Rose (Rosa gallica) Petal Extract Suppress Proliferation, Migration, and Invasion of Human Lung Adenocarcinoma A549 Cells through via the EGFR Signaling Pathway
Abstract
1. Introduction
2. Results
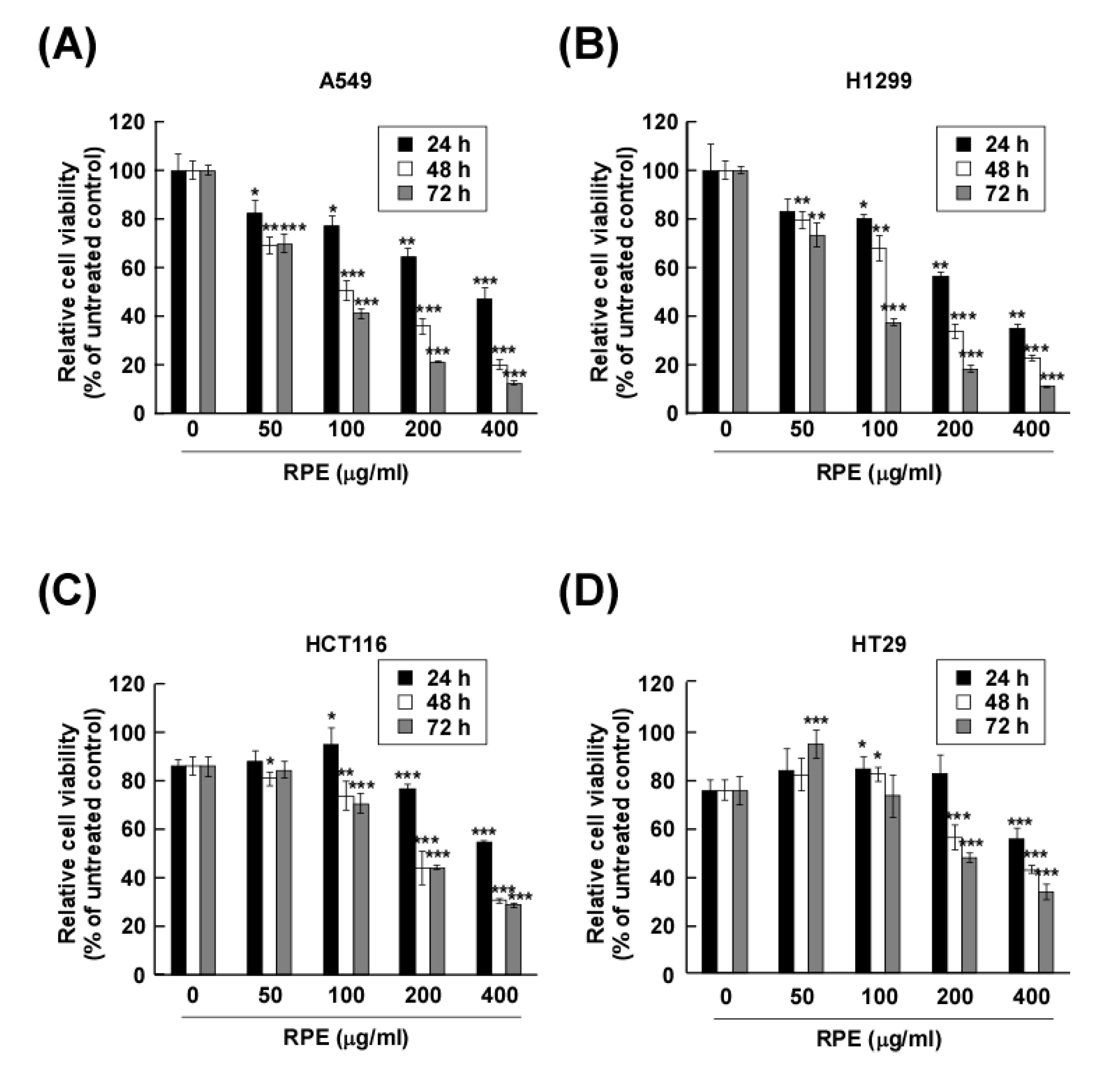
2.1. Rose Petal Extract Suppresses Lung and Colon Cancer Cell Proliferation
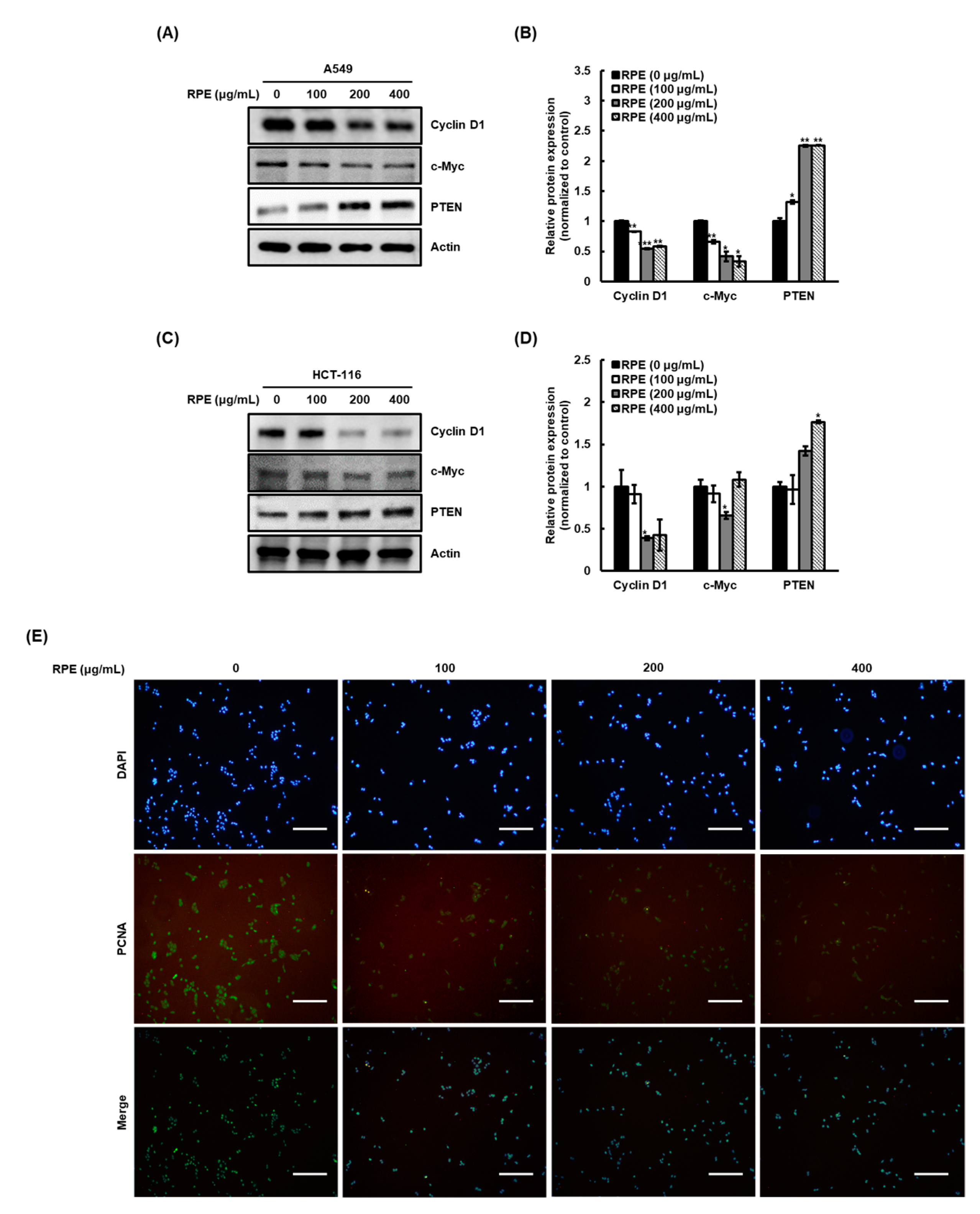
2.2. Rose Petal Extract Reduces Cyclin D1 and c-myc Expression and Enhances PTEN Expression in A549 Cells
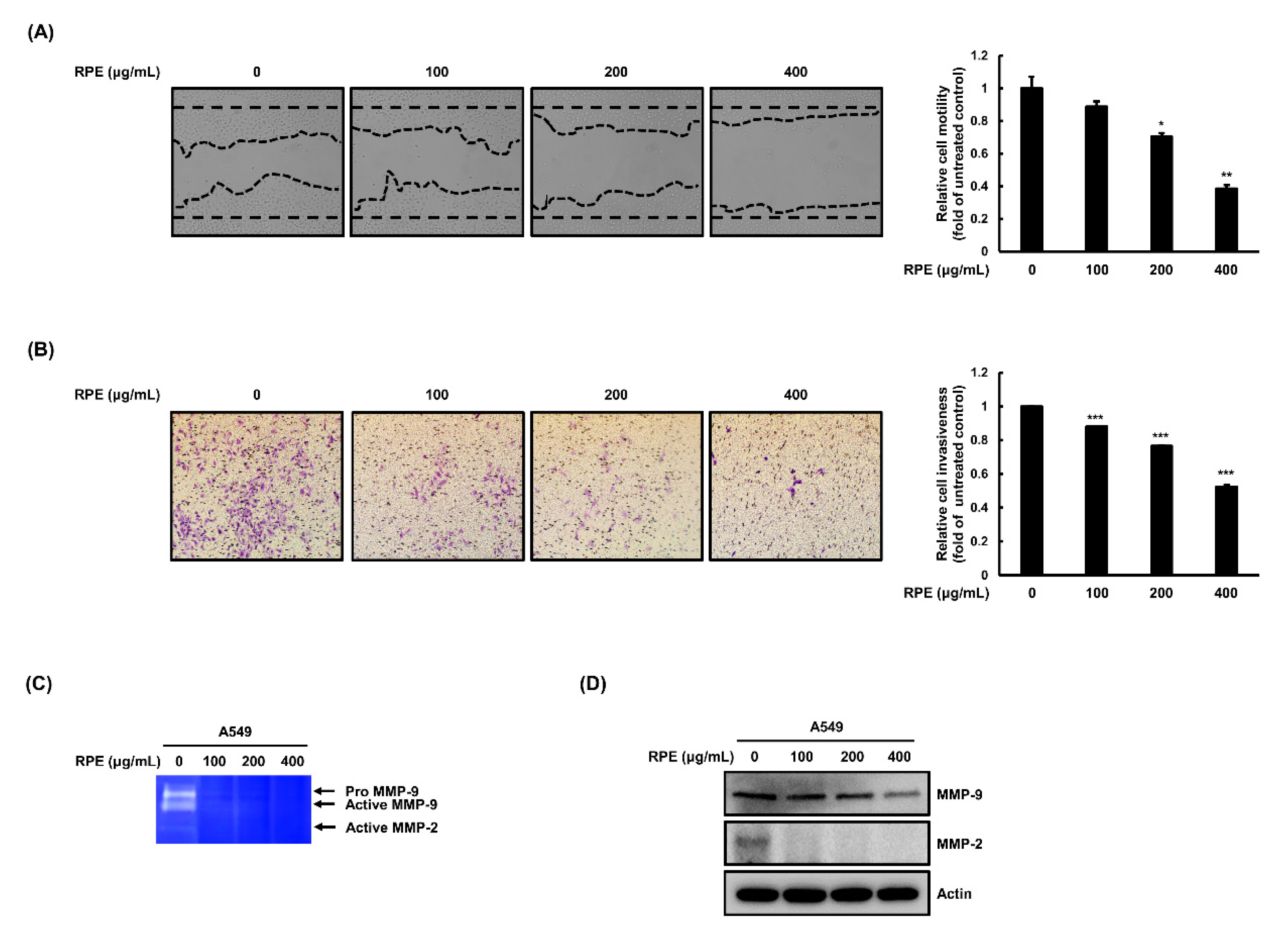
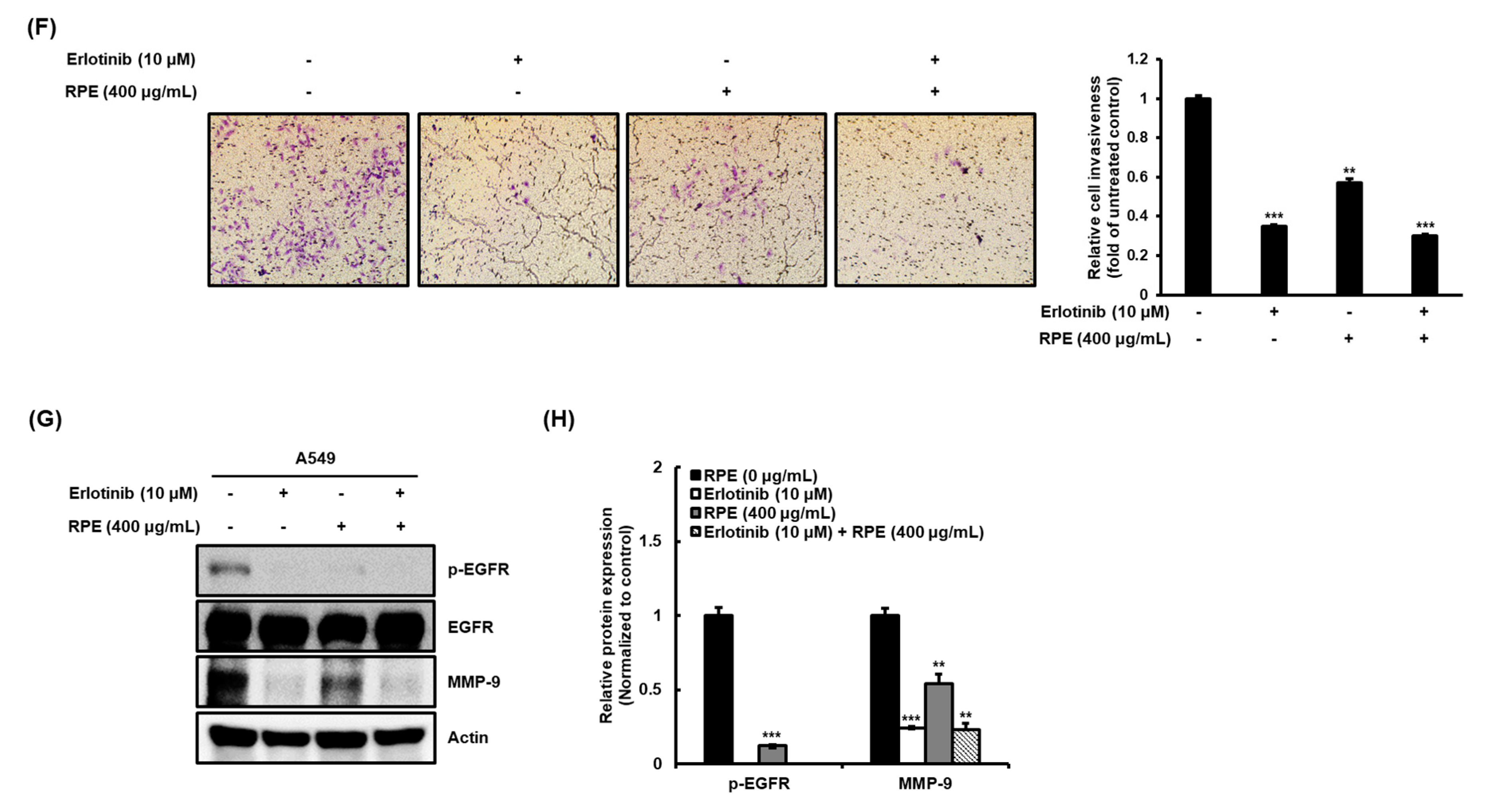
2.3. Rose Petal Extract Inhibits Cell Migration and Invasion by A549 Cells
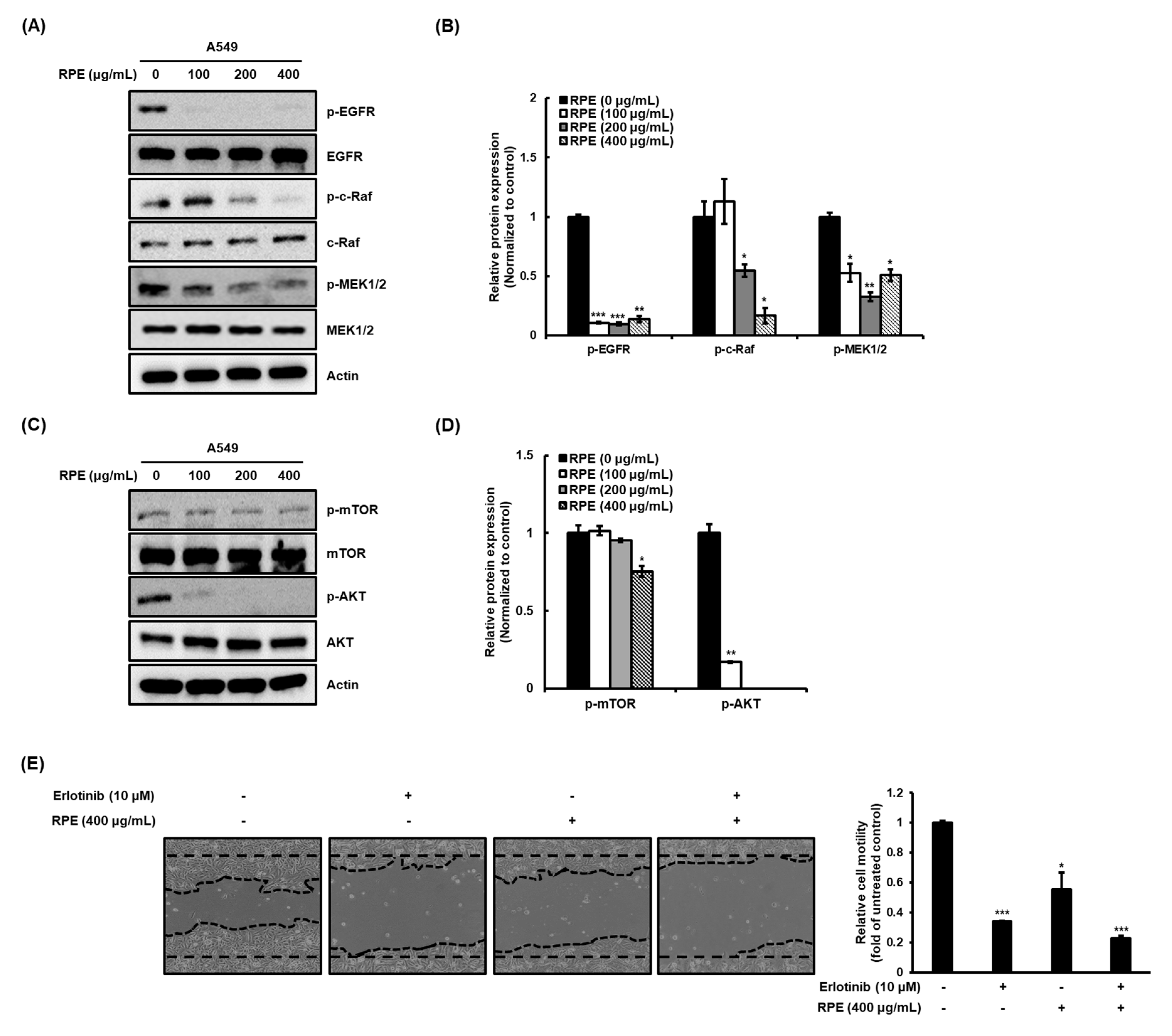
2.4. Rose Petal Extract Regulates EGFR-c-Raf-MEK and the mTOR-Akt Signaling Pathway in A549 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Rose Petal Extract (RPE)
4.3. Cell Culture
4.4. Cell Proliferation Assay
4.5. Immunofluorescence Assay
4.6. Western Blot Analysis
4.7. Migration Assay
4.8. Invasion Assay
4.9. Gelatin Zymography Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhao, B.; Chang, H.; Xiao, M.; Wu, Y.; Liu, Y. Paclitaxel suppresses proliferation and induces apoptosis through regulation of ROS and the AKT/MAPK signaling pathway in canine mammary gland tumor cells. Mol. Med. Rep. 2018, 17, 8289–8299. [Google Scholar] [CrossRef] [PubMed]
