Efficient Synthesis of Novel 1,3,4-Oxadiazoles Bearing a 4-N,N-Dimethylaminoquinazoline Scaffold via Palladium-Catalyzed Suzuki Cross-Coupling Reactions
Abstract
1. Introduction
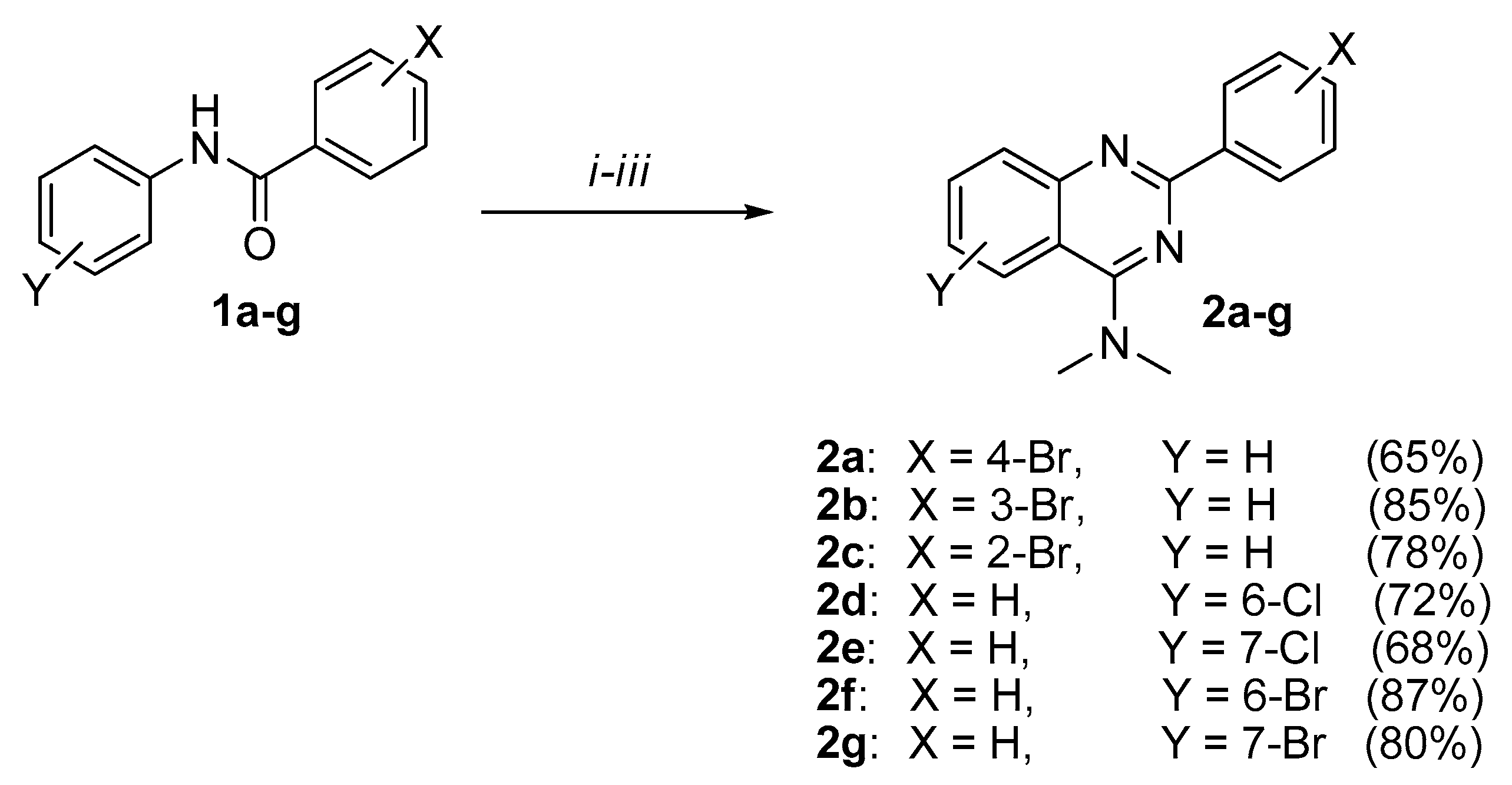
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. General Procedure for Preparing 4-(N,N-dimethylamino)-2-phenylquinazoline Derivatives 2a-g
3.2.2. Preparation of 2-(4-Bromophenyl)-5-phenyl-1,3,4-oxadiazole (5a)—Pathway A
3.2.3. Preparation of 2,5-bis(4-Bromophenyl)-1,3,4-oxadiazole (5b)—Pathway B
3.2.4. Preparation of 2-Phenyl-5-[4-(tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1,3,4-oxadiazole (6)
3.2.5. Preparation of bis[4-(Tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1,3,4-oxadiazole (7)
3.2.6. General Procedure for the Preparation of Unsymmetrical Quinazolinylphenyl-1,3,4-Oxadiazole Derivatives 8a-c, 8f,g via Suzuki Cross-Coupling from Boronic Acid Pinacol Ester 6
3.2.7. General Procedure for Preparing Symmetrical Quinazolinylphenyl-1,3,4-oxadiazole Derivatives 9a-c, 9f,g via Suzuki Cross-Coupling Using Diboronic Acid bis(pinacol) Ester 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Summa, V.; Petrocchi, A.; Bonelli, F.; Crescenzi, B.; Donghi, M.; Ferrara, M.; Fiore, F.; Gardelli, C.; Gonzalez Paz, O.; Hazuda, D.J.; et al. Discovery of Raltegravir, a Potent, Selective Orally Bioavailable HIV-Integrase Inhibitor for the Treatment of HIV-AIDS Infection. Med. Chem. 2008, 51, 5843–5855. [Google Scholar] [CrossRef]
- Tan, T.M.C.; Chen, Y.; Kong, K.H.; Bai, J.; Li, Y.; Lim, S.G.; Ang, T.H.; Lam, Y. Synthesis and the biological evaluation of 2-benzenesulfonylalkyl-5-substituted-sulfanyl-[1,3,4]-oxadiazoles as potential anti-hepatitis B virus agents. Antivir. Res. 2006, 71, 7–14. [Google Scholar] [CrossRef]
- De Oliveira, C.S.; Lira, B.F.; Barbosa-Filho, J.M.; Lorenzo, J.G.F.; De Athayde-Filho, P.F. Synthetic Approaches and Pharmacological Activity of 1,3,4-Oxadiazoles: A Review of the Literature from 2000–2012. Molecules 2012, 17, 10192–10231. [Google Scholar] [CrossRef]
- Rachakonda, V.; Alla, M.; Kotipalli, S.S.; Ummanni, R. Design, diversity-oriented synthesis and structure activity relationship studies of quinolinyl heterocycles as antimycobacterial agents. Eur. J. Med. Chem. 2013, 70, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.-Z.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.-C.; Chen, Q.; Yang, G.-F.; Clough, J. Synthesis and antifungal activity of 3-(1,3,4-oxadiazol-5-yl)-indoles and 3-(1,3,4-oxadiazol-5-yl)methyl-indoles. Eur. J. Med. Chem. 2013, 63, 22–32. [Google Scholar] [CrossRef]
