Combining EXAFS and Computer Simulations to Refine the Structural Description of Actinyls in Water
Abstract
1. Introduction
2. Computational Methods
2.1. Quantum Chemical Calculations
2.2. Molecular Dynamics Simulations
2.3. Simulated XAS Spectra
3. Results and Discussion
3.1. Simulated EXAFS Spectrum Based on A NEVPT2 Force Field for NpO2+ in Aqueous Solution
3.2. Simulated EXAFS Spectrum Based on NEVPT2 Optimized Geometries
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choppin, G.R.; Jensen, M.P. Actinides in Solution: Complexation and Kinetics. In The Chemistry of the Actinide and Transactinide Elements, 3rd ed.; Morss, L.R., Edelstein, N., Fuger, J., Katz, J.J., Eds.; Springer: Dordrecht, The Netherlands, 2008; Volume 4, Chapter 23; pp. 2524–2621. [Google Scholar]
- Cotton, S. Lanthanide & Actinide Chemistry; Wiley: London, UK, 2006. [Google Scholar]
- Bardin, N.; Rubini, P.; Madic, C. Hydration of Actinyl(VI), aq (M = U, Np, Pu). An NMR Study. Radiochim. Acta 1998, 83, 189–194. [Google Scholar] [CrossRef]
- Aaberg, M.; Ferri, D.; Glaser, J.; Grenthe, I. Structure of the hydrated dioxouranium (VI) ion in aqueous solution. An x-ray diffraction and proton NMR study. Inorg. Chem. 1983, 22, 3986–3989. [Google Scholar] [CrossRef]
- Allen, P.G.; Bucher, J.J.; Shuh, D.K.; Edelstein, N.M.; Reich, T. Investigation of aquo and chloro complexes of , , Np4+, and Pu3+ by X-ray absorption fine structure spectroscopy. Inorg. Chem. 1997, 36, 4676–4683. [Google Scholar] [CrossRef] [PubMed]
- Conradson, S.D. Application of X-ray absorption fine structure spectroscopy to materials and environmental science. Appl. Spectrosc. 1998, 52, 252A–279A. [Google Scholar] [CrossRef]
- Wahlgren, U.; Moll, H.; Grenthe, I.; Schimmelpfennig, B.; Maron, L.; Vallet, V.; Gropen, O. Structure of Uranium(VI) in Strong Alkaline Solutions. A Combined Theoretical and Experimental Investigation. J. Phys. Chem. A 1999, 103, 8257–8264. [Google Scholar] [CrossRef]
- Neuefeind, J.; Soderholm, L.; Skanthakumar, S. Experimental Coordination Environment of Uranyl(VI) in Aqueous Solution. J. Phys. Chem. A 2004, 108, 2733–2739. [Google Scholar] [CrossRef]