- Liao, D.J.; Thakur, A.; Wu, J.; Biliran, H.; Sarkar, F.H. Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit. Rev. Oncog. 2007, 13, 93–158. [Google Scholar] [CrossRef] [PubMed]
- Mittal, V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu. Rev. Pathol. 2018, 13, 395–412. [Google Scholar] [CrossRef] [PubMed]
- Siwak, D.R.; Carey, M.; Hennessy, B.T.; Nguyen, C.T.; McGahren Murray, M.J.; Nolden, L.; Mills, G.B. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: Current knowledge and future challenges. J. Oncol. 2010, 2010, 568938. [Google Scholar] [CrossRef] [PubMed]
- Seshacharyulu, P.; Ponnusamy, M.P.; Haridas, D.; Jain, M.; Ganti, A.K.; Batra, S.K. Targeting the EGFR signaling pathway in cancer therapy. Expert Opin. Ther. Targets 2012, 16, 15–31. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Pecio, L.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 2013, 94, 560–567. [Google Scholar] [CrossRef]
- Hajra, A.; Dutta, S.; Mondal, N.K. Mosquito larvicidal activity of cadmium nanoparticles synthesized from petal extracts of marigold (Tagetes sp.) and rose (Rosa sp.) flower. J. Parasit. Dis. 2016, 40, 1519–1527. [Google Scholar] [CrossRef]