- Reddy, G.D.; Park, S.J.; Cho, H.M.; Kim, T.J.; Lee, M.E. Antiallergic activity profile in vitro RBL-2H3 and in vivo passive cutaneous anaphylaxis mouse model of new sila-substituted 1,3,4-oxadiazoles. J. Med. Chem. 2012, 55, 6438–6444. [Google Scholar] [CrossRef]
- Paun, A.; Hadade, N.D.; Paraschivescu, C.C.; Matache, M. 1,3,4-Oxadiazoles as luminescent materials for organic light emitting diodes via cross-coupling reactions. J. Mater. Chem. C 2016, 4, 8596–8610. [Google Scholar] [CrossRef]
- Yang, X.; Xu, X.; Zhou, G. Recent advances of the emitters for high performance deep-blue organic light-emitting diodes. J. Mater. Chem. C 2015, 3, 913–944. [Google Scholar] [CrossRef]
- Han, J. 1,3,4-Oxadiazole based liquid crystals. J. Mater. Chem. C 2013, 1, 7779–7797. [Google Scholar] [CrossRef]
- Zheng, C.; Yuan, A.; Zhang, Z.; Shen, H.; Bai, S.; Wang, H. Synthesis of pyridine-based 1,3,4-oxadiazole derivative as fluorescence turn-on sensor for high selectivity of Ag+. J. Fluoresc. 2013, 23, 785–791. [Google Scholar] [CrossRef]
- Tang, L.; Zheng, Z.; Huang, Z.; Zhong, K.; Bian, Y.; Nandhakumar, R. Multi-analyte, ratiometric and relay recognition of a 2,5-diphenyl-1,3,4-oxadiazole-based fluorescent sensor through modulating ESIPT. RSC Adv. 2015, 5, 10505–10511. [Google Scholar] [CrossRef]
- Dong, Y.-B.; Zhang, Q.; Wang, L.; Ma, J.-P.; Huang, R.-Q.; Shen, D.-Z.; Chen, D.-Z. Organometallic Coordination Polymers Generated from Bent Bis(acetylenylphenyl)oxadiazole Ligands and Ag(I) Salts. Inorg. Chem. 2005, 44, 6591–6608. [Google Scholar] [CrossRef]
- Theocharis, A.B.; Alexandrou, N.E. Synthesis and spectral data of 4,5-bis[5 -aryl-1,3,4-oxadiazol-2-yl]-1-benzyl-1,2,3-triazoles. J. Heterocycl. Chem. 1990, 27, 1685–1688. [Google Scholar] [CrossRef]
- Tandon, V.K.; Chhor, R.B. An efficient one pot synthesis of 1,3,4-oxadiazoles. Synth. Commun. 2001, 31, 1727–1732. [Google Scholar] [CrossRef]
- Stabile, P.; Lamonica, A.; Ribecai, A.; Castoldi, D.; Guercio, G.; Curcuruto, O. Mild and convenient one-pot synthesis of 1,3,4-oxadiazoles. Tetrahedron Lett. 2010, 51, 4801–4805. [Google Scholar] [CrossRef]
- Dobrota, C.; Paraschivescu, C.; Dumitru, I.; Matache, M.; Baciu, I.; Ruta, L.L. Convenient preparation of unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles promoted by Dess–Martin reagent. Tetrahedron Lett. 2009, 50, 1886–1888. [Google Scholar] [CrossRef]