- Soderholm, L.; Skanthakumar, S.; Neuefeind, J. Determination of actinide speciation in solution using high-energy X-ray scattering. Anal. Bioanal. Chem. 2005, 383, 48–55. [Google Scholar] [CrossRef]
- McKibben, J.M. Chemistry of the Purex Process. Radiochim. Acta 1984, 36, 3–15. [Google Scholar] [CrossRef]
- Galbis, E.; Hernández-Cobos, J.; den Auwer, C.; Le Naour, C.; Guillaumont, D.; Simoni, E.; Pappalardo, R.R.; Sánchez Marcos, E. Solving the hydration structure of the heaviest actinide aqua ion known: The californium (III) case. Angew. Chem. Int. Ed. Engl. 2010, 122, 3899–3903. [Google Scholar] [CrossRef]
- Altmaier, M.; Gaona, X.; Fanghänel, T. Recent Advances in Aqueous Actinide Chemistry and Thermodynamics. Chem. Rev. 2013, 113, 901–943. [Google Scholar] [CrossRef]
- Denecke, M.A. Actinide speciation using X-ray absorption fine structure spectroscopy. Coord. Chem. Rev. 2006, 250, 730–754. [Google Scholar] [CrossRef]
- Atta-Fyn, R.; Bylaska, E.J.; Schenter, G.K.; de Jong, W.A. Hydration Shell Structure and Dynamics of Curium(III) in Aqueous Solution: First Principles and Empirical Studies. J. Phys. Chem. A 2011, 115, 4665–4677. [Google Scholar] [CrossRef] [PubMed]
- D’Angelo, P.; Martelli, F.; Spezia, R.; Filipponi, A.; Denecke, M.A. Hydration properties and ionic radii of actinide (III) ions in aqueous solution. Inorg. Chem. 2013, 52, 10318–10324. [Google Scholar]
- Pérez-Conesa, S.; Torrico, F.; Martínez, J.M.; Pappalardo, R.R.; Sánchez Marcos, E. A hydrated ion model of [UO2]2+ in water: Structure, dynamics, and spectroscopy from classical molecular dynamics. J. Chem. Phys. 2016, 145, 224502. [Google Scholar] [CrossRef]
- Spezia, R.; Migliorati, V.; D’Angelo, P. On the Development of Polarizable and Lennard-Jones Force Fields to Study Hydration Structure and Dynamics of Actinide(III) Ions Based on Effective Ionic Radii. J. Chem. Phys. 2017, 147, 161707. [Google Scholar] [CrossRef]