- Jeon, J.H.; Kwon, S.C.; Park, D.; Shin, S.; Jeong, J.H.; Park, S.Y.; Hwang, S.Y.; Kim, Y.B.; Joo, S.S. Anti-allergic effects of white rose petal extract and anti-atopic properties of its hexane fraction. Arch. Pharm. Res. 2009, 32, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Nam, T.G.; Lee, I.; Shin, E.J.; Han, A.R.; Lee, P.; Lee, S.Y.; Lim, T.G. Skin anti-inflammatory activity of rose petal extract (Rosa gallica) through reduction of MAPK signaling pathway. Food Sci. Nutr. 2018, 6, 2560–2567. [Google Scholar] [CrossRef] [PubMed]
- Park, D.; Shin, K.; Choi, Y.; Guo, H.; Cha, Y.; Kim, S.H.; Han, N.S.; Joo, S.S.; Choi, J.K.; Lee, Y.B.; et al. Antimicrobial activities of ethanol and butanol fractions of white rose petal extract. Regul. Toxicol. Pharmacol. 2016, 76, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Yon, J.M.; Kim, Y.B.; Park, D. The Ethanol Fraction of White Rose Petal Extract Abrogates Excitotoxicity-Induced Neuronal Damage In Vivo and In Vitro through Inhibition of Oxidative Stress and Proinflammation. Nutrients 2018, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tai, Y.; Lisanti, M.P.; Liao, D.J. c-Myc induction of programmed cell death may contribute to carcinogenesis: A perspective inspired by several concepts of chemical carcinogenesis. Cancer Biol. Ther. 2011, 11, 615–626. [Google Scholar] [CrossRef]