- Pardeshi, S.P.; Patil, S.S.; Bobade, V.D. N-Chlorosuccinimide/1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU)–Mediated Synthesis of 2,5-Disubstituted 1,3,4-Oxadiazoles. Synth. Commun. 2010, 40, 1601–1606. [Google Scholar] [CrossRef]
- Rapolu, S.; Alla, M.; Bommenaa, V.R.; Murthy, R.; Jain, N.; Bommareddy, V.R.; Bommineni, M.R. Synthesis and biological screening of 5-(alkyl(1H-indol-3-yl))-2-(substituted)-1,3,4-oxadiazoles as antiproliferative and anti-inflammatory agents. Eur. J. Med. Chem. 2013, 66, 91–100. [Google Scholar] [CrossRef]
- Rostamizadeh, S.; Housaini, S.A.G. Microwave assisted syntheses of 2,5-disubstituted 1,3,4-oxadiazoles. Tetrahedron Lett. 2004, 45, 8753–8756. [Google Scholar] [CrossRef]
- Jasiak, K.; Kudelko, A.; Zieliński, W.; Kuźnik, N. Study on DDQ-promoted synthesis of 2,5-disubstituted 1,3,4-oxadiazoles from acid hydrazides and aldehydes. ARKIVOC 2017, 2, 87–106. [Google Scholar] [CrossRef]
- Buscemi, S.; Pace, A.; Pibiri, I.; Vivona, N. Competing Ring-Photoisomerization Pathways in the 1,2,4-Oxadiazole Series. An Unprecedented Ring-Degenerate Photoisomerization. J. Org. Chem. 2002, 67, 6253–6255. [Google Scholar] [CrossRef]
- Shaker, R.M.; Mahmoud, A.F.; Abdel-Latif, F.F. Synthesis and Biological Activities of Novel 1,4-Bridged Bis-1,2,4-Triazoles, Bis-1,3,4-Thiadiazoles and Bis-1,3,4-Oxadiazoles. Phosphorus Sulfur Silicon Relat. Elements 2005, 180, 397–406. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, J.; Yang, P.; Shi, X.; Li, J. Synthesis of 2-amino-1,3,4-oxadiazoles from isoselenocyanates via cyclodeselenization. Tetrahedron 2011, 67, 5369–5374. [Google Scholar] [CrossRef]
- Dupau, P.; Epple, R.; Thomas, A.A.; Fokin, V.V.; Sharpless, K.B. Osmium-Catalyzed Dihydroxylation of Olefins in Acidic Media: Old Process, New Tricks. Adv. Synth. Catal. 2002, 344, 421–433. [Google Scholar] [CrossRef]
- Huisgen, R.; Sauer, J.; Sturm, H.J.; Markgraf, J.H. Ring opening of azoles. II. The formation of 1,3,4-oxadiazoles in the acylation of 5-substituted tetrazoles. Chem. Ber. 1960, 93, 2106–2124. [Google Scholar] [CrossRef]
- Verhaeghe, P.; Azas, N.; Gasquet, M.; Hutter, S.; Ducros, C.; Laget, M.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines. Bioorg. Med. Chem. Lett. 2008, 18, 396–401. [Google Scholar] [CrossRef]
