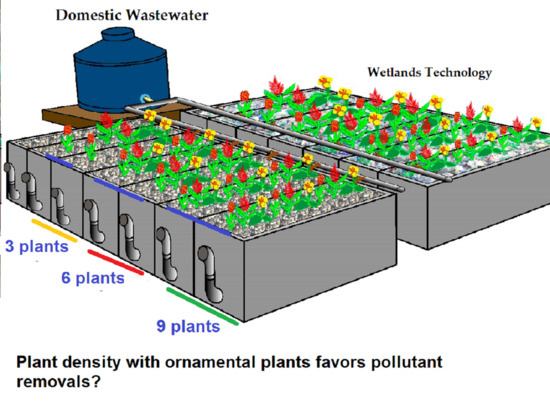
Effects of Ornamental Plant Density and Mineral/Plastic Media on the Removal of Domestic Wastewater Pollutants by Home Wetlands Technology
Abstract
1. Introduction
2. Results and Discussion
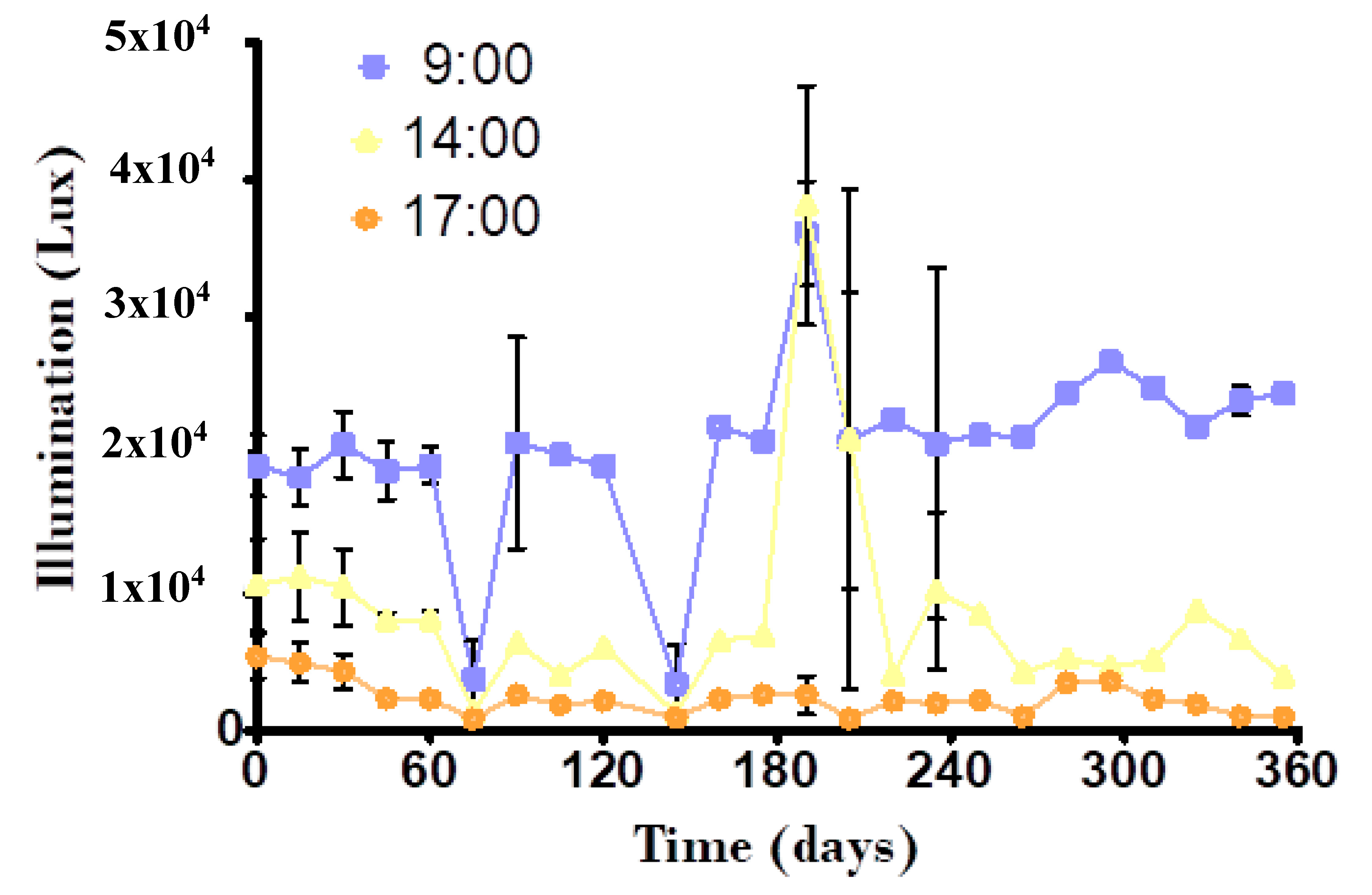
2.1. Control Parameters
2.2. Plant Growth and Flower Production
2.2.1. Alpina purpurata
2.2.2. Hedychium coronarium
2.2.3. Canna hybrids
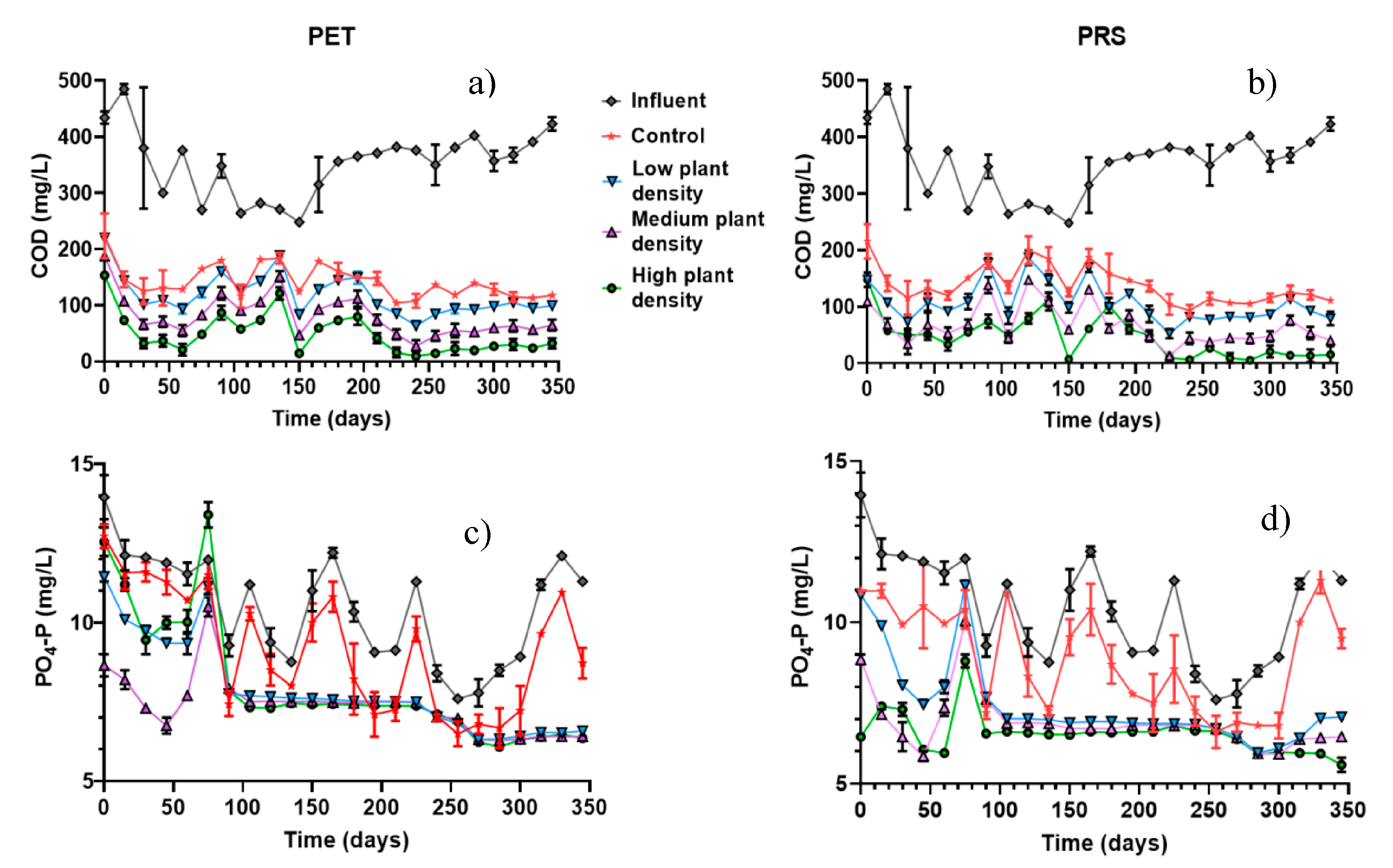
2.3. Contaminants Concentration in Influents and Effluents from CWs
3. Materials and Methods
3.1. Study Area
3.2. Constructed Wetlands (CWs) Systems’ Characteristics
3.3. Sampling and Analysis
3.4. Statistic Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villalobos, A.; Díaz, J.A. Calidad del Ambiente, Vulnerabilidad y Acciones Ante el Cambio Climático: Costa Rica en Perspectiva Comparada. Av. Hacia Política Econ. Sosten. Contexto Cambio Climático Costa Rica 2018, 3, 64. Available online: https://www.academia.edu/36802095/Calidad_del_ambiente_vulnerabilidad_y_acciones_ante_el_cambio_clim%C3%A1tico_Costa_Rica_en_perspectiva_comparada (accessed on 8 October 2020).