- Li, X.F.; Song, B.; Xu, Q.Q.; Yin, S.Q.; Chen, X.; Xu, L.; Zhang, L.; Wen, J.G.; Huang, C.; Meng, X.M.; et al. Over-Expression of PTEN Suppresses the Proliferation and Migration of Fibroblast-Like Synoviocytes in Adjuvant-Induced Arthritis. Cell Physiol. Biochem. 2019, 52, 1446–1462. [Google Scholar]
- Li, S.X.; Chen, S.X.; Jiang, Y.Y.; Liu, J.F.; Yang, X.L.; Quan, S.C. Synergistic interaction between MEK inhibitor and gefitinib in EGFR-TKI-resistant human lung cancer cells. Oncol. Lett. 2015, 10, 2652–2656. [Google Scholar] [CrossRef]
- Ono, M.; Kuwano, M. Molecular mechanisms of epidermal growth factor receptor (EGFR) activation and response to gefitinib and other EGFR-targeting drugs. Clin. Cancer Res. 2006, 12, 7242–7251. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hiroki, K.; Yamashita, Y. The Role of Epidermal Growth Factor Receptor in Cancer Metastasis and Microenvironment. Biomed. Res. Int. 2013. [Google Scholar] [CrossRef]
- von Karstedt, S.; Conti, A.; Nobis, M.; Montinaro, A.; Hartwig, T.; Lemke, J.; Legler, K.; Annewanter, F.; Campbell, A.D.; Taraborrelli, L.; et al. Cancer cell-autonomous TRAIL-R signaling promotes KRAS-driven cancer progression, invasion, and metastasis. Cancer Cell 2015, 27, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, T.; Tahara, S.; Takabayashi, F. Inhibitory effect of natural coumarin compounds, esculetin and esculin, on oxidative DNA damage and formation of aberrant crypt foci and tumors induced by 1,2-dimethylhydrazine in rat colons. Biol. Pharm. Bull. 2007, 30, 2052–2057. [Google Scholar] [CrossRef]
- Nguyen-Ba, G.; Vasseur, P. Epigenetic events during the process of cell transformation induced by carcinogens (review). Oncol. Rep. 1999, 6, 925–932. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Wang, Y.; Thakur, A.; Sun, Y.; Wu, J.; Biliran, H.; Bollig, A.; Liao, D.J. Synergistic effect of cyclin D1 and c-Myc leads to more aggressive and invasive mammary tumors in severe combined immunodeficient mice. Cancer Res. 2007, 67, 3698–3707. [Google Scholar] [CrossRef]
- Papa, A.; Pandolfi, P.P. The PTEN(-)PI3K Axis in Cancer. Biomolecules 2019, 9, 153. [Google Scholar] [CrossRef]
- Dillekas, H.; Rogers, M.S.; Straume, O. Are 90% of deaths from cancer caused by metastases? Cancer Med. 2019, 8, 5574–5576. [Google Scholar] [CrossRef]
