussion
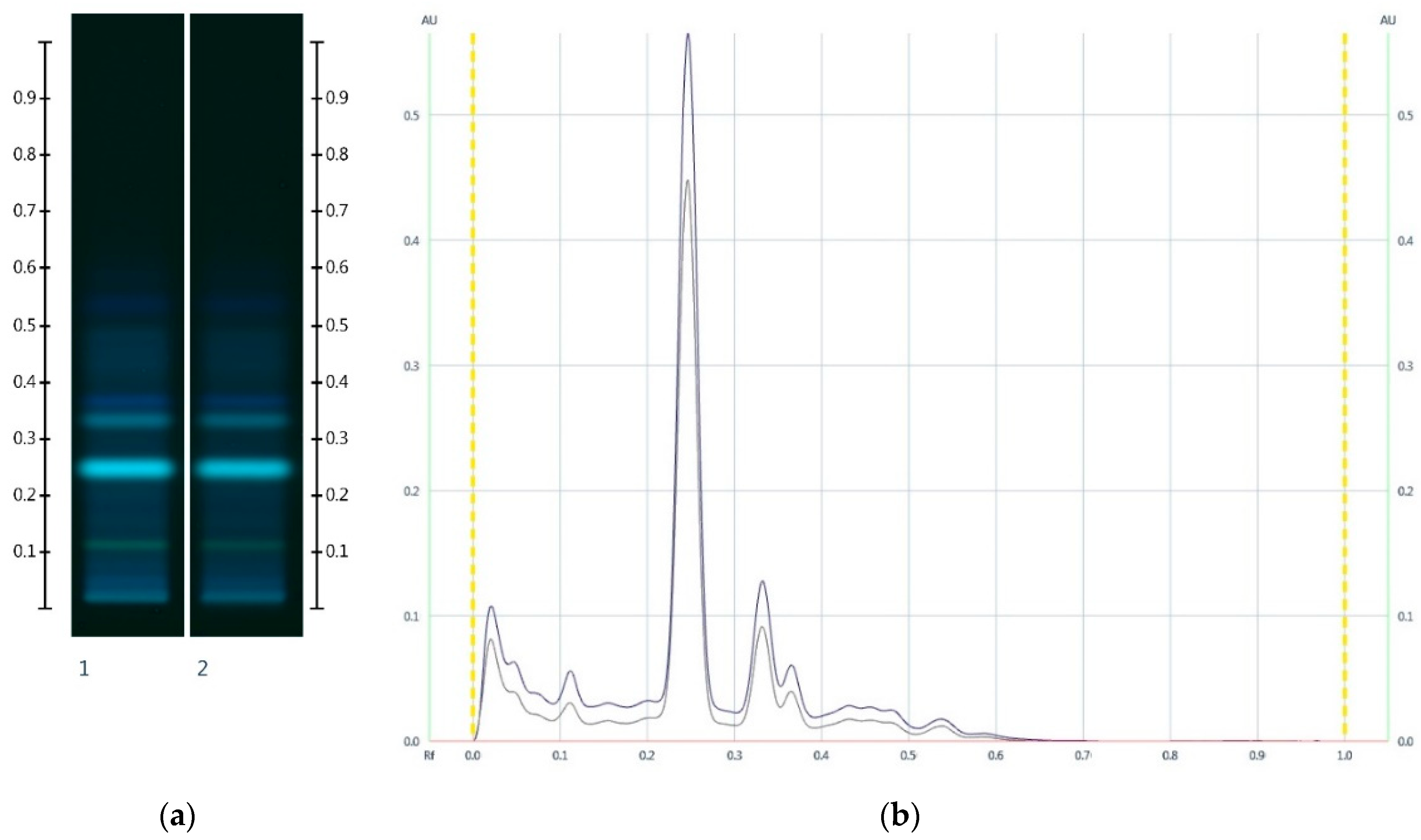
2.1. Sugar Analysis of Honeys and Syrups
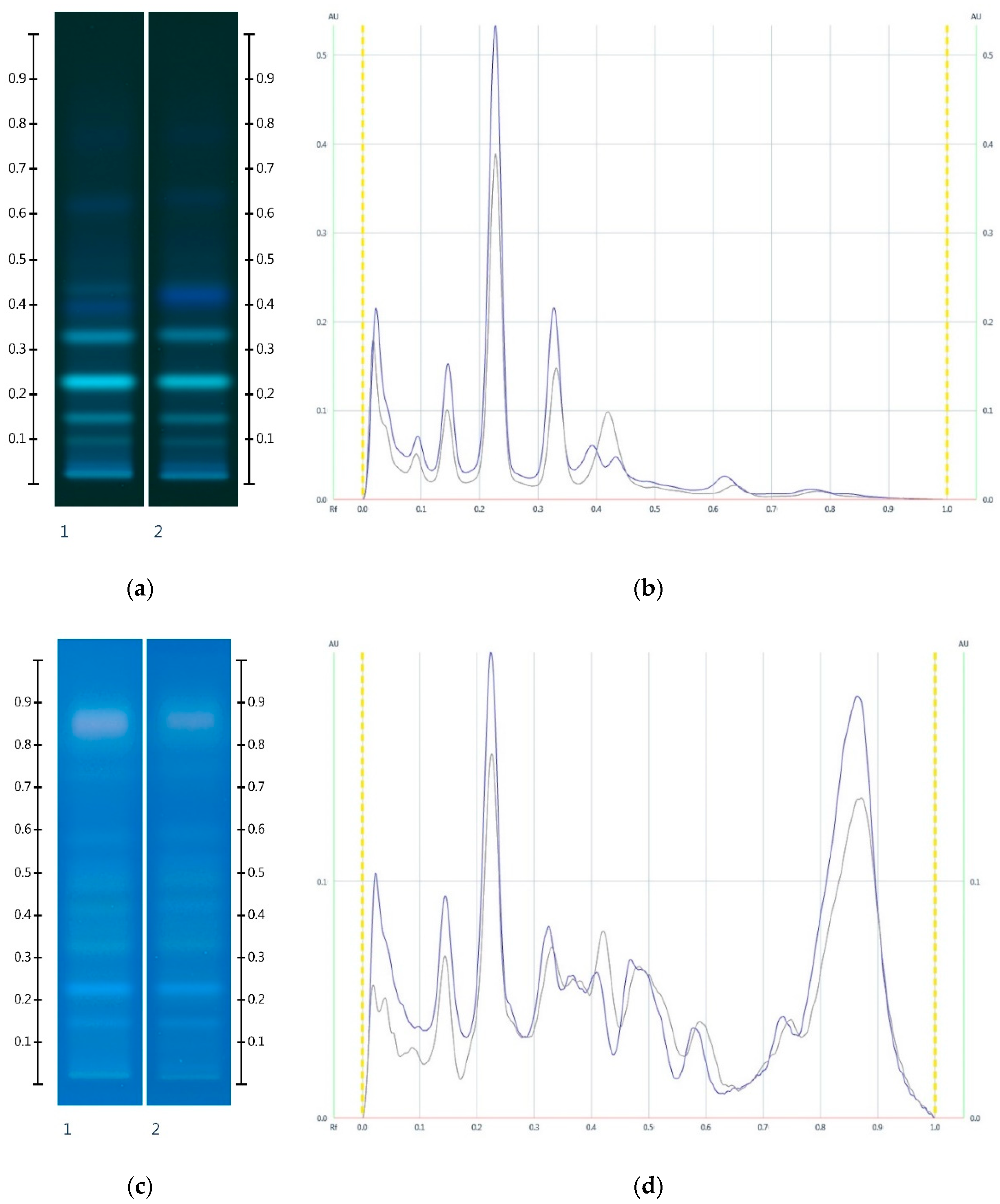
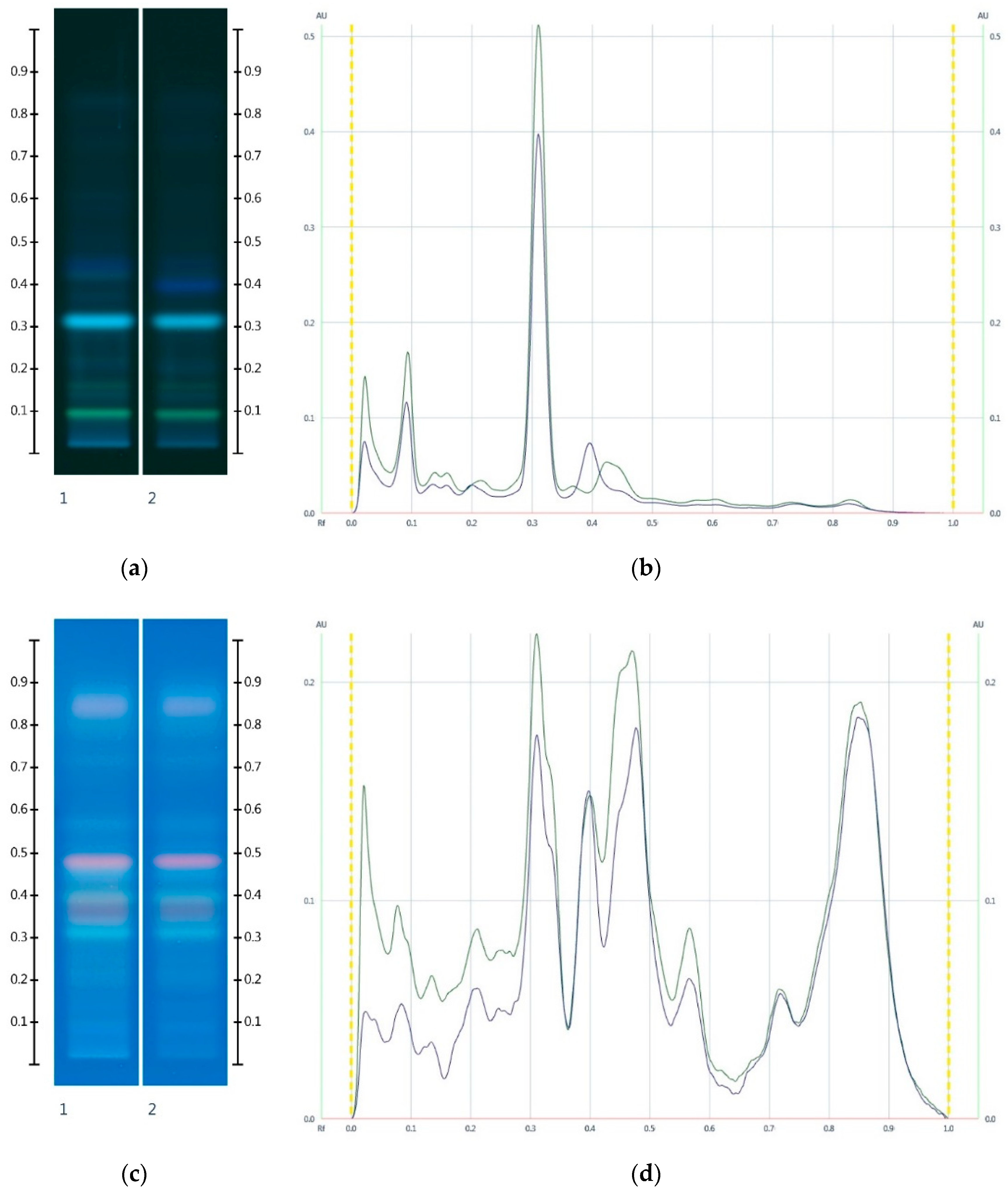
2.2. Sugar Analysis of Adulterated Honeys
2.3. Organic Extract Analysis of Honeys, Syrups and Adulterated Honeys
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Standards and Reagent Preparation
3.3. Sample Preparation
3.4. Instrumentation and High-Performance Thin Layer Chromatography (HPTLC) Method
3.4.1. Sugar Analysis
3.4.2. Organic Extract Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Forrest, R.D. Early history of wound treatment. J. R. Soc. Med. 1982, 75, 198–205. [Google Scholar]
- Kuropatnicki, A.K.; Kłósek, M.; Kucharzewski, M. Honey as medicine: Historical perspectives. J. Apic. Res. 2018, 57, 113–118. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. F 2017, 16, 1072–1100. [Google Scholar] [CrossRef]
- Frew, R.; McComb, K.; Croudis, L.; Clark, D.; Van Hale, R. Modified sugar adulteration test applied to New Zealand honey. Food Chem. 2013, 141, 4127–4131. [Google Scholar] [CrossRef]
- Yang, X.; Guang, P.; Xu, G.; Zhu, S.; Chen, Z.; Huang, F. Manuka honey adulteration detection based on near-infrared spectroscopy combined with aquaphotomics. LWT 2020, 132, 109837. [Google Scholar] [CrossRef]
