Photocatalytic Oxidation of HMF under Solar Irradiation: Coupling of Microemulsion and Lyophilization to Obtain Innovative TiO2-Based Materials
Abstract
1. Introduction
2. Results
2.1. Catalyst Characterization
2.1.1. Characterization of Commercial and Microemulsion-Synthesized TiO2
2.1.2. Characterization of SiO2-TiO2-m Mixed Oxides
2.1.3. Characterization of Ti15Si85 SFD and TiO2-m SFD Supported Au3Cu1 Nanoparticles
2.2. Photocatalytic Tests
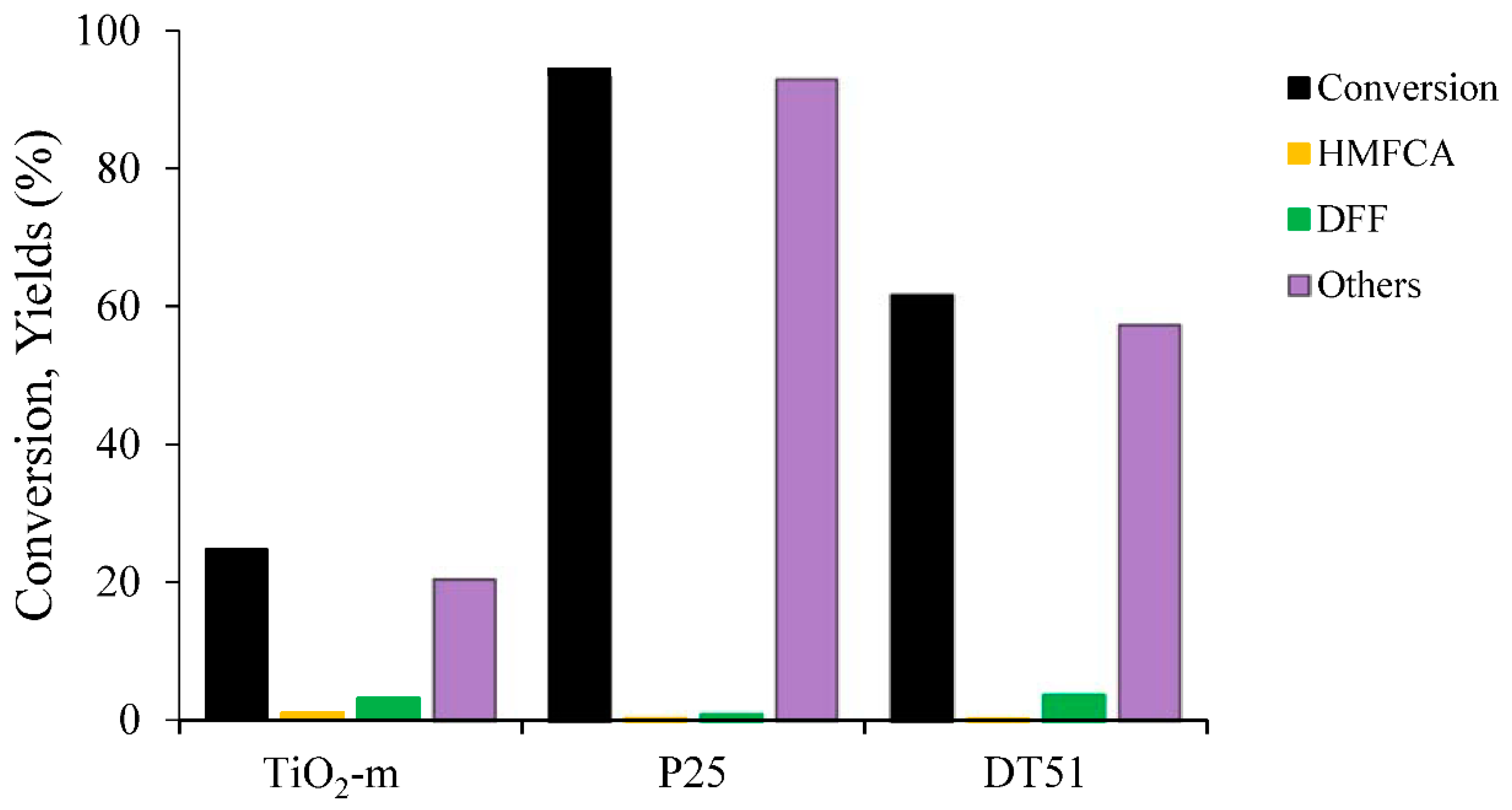
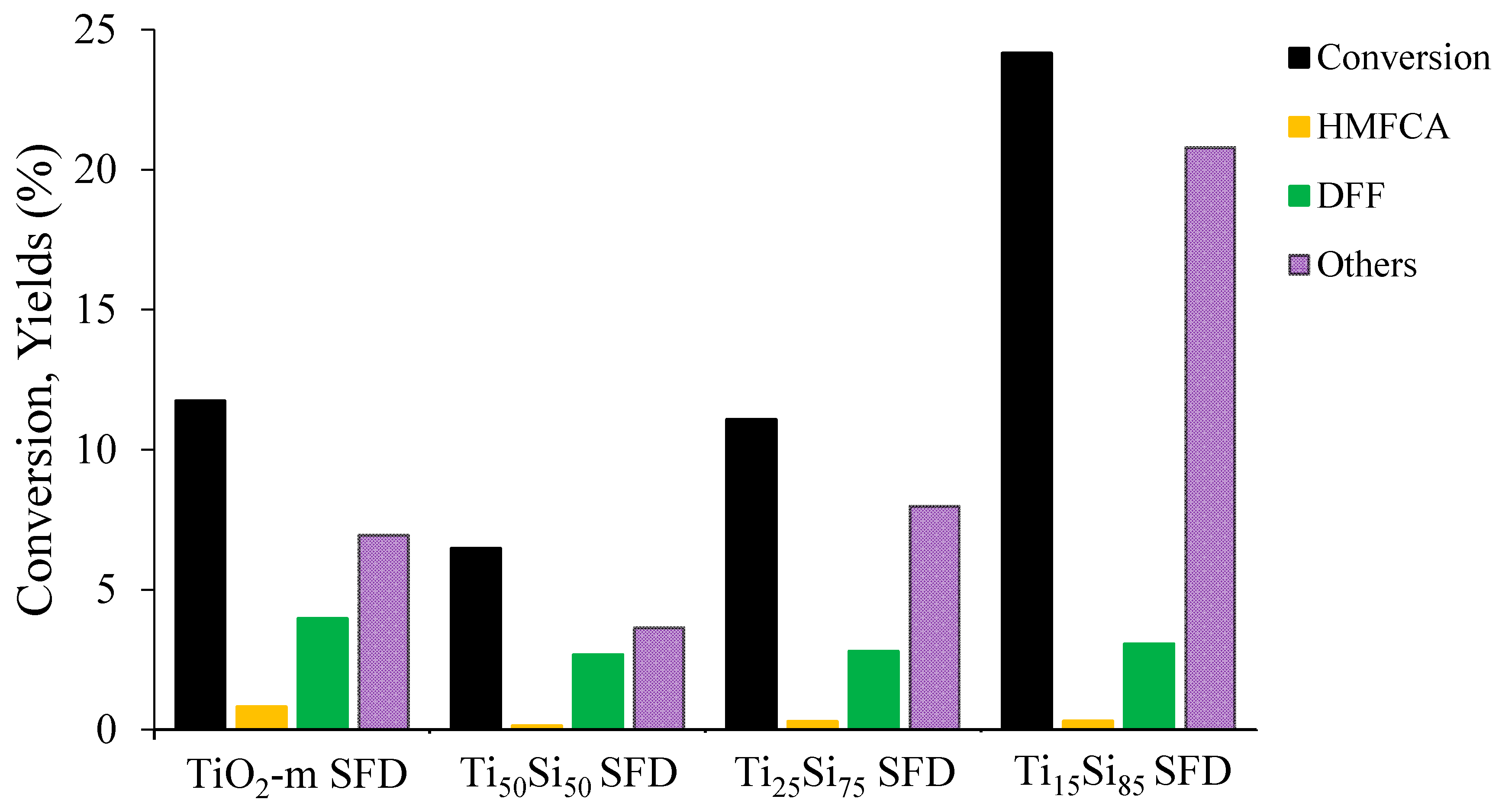
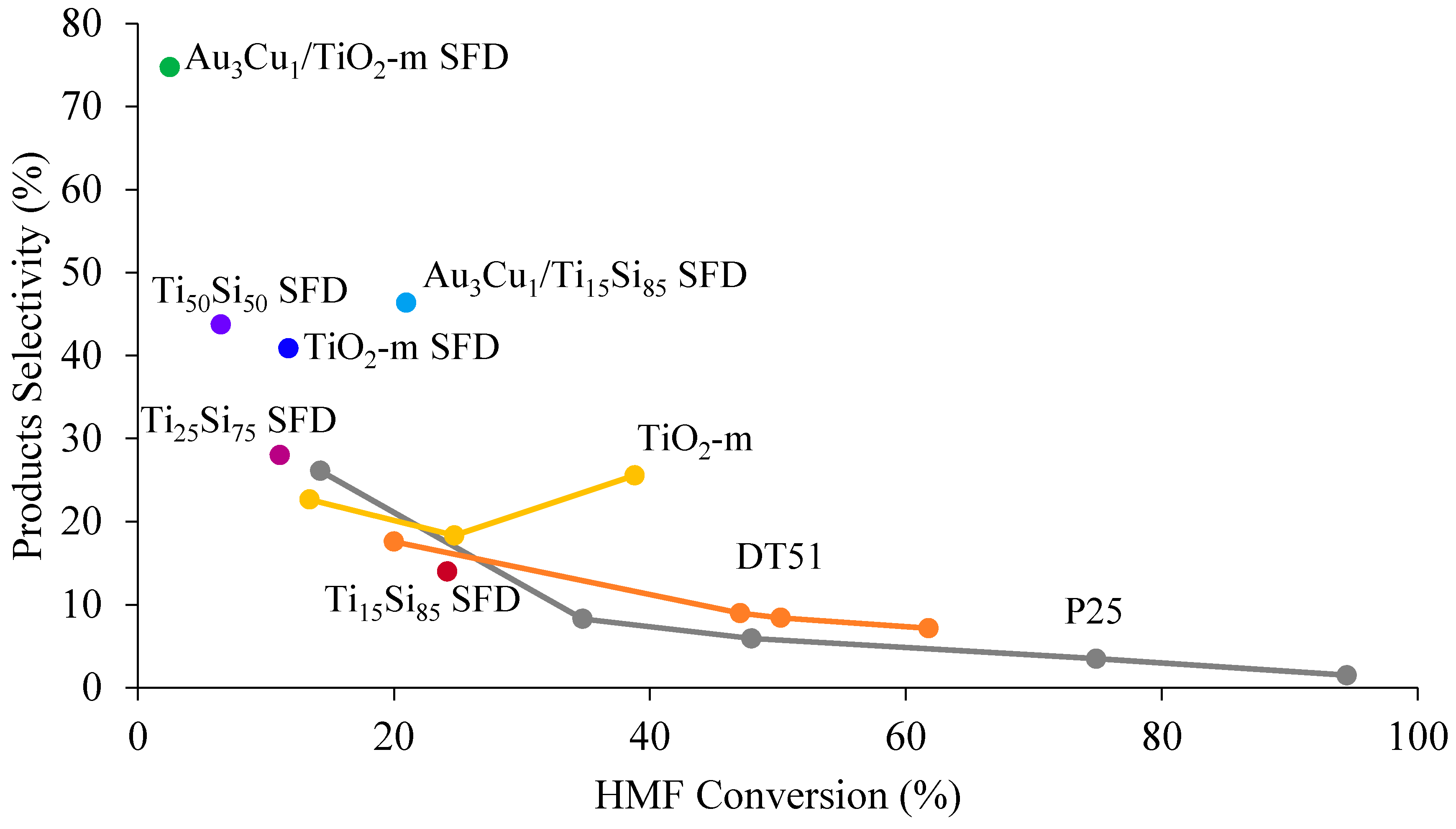
2.2.1. HMF Photo-Oxidation Reactions
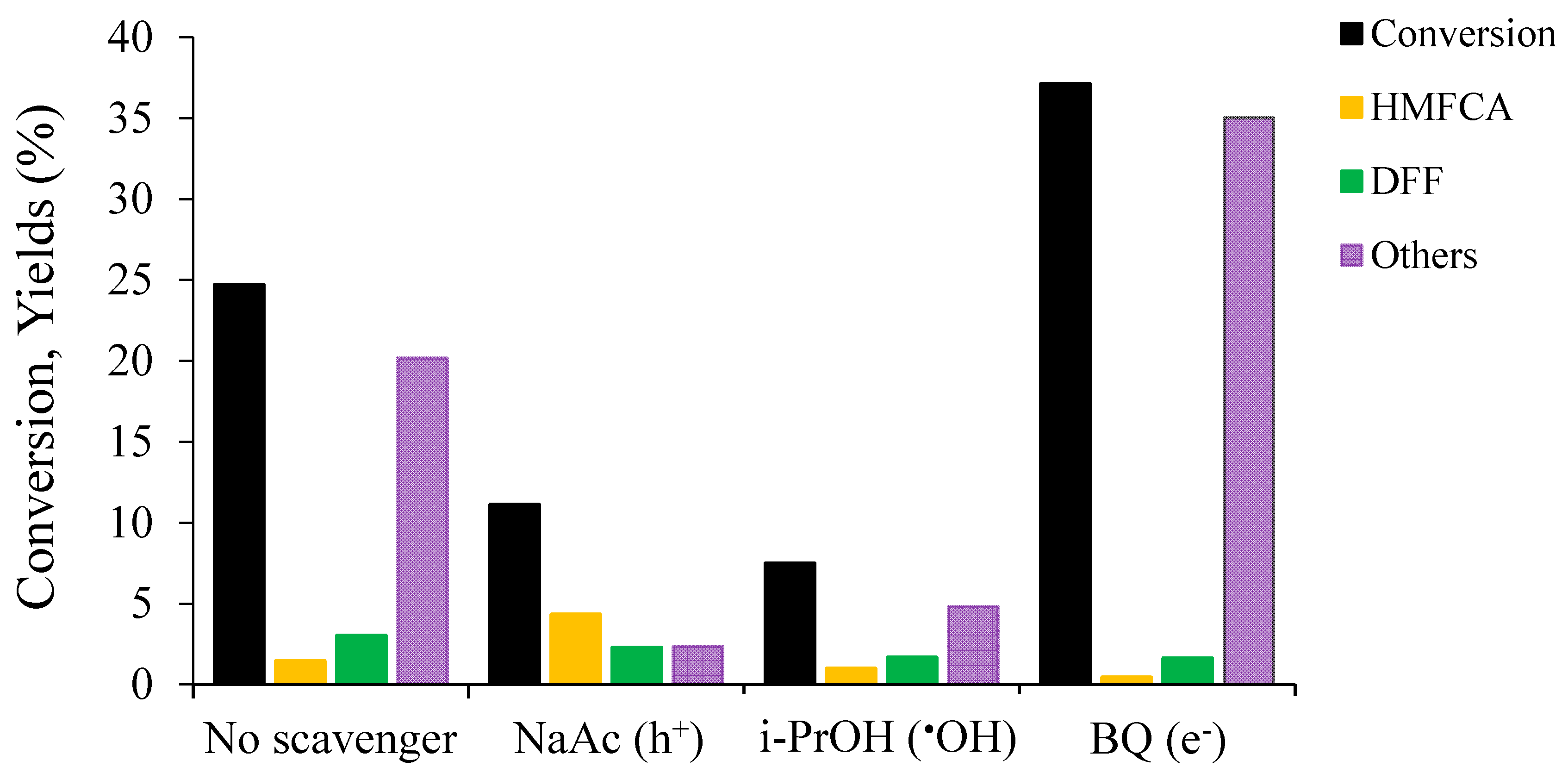
2.2.2. Study of the Mechanism by Tests with Radical Scavengers
3. Materials and Methods
- (a)
- P25 (AEROXIDE TiO2 P25, Evonik, Essen, Germany) and DT51 (TiO2 CrystalACTiV DT51, TRONOX, Stamford, CT, USA) were used as commercially available references as received.