- Birkedal-Hansen, H.; Moore, W.G.; Bodden, M.K.; Windsor, L.J.; Birkedal-Hansen, B.; DeCarlo, A.; Engler, J.A. Matrix metalloproteinases: A review. Crit. Rev. Oral Biol. Med. 1993, 4, 197–250. [Google Scholar] [CrossRef]
- Lim, W.C.; Kim, H.; Kim, Y.J.; Jeon, B.N.; Kang, H.B.; Ko, H. Catechol inhibits epidermal growth factor-induced epithelial-to-mesenchymal transition and stem cell-like properties in hepatocellular carcinoma cells. Sci. Rep. 2020, 10, 7620. [Google Scholar] [CrossRef]
- Schafer, K.A. The cell cycle: A review. Vet. Pathol. 1998, 35, 461–478. [Google Scholar] [CrossRef]
- Han, X.; Liu, C.F.; Gao, N.; Zhao, J.; Xu, J. Kaempferol suppresses proliferation but increases apoptosis and autophagy by up-regulating microRNA-340 in human lung cancer cells. Biomed. Pharm. 2018, 108, 809–816. [Google Scholar] [CrossRef]
- Meng, G.; Chai, K.; Li, X.; Zhu, Y.; Huang, W. Luteolin exerts pro-apoptotic effect and anti-migration effects on A549 lung adenocarcinoma cells through the activation of MEK/ERK signaling pathway. Chem. Biol. Interact. 2016, 257, 26–34. [Google Scholar] [CrossRef]