- Cordella, C.; Militão, J.S.L.T.; Clément, M.-C.; Drajnudel, P.; Cabrol-Bass, D. Detection and quantification of honey adulteration via direct incorporation of sugar syrups or bee-feeding: Preliminary study using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and chemometrics. Anal. Chim. Acta 2005, 531, 239–248. [Google Scholar] [CrossRef]
- Rios-Corripio, M.A.; Rojas-López, M.; Delgado-Macuil, R. Analysis of adulteration in honey with standard sugar solutions and syrups using attenuated total reflectance-Fourier transform infrared spectroscopy and multivariate methods. CyTA J. Food 2012, 10, 119–122. [Google Scholar] [CrossRef]
- Vorlová, L. Adulteration of honey and available methods for detection—A review. Acta Vet. Brno 2015, 83, S85–S102. [Google Scholar]
- Du, B.; Wu, L.; Xue, X.; Chen, L.; Li, Y.; Zhao, J.; Cao, W. Rapid Screening of Multiclass Syrup Adulterants in Honey by Ultrahigh-Performance Liquid Chromatography/Quadrupole Time of Flight Mass Spectrometry. J. Agric. Food Chem. 2015, 63, 6614–6623. [Google Scholar] [CrossRef]
- Amiry, S.; Esmaiili, M.; Alizadeh, M. Classification of adulterated honeys by multivariate analysis. Food Chem. 2017, 224, 390–397. [Google Scholar] [CrossRef]
- Wu, L.; Du, B.; Vander Heyden, Y.; Chen, L.; Zhao, L.; Wang, M.; Xue, X. Recent advancements in detecting sugar-based adulterants in honey—A challenge. TrAC Trend Anal. Chem. 2017, 86, 25–38. [Google Scholar] [CrossRef]
