Modified Spruce Sawdust for Sorption of Hexavalent Chromium in Batch Systems and Fixed-Bed Columns
Abstract
1. Introduction
2. Results and Discussion
2.1. Organosolv Pretreatment
2.2. BET Surface Area
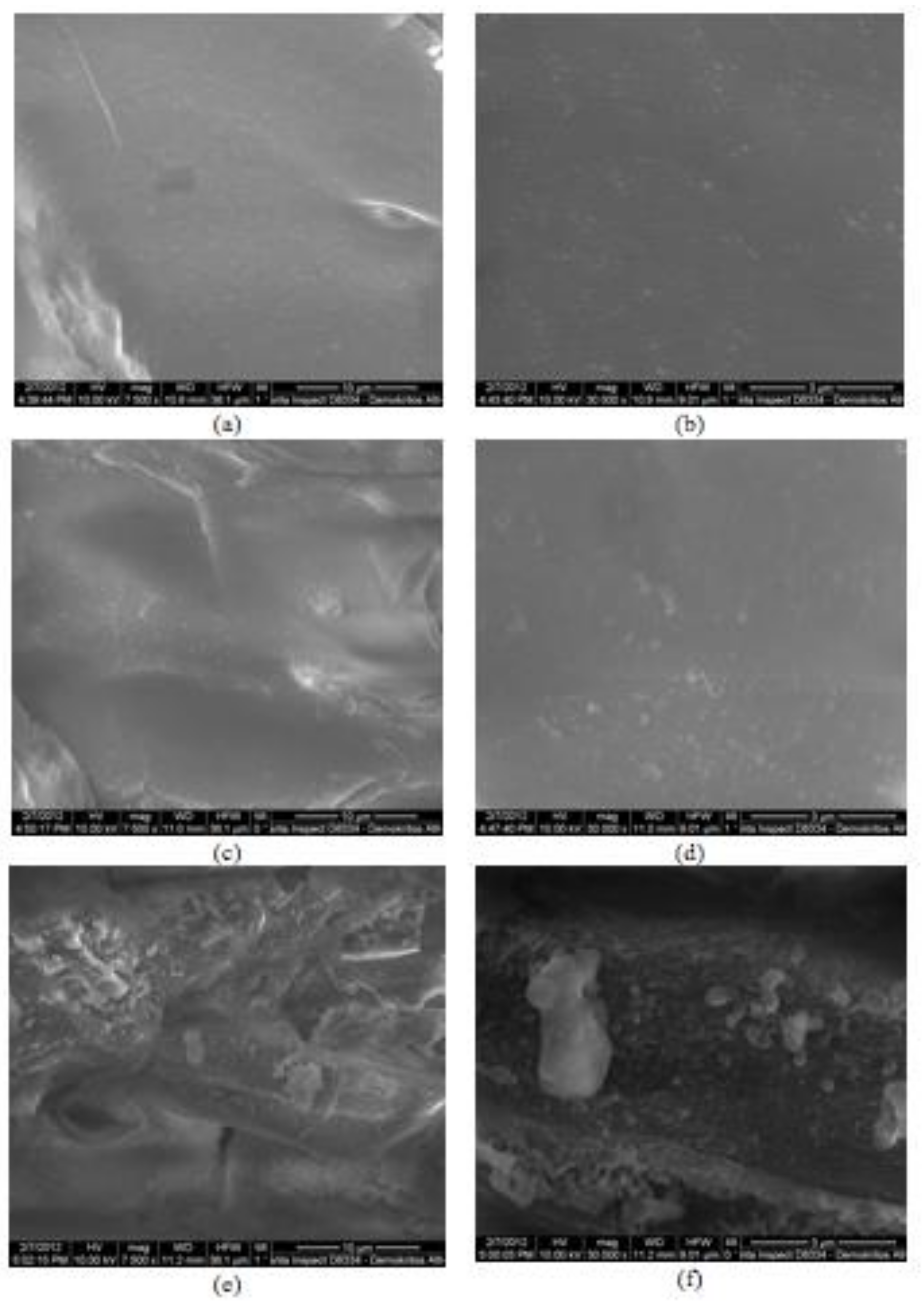
2.3. Microstructure
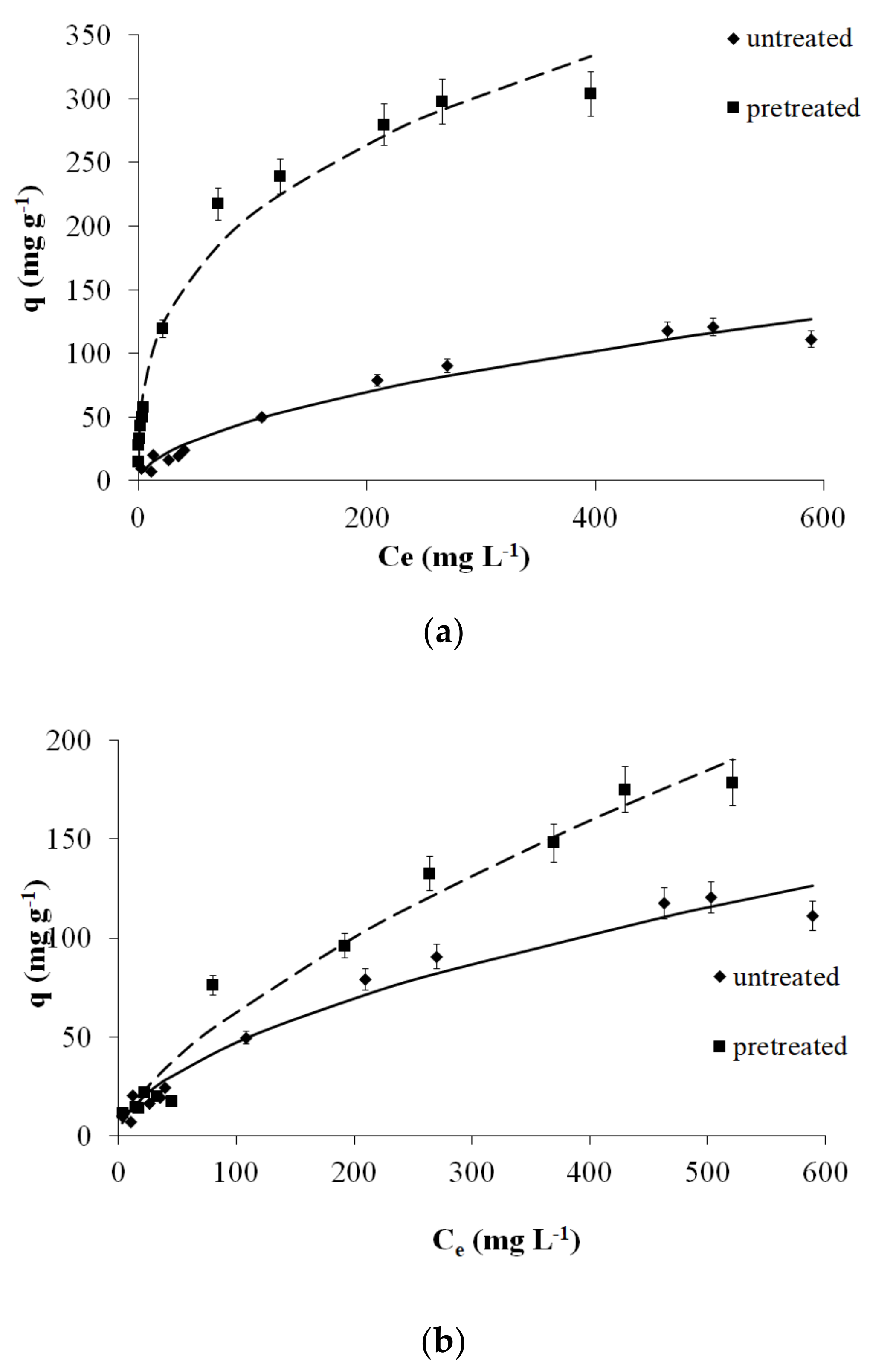
2.4. Adsorption Isotherms
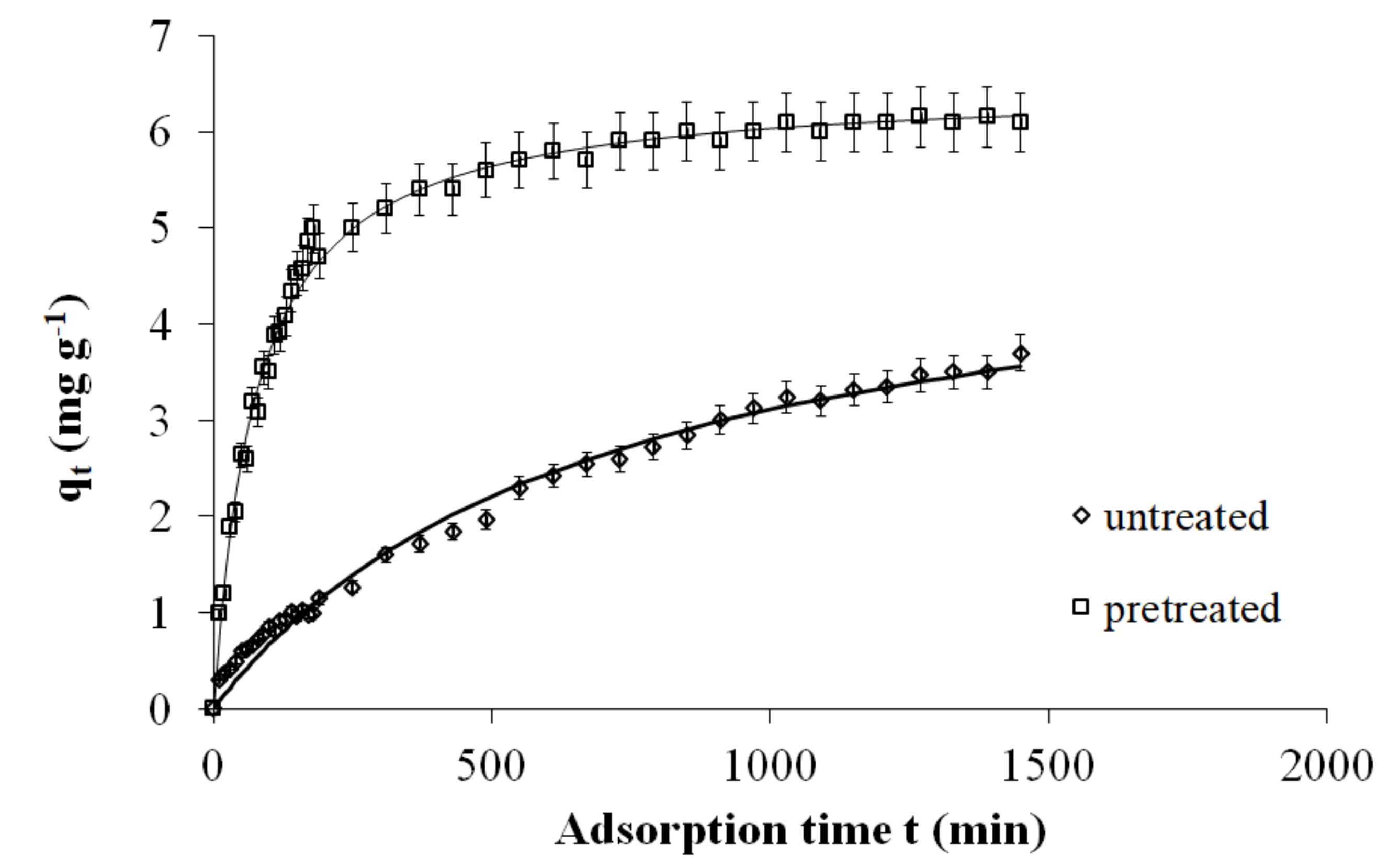
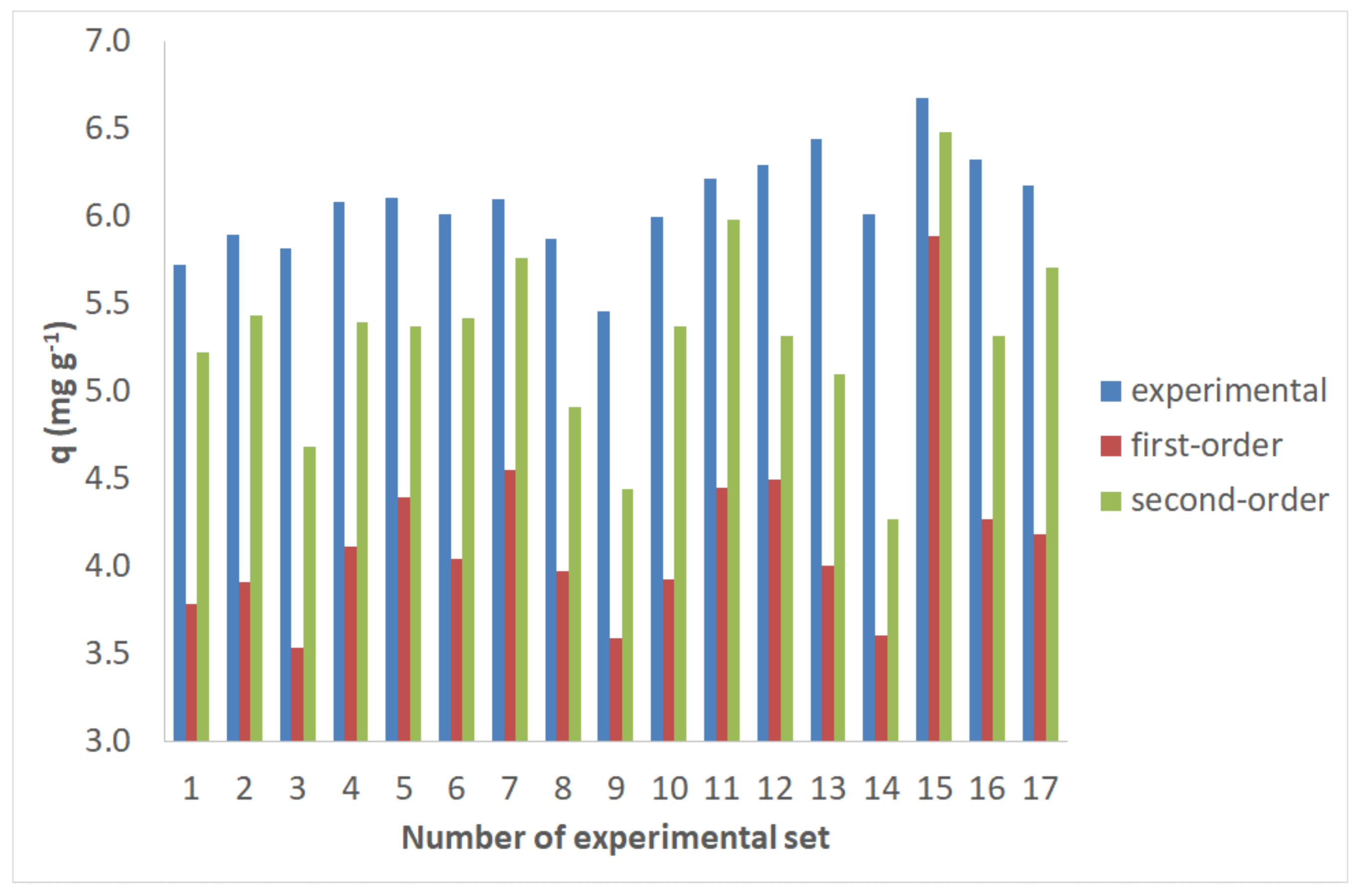
2.5. Adsorption Kinetics
2.6. Adsorption Columns
2.7. Chemical-Physical Mechanisms of the Process
3. Materials and Methods
3.1. Materials
3.2. Organosolv Pretreatment
3.3. Characterization
3.4. Adsorption Isotherm Studies
3.5. Kinetic Adsorption Studies
3.6. Adsorption Column Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ali, H.; Khan, E.; Ilahi, I. Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. J. Chem. N. Y. 2019, 6730305. [Google Scholar] [CrossRef]
- Junaid, M.; Hashmi, M.Z.; Malik, R.N.; Pei, D.-S. Toxicity and oxidative stress induced by chromium in workers exposed from different occupational settings around the globe: A review. Environ. Sci. Pollut. Res. Int. 2016, 20, 20151–20167. [Google Scholar] [CrossRef] [PubMed]
- Mojdeh, O.; Mohamed, K.A.; Wan, A.W.D.; Saeid, B. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water Air Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Mokhtar, M.B. Assessing Cadmium and Chromium Concentrations in Drinking Water to Predict Health Risk in Malaysia. Int. J. Environ. Res. Public Health 2020, 17, 2966. [Google Scholar] [CrossRef]
- Economou-Eliopoulos, M.; Megremi, I.; Vasilatos, C. Factors controlling the heterogeneous distribution of Cr(VI) in soil, plants and groundwater: Evidence from the Assopos basin, Greece. Geochemistry 2011, 71, 39–52. [Google Scholar] [CrossRef]
- Oliveira, H. Chromium as an Environmental Pollutant: Insights on Induced Plant Toxicity. J. Bot. 2012, 375843. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Removal of heavy metals and pollutants by membrane adsorption techniques. Appl. Water Sci. 2018, 8, 19. [Google Scholar] [CrossRef]
- Barakat, M.A. New trends in removing heavy metals from industrial wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Wastewater treatment: An overview. In Book Green Adsorbents for Pollutant Removal; Springer: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E.; Wilson, L.D.; Morin-Crini, N. Adsorption-Oriented Processes Using Conventional and Non-conventional Adsorbents for Wastewater Treatment. In Book Green Adsorbents for Pollutant Removal; Springer: Cham, Switzerland, 2018; pp. 23–71. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sureshkumar, M.V. Removal of chromium(VI) from water and wastewater using surfactant modified coconut coir pith as a biosorbent. Bioresour. Technol. 2008, 99, 2218–2225. [Google Scholar] [CrossRef] [PubMed]
- Tsamo, C.; Bachirou, I.; Samomssa, I.; Fouogoung, T.B. Removal of Hexavalent Chromium from Aqueous Solution Using Unmodified SawDust: Batch and Column Studies. Curr. J. Appl. 2019, 32, 1–16. [Google Scholar] [CrossRef]
- Moussavi, G.; Barikbin, B. Biosorption of chromium(VI) from industrial wastewater onto pistachio hull waste biomass. Chem. Eng. J. 2010, 162, 893–900. [Google Scholar] [CrossRef]
- Vaghetti, J.C.P.; Lima, E.C.; Royer, B.; Brasil, J.L.; Da Cunha, B.M.; Simon, N.M.; Cardoso, N.F.; Zapata-Norena, C.P. Application of Brazilian-pine fruit coat as a biosorbent to removal of Cr(VI) from aqueous solution -Kinetics and equilibrium study. Biochem. Eng. J. 2008, 42, 67–76. [Google Scholar] [CrossRef]
- Tejada-Tovar, C.; Herrera-Barros, A.; Villabona-Ortíz, A. Assessment of Chemically Modified Lignocellulose Waste for the Adsorption of Cr(VI). Rev. Fac. Ing. 2020, 29. [Google Scholar] [CrossRef]