- Zhindón, R.; Cartuche, D.; España, P.; Maldonado, M. Evaluación Ambiental de Aguas Residuales: Estero y Manglar el Macho de la Ciudad de Machala. Conf. Proc. 2018, 2, 2. Available online: http://investigacion.utmachala.edu.ec/proceedings/index.php/utmach/article/view/277/226 (accessed on 10 July 2020).
- Zhang, D.; Jinadasa, K.; Gersberg, R.M.; Liu, Y.; Tan, S.K.; Ng, W.J. Application of constructed wetlands for wastewater treatment in tropical and subtropical regions (2000–2013). J. Environ. Sci. 2015, 30, 30–46. [Google Scholar] [CrossRef] [PubMed]
- Guven, H.; Dereli, R.K.; Ozgun, H.; Ersahin, M.E.; Ozturk, I. Towards sustainable and energy efficient municipal wastewater treatment by up-concentration of organics. Prog. Energy Combust. Sci. 2019, 70, 145–168. [Google Scholar] [CrossRef]
- Leiva, A.M.; Núñez, R.; Gómez, G.; López, D.; Vidal, G. Performance of ornamental plants in monoculture and polyculture horizontal subsurface flow constructed wetlands for treating wastewater. Ecol. Eng. 2018, 120, 116–125. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.Q.; Dong, J.W.; Tan, S.K. Constructed wetlands for wastewater treatment in cold climate—A review. J. Environ. Sci. 2017, 57, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lyu, T.; Zhang, Y.; Button, M.; Arias, C.A.; Weber, K.P.; Brix, H.; Carvalho, P.N. Impacts of design configuration and plants on the functionality of the microbial community of mesocosm-scale constructed wetlands treating ibuprofen. Water Res. 2018, 131, 228–238. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, J.; Ngo, H.H.; Guo, W.; Shuang, L.; Liang, S.; Fan, J.; Liu, H. A review on the sustainability of constructed wetlands for wastewater treatment: Design and operation. Bioresour. Technol. 2015, 175, 594–601. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, X.; Wang, J.; Pei, L. Impacts of different media on constructed wetlands for rural household sewage treatment. J. Clean. Prod. 2016, 127, 325–330. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L. Humedales construidos en México para el tratamiento de aguas residuales, producción de plantas ornamentales y reuso del agua. Agroproductividad 2017, 10, 5. Available online: http://revista-agroproductividad.org/index.php/agroproductividad/article/view/1028/879 (accessed on 15 June 2020).
- Sandoval-Herazo, L.C.; Alvarado-Lassman, A.; Marín-Muñiz, J.L.; Méndez-Contreras, J.M.; Zamora-Castro, S.A. Effects of the Use of Ornamental Plants and Different Substrates in the Removal of Wastewater Pollutants through Microcosms of Constructed Wetlands. Sustainability 2018, 10, 1594. [Google Scholar] [CrossRef]
- Marín-Muñiz, J.L.; García-González, M.C.; Ruelas-Monjardín, L.C.; Moreno-Casasola, P. Influence of Different Porous Media and Ornamental Vegetation on Wastewater Pollutant Removal in Vertical Subsurface Flow Wetland Microcosms. Environ. Eng. Sci. 2018, 35, 88–94. [Google Scholar] [CrossRef]
- Hernández-Vásquez, L.A.; Vallejo-Cantú, N.A.; Alvarado-Lassman, A.; Reyes-Rosas, S. Evaluación de la biodegradabilidad de un inóculo anaerobio pretratado térmicamente. Renew. Energy Biomass Sustain. 2019, 1, 65–71. Available online: https://aldeser.org/uploads/1/3/0/8/130818527/articulo_5_rb_s_vol1_no1.pdf (accessed on 12 May 2020).