- Geana, E.-I.; Ciucure, C.T. Establishing authenticity of honey via comprehensive Romanian honey analysis. Food Chem. 2020, 306, 125595. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.S.M.; Ziena, H.M.; Youssef, M.M. Adulteration of honey with high-fructose corn syrup: Detection by different methods. Food Chem. 1993, 48, 209–212. [Google Scholar] [CrossRef]
- de la Fuente, E.; Ruiz-Matute, A.I.; Valencia-Barrera, R.M.; Sanz, J.; Castro, I.M. Carbohydrate composition of Spanish unifloral honeys. Food Chem. 2011, 129, 1483–1489. [Google Scholar] [CrossRef]
- Rogers, K.M.; Sim, M.; Stewart, S.; Phillips, A.; Cooper, J.; Douance, C.; Pyne, R.; Rogers, P. Investigating C-4 Sugar Contamination of Manuka Honey and Other New Zealand Honey Varieties Using Carbon Isotopes. J. Agric. Food Chem. 2014, 62, 2605–2614. [Google Scholar] [CrossRef]
- Ventura, E.E.; Davis, J.N.; Goran, M.I. Sugar content of popular sweetened beverages based on objective laboratory analysis: Focus on fructose content. Obesity 2011, 19, 868–874. [Google Scholar] [CrossRef]
- Wang, J.; Kliks, M.M.; Jun, S.; Jackson, M.; Li, Q.X. Rapid analysis of glucose, fructose, sucrose, and maltose in honeys from different geographic regions using fourier transform infrared spectroscopy and multivariate analysis. J. Food Sci. 2010, 75, C208–C214. [Google Scholar] [CrossRef]