- Saranya, K.; Palanisami, T.; Mallavarapu, M.; Kadiyala, V.; Yong, B.L.; Ravi, N. Potential of Melaleuca diosmifolia leaf as a low-cost adsorbent for hexavalent chromium removal from contaminated water bodies. Process Saf. Environ. Protect. 2016, 100, 173–182. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.J.; Liu, M.; Wang, X.; Wu, Z.; Yang, L.Z.; Xia, S.Q.; Zhao, J.F. Cr(VI) removal from water using cobalt-coated bamboo charcoal prepared with microwave heating. Ind. Crop. Prod. 2012, 39, 81–88. [Google Scholar] [CrossRef]
- Altun, T.; Pehlivan, E. Removal of Cr(VI) from aqueous solutions by modified walnut shells. Food Chem. 2012, 132, 693–700. [Google Scholar] [CrossRef]
- Pourfadaraki, S.; Jorfi, S.; Ahmadi, M.; Takdastan, A. Experimental data on adsorption of Cr(VI) from aqueous solution using nanosized cellulose fibers obtained from rice husk. Data Brief 2017, 15, 887–895. [Google Scholar] [CrossRef]
- Shi, C.; Qiao, Y.; An, X.; Tian, Y.; Zhou, H. High-capacity adsorption of Cr(VI) by lignin-based composite: Characterization, performance and mechanism. Int. J. Biol. Macromol. 2020, 159, 839–849. [Google Scholar] [CrossRef]
- Mohan, D.; Rajput, S.; Singh, V.K.; Steele, P.H.; Pittman, C.U., Jr. Modeling and evaluation of chromium remediation from water using low cost bio-char, a green adsorbent. J. Hazard. Mater. 2011, 188, 319–333. [Google Scholar] [CrossRef]
- Garg, V.K.; Gupta, R.; Kumar, R.; Gupta, R.K. Adsorption of chromium from aqueous solution on treated sawdust. Bioresour. Technol. 2004, 92, 79–81. [Google Scholar] [CrossRef]
- Lin, C.; Luo, W.; Luo, T.; Zhou, Q.; Li, H.; Jing, L. A study on adsorption of Cr(VI) by modified rice straw: Characteristics, performances and mechanism. J. Clean. Prod. 2018, 196, 626–634. [Google Scholar] [CrossRef]
- Vilardi, G.; Ochando-Pulido, J.M.; Verdone, N.; Stoller, M.; Di Palma, L. On the removal of hexavalent chromium by olive stones coated by iron-based nanoparticles: Equilibrium study and chromium recovery. J. Clean. Prod. 2018, 190, 200–210. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Naimah, I.; Hamidi, A.A.; Mohd, N.A.; Nor Habsah, Md.S.; Ali, A.L.Z.; Shamsul, R.M.K. Removal of chromium (VI) from aqueous solution using treated oil palm fibre. J. Hazard. Mater. 2008, 152, 662–668. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Y.-C.; Sun, R.-C. Fractionational and structural characterization of lignin and its modification as biosorbents for efficient removal of chromium from wastewater: A review. J. Leather Sci. Eng. 2019, 1–5. [Google Scholar] [CrossRef]
- Sidiras, D.; Politi, D.; Batzias, F.; Boukos, N. Efficient removal of hexavalent chromium from aqueous solutions using autohydrolyzed Scots Pine (Pinus Sylvestris) sawdust as adsorbent. Int. J. Environ. Sci. Technol. 2013, 10, 1337–1348. [Google Scholar] [CrossRef][Green Version]
- Sidiras, D.; Batzias, F.; Ranjan, R.; Tsapatsis, M. Simulation and optimization of batch autohydrolysis of wheat straw to monosaccharides and oligosaccharides. Bioresour. Technol. 2011, 102, 10486–10492. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Batzias, F.; Sidiras, D.; Schroeder, E.; Weber, C. Simulation of dye adsorption on hydrolyzed wheat straw in batch and fixed-bed systems. Chem. Eng. J. 2009, 148, 459–472. [Google Scholar] [CrossRef]
- Maarten, A.; Kootstra, J.; Beeftink, H.H.; Scott, E.L.; Sanders, J.P.M. Comparison of dilute mineral and organic acid pretreatment for enzymatic hydrolysis of wheat straw. Biochem. Eng. J. 2009, 46, 126–131. [Google Scholar] [CrossRef]