- (b)
- Titanium(IV) butoxide (97%, Merck, Darmstadt, Germany), Triton X-100 (Alfa Aesar, Ward Hill, MA, USA), 1-hexanol (99%, Alfa Aesar, Ward Hill, MA, USA), Cyclohexane (99%, Alfa Aesar, Ward Hill, MA, USA), Ethanol (99.8%, Merck, Darmstadt, Germany), and HNO3 (65%, Merck, Darmstadt, Germany) were used for the synthesis of titania by reverse microemulsion method.
- (c)
- SiO2 LUDOX HS-40 (40 wt%, W. R. Grace and Company, Columbia, MD, USA) for silica-titania nanocomposites prepared by spray-freeze-drying method in different ratios.
- (d)
- HAuCl4 (99.995%, Merck, Darmstadt, Germany), CuSO4·H2O (99.5%, Merck, Darmstadt, Germany), Polivinilpirrolidone (PVP 25K, Merck, Darmstadt, Germany), NaOH (99%, Merck, Darmstadt, Germany), and β-D-glucose (99.9%, Merck, Darmstadt, Germany) were used for the Au3Cu1 nanoparticles preparation,
- (e)
- 5-hydroxymethyl furfural (HMF 99%, AVA Biochem, Muttenz, Switzerland), 2,5-diformylfurane (DFF 99.99%, Toronto Reserch Chemicals, North York, ON, Canada), 5-hydroxymethyl-2-furancarboxylic acid (HMFCA 99.99%, Toronto Reserch Chemicals, North York, ON, Canada), and 5-formyl-2-furancarboxylic acid (FFCA 99.99%, Toronto Reserch Chemicals, North York, ON, Canada) were used as substrate (in case of HMF) and external standards for HPLC calibrations.
- (f)
- Sodium acetate (99%, Merck, Darmstadt, Germany), 2-propanol (99.5%, Merck, Darmstadt, Germany), and p-benzoquinone (98%, Merck, Darmstadt, Germany) were used as radical scavengers.
- (g)
- All of the above were used without further purification.
3.1. Catalyst Preparation
3.1.1. Preparation of TiO2-m
3.1.2. Synthesis of Au3Cu1 Nanoparticles
3.1.3. Preparation of TiSi Titania-Silica Nanocomposites by Spray-Freeze-Drying (SFD)
3.2. Catalyst Characterisation
3.3. Photocatalytic Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mika, L.T.; Csefalvay, E.; Nemeth, A. Catalytic Conversion of Carbohydrates to Initial Platform Chemicals: Chemistry and Sustainability. Chem. Rev. 2018, 118, 505–613. [Google Scholar] [CrossRef]
- Bicker, M.; Kaiser, D.; Ott, L.; Vogel, H. Dehydration of d -fructose to hydroxymethylfurfural in sub- and supercritical fluids. J. Supercrit. Fluids 2005, 36, 118–126. [Google Scholar] [CrossRef]
- Harlin, A. Biogenic Precursors for Polyphenol, Polyester and Polyurethane Resins. In Handbook of Bioplastics and Biocomposites Engineering Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 511–553. ISBN 9781118203699. [Google Scholar]
- Albonetti, S.; Lolli, A.; Morandi, V.; Migliori, A.; Lucarelli, C.; Cavani, F. Conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over Au-based catalysts: Optimization of active phase and metal-support interaction. Appl. Catal. B Environ. 2015, 163, 520–530. [Google Scholar] [CrossRef]
- Artz, J.; Mallmann, S.; Palkovits, R. Selective Aerobic Oxidation of HMF to 2,5-Diformylfuran on Covalent Triazine Frameworks-Supported Ru Catalysts. ChemSusChem 2015, 8, 672–679. [Google Scholar] [CrossRef] [PubMed]
- de Vries, J.G. Chapter Eight—Green Syntheses of Heterocycles of Industrial Importance. 5-Hydroxymethylfurfural as a Platform Chemical. In Heterocyclic Chemistry in the 21st Century; Scriven, E.F.V., Ramsden, C.A.B.T.-A., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 121, pp. 247–293. ISBN 0065-2725. [Google Scholar]
- Pasini, T.; Piccinini, M.; Blosi, M.; Bonelli, R.; Albonetti, S.; Dimitratos, N.; Lopez-Sanchez, J.A.J.; Sankar, M.; He, Q.; Kiely, C.C.J.; et al. Selective oxidation of 5-hydroxymethyl-2-furfural using supported gold-copper nanoparticles. Green Chem. 2011, 13, 2091–2099. [Google Scholar] [CrossRef]
- Tuteja, J.; Choudhary, H.; Nishimura, S.; Ebitani, K. Direct synthesis of 1,6-hexanediol from HMF over a heterogeneous Pd/ZrP catalyst using formic acid as hydrogen source. ChemSusChem 2014, 7, 96–100. [Google Scholar] [CrossRef]
- European Bioplastics, Nova-Institute. 2019. Available online: https://docs.european-bioplastics.org/publications/EUBP_Facts_and_figures.pdf (accessed on 5 September 2020).