- Palmer, B.J.; Pfund, D.M.; Fulton, J.L. Direct modeling of EXAFS spectra from molecular dynamics simulations. J. Phys. Chem. 1996, 100, 13393–13398. [Google Scholar] [CrossRef]
- Campbell, L.; Rehr, J.; Schenter, G.; McCarthy, M.; Dixon, D. XAFS Debye-Waller factors in aqueous Cr3+ from molecular dynamics. J. Synchrotron. Rad. 1999, 6, 310–312. [Google Scholar] [CrossRef]
- Chilemi, G.; D’Angelo, P.; Pavel, N.V.; Sanna, N.; Barone, V. Development and validation of an intergrated computational approach for the study of ionic species in solution by means of effective two-body potentials. The case case of Zn2+, Ni2+ and Co2+ aqueous solutions. J. Am. Chem. Soc. 2002, 124, 1968–1976. [Google Scholar] [CrossRef]
- Merkling, P.J.; Adela Muñoz-Páez, A.; Sánchez Marcos, E. Exploring the Capabilities of X-ray Absorption Spectroscopy for Determining the Structure of Electrolyte Solutions: Computed Spectra for Cr3+ or Rh3+ in Water Based on Molecular Dynamics. J. Am. Chem. Soc. 2002, 124, 10911–10920. [Google Scholar] [CrossRef]
- Martínez, J.M.; Pappalardo, R.R.; Sánchez Marcos, E. First-Principles Ion-Water Interaction Potentials for Highly Charged Monatomic Cations. Computer Simulations of Al3+, Mg2+, and Be2+ in Water. J. Am. Chem. Soc. 1999, 121, 3175–3184. [Google Scholar] [CrossRef]
- Pérez-Conesa, S.; Torrico, F.; Martínez, J.M.; Pappalardo, R.R.; Sánchez Marcos, E. A general study of actinyl hydration by molecular dynamics simulations using ab initio force fields. J. Chem. Phys. 2019, 150, 104504. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Conesa, S.; Martínez, J.M.; Pappalardo, R.R.; Sánchez Marcos, E. Extracting the Americyl Hydration from an Americium Cationic Mixture in Solution: A Combined X-ray Absorption Spectroscopy and Molecular Dynamics Study. Inorg. Chem. 2018, 57, 8089–8097. [Google Scholar] [CrossRef] [PubMed]
- Riddle, C.; Czerwinski, K.; Kim, E.; Paviet, P.; Weck, P.; Poineau, F.; Conradson, S. Characterization of pentavalent and hexavalent americium complexes in nitric acid using X-ray absorption fine structure spectroscopy and first-principles modeling. J. Radioanal. Nucl. 2016, 309, 1087–1095. [Google Scholar] [CrossRef]