- Zhang, K.; Pei, Z.; Wang, D. Organic solvent pretreatment of lignocellulosic biomass for biofuels and biochemicals: A review. Bioresour. Techno. 2016, 199, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Salapa, I.; Topakas, E.; Sidiras, D. Simulation and optimization of barley straw organosolv pretreatment. Ind. Crops Prod. 2018, 133, 80–88. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Über die adsorption in lösungen, Zeitschrift für Physikalische Chemie. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Sips, R. Structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Radke, C.J.; Prausnitz, J.M. Adsorption of Organic Solutes from Dilute Aqueous Solution on Activated Carbon. Ind. Eng. Chem. Fundam. 1972, 11, 445–451. [Google Scholar] [CrossRef]
- Chern, J.M.; Wu, C.Y. Desorption of dye from activated carbon beds: Effects of temperature, pH, and alcohol. Wat. Res. 2001, 35, 4159–4165. [Google Scholar] [CrossRef]
- Toth, J. Calculation of the BET-compatible surface area from any Type I isotherms measured above the critical temperature. J. Colloid Interface Sci. 2000, 225, 378–383. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, L.; Qin, Y.; Xu, M.; Jia, X.; Chen, Z. Removal of aqueous Cr(VI) by a magnetic biochar derived from Melia azedarach wood. Bioresour. Technol. 2018, 256, 1–10. [Google Scholar] [CrossRef]
- Rai, M.K.; Shahi, G.; Meena, V.; Meena, R.; Chakraborty, S.; Singh, R.S.; Rai, B.N. Removal of hexavalent chromium Cr(VI) using activated carbon prepared from mango kernel activated with H3PO4. Resour. Effic. Technol. 2016, 2, 63–70. [Google Scholar] [CrossRef]
- Gupta, A.; Balomajumder, C. Simultaneous adsorption of Cr(VI) and phenol onto tea waste biomass from binary mixture: Multicomponent adsorption, thermodynamic and kinetic study. J. Environ. Chem. Eng. 2015, 3, 785–796. [Google Scholar] [CrossRef]
- Wang, X.S.; Chen, L.F.; Li, F.Y.; Chen, K.L.; Wan, W.Y.; Tang, Y.J. Removal of Cr(VI) with wheat-residue derived black carbon: Reaction mechanism and adsorption performance. J. Hazard. Mater. 2010, 175, 816–822. [Google Scholar] [CrossRef]
- Danish, M.; Hashim, R.; Ibrahim, M.N.M.; Rafatullah, M.; Sulaiman, O. Surface characterization and comparative adsorption properties of Cr(VI) on pyrolysed adsorbents of Acacia mangium wood and Phoenix dactylifera L. stone carbon. J. Anal. Appl. Pyrolysis. 2012, 97, 19–28. [Google Scholar] [CrossRef]
- Xu, X.; Gao, B.-Y.; Tang, X.; Yue, Q.-Y.; Zhong, Q.-Q.; Li, Q. Characteristics of cellulosic amine-crosslinked copolymer and its sorption properties for Cr(VI) from aqueous solutions. J. Hazard. Mater. 2011, 189, 420–426. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption gelöster stoffe. Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutants sorption by biosorbents: Review. Sep. Purif. Methods. 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar]
- Norouzi, S.; Heidari, M.; Alipour, V.; Rahmanian, O.; Fazlzadeh, M.; Mohammadi-moghadam, F.; Nourmoradi, H.; Goudarzi, B.; Dindarloo, K. Preparation, characterization and Cr(VI) adsorption evaluation of NaOH—activated carbon produced from Date Press Cake; an agro-industrial waste. Bioresour. Technol. 2018, 258, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Bouaziz, F.; Koubaa, M.; Kallel, F.; Chaari, F.; Driss, D.; Ghorbel, R.E.; Chaabouni, S.E. Efficiency of almond gum as a low-cost adsorbent for methylene blue dye removal from aqueous solutions. Ind. Crops Prod. 2015, 74, 903–911. [Google Scholar] [CrossRef]
- Bohart, G.; Adams, E.N. Some aspects of the behavior of charcoal with respect to chlorine. J. Am. Chem. Soc. 1920, 42, 523–544. [Google Scholar] [CrossRef]
- Thomas, H.C. Heterogeneous ion exchange in a flowing system. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Chu, K.H. Fixed bed sorption: Setting the record straight on the Bohart–Adams and Thomas models. J. Hazard. Mater. 2010, 177, 1006–1012. [Google Scholar] [CrossRef]