- Zurita, F.; De Anda, J.; Belmont, M. Treatment of domestic wastewater and production of commercial flowers in vertical and horizontal subsurface-flow constructed wetlands. Ecol. Eng. 2009, 35, 861–869. [Google Scholar] [CrossRef]
- Hernández, M.E.A. Humedales ornamentales con participación comunitaria para el saneamiento de aguas municipales en México. Rinderesu 2016, 1, 1–12. Available online: http://www.rinderesu.com/index.php/rinderesu/article/view/16/32 (accessed on 20 May 2020).
- Wu, S.; Austin, D.; Liu, L.; Dong, R. Performance of integrated household constructed wetland for domestic wastewater treatment in rural areas. Ecol. Eng. 2011, 37, 948–954. [Google Scholar] [CrossRef]
- Hernández, M.E.; Galindo-Zetina, M.; Carlos, H.-H.J. Greenhouse gas emissions and pollutant removal in treatment wetlands with ornamental plants under subtropical conditions. Ecol. Eng. 2018, 114, 88–95. [Google Scholar] [CrossRef]
- Morvannou, A.; Forquet, N.; Vanclooster, M.; Molle, P. Characterizing hydraulic properties of filter material of a vertical flow constructed wetland. Ecol. Eng. 2013, 60, 325–335. [Google Scholar] [CrossRef]
- Wastewater engineering: Treatment, disposal and reuse. Adv. Water Resour. 1980, 3, 146. Available online: https://doi.org/10.1016/0309-1708(80)90067-6 (accessed on 8 October 2020). [CrossRef]
- Pech, O.M.S.; Ocaña, G.L. Tratamiento de aguas residuales mediante humedales artificiales. Kuxulkab 2014, 19, 36. Available online: http://revistas.ujat.mx/index.php/kuxulkab/article/view/337 (accessed on 1 June 2020).
- Díaz, F.J.; O′geen, A.T.; Dahlgren, R.A. Agricultural pollutant removal by constructed wetlands: Implications for water management and design. Agric. Water Manag. 2012, 104, 171–183. [Google Scholar] [CrossRef]
- Zurita, F.; White, J.R. Comparative Study of Three Two-Stage Hybrid Ecological Wastewater Treatment Systems for Producing High Nutrient, Reclaimed Water for Irrigation Reuse in Developing Countries. Water 2014, 6, 213–228. [Google Scholar] [CrossRef]
- He, K.; Lv, T.; Wu, S.; Guo, L.; Ajmal, Z.; Luo, H.; Dong, R. Treatment of Alkaline Stripped Effluent in Aerated Constructed Wetlands: Feasibility Evaluation and Performance Enhancement. Water 2016, 8, 386. [Google Scholar] [CrossRef]
- Sun, H.; Xu, S.; Wu, S.; Wang, R.; Zhuang, G.; Bai, Z.; Deng, Y.; Zhuang, X. Enhancement of facultative anaerobic denitrifying communities by oxygen release from roots of the macrophyte in constructed wetlands. J. Environ. Manag. 2019, 246, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Białowiec, A.; Sobieraj, K.; Pilarski, G.; Manczarski, P. The Oxygen Transfer Capacity of Submerged Plant Elodea densa in Wastewater Constructed Wetlands. Water 2019, 11, 575. [Google Scholar] [CrossRef]
- Lazcano, C.; Tsang, A.; Doane, T.A.; Pettygrove, G.S.; Horwáth, W.R.; Burger, M. Soil nitrous oxide emissions in forage systems fertilized with liquid dairy manure and inorganic fertilizers. Agric. Ecosyst. Environ. 2016, 225, 160–172. [Google Scholar] [CrossRef]
- USEPA. A Handbook of Constructed Wetlands. A Guide to Creating Wetlands for Agricultural Wastewater, Domestic Wastewater, Coal Mine Drainage, Stormwater in the MidAtlantic Region. General Considerations; United States Environmental Protection Agency: Washintong, DC, USA, 2000; Volume 1, p. 32. Available online: http://biblioteca.cehum.org/bitstream/CEHUM2018/1287/1/Davis.%20A%20Handbook%20of%20Constructed%20Wetlands%2C%20Volume%201%2C%20General%20Considerations.pdf (accessed on 2 June 2020).