- Hussain Motagamwala, A.; Won, W.; Sener, C.; Alonso, D.M.; Maravelias, C.T.; Dumesic, J.A. Toward biomass-derived renewable plastics: Production of 2,5-furandicarboxylic acid from fructose. Sci. Adv. 2018, 4, 1–8. [Google Scholar] [CrossRef]
- Wojcieszak, R.; Ferraz, C.P.; Sha, J.; Houda, S.; Rossi, L.M.; Paul, S. Advances in base-free oxidation of bio-based compounds on supported gold catalysts. Catalysts 2017, 7, 352. [Google Scholar] [CrossRef]
- Lolli, A.; Albonetti, S.; Utili, L.; Amadori, R.; Ospitali, F.; Lucarelli, C.; Cavani, F. Insights into the reaction mechanism for 5-hydroxymethylfurfural oxidation to FDCA on bimetallic Pd-Au nanoparticles. Appl. Catal. A 2015, 504, 408–419. [Google Scholar] [CrossRef]
- Albonetti, S.; Pasini, T.; Lolli, A.; Blosi, M.; Piccinini, M.; Dimitratos, N.; Lopez-sanchez, J.A.; Morgan, D.J.; Carley, A.F.; Hutchings, G.J.; et al. Selective oxidation of 5-hydroxymethyl-2-furfural over TiO2-supported gold—Copper catalysts prepared from preformed nanoparticles: Effect of Au/Cu ratio. Catal. Today 2012, 195, 120–126. [Google Scholar] [CrossRef]
- Lolli, A.; Amadori, R.; Lucarelli, C.; Cutrufello, M.G.; Rombi, E.; Cavani, F.; Albonetti, S. Hardlate preparation of Au/CeO2 mesostructured catalysts and their activity for the selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid. Microporous Mesoporous Mater. 2016, 226, 466–475. [Google Scholar] [CrossRef]
- Bonincontro, D.; Lolli, A.; Villa, A.; Prati, L.; Dimitratos, N.; Veith, G.M.; Botton, G.; Chinchilla, L.E.; Cavani, F.; Albonetti, S. AuPd-nNiO as effective catalyst for the base -free oxidation of HMF at mild reaction conditions. Green Chem. 2019. [Google Scholar] [CrossRef]
- Davis, S.E.; Zope, B.N.; Davis, R.J. On the mechanism of selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over supported Pt and Au catalysts. Green Chem. 2012, 14, 143–147. [Google Scholar] [CrossRef]
- Yi, G.; Teong, S.P.; Zhang, Y. Base-free conversion of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid over a Ru/C catalyst. Green Chem. 2016, 18, 979–983. [Google Scholar] [CrossRef]
- Xu, C.; Paone, E.; Rodríguez-Padrón, D.; Luque, R.; Mauriello, F. Recent catalytic routes for the preparation and the upgrading of biomass derived furfural and 5-hydroxymethylfurfural. Chem. Soc. Rev. 2020, 49, 4273–4306. [Google Scholar] [CrossRef]
- Yang, B.; Hu, W.; Wan, F.; Zhang, C.; Fu, Z.; Su, A.; Chen, M.; Liu, Y. Adjusting effect of additives on decatungstate-photocatalyzed HMF oxidation with molecular oxygen under visible light illumination. Chem. Eng. J. 2020, 396, 125345. [Google Scholar] [CrossRef]
- Ulyankina, A.; Mitchenko, S.; Smirnova, N. Selective photocatalytic oxidation of 5-HMF in water over electrochemically synthesized TiO2 nanoparticles. Processes 2020, 8, 647. [Google Scholar] [CrossRef]
- Gonzalez-Casamachin, D.A.; Rivera De la Rosa, J.; Lucio–Ortiz, C.J.; Sandoval-Rangel, L.; García, C.D. Partial oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid using O2 and a photocatalyst of a composite of ZnO/PPy under visible-light: Electrochemical characterization and kinetic analysis. Chem. Eng. J. 2020, 393, 124699. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Cheng, L.; Ismael, M.; Feng, Z.; Wu, Y. Novel application of g-C3N4/NaNbO3 composite for photocatalytic selective oxidation of biomass-derived HMF to FFCA under visible light irradiation. Adv. Powder Technol. 2020, 31, 1148–1159. [Google Scholar] [CrossRef]
- García-López, E.I.; Pomilla, F.R.; Bloise, E.; Lü, X.F.; Mele, G.; Palmisano, L.; Marcì, G. C3N4 Impregnated with Porphyrins as Heterogeneous Photocatalysts for the Selective Oxidation of 5-Hydroxymethyl-2-Furfural Under Solar Irradiation. Top. Catal. 2020. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, Q.; Guo, C.; Wu, Y.; Wu, T. Photocatalytic Selective Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran over Nb2O5 under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 3517–3523. [Google Scholar] [CrossRef]
- Ma, B.; Wang, Y.; Guo, X.; Tong, X.; Liu, C.; Wang, Y.; Guo, X. Photocatalytic synthesis of 2,5-diformylfuran from 5-hydroxymethyfurfural or fructose over bimetallic Au-Ru nanoparticles supported on reduced graphene oxides. Appl. Catal. A Gen. 2018, 552, 70–76. [Google Scholar] [CrossRef]
- Giannakoudakis, D.A.; Nair, V.; Khan, A.; Deliyanni, E.A.; Colmenares, J.C.; Triantafyllidis, K.S. Additive-free photo-assisted selective partial oxidation at ambient conditions of 5-hydroxymethylfurfural by manganese (IV) oxide nanorods. Appl. Catal. B Environ. 2019, 256, 117803. [Google Scholar] [CrossRef]
- Krivtsov, I.; García-lópez, E.I.; Marcì, G.; Palmisano, L.; Díaz, E.; Amghouz, Z.; García, J.R.; Ordó, S. Selective photocatalytic oxidation of 5-hydroxymethyl-2-furfural to 2,5-furandicarboxyaldehyde in aqueous suspension of g-C3N4. Appl. Catal. B Environ. 2017, 204, 430–439. [Google Scholar] [CrossRef]
- Xu, S.; Zhou, P.; Zhang, Z.; Yang, C.; Zhang, B.; Deng, K.; Bottle, S.; Zhu, H. Selective Oxidation of 5—Hydroxymethylfurfural to 2,5—Furandicarboxylic Acid Using O2 and a Photocatalyst of Co—Thioporphyrazine Bonded to g-C3N4. J. Am. Chem. Soc. 2017, 139, 14775–14782. [Google Scholar] [CrossRef] [PubMed]
- Ilkaeva, M.; Krivtsov, I.; García, J.R.; Díaz, E.; Ordóñez, S.; García-lópez, E.I.; Marcì, G.; Palmisano, L.; Maldonado, M.I.; Malato, S. Selective photocatalytic oxidation of 5-hydroxymethyl-2-furfural in aqueous suspension of polymeric carbon nitride and its adduct with H2O2 in a solar pilot plant. Catal. Today 2018, 315, 138–148. [Google Scholar] [CrossRef]
- Ilkaeva, M.; Krivtsov, I.; García-lópez, E.I.; Marcì, G.; Khainakova, O.; García, J.R.; Palmisano, L. Selective photocatalytic oxidation of 5-hydroxymethylfurfural to 2, 5-furandicarboxaldehyde by polymeric carbon nitride-hydrogen peroxide adduct. J. Catal. 2018, 359, 212–222. [Google Scholar] [CrossRef]
- Battula, V.R.; Jaryal, A.; Kailasam, K. Visible light-driven simultaneous H2 production by water splitting coupled with selective oxidation of HMF to DFF catalyzed by porous carbon nitride. J. Mater. Chem. A 2019, 7, 5643–5649. [Google Scholar] [CrossRef]
- Zhang, H.; Feng, Z.; Zhu, Y.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived 5-hydroxymethylfurfural to 2,5-diformylfuran on WO3/g-C3N4 composite under irradiation of visible light. J. Photochem. Photobiol. A Chem. 2019, 371, 1–9. [Google Scholar] [CrossRef]
- Wu, Q.; He, Y.; Zhang, H.; Feng, Z.; Wu, Y.; Wu, T. Photocatalytic selective oxidation of biomass-derived under visible light irradiation. Mol. Catal. 2017, 436, 10–18. [Google Scholar] [CrossRef]