- Schievano, E.; Morelato, E.; Facchin, C.; Mammi, S. Characterization of markers of botanical origin and other compounds extracted from unifloral honeys. J. Agric. Food Chem. 2013, 61, 1747–1755. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, S.; Li, H.; Han, X.; Ouyang, Q.; Zhao, J. Determination of rice syrup adulterant concentration in honey using three-dimensional fluorescence spectra and multivariate calibrations. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 131, 177–182. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.-U. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Sesta, G. Determination of sugars in royal jelly by HPLC. Apidologie 2006, 37, 84–90. [Google Scholar] [CrossRef]
- Xu, W.; Liang, L.; Zhu, M. Determination of Sugars in Molasses by HPLC Following Solid-Phase Extraction. Int. J. Food Prop. 2014, 18, 547–557. [Google Scholar] [CrossRef]
- Luo, P.; Luo, M.Z.; Baldwin, R.P. Determination of sugars in food products: Using HPLC and electrochemical detection at a Cu electrode. J. Chem. Educ. 1993, 70, 679. [Google Scholar] [CrossRef]
- Ballin, N.Z.; Laursen, K.H. To target or not to target? Definitions and nomenclature for targeted versus non-targeted analytical food authentication. Trends Food Sci. Technol. 2019, 86, 537–543. [Google Scholar] [CrossRef]