- Méndez-Mendoza, A.S.; Bello-Mendoza, R.; Herrera-López, D.; Mejía-González, G.; Calixto-Romo, A. Performance of constructed wetlands with ornamental plants in the treatment of domestic wastewater under the tropical climate of South Mexico. Water Pr. Technol. 2015, 10, 110–123. [Google Scholar] [CrossRef]
- Randall, R.P. Un Compendio Global de Malas Hierbas; Departamento de Agricultura y Alimentación de Australia Occidental: Perth, Australia, 2012; p. 1124. Available online: https://www.cabdirect.org/cabdirect/abstract/20173071957 (accessed on 2 June 2020).
- Govaerts, R. Lista de Verificación Mundial de Zingiberaceae. Richmond, Londres: Royal Botanic Gardens, Kew 2013. Available online: https://www.cabdirect.org/cabdirect/abstract/20053205919 (accessed on 15 July 2020).
- Kress, W.J.; Liu, A.-Z.; Newman, M.; Li, Q. The molecular phylogeny of Alpinia (Zingiberaceae): A complex and polyphyletic genus of gingers. Am. J. Bot. 2005, 92, 167–178. [Google Scholar] [CrossRef]
- Ovando-Medina, I.; Adriano-Anaya, L.; Chávez-Aguilar, A.; Oliva-Llaven, A.; Ayora-Talavera, T.; Dendooven, L.; Gutiérrez-Miceli, F.; Salvador-Figueroa, M. Ex vitro Survival and Early Growth of Alpinia purpurata Plantlets Inoculated with Azotobacter and Azospirillum. Pak. J. Biol. Sci. 2007, 10, 3454–3457. [Google Scholar] [CrossRef]
- Baltazar-Bernal, O.; Zavala-Ruiz, J. Cultivo de Maracas (Zingiber spp.) en la Floricultura Tropical. AGROProductividad 2012, 5, 20–28. Available online: https://go.gale.com/ps/anonymous?id=GALE%7CA382318766&sid=googleScholar&v=2.1&it=r&linkaccess=fulltext&issn=&p=IFME&sw=w (accessed on 15 July 2020).
- Loges, V.; Teixeira, M.D.C.F.; De Castro, A.C.R.; Costa, A.S. Colheita, pós-colheita e embalagem de flores tropicais em Pernambuco. Hortic. Bras. 2005, 23, 699–702. [Google Scholar] [CrossRef][Green Version]
- Prince, L.M. Phylogenetic relationships and species delimitation in Canna (Cannaceae). Divers. Phylogeny Evol. Monocotyledons 2010, 307–331. Available online: https://www.academia.edu/download/33163564/Prince_2010.pdf (accessed on 15 July 2020).
- Guzmán, A.B.M.; Palenius, H.G.N. Multiplicar Plantas Ornamentales De Agave Victoriae-Reginae (T. Moore). Jóvenes Cienc. 2017, 2, 1429–1433. Available online: http://148.214.90.90/index.php/jovenesenlaciencia/article/view/1283 (accessed on 17 July 2020).
- Konnerup, D.; Brix, H. Nitrogen nutrition of Canna indica: Effects of ammonium versus nitrate on growth, biomass allocation, photosynthesis, nitrate reductase activity and N uptake rates. Aquat. Bot. 2010, 92, 142–148. [Google Scholar] [CrossRef]
- Gupta, A.; Maurya, R.; Roy, R.; Sawant, S.V.; Yadav, H.K. AFLP based genetic relationship and population structure analysis of Canna—An ornamental plant. Sci. Hortic. 2013, 154, 1–7. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Xu, Y.; Shutes, R.; Yan, B.; Zhou, Q. Effect of Aeration Modes and COD/N Ratios on Organic Matter and Nitrogen Removal in Horizontal Subsurface Flow Constructed Wetland Mesocosms. Water 2018, 10, 1530. [Google Scholar] [CrossRef]
- Abdalla, K.Z.; Hammam, G. Correlation between biochemical oxygen demand and chemical oxygen demand for various wastewater treatment plants in Egypt to obtain the biodegradability indices. Int. J. Sci. Basic Appl. Res. 2014, 13, 42–48. Available online: https://pdfs.semanticscholar.org/2629/9e0f5b734f1b1b14de684c79caa4d91f081b.pdf (accessed on 20 July 2020).