- Yan, G.; Viraraghavan, T.; Chen, M. A new model for heavy metal removal in a biosorption column. Adsorpt. Sci. Technol. 2001, 19, 25–43. [Google Scholar] [CrossRef]
- Gokhale, S.V.; Jyoti, K.K.; Lele, S.S. Modeling of chromium(VI) biosorption by immobilized Spirulina platensis in packed column. J. Hazard. Mater. 2009, 170, 735–743. [Google Scholar] [CrossRef]
- Nakkeeran, E.; Patra, C.; Shahnaz, T.; Rangabhashiyam, S.; Selvaraju, N. Continuous biosorption assessment for the removal of hexavalent chromium from aqueous solutions using Strychnos nux vomica fruit shell. Bioresour. Technol. Rep. 2018, 3, 256–260. [Google Scholar] [CrossRef]
- Sidiras, D.; Politi, D. Simulation and optimization of hexavalent chromium adsorption on autohydrolyzed Scots Pine (Pinus Sylvestris) sawdust in batch and fixed-bed systems. In Proceedings of the Second International Conference on Advances in Bio-Informatics, Bio-Technology and Environmental Engineering-ABBE, Birmingham, UK, 16–17 November 2014; pp. 83–89. [Google Scholar] [CrossRef]
- Chen, S.; Yue, Q.; Gao, B.; Li, Q.; Xu, X.; Fu, K. Adsorption of hexavalent chromium from aqueous solution by modified corn stalk: A fixed-bed column study. Bioresour. Technol. 2012, 113, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Agrawal, S.B.; Mondal, M.K. Fixed Bed Column Adsorption of Cr(VI) from Aqueous Solution Using Nanosorbents Derived from Magnetite Impregnated Phaseolus vulgaris Husk. Environ. Prog. Sustain. Energy 2018, 38, 68–76. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, P.; Li, Z.; Tong, Z. Adsorption behavior of chromium(VI) on activated carbon from eucalyptus sawdust prepared by microwave-assisted activation with ZnCl2. Desalin. Water Treat. 2016, 57, 12572–12584. [Google Scholar] [CrossRef]
- Gupta, S.; Babu, B.V. Removal of toxic metal Cr(VI) from aqueous solutions using sawdust as adsorbent: Equilibrium, kinetics and regeneration studies. Chem. Eng. J. 2009, 150, 352–365. [Google Scholar] [CrossRef]
- Pan, S.Y.; Syu, W.J.; Chang, T.K.; Lee, C.H. A multiple model approach for evaluating the performance of time-lapse capsules in trapping heavy metals from water bodies. RSC Adv. 2020, 10, 16490. [Google Scholar] [CrossRef]
- Karthikeyan, T.; Rajgopal, S.; Miranda, L.R. Chromium(VI) adsorption from aqueous solution by Hevea Brasilinesis sawdust activated carbon. J. Hazard. Mater. 2005, 124, 192–199. [Google Scholar] [CrossRef]
- Albadarina, A.B.; Al-Muhtasebb, A.H.; Al-laqtaha, N.A.; Walker, G.M.; Allena, S.J.; Ahmada, M.N.M. Biosorption of toxic chromium from aqueous phase by lignin: Mechanism, effect of other metal ions and salts. Chem. Eng. J. 2011, 169, 20–30. [Google Scholar] [CrossRef]
- Gurgel, L.V.; Perin de Melo, J.C.; de Lena, J.C.; Gil, L.F. Adsorption of chromium (VI) ion from aqueous solution by succinylated mercerized cellulose functionalized with quaternary ammonium groups. Bioresour. Technol. 2009, 100, 3214–3220. [Google Scholar] [CrossRef]
- Demirbaş, A. Adsorption of Cr(III) and Cr(VI) Ions from Aqueous Solutions on to Modified Lignin. Energy Sources 2005, 27, 1449–1455. [Google Scholar] [CrossRef]
- Tazrouti, N.; Amrani, M. Chromium (Vi) Adsorption onto Activated Kraft Lignin Produced from Alfa Grass (Stipa Tenacissima). Bioresources 2009, 4, 740–755. [Google Scholar]
- Zhuang, J.; Li, M.; Pu, Y.; Ragauskas, A.J.; Yoo, C.G. Observation of Potential Contaminants in Processed Biomass Using Fourier Transform Infrared Spectroscopy. Appl. Sci. 2020, 10, 4345. [Google Scholar] [CrossRef]
- Ahmad, A.; Rafatullah, M.; Sulaiman, O.; Ibrahim, M.H.; Hashim, R. Scavenging behaviour of meranti sawdust in the removal of methylene blue from aqueous solution. J. Hazard. Mater. 2009, 170, 357–365. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Removal of cadmium (II) from aqueous solutions by adsorption using meranti wood. Wood. Sci. Technol. 2012, 46, 221–241. [Google Scholar] [CrossRef]