- Esslinger, S.; Riedl, J.; Fauhl-Hassek, C. Potential and limitations of non-targeted fingerprinting for authentication of food in official control. Food Res. Int. 2014, 60, 189–204. [Google Scholar] [CrossRef]
- Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. A validated method for the quantitative determination of sugars in honey using high-performance thin-layer chromatography. JPC J. Planar Chromat. 2020, 1–11. [Google Scholar] [CrossRef]
- Locher, C.; Neumann, J.; Sostaric, T. Authentication of honeys of different floral origins via high-performance thin-layer chromatographic fingerprinting. JPC J Planar Chromat. 2017, 30, 57–62. [Google Scholar] [CrossRef]
- Locher, C.; Tang, E.; Neumann, J.; Sostaric, T. High-performance thin-layer chromatography profiling of Jarrah and Manuka honeys. JPC J. Planar Chromat. 2018, 31, 181–189. [Google Scholar] [CrossRef]
- Al-Habsi, N.A.; Davis, F.J.; Niranjan, K. Development of novel methods to determine crystalline glucose content of honey based on DSC, HPLC, and Viscosity measurements, and their use to examine the setting propensity of honey. J. Food Sci. 2013, 78, E845–E852. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.; Li, H.; Li, Y.; Hu, J.; Li, J.; Wang, H.; Deng, Z. Chemical and molecular dynamics analysis of crystallization properties of honey. Int. J. Food Prop. 2017, 20, 725–733. [Google Scholar] [CrossRef]