- Valente, J.P.S.; Padilha, P.M.; Silva, A.M.M. Oxigênio dissolvido (OD), demanda bioquímica de oxigênio (DBO) e demanda química de oxigênio (DQO) como parâmetros de poluição no ribeirão Lavapés/Botucatu - SP. Eclética Química J. 1997, 22, 49–66. [Google Scholar] [CrossRef]
- Glenn, E.P.; Flessa, K.W.; Pitt, J. Restoration potential of the aquatic ecosystems of the Colorado River Delta, Mexico: Introduction to special issue on “Wetlands of the Colorado River Delta ”. Ecol. Eng. 2013, 59, 1–6. [Google Scholar] [CrossRef]
- Gagnon, V.; Chazarenc, F.; Comeau, Y.; Brisson, J. Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci. Technol. 2007, 56, 249–254. [Google Scholar] [CrossRef]
- Casierra-Martínez, H.A.; Charris-Olmos, J.C.; Caselles-Osorio, A.; Parody-Muñoz, A. Organic Matter and Nutrients Removal in Tropical Constructed Wetlands Using Cyperus ligularis (Cyperaceae) and Echinocloa colona (Poaceae). Water Air Soil Pollut. 2017, 228, 338. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, J.; Hu, Z.; Ji, M.; Zhang, X.; Li, F.; Yao, G. Phosphorus removal enhancement of magnesium modified constructed wetland microcosm and its mechanism study. Chem. Eng. J. 2018, 335, 209–214. [Google Scholar] [CrossRef]
- Vymazal, J. Removal of nutrients in various types of constructed wetlands. Sci. Total. Environ. 2007, 380, 48–65. [Google Scholar] [CrossRef] [PubMed]
- Rugaika, A.M.; Van Deun, R.; Njau, K.N.; Van Der Bruggen, B. Phosphorus recovery as calcium phosphate by a pellet reactor pre-treating domestic wastewater before entering a constructed wetland. Int. J. Environ. Sci. Technol. 2019, 16, 3851–3860. [Google Scholar] [CrossRef]
- Torres Bojorges, Á.X.; Hernández Razo, N.A.; Fausto Urquieta, A.A.; Zurita, F. Evaluación de tres sistemas de humedales híbridos a escala piloto para la remoción de nitrógeno. Rev. Int. Contam. Ambient. 2017, 33, 37–47. [Google Scholar] [CrossRef]
- Han, W.; Luo, G.; Luo, B.; Yu, C.; Wang, H.; Chang, J.; Ge, Y. Effects of plant diversity on greenhouse gas emissions in microcosms simulating vertical constructed wetlands with high ammonium loading. J. Environ. Sci. 2019, 77, 229–237. [Google Scholar] [CrossRef]
- Shiwei, C.; Zhaoqian, J.; Peng, Y.; Yue, W.; Yin, W.; Wang, Y.; Wang, Y. Performance of constructed wetlands with different substrates for the treated effluent from municipal sewage plants. J. Water Reuse Desalination 2019, 9, 452–462. [Google Scholar] [CrossRef]
- Correa-Torres, S.N.; Gamarra, Y.; Salazar, A.A.; Pitta, N.M. Evaluación de la Remoción de Nitrógeno, Fósforo y Sulfuros en Agua Residual Doméstica, Utilizando Phragmites australis en Bioreactores. Inf. Tecnológica 2015, 26, 89–98. [Google Scholar] [CrossRef][Green Version]
- Lee, C.-G.; Fletcher, T.D.; Sun, G. Nitrogen removal in constructed wetland systems. Eng. Life Sci. 2009, 9, 11–22. [Google Scholar] [CrossRef]