- González-Peña, M.M.; Hale, M.D.C. Rapid assessment of physical properties and chemical composition of thermally modified wood by mid-infrared spectroscopy. Wood Sci. Technol. 2011, 45, 83–102. [Google Scholar] [CrossRef]
- Fackler, K.; Stevanic, J.S.; Ters, T.; Hinterstoisser, B.; Schwanninger, M.; Salmen, L. Localisation and characterisation of incipient brown-rot decay within spruce wood cell walls using FT-IR imaging microscopy. Enzym. Microb. Technol. 2010, 47, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Saeman, J.F.; Bubl, J.F.; Harris, E.E. Quantitative saccharification of wood and cellulose. Ind. Eng. Chem. Anal. Ed. 1945, 17, 35–37. [Google Scholar] [CrossRef]
- Salapa, I.; Katsimpouras, C.; Topakas, E.; Sidiras, D. Organosolv pretreatment of wheat straw for efficient ethanol production using various solvents. Biomass Bioenergy 2017, 100, 10–16. [Google Scholar] [CrossRef]
- Tappi Standards. Tappi Tests Methods, T222 om-88 Atlanta. 1997. Available online: https://www.tappi.org/content/SARG/T222.pdf (accessed on 4 November 2020).
- DIN 66132: Determination of Specific Surface Area of Solids by Adsorption of Nitrogen; Single Point Differential Method According to Haul and Dümbgen; German Institute for Standardisation: Berlin, Germany, 1975.


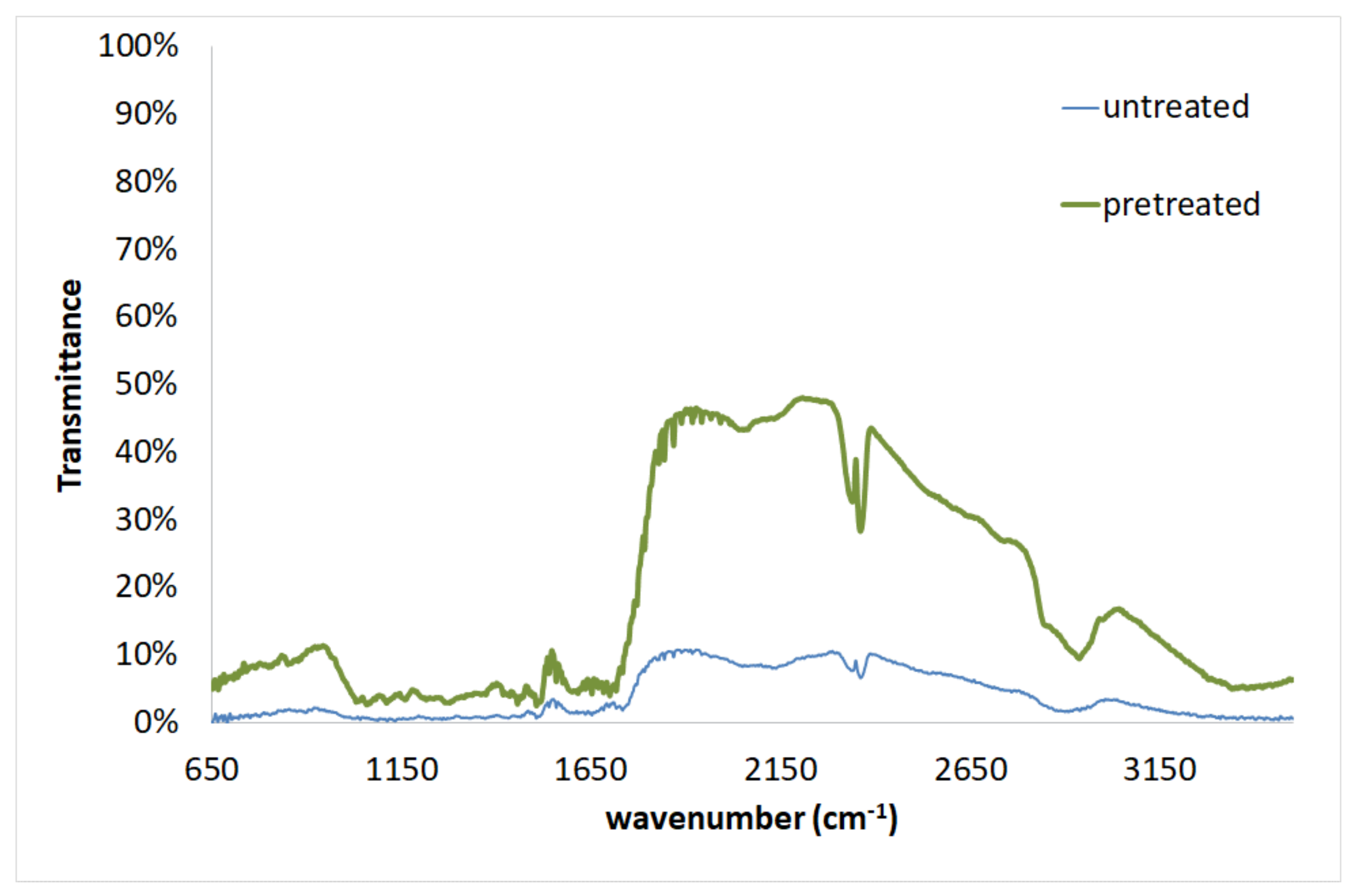
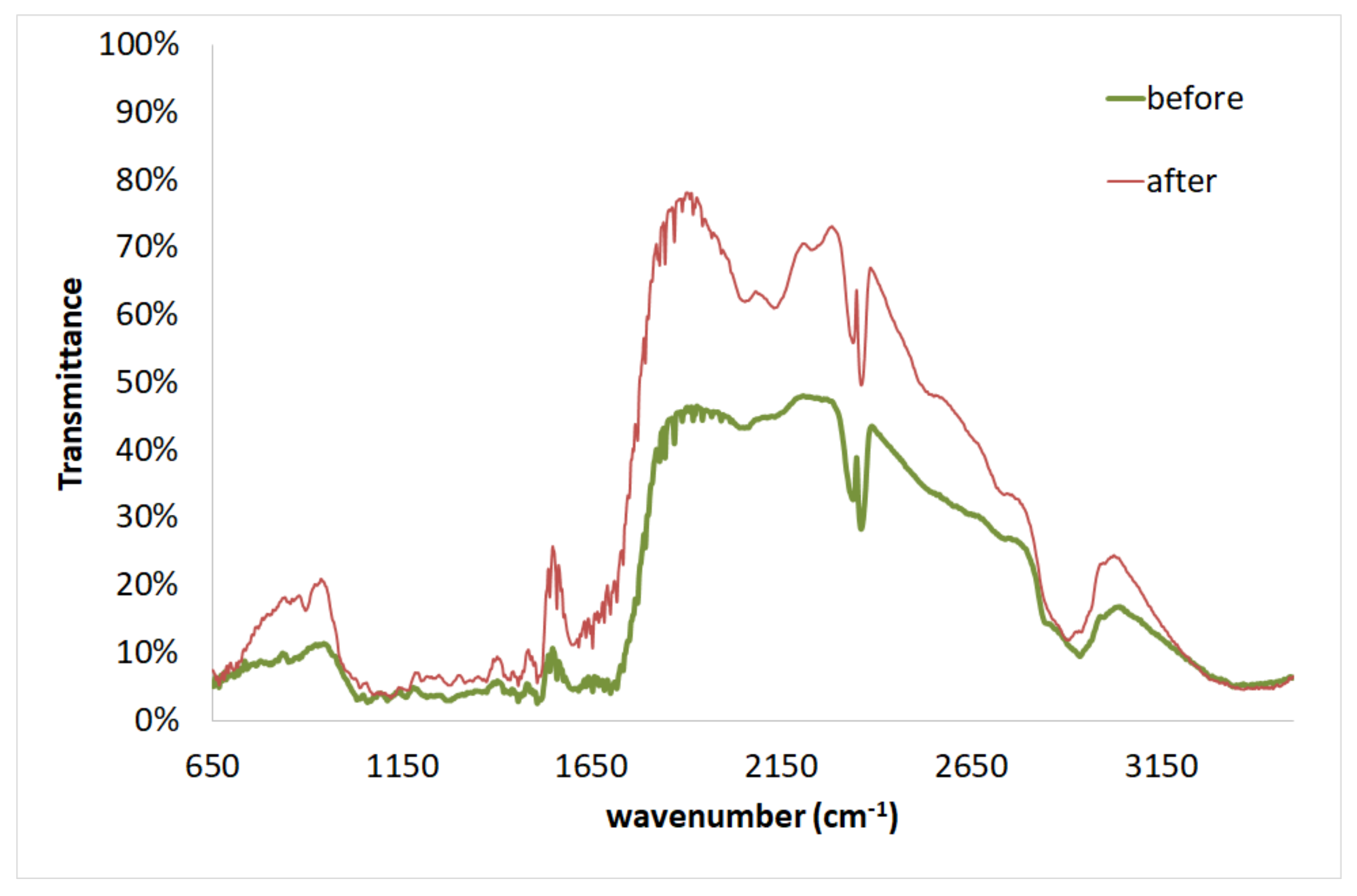
| No | Temperature Tp (°C) | Time tp (min) | H2SO4 (N) | Cellulose (%) | Hemicelluloses (%) | Xylan (%) | Arabinan (%) | Mannan (%) | Lignin (%) | Other Components (%) | SRY (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Untreated | 38.10 | 16.96 | 4.74 | 0.86 | 11.37 | 29.44 | 15.19 | 100 | ||
| 2 | 160 | 0 | - | 40.88 | 16.71 | 3.42 | 0.76 | 12.91 | 26.87 | 15.54 | 92.13 |
| 3 | 180 | 0 | - | 41.45 | 15.86 | 2.80 | 0.19 | 12.91 | 27.01 | 15.38 | 86.72 |
| 4 | 200 | 0 | - | 44.33 | 11.87 | 2.47 | - | 9.40 | 28.59 | 14.91 | 80.17 |
| 5 | 220 | 0 | - | 50.47 | 7.79 | 1.90 | - | 5.89 | 30.16 | 11.28 | 70.18 |
| 6 | 160 | 50 | - | 44.72 | 14.43 | 3.04 | 0.19 | 10.82 | 28.34 | 12.51 | 85.85 |
| 7 | 180 | 50 | - | 45.87 | 11.58 | 2.47 | - | 9.12 | 31.27 | 10.97 | 75.18 |
| 8 | 200 | 50 | - | 54.70 | 7.03 | 1.52 | - | 5.51 | 32.24 | 5.73 | 67.24 |
| 9 | 220 | 50 | - | 54.89 | 1.71 | - | - | 1.71 | 38.87 | 4.23 | 61.76 |
| 10 | 160 | 0 | 0.045 | 48.08 | 6.67 | 1.99 | - | 4.67 | 31.05 | 13.91 | 68.54 |
| 11 | 180 | 0 | 0.045 | 54.56 | 1.94 | 0.55 | - | 1.39 | 34.12 | 9.08 | 60.10 |
| 12 | 200 | 0 | 0.045 | 54.74 | 0.55 | - | - | 0.55 | 37.76 | 6.66 | 48.00 |
| 13 | 220 | 0 | 0.045 | 32.00 | 0.72 | - | - | 0.72 | 61.62 | 4.94 | 28.40 |
| 14 | 160 | 50 | 0.045 | 56.83 | 0.99 | - | - | 0.99 | 33.74 | 8.15 | 60.08 |
| 15 | 180 | 50 | 0.045 | 54.73 | 0.65 | - | - | 0.65 | 38.83 | 5.50 | 57.30 |
| 16 | 200 | 50 | 0.045 | 52.62 | 0.61 | - | - | 0.61 | 43.92 | 2.55 | 35.00 |
| 17 | 220 | 50 | 0.045 | 25.00 | 0.72 | - | - | 0.72 | 70.71 | 3.38 | 25.69 |
| Temperature Tp (°C) | Time tp (min) | H2SO4 (N) | BET Surface Area (m2/g) |
|---|---|---|---|
| Untreated | 0.703 | ||
| 160 | 0 | - | 0.812 |
| 180 | 0 | - | 1.567 |
| 200 | 0 | - | 2.600 |
| 220 | 0 | - | 2.849 |
| 160 | 50 | - | 2.893 |
| 180 | 50 | - | 2.594 |
| 200 | 50 | - | 2.968 |
| 220 | 50 | - | 4.078 |
| 160 | 0 | 0.045 | 0.517 |
| 180 | 0 | 0.045 | 2.323 |
| 200 | 0 | 0.045 | 8.613 |
| 220 | 0 | 0.045 | 9.392 |
| 160 | 50 | 0.045 | 1.248 |
| 180 | 50 | 0.045 | 2.026 |
| 200 | 50 | 0.045 | 8.700 |
| 220 | 50 | 0.045 | 11.335 |
| Freundlich | Langmuir | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature Tp (°C) | Time tp (min) | H2SO4 (N) | KF [(mg g−1)(L mg−1)1/n] | n | SEE | qm (mg g−1) | KL (L mg−1) | SEE |