- Chandrika, P.M.; Yakaiah, T.; Rao, A.R.R.; Narsaiah, B.; Reddy, N.C.; Sridhar, V.; Rao, J.V. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Giardin, D.; Martarelli, D.; Sagratini, G.; Angeli, P.; Ballinari, D.; Gulini, U.; Melchiorre, C.; Poggesi, E.; Pompei, P. Doxazosin-Related α1-Adrenoceptor Antagonists With Prostate Antitumor Activity. J. Med. Chem. 2009, 52, 4951–4954. [Google Scholar] [CrossRef]
- Shallal, H.M.; Russu, W.A. Discovery, synthesis, and investigation of the antitumor activity of novel piperazinylpyrimidine derivatives. Eur. J. Med. Chem. 2011, 46, 2043–2057. [Google Scholar] [CrossRef]
- El-Azab, A.S.; Abdel-Hamide, S.G.; Sayed-Ahmed, M.M.; Hassan, G.S.; El-Hadiyah, T.M.; Al-Shabanah, O.A.; Al-Deeb, O.A.; El-Subbagh, H.I. Novel 4(3H)-Quinazolinone Analogues: Synthesis and Anticonvulsant Activity. Med. Chem. Res. 2013, 22, 2815–2827. [Google Scholar] [CrossRef]
- Elshahawi, M.M.; El-Ziaty, A.K.; Morsy, J.M.; Aly, A.F. Synthesis and Insecticidal Efficacy of Novel Bis Quinazolinone Derivatives. J. Heterocycl. Chem. 2015, 53, 1443–1448. [Google Scholar] [CrossRef]
- Bowen Li, B.; Wang, Z.; Su, S.-J.; Guo, F.; Cao, Y.; Zhang, Y. Quinazoline-Based Thermally Activated Delayed Fluorecence for High-Performance OLEDs with External Quantum Efficiencies Exceeding 20%. Adv. Opt. Mater. 2019, 1801496. [Google Scholar] [CrossRef]
- Jiang, J.B.; Hesson, D.P.; Dusak, B.A.; Dexter, D.L.; Kang, G.L.; Hamel, E. Synthesis and biological evaluation of 2-styrylquinazolin-4(3H)-ones, a new class of antimitotic anticancer agents which inhibit tubulin polymerization. J. Med. Chem. 1990, 33, 1721–1728. [Google Scholar] [CrossRef] [PubMed]
- Rad-Moghadam, K.; Mohseni, M. An Expeditious and Solvent-Free Route to the Synthesis of 2-Substituted Quinazolin-4(3H)-Ones Under Microwave Conditions. J. Chem. Res. 2003, 8, 487–488. [Google Scholar] [CrossRef]
- Bergman, J.; Brynolf, A. Synthesis of Chrysogine, a Metabolite of Penicillium chrysogenum and some related 2-substituted 4-(3H)-Quinazolinones. Tetrahedron Lett. 1990, 46, 1295–1310. [Google Scholar] [CrossRef]
- Witt, A.; Bergman, J. Synthesis and Reactions of some 2-Vinyl-3H-quinazolin-4-ones. Tetrahedron 2000, 56, 7245–7253. [Google Scholar] [CrossRef]
- Connolly, D.J.; Guiry, P.J. A Facile and Versatile Route to 2-Substituted-4(3H)-Quinazolinones and Quinazolines. Synlett 2001, 11, 1707–1710. [Google Scholar] [CrossRef]