- Xue, J.; Huang, C.; Zong, Y.; Gu, J.; Wang, M.; Ma, S. Fe (III)-grafted Bi2MoO6 nanoplates for enhanced photocatalytic activities on tetracycline degradation and HMF oxidation. Appl. Organomet. Chem. 2019, 33, 1–10. [Google Scholar] [CrossRef]
- Augugliaro, V.; Loddo, V.; Palmisano, G.; Palmisano, L. Photocatalytic Selective Oxidation of 5-(Hydroxymethyl)-2- furaldehyde to 2,5-Furandicarbaldehyde in Water by Using Anatase, Rutile, and Brookite TiO2 Nanoparticles. ACS Sustain. Chem. Eng 2013, 1, 456–461. [Google Scholar]
- Krivtsov, I.; Ilkaeva, M.; Salas-colera, E.; Amghouz, Z.; Garc, J.R.; Eva, D.; Ordo, S.; Villar-rodil, S. Consequences of Nitrogen Doping and Oxygen Enrichment on Titanium Local Order and Photocatalytic Performance of TiO2 Anatase. J. Phys. Chem. C 2017, 121, 6770–6780. [Google Scholar] [CrossRef]
- Lolli, A.; Maslova, V.; Bonincontro, D.; Basile, F.; Ortelli, S.; Albonetti, S. Selective oxidation of HMF via catalytic and photocatalytic processes using metal-supported catalysts. Molecules 2018, 23, 2792. [Google Scholar] [CrossRef]
- Augugliaro, V.; Bellardita, M.; Loddo, V.; Palmisano, G.; Palmisano, L.; Yurdakal, S. Overview on oxidation mechanisms of organic compounds by TiO2 in heterogeneous photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 224–245. [Google Scholar] [CrossRef]
- Ibrahim, U.; Halim, A. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: A review of fundamentals, progress and problems. J. Photochem. Photobiol. C Photochem. Rev. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Zhou, B.; Song, J.; Zhang, Z.; Jiang, Z.; Zhang, P.; Han, B. Highly selective photocatalytic oxidation of biomass-derived chemicals to carboxyl compounds over Au/TiO2. Green Chem. 2017, 19, 1075–1081. [Google Scholar] [CrossRef]
- Lolli, A.; Blosi, M.; Ortelli, S.; Costa, A.L.; Zanoni, I.; Bonincontro, D.; Carella, F.; Albonetti, S. Innovative synthesis of nanostructured composite materials by a spray-freeze drying process: Efficient catalysts and photocatalysts preparation. Catal. Today 2019, 334, 193–202. [Google Scholar] [CrossRef]
- Martínez, L.; Benito, M.; Mata, I.; Soler, L.; Molins, E.; Llorca, J. Preparation and photocatalytic activity of Au/TiO2 lyogels for hydrogen production. Sustain. Energy Fuels 2018, 2, 2284–2295. [Google Scholar] [CrossRef]
- Tbessi, I.; Benito, M.; Llorca, J.; Molins, E.; Sayadi, S.; Najjar, W. Silver and manganese co-doped titanium oxide aerogel for effective diclofenac degradation under UV-A light irradiation. J. Alloys Compd. 2019, 779, 314–325. [Google Scholar] [CrossRef]
- Das, D.; Shivhare, A.; Saha, S.; Ganguli, A.K. Room temperature synthesis of mesoporous TiO2 nanostructures with high photocatalytic efficiency. Mater. Res. Bull. 2012, 47, 3780–3785. [Google Scholar] [CrossRef]
- Carbajo, J.; Bahamonde, A.; Faraldos, M. Photocatalyst performance in wastewater treatment applications: Towards the role of TiO2 properties. Mol. Catal. 2017, 434, 167–174. [Google Scholar] [CrossRef]
- Basile, F.; Mafessanti, R.; Fasolini, A.; Fornasari, G.; Lombardi, E.; Vaccari, A. Effect of synthetic method on CeZr support and catalytic activity of related Rh catalyst in the oxidative reforming reaction. J. Eur. Ceram. Soc. 2019, 39, 41–52. [Google Scholar] [CrossRef]
- Maslova, V.; Fasolini, A.; Offidani, M.; Albonetti, S.; Basile, F. Solar-driven valorization of glycerol towards production of chemicals and hydrogen. Submitted.
- Wang, J.; Wang, Z.; Huang, B.; Ma, Y.; Liu, Y.; Qin, X.; Zhang, X.; Dai, Y. Oxygen vacancy induced band-gap narrowing and enhanced visible light photocatalytic activity of ZnO. ACS Appl. Mater. Interfaces 2012, 4, 4024–4030. [Google Scholar] [CrossRef]
- Xin, X.; Xu, T.; Yin, J.; Wang, L.; Wang, C. Management on the location and concentration of Ti3+ in anatase TiO2 for defects-induced visible-light photocatalysis. Appl. Catal. B Environ. 2015, 176–177, 354–362. [Google Scholar] [CrossRef]
- Fiorenza, R.; Sciré, S.; D’Urso, L.; Compagnini, G.; Bellardita, M.; Palmisano, L. Efficient H2 production by photocatalytic water splitting under UV or solar light over variously modified TiO2-based catalysts. Int. J. Hydrogen Energy 2019, 44, 14796–14807. [Google Scholar] [CrossRef]
- Carbajo, J.; Tolosana-Moranchel, A.; Casas, J.A.; Faraldos, M.; Bahamonde, A. Analysis of photoefficiency in TiO2 aqueous suspensions: Effect of titania hydrodynamic particle size and catalyst loading on their optical properties. Appl. Catal. B Environ. 2018, 221, 1–8. [Google Scholar] [CrossRef]
- Ortelli, S.; Costa, A.L. Nanoencapsulation techniques as a “safer by (molecular) design” tool. Nano-Struct. Nano-Objects 2018, 13, 155–162. [Google Scholar] [CrossRef]
- Blosi, M.; Ortelli, S.; Costa, A.; Dondi, M.; Lolli, A.; Andreoli, S.; Benito, P.; Albonetti, S. Bimetallic Nanoparticles as Efficient Catalysts: Facile and Green Microwave Synthesis. Materials (Basel) 2016, 9, 550. [Google Scholar] [CrossRef]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Kubacka, A.; Fernández-García, M.; Colón, G. Advanced nanoarchitectures for solar photocatalytic applications. Chem. Rev. 2012, 112, 1555–1614. [Google Scholar] [CrossRef] [PubMed]
- Oguma, J.; Kakuma, Y.; Murayama, S.; Nosaka, Y. Effects of silica coating on photocatalytic reactions of anatase titanium dioxide studied by quantitative detection of reactive oxygen species. Appl. Catal. B Environ. 2013, 129, 282–286. [Google Scholar] [CrossRef]
- Bonmatí, E.; Casanovas, A.; Angurell, I.; Llorca, J. Hydrogen Photoproduction from Ethanol-Water Mixtures over Au-Cu Alloy Nanoparticles Supported on TiO2. Top. Catal. 2015, 58, 77–84. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, T.; Li, P.; Liu, L.; Chang, K.; Li, M.; Ye, J. Photocatalytic Reduction of Carbon Dioxide by Hydrous Hydrazine over Au-Cu Alloy Nanoparticles Supported on SrTiO3/TiO2 Coaxial Nanotube Arrays. Angew. Chem. Int. Ed. 2015, 127, 855–859. [Google Scholar] [CrossRef]
- Henderson, M.A.; Shen, M. Electron-Scavenging Chemistry of Benzoquinone on TiO2(110). Top. Catal. 2017, 60, 440–445. [Google Scholar] [CrossRef]
- Qourzal, S.; Barka, N.; Belmouden, M.; Abaamrane, A.; Alahiane, S.; Elouardi, M.; Assabbane, A.; Ait-Ichou, Y. Heterogeneous photocatalytic degradation of 4-nitrophenol on suspended titania surface in a dynamic photoreactor. Fresenius Environ. Bull. 2012, 21, 1972–1981. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Maslova, V.; Quadrelli, E.A.; Gaval, P.; Fasolini, A.; Albonetti, S.; Basile, F. Highly-dispersed ultrafine Pt nanoparticles on microemulsion-mediated TiO2 for production of hydrogen and valuable chemicals via oxidative photo-dehydrogenation of glycerol Valeriia Maslova. Submitted.