| Untreated | 3.70 | 1.81 | 8.02 | 168.45 | 0.0043 | 6.16 | ||
| 160 | 0 | - | 4.80 | 2.14 | 6.44 | 110.68 | 0.0069 | 7.75 |
| 180 | 0 | - | 4.23 | 1.95 | 10.75 | 201.19 | 0.0022 | 11.84 |
| 200 | 0 | - | 7.48 | 2.32 | 3.97 | 120.95 | 0.0109 | 8.01 |
| 220 | 0 | - | 7.88 | 2.17 | 8.34 | 161.38 | 0.0089 | 5.78 |
| 160 | 50 | - | 6.21 | 2.26 | 9.38 | 113.64 | 0.0092 | 8.33 |
| 180 | 50 | - | 7.77 | 2.32 | 5.95 | 129.31 | 0.0101 | 7.08 |
| 200 | 50 | - | 4.78 | 1.70 | 8.17 | 265.31 | 0.0039 | 10.36 |
| 220 | 50 | - | 2.88 | 1.49 | 11.73 | 308.51 | 0.0027 | 10.30 |
| 160 | 0 | 0.045 | 5.97 | 2.09 | 6.18 | 142.83 | 0.0072 | 8.66 |
| 180 | 0 | 0.045 | 10.80 | 2.26 | 19.50 | 200.95 | 0.0082 | 19.62 |
| 200 | 0 | 0.045 | 14.25 | 2.31 | 7.78 | 225.20 | 0.0113 | 12.32 |
| 220 | 0 | 0.045 | 47.09 | 3.35 | 16.00 | 257.18 | 0.0705 | 20.82 |
| 160 | 50 | 0.045 | 6.21 | 1.88 | 8.10 | 216.42 | 0.0058 | 5.05 |
| 180 | 50 | 0.045 | 43.61 | 2.94 | 17.53 | 318.31 | 0.0333 | 18.01 |
| 200 | 50 | 0.045 | 19.11 | 2.81 | 17.58 | 170.35 | 0.0245 | 11.64 |
| 220 | 50 | 0.045 | 10.07 | 2.43 | 11.36 | 139.32 | 0.0143 | 6.82 |
| KL (L mg−1) | qm (mg g−1) | KF [(mg g−1)(L mg−1)1/n] | n | SEE | |
|---|---|---|---|---|---|
| Untreated Spruce Sawdust | |||||
| Freundlich | 3.702 | 1.807 | 8.017 | ||
| Langmuir | 0.00426 | 168.45 | 6.157 | ||
| Sips | 0.00367 | 179.37 | 1.060 | 6.4541 | |
| Radle-Prausniz | 0.00110 | 563.44 | 0.846 | 6.408 | |
| ModifiedRadle-Prausniz | 0.00425 | 168.51 | 0.995 | 6.489 | |
| Toth | 0.00430 | 168.00 | 1.0006 | 6.491 | |
| UNILAN | 0.00425 | 168.45 | −0.0016 | 6.489 | |
| Pretreated Spruce Sawdust with 50% Diethylene Glycol, 50% Water, 220 °C, 50 min | |||||
| Freundlich | 2.884 | 1.494 | 11.725 | ||
| Langmuir | 0.00272 | 308.52 | 10.301 | ||
| Sips | 0.00272 | 308.22 | 1.003 | 10.857 | |
| Radle-Prausniz | 0.00561 | 159.55 | 1.111 | 10.855 | |
| ModifiedRadle-Prausniz | 0.00272 | 308.51 | 1.001 | 10.858 | |
| Toth | 0.00293 | 311.04 | 1.013 | 10.856 | |
| UNILAN | 0.00272 | 308.47 | 0.212 | 10.857 | |
| Pretreated Spruce Sawdust with 50% Diethylene Glycol, 50% Water, 0.045 N Sulfuric Acid, 180 °C, 50 min | |||||
| Freundlich | 43.608 | 2.943 | 17.5321 | ||
| Langmuir | 0.0333 | 318.31 | 18.010 | ||
| Sips | 0.01161 | 436.48 | 1.651 | 10.8906 | |
| Radle-Prausniz | 0.24487 | 101.65 | 1.242 | 14.175 | |
| ModifiedRadle-Prausniz | 0.15610 | 131.80 | 1.274 | 14.848 | |
| Toth | 0.47094 | 565.35 | 2.374 | 12.414 | |
| UNILAN | 0.01251 | 432.44 | 3.238 | 13.880 | |
| Freundlich | Langmuir | ||||||
|---|---|---|---|---|---|---|---|
| Materials | KF [(mg g−1)(L mg−1)1/n] | n | qm (mg g−1) | KL (L mg−1) | pH | T (oC) | References |
| Magnetic biochar prepared from Melia azedarach wood | 3.38 | 2.47 | 25.27 | 0.047 | 3 | - | [41] |
| Mango kernel activated carbon | 1.198 | 0.76 | 7.96 | 0.2634 | 2 | 35 | [42] |
| Tea waste biomass | 9.832 | 5621 | 33.33 | 0.0653 | 8 | 30 | [43] |
| Wheat-residue black carbon | 2.35 | 2.40 | 21.34 | 0.0288 | 1 | 30 | [44] |
| Physically activated wood carbon | 5.436 | 1.435 | 46.95 | 0.118 | 2 | 20 | [45] |
| Physically activated date stone carbon | 6.844 | 2.008 | 43.10 | 0.132 | 20 | [45] | |
| Virgin bamboo charcoal | 1.524 | 1.258 | 7.58 | 0.013 | 2 | 25 | [17] |
| Bamboo charcoal-based, cobalt-coated adsorbent | 1.928 | 2.369 | 38.46 | 0.080 | 2 | 25 | [17] |
| Cotton stalk peel | 2.9 | 2.99 | 13.8 | 0.014 | 5.12 | 20 | [46] |
| Cotton stalk peel (amine-cross linked) | 0.36 | 3.74 | 117.9 | 0.024 | 5.12 | 20 | [46] |
| Oak bark chars | 0.523 | 2.016 | 4.619 | 0.073 | 2 | 25 | [21] |
| Oak wood char | 0.436 | 2.475 | 3.031 | 0.051 | 2 | 25 | [21] |