- Nichols, P.; Bylaska, E.J.; Schenter, G.K.; de Jong, W. Equatorial and apical solvent shells of the ion. J. Chem. Phys. 2008, 128, 124507. [Google Scholar] [CrossRef]
- Duvail, M.; Dumas, T.; Paquet, A.; Coste, A.; Berthon, L.; Guilbaud, P. structure in solvent extraction phases resolved at molecular and supramolecular scales: A combined molecular dynamics, EXAFS and SWAXS approach. Phys. Chem. Chem. Phys. 2019, 21, 7894–7906. [Google Scholar] [CrossRef]
- Sukhbaatar, T.; Duvail, M.; Dumas, T.; Dourdain, S.; Arrachart, G.; Solari, P.L.; Guilbaud, P.; Pellet-Rostainga, S. Probing the existence of uranyl trisulfate structures in the AMEX solvent extraction process. Chem. Commun. 2019, 55, 7583–7586. [Google Scholar] [CrossRef]
- den Auwer, C.; Drot, R.; Simoni, E.; Conradson, S.D.; Gailhanou, M.; Mustre de Leon, J. Grazing incidence XAFS spectroscopy of uranyl sorbed onto TiO2 rutile surfaces. New J. Chem. 2003, 27, 648–655. [Google Scholar] [CrossRef]
- Hennig, C.; Schmeide, K.; Brendler, V.; Moll, H.; Tsushima, S.; Scheinost, A.C. EXAFS investigation of U (VI), U (IV), and Th (IV) sulfato complexes in aqueous solution. Inorg. Chem. 2007, 46, 5882–5892. [Google Scholar] [CrossRef]
- Reich, T.; Bernhard, G.; Geipel, G.; Funke, H.; Hennig, C.; Roßberg, A.; Matz, W.; Schell, N.; Nitsche, H. The Rossendorf Beam Line ROBL—A dedicated experimental station for XAFS measurements of actinides and other radionuclides. Radiochim. Acta 2000, 88, 633–637. [Google Scholar] [CrossRef]
- Ikeda-Ohno, A.; Hennig, C.; Rossberg, A.; Funke, H.; Scheinost, A.C.; Bernhard, G.; Yaita, T. Electrochemical and complexation behavior of neptunium in aqueous perchlorate and nitrate solutions. Inorg. Chem. 2008, 47, 8294–8305. [Google Scholar] [CrossRef]
- Reich, T.; Geipel, G.; Funke, H.; Hennig, C.; Roßberg, A.; Bernhard, G. Plutonium. XAFS Measurements of Plutonium Hydrates; ESRF Highlights (Biannual Report 1999/2000); Project-Group ESRF-Beamline: Dresden, Germany, 2001; pp. 27–32. [Google Scholar]