- Tappi, S.; Laghi, L.; Dettori, A.; Piana, L.; Ragni, L.; Rocculi, P. Investigation of water state during induced crystallization of honey. Food Chem. 2019, 294, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Stanek, N.; Jasicka-Misiak, I. HPTLC Phenolic Profiles as Useful Tools for the Authentication of Honey. Food Anal. Methods 2018, 11, 2979. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
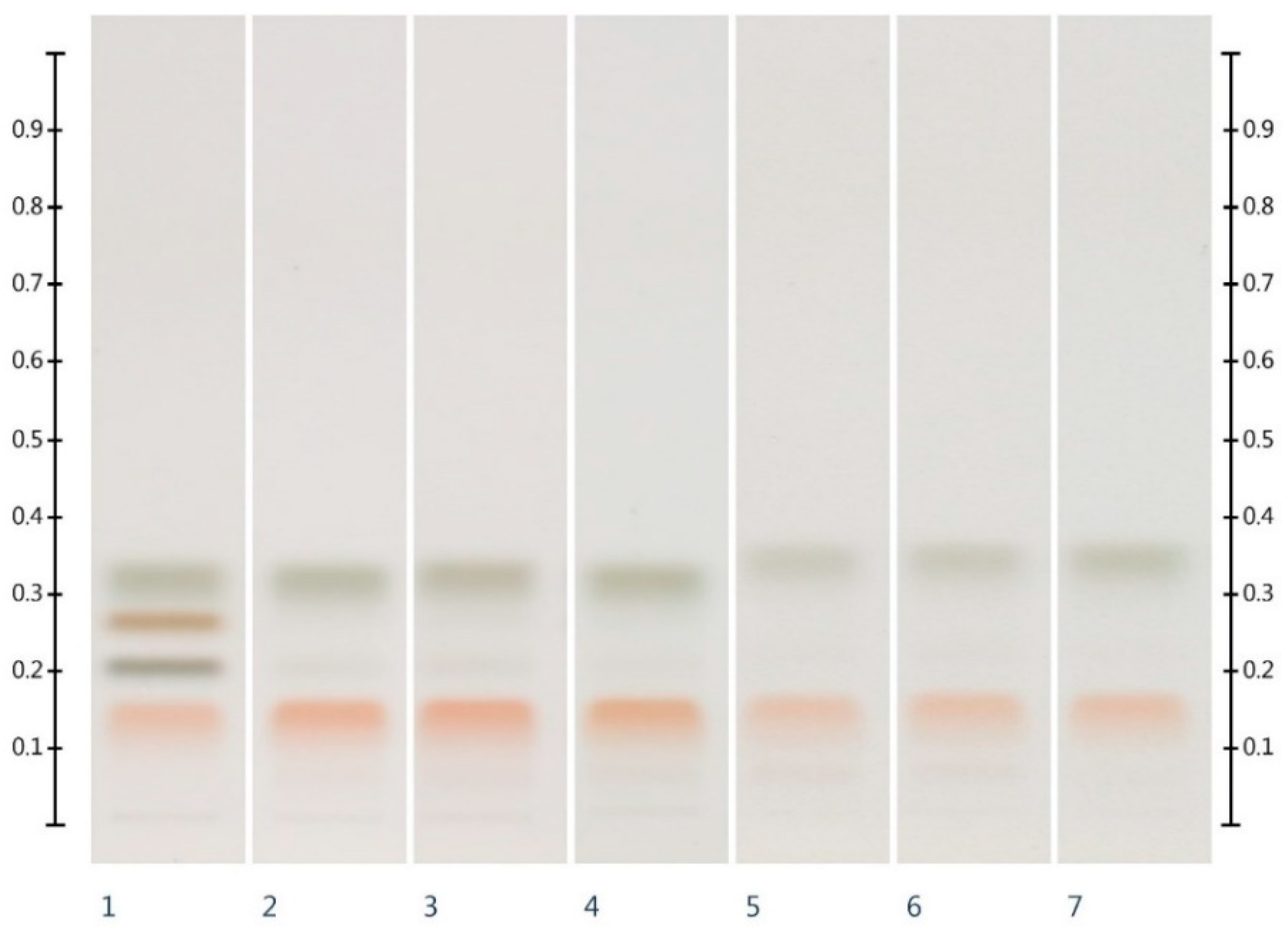
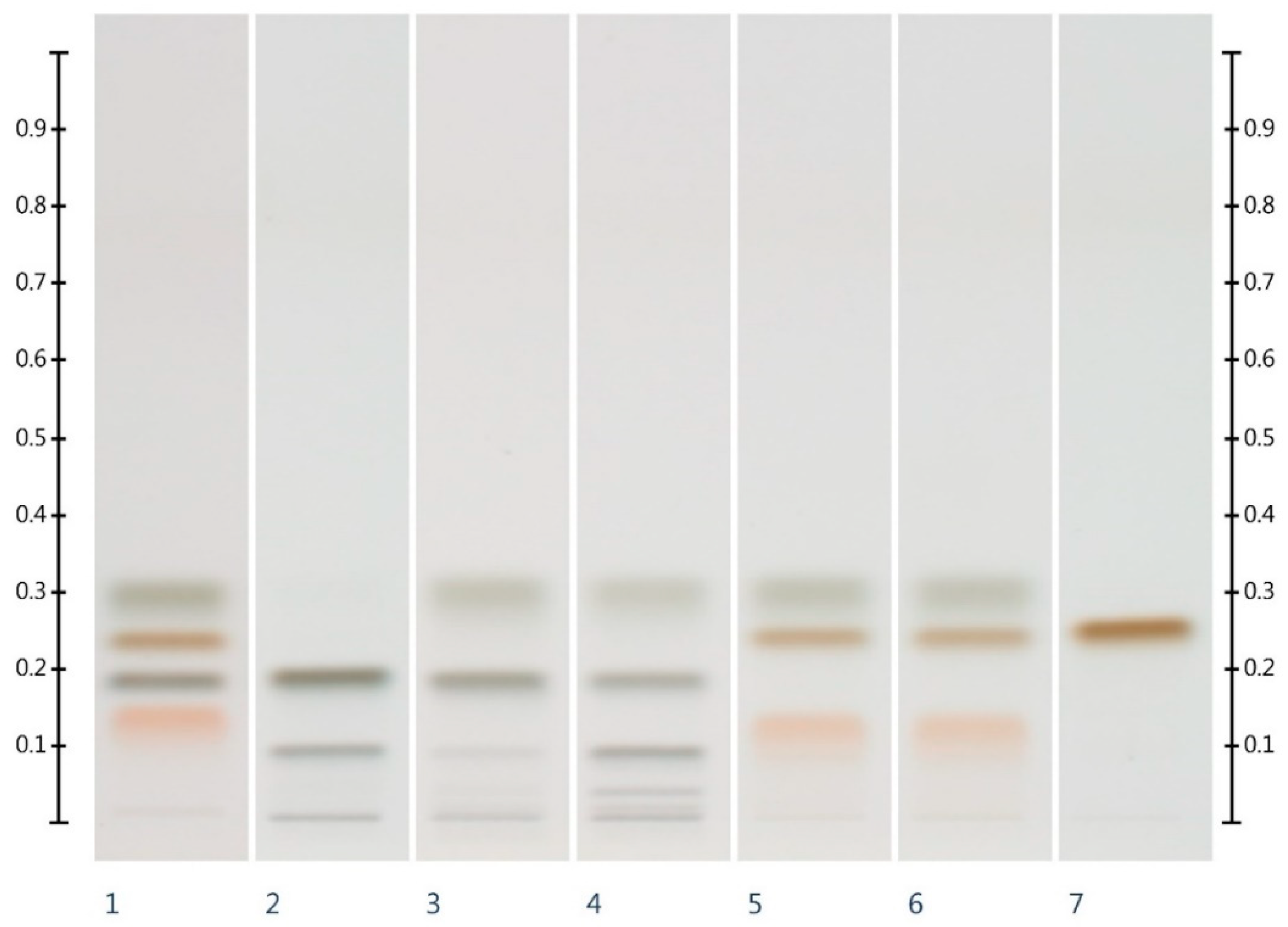

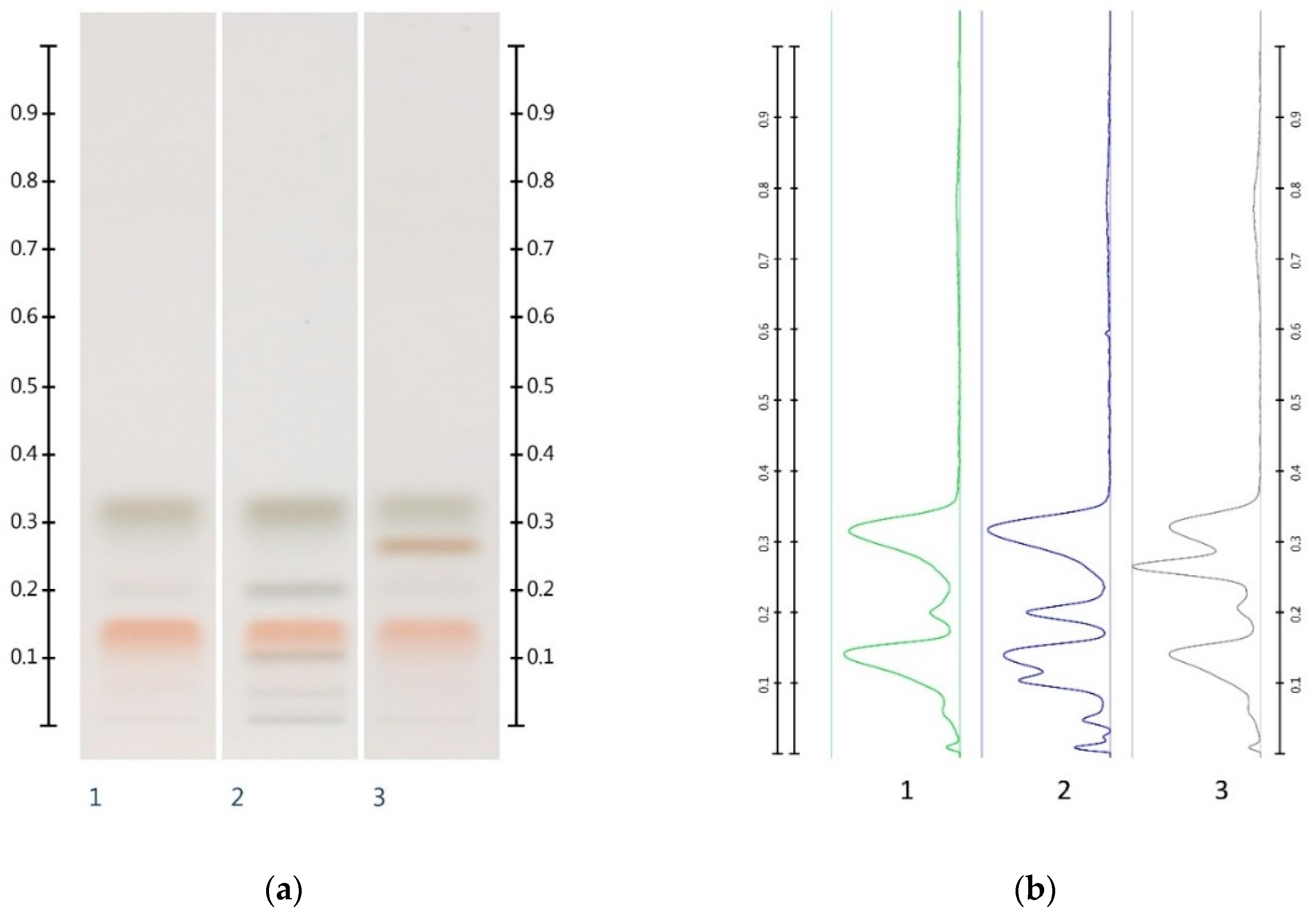
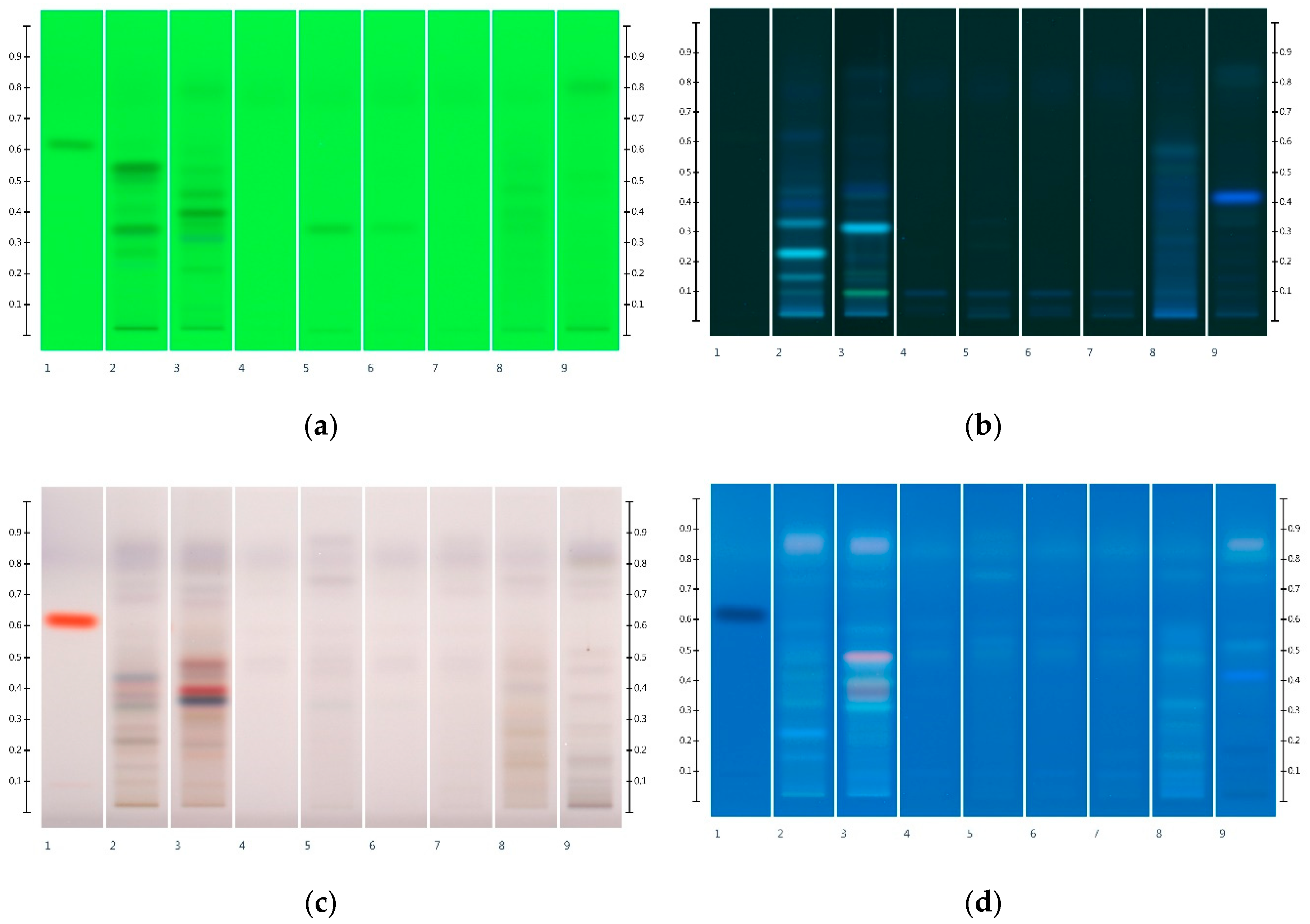
| Honey | ID | Fructose (mg/g) | Glucose (mg/g) | F:G Ratio |
|---|---|---|---|---|
| Manuka | MAN | 392 | 307 | 1.3 |
| Jarrah | JAR | 426 | 337 | 1.3 |
| Marri | MAR | 439 | 269 | 1.6 |
| Karri | KAR | 387 | 249 | 1.5 |
| Peppermint | PEP | 404 | 257 | 1.5 |
| White Gum | WHG | 415 | 323 | 1.3 |
| Syrup | ID | Glucose (mg/g) | Fructose (mg/g) | Sucrose (mg/g) | Maltose (mg/g) | Remarks |
|---|---|---|---|---|---|---|
| Corn | COR | - | - | - | 372 | Contains additional unidentified compounds |
| Rice | RIC | 250 | - | - | 242 | Contains additional unidentified compounds |
| Glucose | GLU | 178 | - | - | 148 | Contains additional unidentified compounds |
| Golden | GOL | 200 | 189 | 206 | - | - |
| Treacle | TRE | 191 | 185 | 204 | - | - |
| Maple | MAP | - | - | 486 | - | - |
| Samples | Glucose (mg/g) | Fructose (mg/g) | Sucrose (mg/g) | Maltose (mg/g) | F:G Ratio |