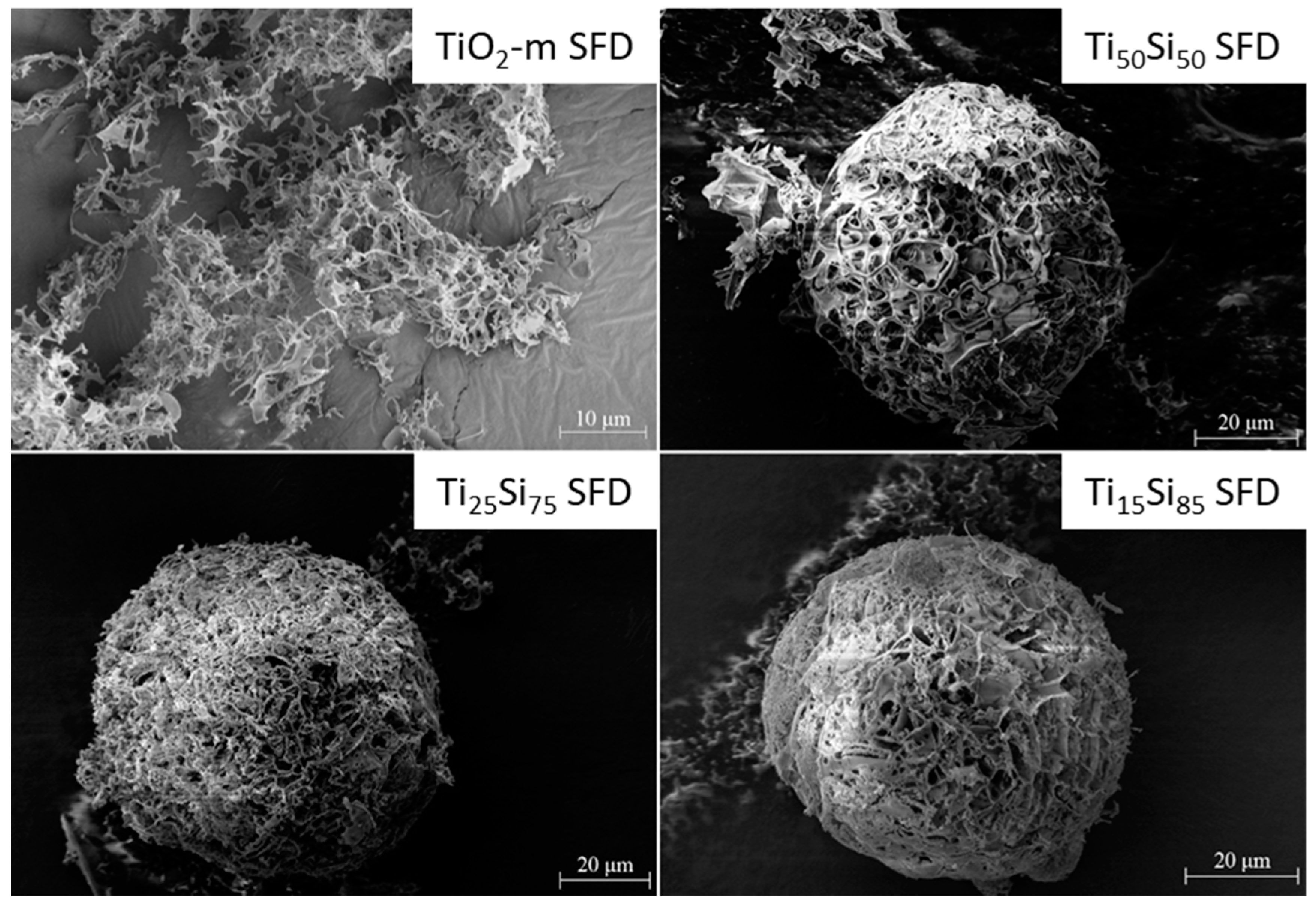
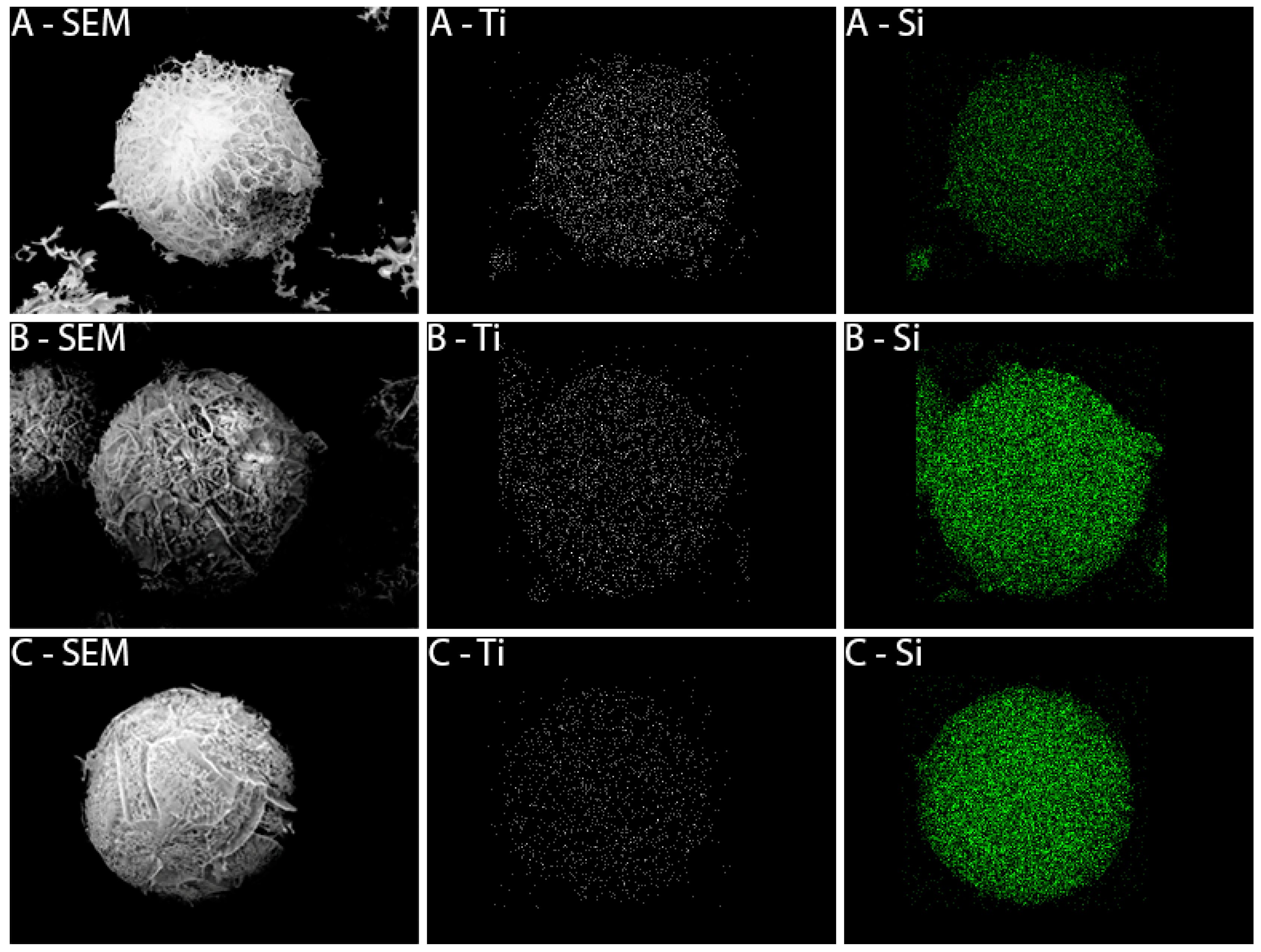
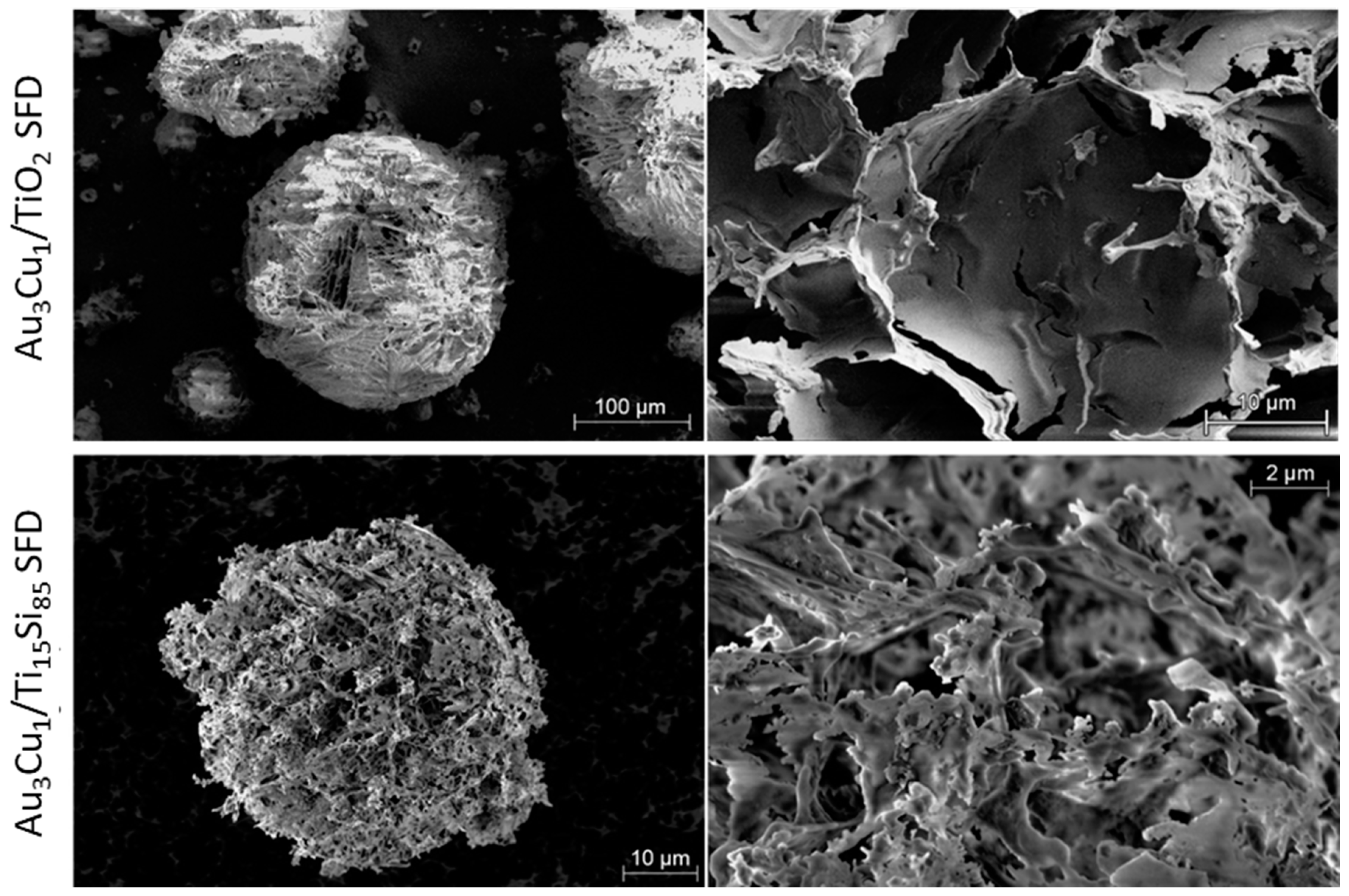
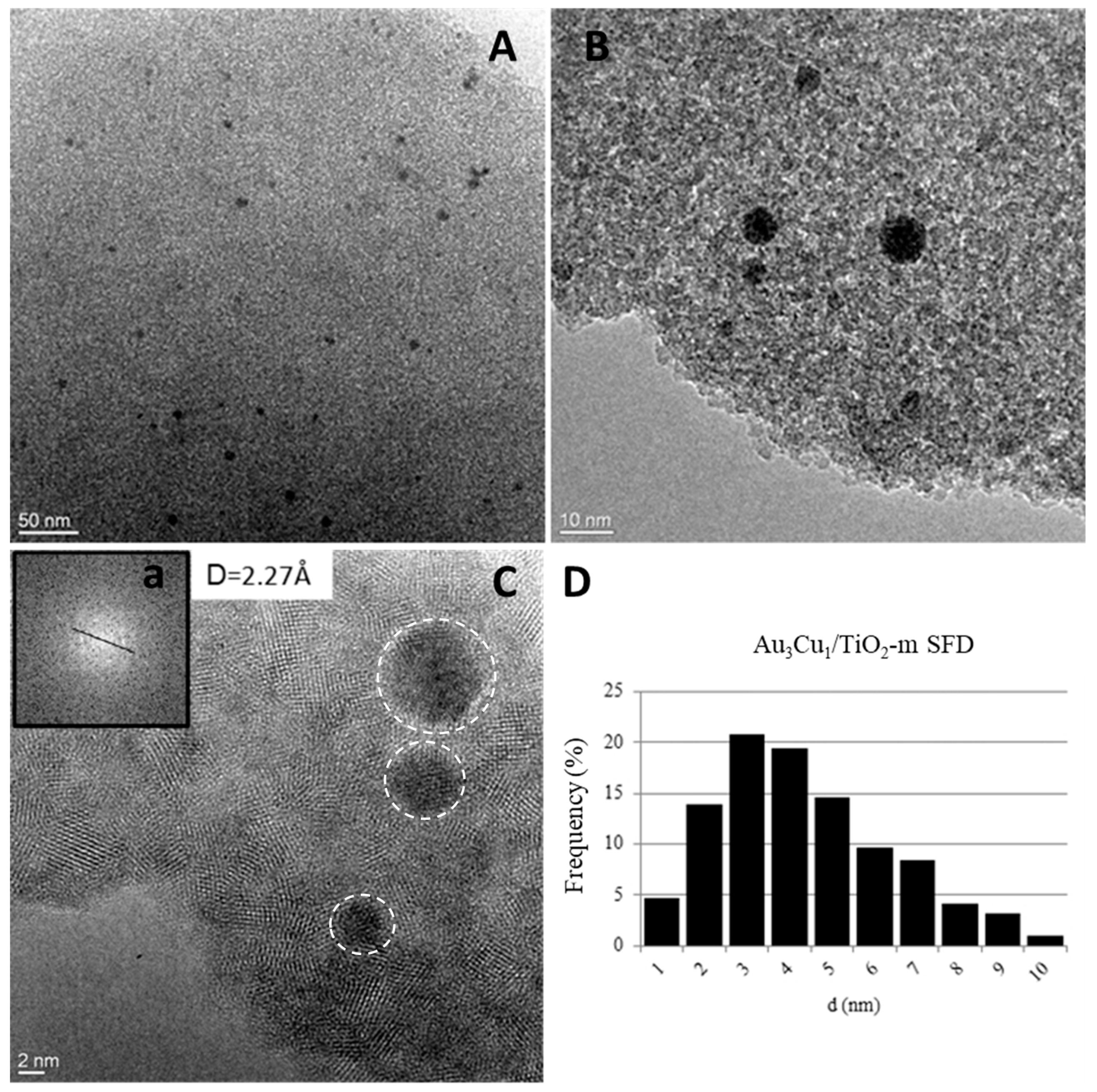

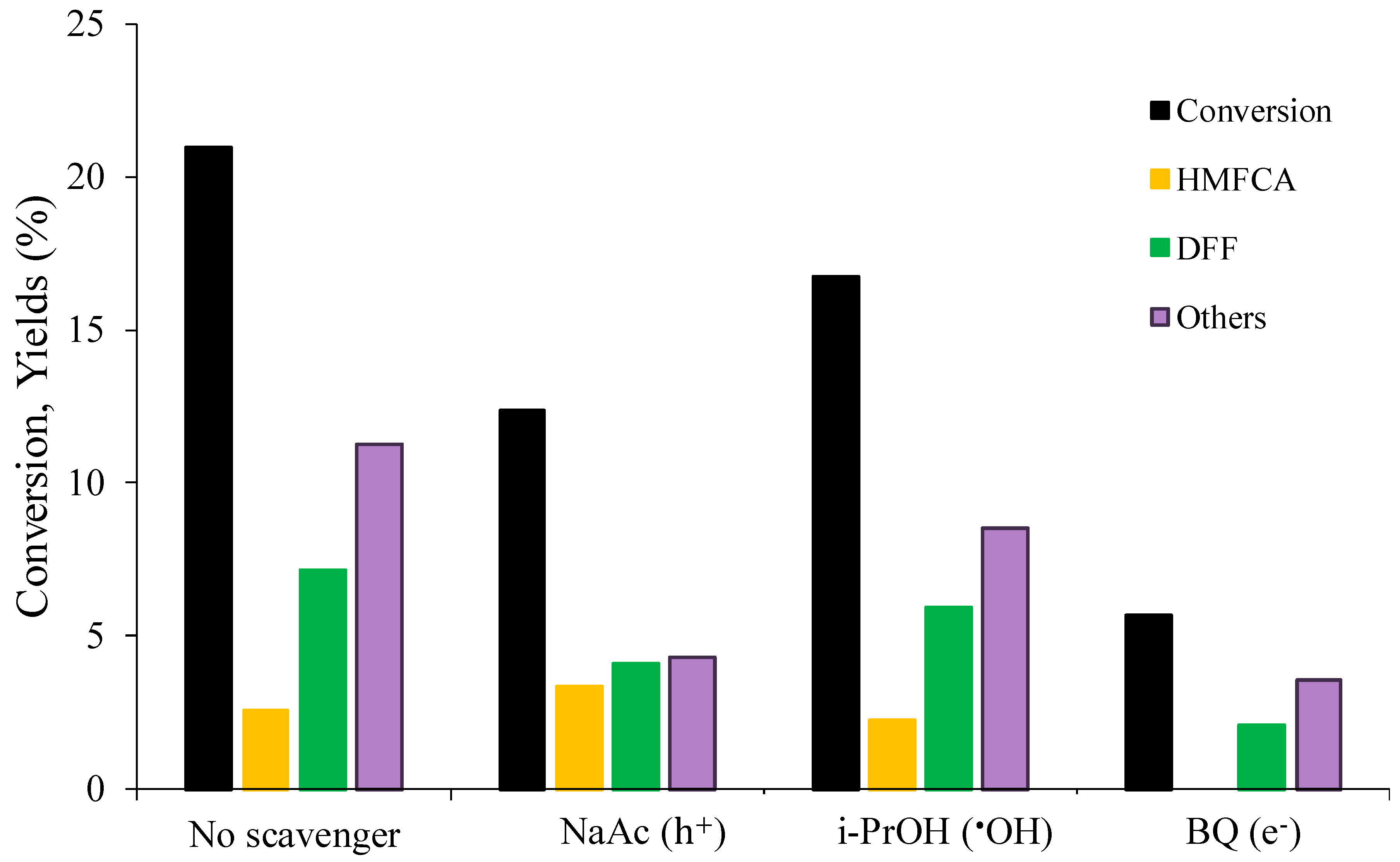
| Catalyst | Crystalline Phase | SSABET (m2/g) | ρ (g/cm3) | d (nm) | Eg (eV) |
|---|---|---|---|---|---|
| P25 | 80% Anatase 20% Rutile | 49 | 0.10 | 23 | 3.10 |
| DT51 | Anatase | 80 | 0.36 | 20 | 3.26 |
| TiO2-m | Anatase | 130 | 1.30 | 8 | 3.08 |
| TiO2-m SFD | 230 | 0.10 | 3 | 3.04 | |
| Ti50Si50 SFD | 250 | 0.05 | 3 | 2.92 | |
| Ti25Si75 SFD | 240 | 0.04 | 3 | 3.48 | |
| Ti15Si85 SFD | 210 | 0.03 | 2 | 3.56 | |
| Au3Cu1/Ti15Si85 SFD | 140 | 0.04 | 5.0 * | 3.56 | |
| Au3Cu1/TiO2-m SFD | 180 | 0.09 | 4.6 * | 3.04 |
| Catalyst | Conversion HMF (%) | Yield HMFCA (%) | Selectivity HMFCA (%) | Yield DFF (%) | Selectivity DFF (%) | Others (%) |
|---|---|---|---|---|---|---|