- Hess, H.-J.; Cronin, T.H.; Scriabine, A. Antihypertensive 2-amino-4(3H)-quinazolinones. J. Med. Chem. 1968, 11, 130–136. [Google Scholar] [CrossRef]
- Connolly, D.J.; Cusack, D.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014, 2014, 395637. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, D.; Yu, C.; Wan, C.; Wang, Z. A Simple and Efficient Approach to the Synthesis of 2-Phenylquinazolines via sp3 C−H Functionalization. Org. Lett. 2010, 12, 2841–2843. [Google Scholar] [CrossRef]
- Karnakar, K.; Shangkar, J.; Murthy, S.N.; Ramesch, K.; Nageshwar, Y.V.D. An Efficient Protocol for the Synthesis of 2-Phenylquinazolines Catalyzed by Ceric Ammonium Nitrate (CAN). Synlett 2011, 8, 1089–1096. [Google Scholar] [CrossRef]
- Kraft, A.; Grimsdale, A.C.; Holmes, A.B. Electroluminescent Conjugated Polymers—Seeing Polymers in a New Light. Angew. Chem. Int. Ed. 1998, 37, 402–428. [Google Scholar] [CrossRef]
- Mitschke, U.; Bauerle, P. The electroluminescence of organic materials. J. Mater. Chem. 2000, 10, 1471–1507. [Google Scholar] [CrossRef]
- Wang, C.; Jung, G.-Y.; Hua, Y.; Pearson, C.; Bryce, M.R.; Petty, M.C.; Batsanov, A.S.; Goeta, A.E.; Howard, J.A.K. An Efficient Pyridine- and Oxadiazole-Containing Hole-Blocking Material for Organic Light-Emitting Diodes: Synthesis, Crystal Structure, and Device Performance. Chem. Mater. 2001, 13, 1167–1173. [Google Scholar] [CrossRef]
- Wang, C.; Jung, G.-Y.; Batsanov, A.S.; Bryce, M.R.; Petty, M.C. New electron-transporting materials for light emitting diodes: 1,3,4-oxadiazole–pyridine and 1,3,4-oxadiazole–pyrimidine hybrids. J. Mater. Chem. 2002, 12, 173–180. [Google Scholar] [CrossRef]
- Waśkiewicz, K.; Gabański, R.; Żak, J.; Suwiński, J. Electrochemical Isomerization and Polymerization of Three Stereoisomers of a Novel Photoluminescent Thienylene-PPV Derivative. Electrochem. Solid-State Lett. 2005, 8, E24–E26. [Google Scholar] [CrossRef]
- Fuks-Janczarek, I.; Reshak, A.H.; Kuźnik, N.; Kityk, I.V.; Gabański, R.; Łapkowski, M.; Motyka, R.; Suwiński, J. UV–vis absorption spectra of 1,4-dialkoxy-2,5-bis[2-(thien-2-yl)ethenyl]benzenes. Spectrochim. Acta Part A 2009, 72, 394–398. [Google Scholar] [CrossRef]
- Tamoto, N.; Adachi, C.; Nagai, K. Electroluminescence of 1,3,4-Oxadiazole and Triphenylamine-Containing Molecules as an Emitter in Organic Multilayer Light Emitting Diodes. Chem. Mater. 1997, 9, 1077–1085. [Google Scholar] [CrossRef]