- Rai, N.; Tiwari, S.P.; Maginn, E.J. Force field development for actinyl ions via quantum mechanical calculations: An approach to account for many body solvation effects. J. Phys. Chem. B 2012, 116, 10885–10897. [Google Scholar] [CrossRef] [PubMed]
- Pomogaev, V.; Tiwari, S.P.; Rai, N.; Goff, G.S.; Runde, W.; Schneider, W.F.; Maginn, E.J. Development and application of effective pairwise potentials for, , , and (n= 1, 2) ions with water. Phys. Chem. Chem. Phys. 2013, 15, 15954–15963. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Evangelisti, S.; Leininger, T.; Malrieu, J.P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 2001, 114, 10252–10264. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.P. N-electron valence state perturbation theory: A fast implementation of the strongly contracted variant. Chem. Phys. Lett. 2001, 350, 297–305. [Google Scholar] [CrossRef]
- Angeli, C.; Cimiraglia, R.; Malrieu, J.P. n-electron valence state perturbation theory: A spinless formulation and an efficient implementation of the strongly contracted and of the partially contracted variants. J. Chem. Phys. 2002, 117, 9138–9153. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H., Jr. Gaussian basis sets for use in correlated molecular calculations. III. The atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Küchle, W.; Dolg, M.; Stoll, H.; Preuss, J. Energy-adjusted pseudopotentials for the actinides. Parameter sets and test calculations for thorium and thorium monoxide. J. Chem. Phys. 1994, 100, 7535–7542. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09 Revision D.01; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Siboulet, B.; Marsden, C.J.; Vitorge, P. A theoretical study of uranyl solvation: Explicit modelling of the second hydration sphere by quantum mechanical methods. Chem. Phys. 2006, 326, 289–296. [Google Scholar] [CrossRef]