| P25 | 94 | 1 | 1 | 1 | 1 | 92 |
| DT51 | 62 | 1 | 1 | 4 | 6 | 57 |
| TiO2-m | 25 | 1 | 4 | 3 | 13 | 21 |
| TiO2-m SFD | 12 | 1 | 7 | 4 | 34 | 7 |
| Ti50Si50 SFD | 7 | 1 | 2 | 2 | 42 | 4 |
| Ti25Si75 SFD | 11 | 1 | 3 | 3 | 25 | 7 |
| Ti15Si85 SFD | 24 | 1 | 2 | 3 | 13 | 20 |
| Au3Cu1/TiO2-m SFD | 3 | 0 | 0 | 2 | 67 | 1 |
| Au3Cu1/Ti15Si85 SFD | 21 | 3 | 12 | 7 | 34 | 11 |
Sample Availability: Samples of the compounds are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allegri, A.; Maslova, V.; Blosi, M.; Costa, A.L.; Ortelli, S.; Basile, F.; Albonetti, S. Photocatalytic Oxidation of HMF under Solar Irradiation: Coupling of Microemulsion and Lyophilization to Obtain Innovative TiO2-Based Materials. Molecules 2020, 25, 5225. https://doi.org/10.3390/molecules25225225
Allegri A, Maslova V, Blosi M, Costa AL, Ortelli S, Basile F, Albonetti S. Photocatalytic Oxidation of HMF under Solar Irradiation: Coupling of Microemulsion and Lyophilization to Obtain Innovative TiO2-Based Materials. Molecules. 2020; 25(22):5225. https://doi.org/10.3390/molecules25225225
Chicago/Turabian StyleAllegri, Alessandro, Valeriia Maslova, Magda Blosi, Anna Luisa Costa, Simona Ortelli, Francesco Basile, and Stefania Albonetti. 2020. "Photocatalytic Oxidation of HMF under Solar Irradiation: Coupling of Microemulsion and Lyophilization to Obtain Innovative TiO2-Based Materials" Molecules 25, no. 22: 5225. https://doi.org/10.3390/molecules25225225
APA StyleAllegri, A., Maslova, V., Blosi, M., Costa, A. L., Ortelli, S., Basile, F., & Albonetti, S. (2020). Photocatalytic Oxidation of HMF under Solar Irradiation: Coupling of Microemulsion and Lyophilization to Obtain Innovative TiO2-Based Materials. Molecules, 25(22), 5225. https://doi.org/10.3390/molecules25225225