| Pine sawdust (Autohydrolyzed) | 8.928 | 4.776 | 345.9 | 0.00696 | 2 | 23 | [28] |
| Spruce sawdust (diethylene glycol, water) | 2.88 | 1.49 | 308.51 | 0.0027 | 2 | 23 | In this work |
| Spruce sawdust (diethylene glycol, water, sulfuric acid) | 43.61 | 2.94 | 318.31 | 0.0333 | 2 | 23 | In this work |
| Pseudo-First-Order Model | Pseudo-Second-Order Model | |||||||
|---|---|---|---|---|---|---|---|---|
| Temperature Tp (oC) | Time tp (min) | H2SO4 (N) | k (min−1) | q (mg g−1) | SEE | k2 (g mg−1min−1) | q (mg g−1) | SEE |
| Untreated | 0.0018 | 3.783 | 0.156 | 0.0003 | 5.225 | 0.133 | ||
| 160 | 0 | 0.0020 | 3.912 | 0.109 | 0.0003 | 5.438 | 0.100 | |
| 180 | 0 | - | 0.0024 | 3.539 | 0.157 | 0.0005 | 4.686 | 0.131 |
| 200 | 0 | - | 0.0024 | 4.114 | 0.116 | 0.0004 | 5.400 | 0.079 |
| 220 | 0 | - | 0.0038 | 4.396 | 0.192 | 0.0007 | 5.376 | 0.139 |
| 160 | 50 | - | 0.0022 | 4.042 | 0.152 | 0.0003 | 5.419 | 0.136 |
| 180 | 50 | - | 0.0031 | 4.551 | 0.177 | 0.0005 | 5.768 | 0.144 |
| 200 | 50 | - | 0.0036 | 3.978 | 0.211 | 0.0007 | 4.914 | 0.139 |
| 220 | 50 | - | 0.0034 | 3.588 | 0.244 | 0.0008 | 4.443 | 0.181 |
| 160 | 0 | 0.045 | 0.0019 | 3.926 | 0.196 | 0.0003 | 5.376 | 0.175 |
| 180 | 0 | 0.045 | 0.0023 | 4.454 | 0.137 | 0.0003 | 5.987 | 0.106 |
| 200 | 0 | 0.045 | 0.0049 | 4.500 | 0.285 | 0.0010 | 5.318 | 0.178 |
| 220 | 0 | 0.045 | 0.0032 | 4.009 | 0.191 | 0.0006 | 5.096 | 0.141 |
| 160 | 50 | 0.045 | 0.0049 | 3.605 | 0.182 | 0.0013 | 4.273 | 0.109 |
| 180 | 50 | 0.045 | 0.0097 | 5.892 | 0.216 | 0.0021 | 6.486 | 0.150 |
| 200 | 50 | 0.045 | 0.0034 | 4.273 | 0.244 | 0.0006 | 5.321 | 0.163 |
| 220 | 50 | 0.045 | 0.0021 | 4.188 | 0.149 | 0.0003 | 5.713 | 0.126 |
| Intra-Particle Diffusion Model | |||||
|---|---|---|---|---|---|
| Temperature Tp (oC) | Time tp (min) | H2SO4 (N) | kp (mg g−1min−1/2) | c (mg g−1) | SEE |
| Untreated | 0.1021 | −0.1831 | 0.091 | ||
| 160 | 0 | 0.1133 | −0.281 | 0.160 | |
| 180 | 0 | - | 0.1011 | −0.071 | 0.128 |
| 200 | 0 | - | 0.1203 | −0.141 | 0.147 |
| 220 | 0 | - | 0.1655 | −0.128 | 0.163 |
| 160 | 50 | - | 0.1147 | −0.146 | 0.151 |
| 180 | 50 | - | 0.1541 | −0.155 | 0.185 |
| 200 | 50 | - | 0.1527 | −0.182 | 0.166 |
| 220 | 50 | - | 0.1198 | −0.002 | 0.190 |
| 160 | 0 | 0.045 | 0.1069 | −0.142 | 0.144 |
| 180 | 0 | 0.045 | 0.1399 | −0.324 | 0.149 |
| 200 | 0 | 0.045 | 0.1721 | −0.148 | 0.203 |
| 220 | 0 | 0.045 | 0.1563 | −0.356 | 0.200 |
| 160 | 50 | 0.045 | 0.1662 | −0.150 | 0.122 |
| 180 | 50 | 0.045 | 0.3768 | −0.180 | 0.139 |
| 200 | 50 | 0.045 | 0.1837 | −0.388 | 0.166 |
| 220 | 50 | 0.045 | 0.1233 | −0.287 | 0.178 |
| Untreated | 160 °C, 50 min | 180 °C, 50 min | 200 °C, 50 min | 220 °C, 50 min | |
|---|---|---|---|---|---|
| Bohart-Adams Model | |||||
| N (mg L−1) | 662 | 1154 | 7814 | 1665 | 998 |
| K (L mg−1 min−1) | 0.00012 | 0.000033 | 0.000016 | 0.000050 | 0.000053 |
| SEE | 3.593 | 4.530 | 1.123 | 2.947 | 4.063 |
| Thomas Model | |||||
| q0 (mg g−1) | 2.437 | 5.148 | 31.08 | 7.425 | 4.452 |
| kT (L mg−1 min−1) | 0.00012 | 0.000033 | 0.000016 | 0.000050 | 0.000053 |
| SEE | 3.593 | 4.530 | 1.123 | 2.947 | 4.063 |
| Modified Dose-Response Model | |||||
| q0 (mg g−1) | 2.222 | 4.377 | 30.13 | 6.895 | 3.988 |
| amdr | 2.484 | 1.425 | 4.029 | 2.315 | 1.487 |
| SEE | 1.976 | 3.138 | 1.682 | 2.661 | 2.942 |
| Materials | kT (L mg−1 min−1) | q0 (mg g−1) | Reference |
|---|---|---|---|
| Spirulina platensis | 0.00178 | 6.087 | [56] |
| Strychnos nux vomica tree fruit shell | 0.00018 | 101.8 | [57] |
| Auto-hydrolyzed pine sawdust | 0.00024 | 18.87 | [58] |
| Modified corn stalk | 0.00095 | 152.3 | [59] |
| Nanosorbents from magnetite Impregnated Phaseolus vulgaris husk | 0.00018 | 53.01 | [60] |
| Spruce sawdust (diethylene glycol, water, sulfuric acid) | 0.000016 | 31.08 | In this work |