- Vallet, V.; Macak, P.; Wahlgren, U.; Grenthe, I. Actinide chemistry in solution, quantum chemical methods and models. Theor. Chem. Acc. 2006, 115, 145–160. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Cao, Z. Theoretical Studies on Structures of Neptunyl Carbonates: NpO2(CO3)m(H2 (m= 1–3, n= 0–3) in Aqueous Solution. Inorg. Chem. 2007, 46, 10510–10519. [Google Scholar] [CrossRef] [PubMed]
- Clavaguéra-Sarrio, C.; Vallet, V.; Maynau, D.; Marsden, C.J. Can density functional methods be used for open-shell actinide molecules? Comparison with multiconfigurational spin-orbit studies. J. Chem. Phys. 2004, 121, 5312–5321. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, D.; Balasubramanian, K.; Nitsche, H. A comparative theoretical study of bonding in , , UO2, , OUCO, O2U(CO)2 and UO2CO3. Chem. Phys. Lett. 2002, 361, 143–151. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Zheng, J.; Xu, X.; Truhlar, D. Minimally augmented Karlsruhe basis sets. Theor. Chem. Acc. 2011, 128, 295–305. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Cao, X.; Dolg, M.; Stoll, H. Valence basis sets for relativistic energy-consistent small-core actinide pseudopotentials. J. Chem. Phys. 2003, 118, 487–496. [Google Scholar] [CrossRef]
- Todorov, I.T.; Smith, W.; Trachenko, K.; Dove, M.T. DL_POLY_3: New dimensions in molecular dynamics simulations via massive parallelism. J. Mat. Chem. 2006, 16, 1911–1918. [Google Scholar] [CrossRef]
- Muñoz-Páez, A.; Sánchez Marcos, E. Molecular Structure of Solvates and Coordination Complexes in Solution as Determined with {EXAFS} and {XANES}. In Comprehensive Inorganic Chemistry II: From Elements to Applications, 2nd ed.; Reedijk, J., Poeppelmeier, K., Eds.; Elsevier: Leiden, The Netherlands, 2013; Volume 9, pp. 133–159. [Google Scholar]
- Rehr, J.J.; Kas, J.J.; Vila, F.D.; Prange, M.P.; Jorissen, K. Parameter-free calculations of X-ray spectra with FEFF9. Phys. Chem. Chem. Phys. 2010, 12, 5503–5513. [Google Scholar] [CrossRef]
- Hudson, E.; Rehr, J.; Bucher, J.J. A hydrated ion model of [UO2]2+ in water: Structure, dynamics, and spectroscopy from classical molecular dynamics. Phys. Rev. B 1995, 52, 13815–13826. [Google Scholar] [CrossRef] [PubMed]