|---|---|---|---|---|---|
| MAN Corn 30% | 196 (−9%) | 185 (−33%) | - | 124 (+11%) | 0.9 |
| MAN Rice 30% | 281 (−3%) | 216 (−21%) | - | 90 (+24%) | 0.8 |
| MAN Glucose 30% | 348 (+ 30%) | 262 (−4%) | - | 79 (+79%) | 0.8 |
| MAN Golden 30% | 243 (−12%) | 332 (0%) | 68 (+11%) | - | 1.4 |
| MAN Treacle 30% | 262 (−4%) | 332 (+1%) | 67 (+10%) | - | 1.3 |
| MAN Maple 30% | 176 (−18%) | 269 (−2%) | 166 (+14%) | - | 1.5 |
| JAR Corn 30% | 204 (−14%) | 210 (−29%) | - | 123 (+10%) | 1.0 |
| JAR Rice 30% | 302 (−3%) | 234 (−21%) | - | 93 (+29%) | 0.8 |
| JAR Glucose 30% | 257 (−11%) | 206 (−31%) | - | 58 (+31%) | 0.8 |
| JAR Golden 30% | 242 (−18%) | 358 (+1%) | 72 (+17%) | - | 1.5 |
| JAR Treacle 30% | 234 (−20%) | 333 (−6%) | 77 (+25%) | - | 1.4 |
| JAR Maple 30% | 215 (−9%) | 338 (+13%) | 153 (+5%) | - | 1.6 |
| Type | Label and Packaging Information | Sample ID | |
|---|---|---|---|
| Honey | Manuka | Australian Manuka (MGO 514+) Barnes Naturals Pty Ltd. | MAN |
| Jarrah | Boyanup Jarrah Sweet As Apiary, WA | JAR | |
| Marri | Pooled Sample 1 (n = 12) | MAR | |
| Karri | Pooled Sample 1 (n = 9) | KAR | |
| Peppermint | Pooled Sample 1 (n = 4) | PEP | |
| White Gum | Pooled Sample 1 (n = 7) | WHG | |
| Syrups | Rice | Organic Rice Syrup Pureharvest, VIC | RIC |
| Corn | Corn Malt Syrup Korea Connections Ptl Ltd. | COR | |