- Kujala, K.; Karlsson, T.; Nieminen, S.; Ronkanen, A.-K. Design parameters for nitrogen removal by constructed wetlands treating mine waters and municipal wastewater under Nordic conditions. Sci. Total. Environ. 2019, 662, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Zamora, S.; Marin-Muñiz, J.L.; Sandoval, L.; Vidal-Álvarez, M.; Carrión-Delgado, J.M. Effect of Ornamental Plants, Seasonality, and Filter Media Material in Fill-and-Drain Constructed Wetlands Treating Rural Community Wastewater. Sustainability 2019, 11, 2350. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]




| Wetlands Plants in Different Substrates | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Parameters | Low Density PET | Medium Density PET | High Density PET | Low Density PRS | Medium Density PRS | High Density PSR | Control PET | Control PRS | |
| Water temperature (°C) | |||||||||
| Influent | 26.45 ± 0.29 | ||||||||
| Effluent | 24.62 ± 0.22 | 25.13 ± 0.20 | 24.83 ± 0.21 | 24.92 ± 0.21 | 25.105 ± 0.22 | 25.01 ± 0.23 | 24.88 ± 0.27 | 24.70 ± 0.24 | |
| pH | |||||||||
| Influent | 6.80 ± 0.47 | ||||||||
| Effluent | 7.72 ± 0.43 | 7.70 ± 0.37 | 7.65 ± 0.41 | 7.69 ± 0.35 | 7.60 ± 0.42 | 7.63 ± 0.36 | 7.89 ± 0.32 | 7.76 ± 0.38 | |
| EC | (µS/cm) | ||||||||
| Influent | 1601.33 ± 291.15 | ||||||||
| Effluent | 1116.63 ± 41.39 | 1128.71 ± 38.66 | 1028.23 ± 47.33 | 1079.62 ± 45.18 | 1014.46 ± 40.32 | 1190.70 ± 178.96 | 1041.62 ± 58.85 | 1064.25 ± 56.67 | |
| DO (mg/L) | |||||||||
| Influent | 1.7 ± 0.42 | ||||||||
| Effluent | 3.6 ± 0.51 | 3.9 ± 0.26 | 6.7 ± 0.13 | 3.4 ± 0.60 | 4.1 ± 0.34 | 5.8 ± 0.72 | 2.4 ± 0.54 | 2.1 ± 0.21 | |
| TDS (mg/L) | |||||||||
| Influent | 670.96 ± 14.79 | ||||||||
| Effluent | 529.08 ± 15.13 | 507.77 ± 16.39 | 505.11 ± 19.59 | 480.61 ± 18.66 | 496.01 ± 20.23 | 458.71 ± 18.13 | 585.01 ± 13.89 | 595.63 ± 11.75 | |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandoval-Herazo, L.C.; Alvarado-Lassman, A.; López-Méndez, M.C.; Martínez-Sibaja, A.; Aguilar-Lasserre, A.A.; Zamora-Castro, S.; Marín-Muñiz, J.L. Effects of Ornamental Plant Density and Mineral/Plastic Media on the Removal of Domestic Wastewater Pollutants by Home Wetlands Technology. Molecules 2020, 25, 5273. https://doi.org/10.3390/molecules25225273
Sandoval-Herazo LC, Alvarado-Lassman A, López-Méndez MC, Martínez-Sibaja A, Aguilar-Lasserre AA, Zamora-Castro S, Marín-Muñiz JL. Effects of Ornamental Plant Density and Mineral/Plastic Media on the Removal of Domestic Wastewater Pollutants by Home Wetlands Technology. Molecules. 2020; 25(22):5273. https://doi.org/10.3390/molecules25225273
Chicago/Turabian StyleSandoval-Herazo, Luis Carlos, Alejandro Alvarado-Lassman, María Cristina López-Méndez, Albino Martínez-Sibaja, Alberto A. Aguilar-Lasserre, Sergio Zamora-Castro, and José Luis Marín-Muñiz. 2020. "Effects of Ornamental Plant Density and Mineral/Plastic Media on the Removal of Domestic Wastewater Pollutants by Home Wetlands Technology" Molecules 25, no. 22: 5273. https://doi.org/10.3390/molecules25225273
APA StyleSandoval-Herazo, L. C., Alvarado-Lassman, A., López-Méndez, M. C., Martínez-Sibaja, A., Aguilar-Lasserre, A. A., Zamora-Castro, S., & Marín-Muñiz, J. L. (2020). Effects of Ornamental Plant Density and Mineral/Plastic Media on the Removal of Domestic Wastewater Pollutants by Home Wetlands Technology. Molecules, 25(22), 5273. https://doi.org/10.3390/molecules25225273