- Kudelko, A.; Wróblowska, M.; Jarosz, T.; Łaba, K.; Łapkowski, M. Synthesis, spectral characteristics and electrochemistry of symmetrically substituted hybrids derived from 2,5-bis(4-bromophenyl)-1,3,4-oxadiazole under Suzuki cross-coupling reaction. ARKIVOC 2015, 5, 287–302. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Łapkowski, M. Efficient Synthesis of Conjugated 1,3,4-Thiadiazole Hybrids through Palladium-Catalyzed Cross-Coupling of 2,5-Bis(4-bromophenyl)-1,3,4-thiadiazole with Boronic Acids. Synlett 2015, 26, 2127–2130. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Synthesis of 4-alkyl-4H-1,2,4-triazole derivatives by Suzuki cross-coupling reactions and their luminescence properties. Molecules 2019, 24, 652. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions via organoboranes. J. Organomet. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Miyaura, N. Metal-Catalyzed Cross-Coupling Reactions of Organoboron Compounds with Organic Halides. In Metal-Catalyzed Cross-Coupling Reactions; de Meijere, A., Diederich, F., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 41–123. [Google Scholar]
- Zieliński, W.; Kudelko, A.; Holt, E.M. Synthesis of 2,4-Diaminoquinazoline Derivatives. Heterocycles 1998, 48, 319–328. [Google Scholar] [CrossRef]
- Zieliński, W.; Kudelko, A. The synthesis of 8-hydroxyquinazoline derivatives and their acid-base interactions. J. Heterocycl. Chem. 2004, 41, 247–251. [Google Scholar] [CrossRef]
- Zieliński, W.; Kudelko, A. Synthesis and Basicity of 4-Amino-2-phenylquinazolines. Monatshefte Chem. 2000, 131, 895–899. [Google Scholar] [CrossRef]
- Jose, D.E.; Kanchana, U.S.; Mathew, T.V.; Anilkumar, G. Recent studies in Suzuki-Miyaura cross-coupling reactions with the aid of phase transfer catalysts. J. Organomet. Chem. 2020, 927, 121538. [Google Scholar] [CrossRef]
- Castanet, A.-S.; Colobert, F.; Desmurs, J.-R.; Schlama, T. Biaryl synthesis via Suzuki coupling promoted by catalytic amounts of quaternary ammonium salts. J. Mol. Catal. A Chem. 2002, 182–183, 481–487. [Google Scholar] [CrossRef]
- Polackova, V.; Hut’ka, M.; Toma, S. Ultrasound effect on Suzuki reactions. 1. Synthesis of unsymmetrical biaryls. Ultrason. Sonochem. 2005, 12, 99–102. [Google Scholar] [CrossRef]
- Badone, D.; Baroni, M.; Cardamone, R.; Ielmini, A.; Guzzi, U. Highly Efficient Palladium-Catalyzed Boronic Acid Coupling Reactions in Water: Scope and Limitations. J. Org. Chem. 1997, 62, 7170–7173. [Google Scholar] [CrossRef] [PubMed]