| Wavenumber [cm−1] | Assignment | Components | ||
|---|---|---|---|---|
| Untreated | Pretreated | Differences | ||
| 3464 | 3347 | 117 | O-H stretching | Cellulose, Hemicelluloses, Lignin |
| 2909 | 2942 | −33 | C-H stretching | Cellulose, Hemicelluloses, Lignin |
| 2362 | 2364 | −2 | N-H stretching | Cellulose, Hemicelluloses, Lignin |
| 1735 | 1700 | 35 | C=O stretching | Hemicelluloses, Lignin |
| 1654 | 1653 | 1 | Aromatic skeletal vibration, C=O stretching, adsorbed O-H | Hemicelluloses, Lignin |
| - | 1616 | - | C=C stretching of phenol group | Cellulose, Hemicelluloses, Lignin |
| 1507 | 1509 | −2 | C=C-C aromatic ring stretching and vibration | Lignin |
| 1435 | 1457 | −22 | C-H deformation (in methyl and methylene) | Lignin |
| 1374 | 1376 | −2 | C-H bending, C-H stretching in CH3 | Cellulose, Hemicelluloses, Lignin |
| 1335 | 1320 | 15 | CH2 wagging, C-O stretching of C5 substituted aromatic units | Cellulose, Hemicelluloses, Lignin |
| 1268 | 1281 | −13 | C-O stretching of guaiacyl unit | Lignin |
| 1134 | 1132 | 2 | C-O-C stretching | Cellulose, Hemicelluloses |
| 1043 | 1068 | −25 | C-OH stretching vibration, C-O deformation | Cellulose, Hemicelluloses, Lignin |
| - | 1039 | - | C-O stretching, aromatic C-H in plane deformation | Cellulose, Lignin |
| 902 | 855 | 47 | C-O-C stretching | Cellulose, Hemicelluloses |
| 805 | 851 | −46 | Aromatic C-H out of plane bending | Lignin |
| Wavenumber [cm−1] | Assignment | Components | ||
|---|---|---|---|---|
| Untreated | Pretreated | Differences | ||
| 3347 | 3410 | −63 | O-H stretching | Cellulose, Hemicelluloses, Lignin |
| 2942 | 2939 | 3 | C-H stretching | Cellulose, Hemicelluloses, Lignin |
| 2364 | 2365 | −1 | N-H stretching | Cellulose, Hemicelluloses, Lignin |
| 1700 | 1684 | 16 | C=O stretching | Hemicelluloses, Lignin |
| 1653 | 1651 | 2 | Aromatic skeletal vibration, C=O stretching, adsorbed O-H | Hemicelluloses, Lignin |
| 1616 | 1617 | −1 | C=C stretching of phenol group | Cellulose, Hemicelluloses, Lignin |
| 1509 | 1508 | 1 | C=C-C aromatic ring stretching and vibration | Lignin |
| 1457 | 1455 | 2 | C-H deformation (in methyl and methylene) | Lignin |
| 1376 | 1375 | 1 | C-H bending, C-H stretching in CH3 | Cellulose, Hemicelluloses, Lignin |
| 1320 | 1314 | 6 | CH2 wagging, C-O stretching of C5 substituted aromatic units | Cellulose, Hemicelluloses, Lignin |
| 1281 | 1283 | −2 | C-O stretching of guaiacyl unit | Lignin |
| 1132 | 1133 | −1 | C-O-C stretching | Cellulose, Hemicelluloses |
| 1068 | 1076 | −8 | C-OH stretching vibration, C-O deformation | Cellulose, Hemicelluloses, Lignin |
| 1039 | 1038 | 1 | C-O stretching, aromatic C-H in plane deformation | Cellulose, Lignin |
| 855 | 899 | −44 | C-O-C stretching | Cellulose, Hemicelluloses |
| 851 | 850 | 1 | Aromatic C-H out of the plane bending | Lignin |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Politi, D.; Sidiras, D. Modified Spruce Sawdust for Sorption of Hexavalent Chromium in Batch Systems and Fixed-Bed Columns. Molecules 2020, 25, 5156. https://doi.org/10.3390/molecules25215156
Politi D, Sidiras D. Modified Spruce Sawdust for Sorption of Hexavalent Chromium in Batch Systems and Fixed-Bed Columns. Molecules. 2020; 25(21):5156. https://doi.org/10.3390/molecules25215156
Chicago/Turabian StylePoliti, Dororthea, and Dimitrios Sidiras. 2020. "Modified Spruce Sawdust for Sorption of Hexavalent Chromium in Batch Systems and Fixed-Bed Columns" Molecules 25, no. 21: 5156. https://doi.org/10.3390/molecules25215156
APA StylePoliti, D., & Sidiras, D. (2020). Modified Spruce Sawdust for Sorption of Hexavalent Chromium in Batch Systems and Fixed-Bed Columns. Molecules, 25(21), 5156. https://doi.org/10.3390/molecules25215156