- Infante, I.; Severo Pereira Gomes, A.; Vissche, L. On the performance of the intermediate Hamiltonian Fock-space coupled-cluster method on linear triatomic molecules: The electronic spectra of , , . J. Chem. Phys. 2006, 125, 074301. [Google Scholar] [CrossRef] [PubMed]
- Infante, I.; Kovacs, A.; La Macchia, G.; Shahi, A.R.M.; Gibson, J.K.; Gagliardi, L. Ionization Energies for the Actinide Mono- and Dioxides Series, from Th to Cm: Theory versus Experiment. J. Phys. Chem. A 2010, 114, 6007–6015. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Glendening, E.D.; Peterson, K.A. Actinyl cation–cation interactions in the gas phase: An accurate thermochemical study. Phys. Chem. Chem. Phys. 2019, 21, 7953–7964. [Google Scholar] [CrossRef]
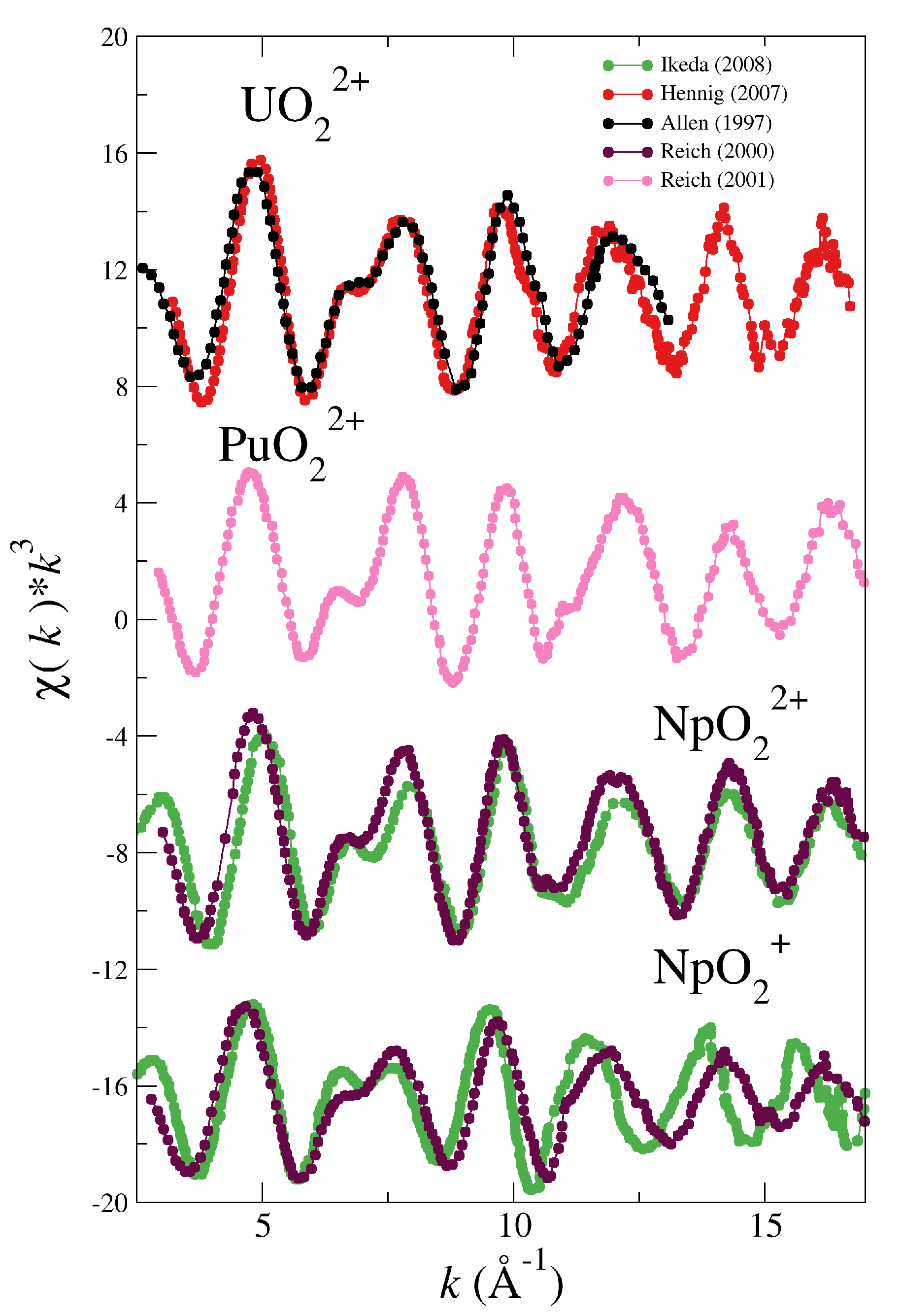
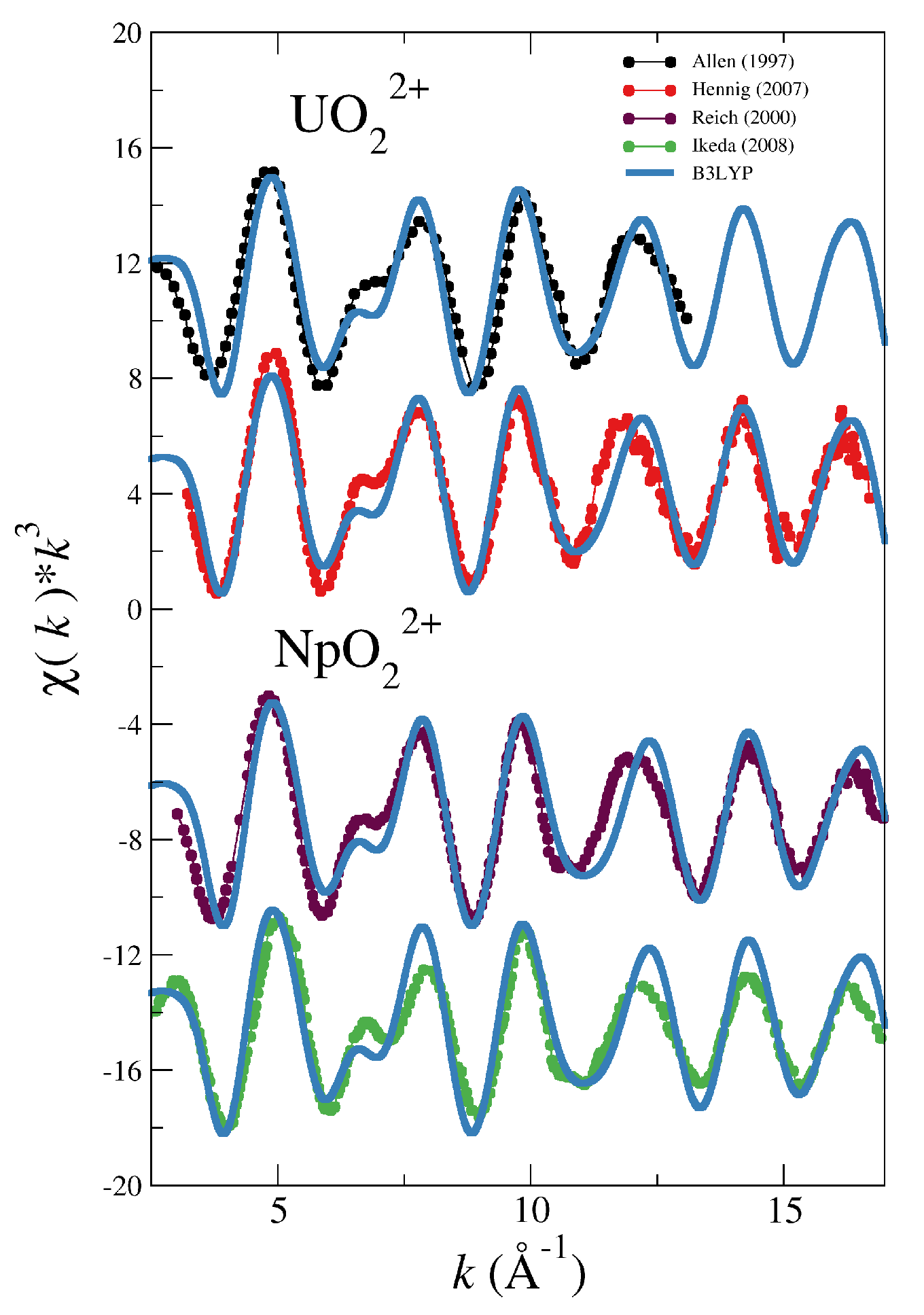
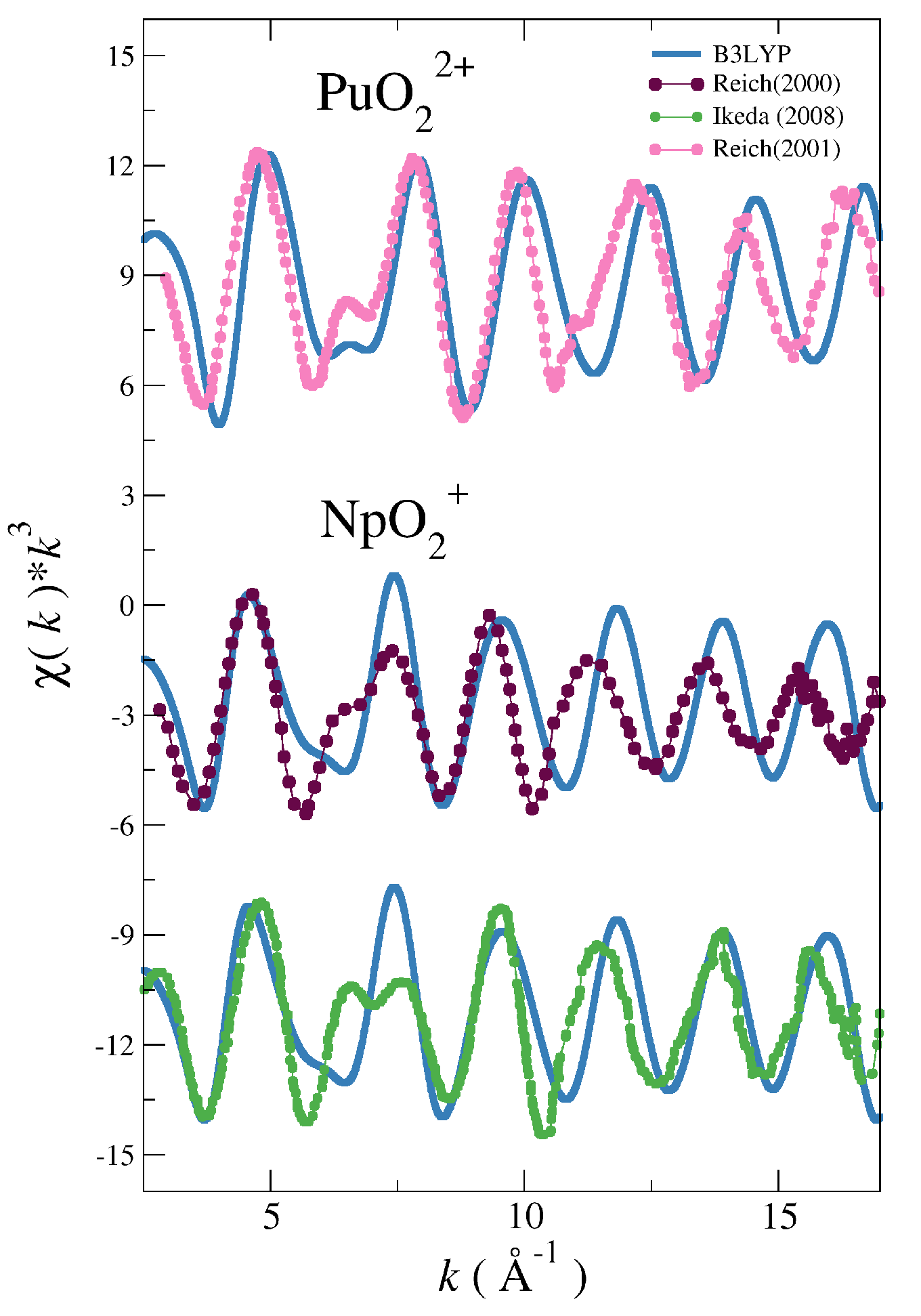

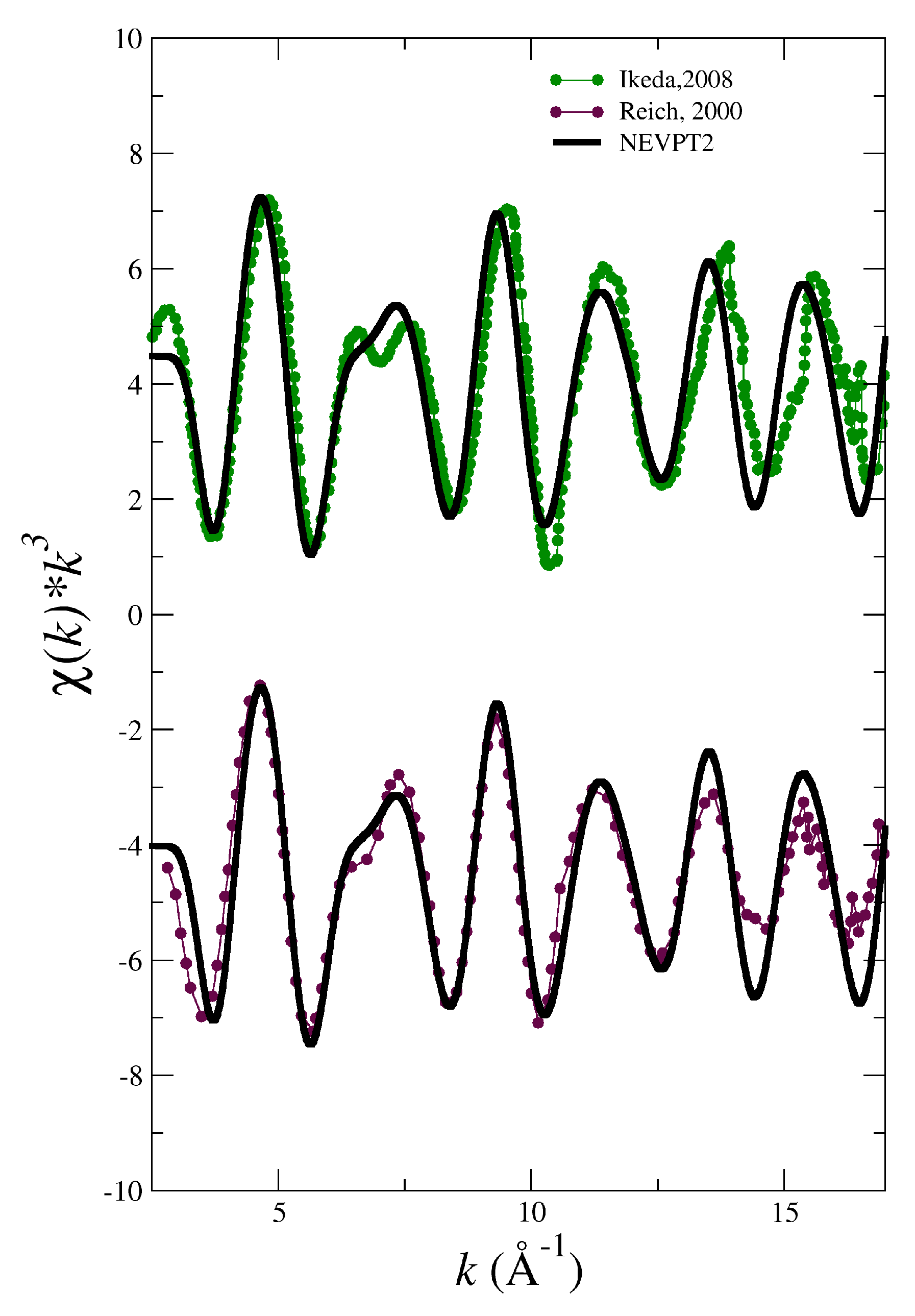
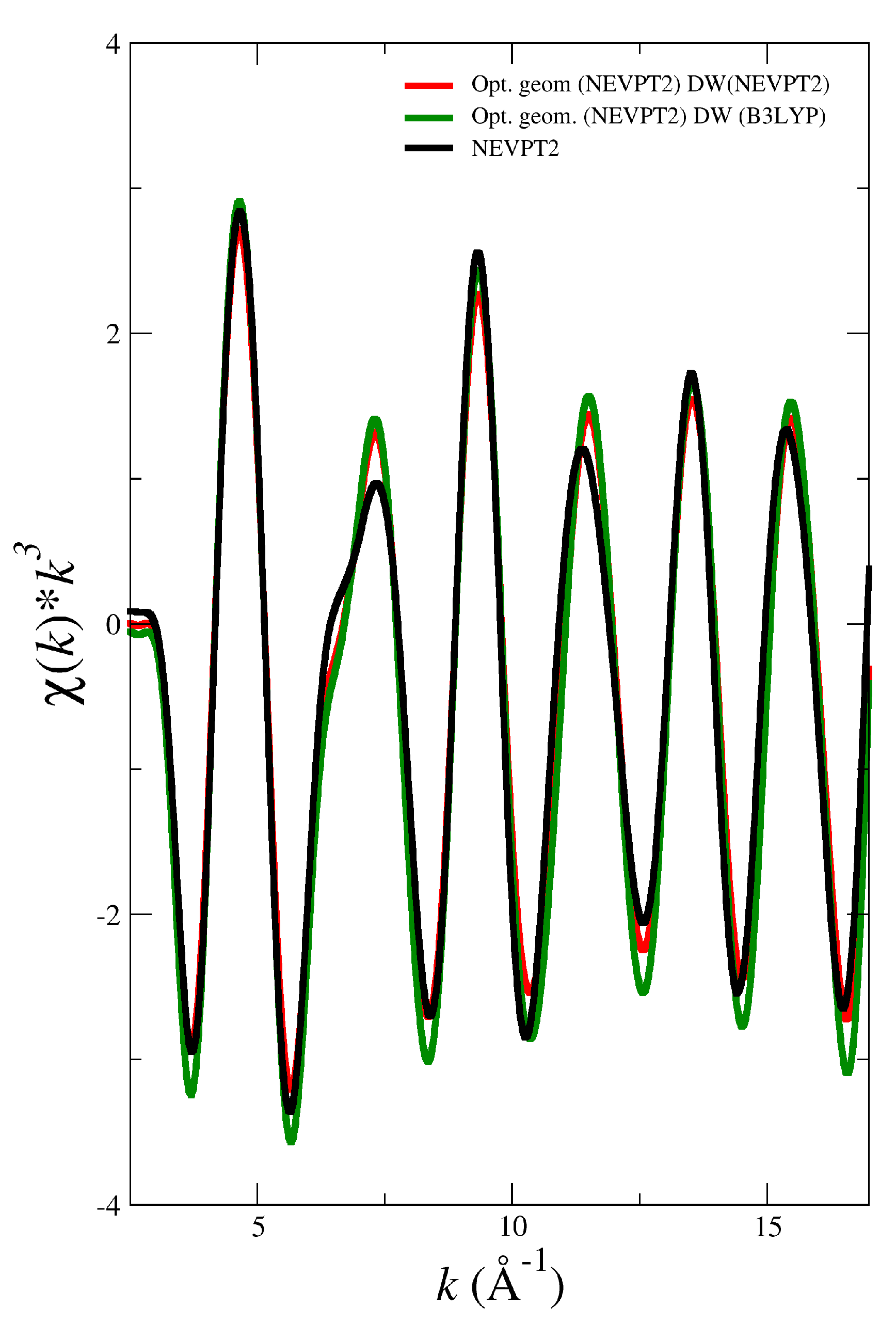
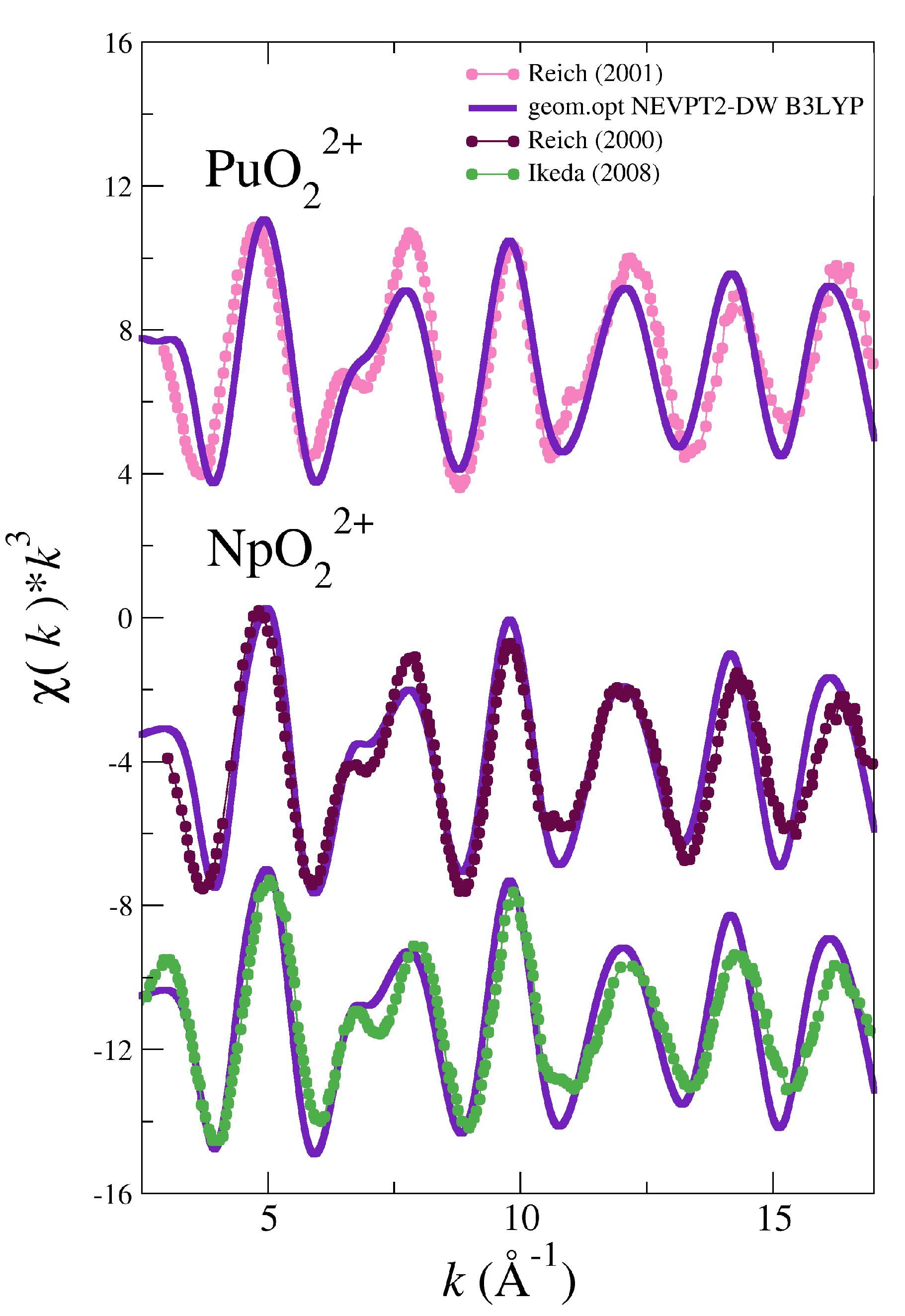
| System | |||||
|---|---|---|---|---|---|
| [UO2]2+(aq) | |||||
| Quantum Mechanics | |||||
| B3LYP-opt | 1.74 | 2.49 | |||
| MP2-opt | 1.77 | 2.50 | |||
| Force Field | |||||
| B3LYPPOT-opt | 1.76 | 2.49 | |||
| B3LYP-MD | 1.76 | 0.0004 | 2.48 | 0.007 | |
| Experimental | |||||
| Hennig 2007 [30] | 1.76 ± 0.02 | 0.002 | 2.41 ± 0.02 | 0.007 | |
| Allen 1997 [5] | 1.76 ± 0.01 | 0.002 | 2.41 ± 0.01 | 0.007 | |
| [NpO2]2+(aq) | |||||
| Quantum Mechanics | |||||
| B3LYP-opt | 1.73 | 2.48 | |||
| NEVPT2-opt | 1.77 | 2.42 | |||
| Force Field | |||||
| B3LYPPOT-opt | 1.74 | 2.47 | |||
| B3LYP-MD | 1.74 | 0.0004 | 2.46 | 0.007 | |
| Experimental | |||||
| Ikeda 2008 [32] | 1.76 ± 0.01 | 0.002 | 2.42 ± 0.01 | 0.006 | |
| Reich 2000 [31] | 1.754 ± 0.003 | 0.002 | 2.414 ± 0.006 | 0.006 | |
| [NpO2]+(aq) | |||||
| Quantum Mechanics | |||||
| B3LYP-opt | 1.78 | 2.59 | |||
| NEVPT2-opt | 1.83 | 2.52 | |||
| Force Field | |||||
| B3LYPPOT-opt | 1.78 | 2.59 | |||
| NEVPOT-opt | 1.83 | 2.52 | |||
| B3LYP-MD | 1.79 | 0.0007 | 2.61 | 0.012 | |
| NEVPT2-MD | 1.84 | 0.0007 | 2.54 | 0.011 | |