| Golden | CSR Golden Syrup Sugar Australia Pty Ltd. | GOL | |
| Treacle | CSR Treacle Syrup Sugar Australia Pty Ltd. | TRE | |
| Glucose | Queen Glucose Syrup Queen Fine Foods Pty Ltd., QLD | GLU | |
| Maple | Queen Maple Syrup Dr. Oetker Queen Australia, QLD | MAP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.K.; Sostaric, T.; Lim, L.Y.; Hammer, K.; Locher, C. Sugar Profiling of Honeys for Authentication and Detection of Adulterants Using High-Performance Thin Layer Chromatography. Molecules 2020, 25, 5289. https://doi.org/10.3390/molecules25225289
Islam MK, Sostaric T, Lim LY, Hammer K, Locher C. Sugar Profiling of Honeys for Authentication and Detection of Adulterants Using High-Performance Thin Layer Chromatography. Molecules. 2020; 25(22):5289. https://doi.org/10.3390/molecules25225289
Chicago/Turabian StyleIslam, Md Khairul, Tomislav Sostaric, Lee Yong Lim, Katherine Hammer, and Cornelia Locher. 2020. "Sugar Profiling of Honeys for Authentication and Detection of Adulterants Using High-Performance Thin Layer Chromatography" Molecules 25, no. 22: 5289. https://doi.org/10.3390/molecules25225289
APA StyleIslam, M. K., Sostaric, T., Lim, L. Y., Hammer, K., & Locher, C. (2020). Sugar Profiling of Honeys for Authentication and Detection of Adulterants Using High-Performance Thin Layer Chromatography. Molecules, 25(22), 5289. https://doi.org/10.3390/molecules25225289