- Suzuki. K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef]
- Dahl, K.; Biswas, R.; Maroncelli, M. The Photophysics and Dynamics of Diphenylbutadiene in Alkane and Perfluoroalkane Solvents. J. Phys. Chem. B 2003, 107, 7838–7853. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Quenching of Fluorescence. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Boston, MA, USA, 2006; Chapter 8; pp. 277–330. [Google Scholar] [CrossRef]
- Chen, X.; Yang, Q.; Zhou, Y.; Deng, Z.; Mao, X.; Peng, Y. Synthesis of 4-(Dimethylamino)quinazoline via Direct Amination of Quinazolin-4(3H)-one Using N,N-Dimethylformamide as a Nitrogen Source at Room Temperature. Synthesis 2015, 47, 2055–2062. [Google Scholar] [CrossRef]
- Zieliński, W.; Mazik, M. Synthesis of 4-aminoquinazoline derivatives. Pol. J. Chem. 1994, 68, 487–489. [Google Scholar]
- Kawano, T.; Yoshizumi, T.; Hirano, K.; Satoh, T.; Miura, M. Copper-Mediated Direct Arylation of 1,3,4-Oxadiazoles and 1,2,4-Triazoles with Aryl Iodides. Org. Lett. 2009, 11, 3072–3075. [Google Scholar] [CrossRef]
- Zhan, X.; Liu, Y.; Wu, X.; Wang, S.; Zhu, D. New Series of Blue-Emitting and Electron-Transporting Copolymers Based on Fluorene. Macromolecules 2002, 35, 2529–2537. [Google Scholar] [CrossRef]





| Entry | Catalyst | Base | Solvent | PTC Catalyst | Yield [%] a |
|---|---|---|---|---|---|
| 1 | Pd(PPh3)4 | Na2CO3 | Toluene/water | Aliquat 336 | 63 |
| 2 | Pd(PPh3)4 | Na2CO3 | Toluene/water | BnEt3NCl | 61 |
| 3 | Pd(PPh3)4 | Na2CO3 | Toluene/water | Bu4NCl | 65 |
| 4 | Pd(PPh3)4 | Na2CO3 | Toluene/water | Bu4NBr | 68 |
| 5 | Pd(t-Bu3P)2 | Na2CO3 | Toluene/water | Bu4NBr | 68 |
| 6 | Pd(dppf)Cl2 | Na2CO3 | Toluene/water | Bu4NBr | 85 |
| 7 | Pd(PPh3)2Cl2 | Na2CO3 | Toluene/water | Bu4NBr | 77 |
| 8 | Pd(dppf)Cl2 | K2CO3 | Toluene/water | Bu4NBr | 60 |
| 9 | Pd(dppf)Cl2 | t-BuOK | Toluene/water | Bu4NBr | 70 |
| 10 | Pd(dppf)Cl2 | AcONa | Toluene/water | Bu4NBr | 66 |
| 11 | Pd(dppf)Cl2 | Na2CO3 | Dioxane | - | 74 |
| 12 | Pd(dppf)Cl2 | Na2CO3 | EtOH | - | 77 |
| 13 | Pd(dppf)Cl2 | Na2CO3 | DME | - | 61 |
| 14 | Pd(dppf)Cl2 | Na2CO3 | DMF | - | 64 |
| Entry | Substrate | Product | Yield [%] a | ||
|---|---|---|---|---|---|
| 1. | 2a |  | 8a |  | 82 a |
| 2. | 2b |  | 8b |  | 74 a |
| 3. | 2c |  | 8c |  | 86 a |
| 4. | 2d |  | No reaction a,b | - | |
| 5. | 2e |  | No reaction a,b | - | |
| 6. | 2f |  | 8f |  | 75 a |
| 7. | 2g |  | 8g |  | 72 a |