| Experimental | |||||
| Ikeda 2008 [32] | 1.84 ± 0.01 | 0.002 | 2.49 ± 0.01 | 0.007 | |
| Reich 2000 [31] | 1.822 ± 0.003 | 0.002 | 2.488 ± 0.009 | 0.006 | |
| [PuO2]2+(aq) | |||||
| Quantum Mechanics | |||||
| B3LYP-opt | 1.71 | 2.46 | |||
| NEVPT2-opt | 1.76 | 2.43 | |||
| Force Field | |||||
| B3LYPPOT-opt | 1.71 | 2.47 | |||
| B3LYP-MD | 1.71 | 0.0006 | 2.45 | 0.009 | |
| Experimental | |||||
| Reich 2001 [33] | 1.74 ± 0.01 | 0.001 | 2.42 ± 0.01 | 0.005 |
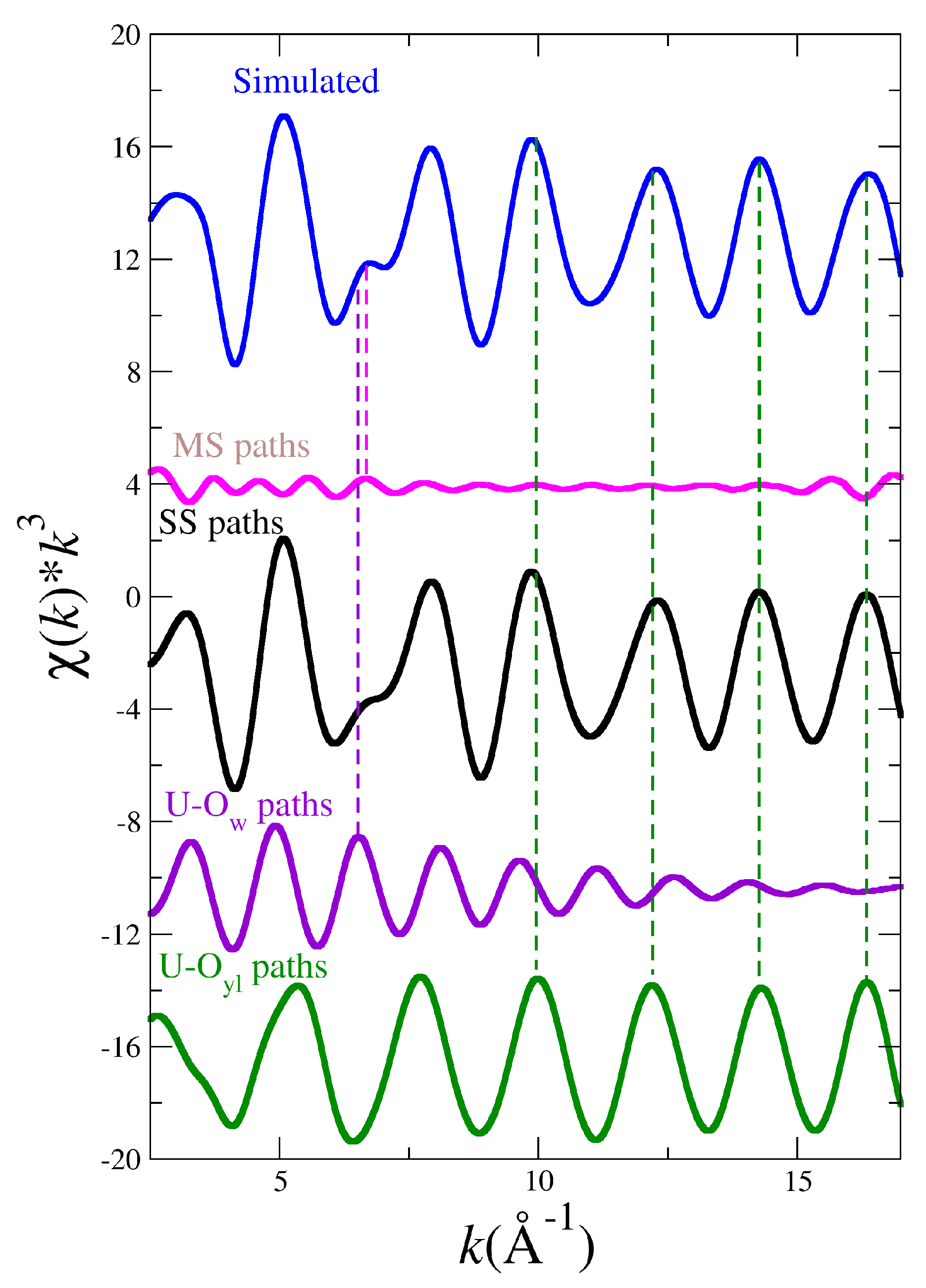
| (AnO) × 10 (MD Simulation) | ||||||
|---|---|---|---|---|---|---|
| Path Index | Path Type | NpO2+ (B3LYP) | NpO2+ (NEVPT2) | NpO22+ (B3LYP) | UO22+ (B3LYP) | PuO22+ (B3LYP) |
| 1 | O-An | 0.70 | 0.73 | 0.39 | 0.59 | 0.41 |
| 2 | O-An | 10.8 | 10.5 | 6.29 | 9.05 | 7.15 |
| 3 | O-O-An | 1.31 | 1.57 | 0.74 | 1.36 | 0.89 |
| 4 | O-An-O-An | 2.79 | 2.91 | 1.54 | 2.35 | 1.64 |
| 5 | O-An-O-An | 1.33 | 1.54 | 0.74 | 1.35 | 0.86 |
| 9 | O-An-O-An | 15.8 | 14.9 | 9.53 | 12.1 | 9.45 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Conesa, S.; Martínez, J.M.; Pappalardo, R.R.; Marcos, E.S. Combining EXAFS and Computer Simulations to Refine the Structural Description of Actinyls in Water. Molecules 2020, 25, 5250. https://doi.org/10.3390/molecules25225250
Pérez-Conesa S, Martínez JM, Pappalardo RR, Marcos ES. Combining EXAFS and Computer Simulations to Refine the Structural Description of Actinyls in Water. Molecules. 2020; 25(22):5250. https://doi.org/10.3390/molecules25225250
Chicago/Turabian StylePérez-Conesa, Sergio, José M. Martínez, Rafael R. Pappalardo, and Enrique Sánchez Marcos. 2020. "Combining EXAFS and Computer Simulations to Refine the Structural Description of Actinyls in Water" Molecules 25, no. 22: 5250. https://doi.org/10.3390/molecules25225250
APA StylePérez-Conesa, S., Martínez, J. M., Pappalardo, R. R., & Marcos, E. S. (2020). Combining EXAFS and Computer Simulations to Refine the Structural Description of Actinyls in Water. Molecules, 25(22), 5250. https://doi.org/10.3390/molecules25225250