| Entry | Substrate | Product | Yield [%] a | |
|---|---|---|---|---|
| 1. | 2a | 9a |  | 70 |
| 2. | 2b | 9b |  | 50 |
| 3. | 2c | 9c |  | 60 |
| 4. | 2d | No reaction | - | |
| 5. | 2e | No reaction | - | |
| 6. | 2f | 9f |  | 52 |
| 7. | 2g | 9g |  | 65 |
| Entry | Compound | Absorption Maximum λmax [nm] (ε.10−4 m3/(mol.cm)]) | Excitation Wavelength λex [nm] | Emission Wavelength λem [nm] | Stokes Shift a ∆ [nm] | Quantum Yield b Φf |
|---|---|---|---|---|---|---|
| 1. | 6 | 291 (3.02) | 290 | 343 (max), 355 | 52 | 0.68 |
| 2. | 7 | 298 (2.97) | 300 | 333, 349 (max), 364 | 51 | 0.91 |
| 3. | 8a | 329 (4.98) | 330 | 414 | 85 | 0.01 |
| 4. | 8b | 305 (4.37) | 310 | 365 (max), 385 | 60 | 0.02 |
| 5. | 8c | 302 (2.94) | 305 | 366 (max), 384 | 64 | 0.13 |
| 6. | 8f | 297 (5.63) | 300 | 429 | 131 | 0.61 |
| 7. | 8g | 314 (7.44) | 315 | 419 | 105 | 0.08 |
| 8. | 9a | 342 (6.91) | 340 | 424 | 82 | 0.05 |
| 9. | 9b | 320 (8.46) | 320 | 378 (max), 397 | 58 | 0.01 |
| 10. | 9c | 319 (4.78) | 320 | 427 | 108 | 0.01 |
| 11. | 9f | 292 (5.07) | 295 | 424 | 70 | 0.98 |
| 12. | 9g | 329 (11.04) | 330 | 421 | 92 | 0.06 |
Sample Availability: Samples of the compounds 8a-c, 8f,g, 9a-c, 9f,g are available from the authors | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołek, B.; Werłos, M.; Komander, M.; Kudelko, A. Efficient Synthesis of Novel 1,3,4-Oxadiazoles Bearing a 4-N,N-Dimethylaminoquinazoline Scaffold via Palladium-Catalyzed Suzuki Cross-Coupling Reactions. Molecules 2020, 25, 5150. https://doi.org/10.3390/molecules25215150
Wołek B, Werłos M, Komander M, Kudelko A. Efficient Synthesis of Novel 1,3,4-Oxadiazoles Bearing a 4-N,N-Dimethylaminoquinazoline Scaffold via Palladium-Catalyzed Suzuki Cross-Coupling Reactions. Molecules. 2020; 25(21):5150. https://doi.org/10.3390/molecules25215150
Chicago/Turabian StyleWołek, Barbara, Mateusz Werłos, Magdalena Komander, and Agnieszka Kudelko. 2020. "Efficient Synthesis of Novel 1,3,4-Oxadiazoles Bearing a 4-N,N-Dimethylaminoquinazoline Scaffold via Palladium-Catalyzed Suzuki Cross-Coupling Reactions" Molecules 25, no. 21: 5150. https://doi.org/10.3390/molecules25215150
APA StyleWołek, B., Werłos, M., Komander, M., & Kudelko, A. (2020). Efficient Synthesis of Novel 1,3,4-Oxadiazoles Bearing a 4-N,N-Dimethylaminoquinazoline Scaffold via Palladium-Catalyzed Suzuki Cross-Coupling Reactions. Molecules, 25(21), 5150. https://doi.org/10.3390/molecules25215150