- Milligan, S.A.; Burke, P.; Coleman, D.T.; Bigelow, R.L.; Steffan, J.J.; Carroll, J.L.; Williams, B.J.; Cardelli, J.A. The green tea polyphenol EGCG potentiates the antiproliferative activity of c-Met and epidermal growth factor receptor inhibitors in non-small cell lung cancer cells. Clin. Cancer Res. 2009, 15, 4885–4894. [Google Scholar] [CrossRef]
- Grandis, J.R.; Sok, J.C. Signaling through the epidermal growth factor receptor during the development of malignancy. Pharmacol. Ther. 2004, 102, 37–46. [Google Scholar] [CrossRef]
- Wells, A. EGF receptor. Int. J. Biochem. Cell Biol. 1999, 31, 637–643. [Google Scholar] [CrossRef]
- Yarden, Y.; Sliwkowski, M.X. Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2001, 2, 127–137. [Google Scholar] [CrossRef]
- Lee, K.W.; Kang, N.J.; Oak, M.H.; Hwang, M.K.; Kim, J.H.; Schini-Kerth, V.B.; Lee, H.J. Cocoa procyanidins inhibit expression and activation of MMP-2 in vascular smooth muscle cells by direct inhibition of MEK and MT1-MMP activities. Cardiovasc. Res. 2008, 79, 34–41. [Google Scholar] [CrossRef]
- He, S.; Liao, T.T.; Chen, Y.T.; Kuo, H.M.; Lin, Y.L. Glutathione-S-transferase enhances proliferation-migration and protects against shikonin-induced cell death in breast cancer cells. Kaohsiung J. Med Sci. 2011, 27, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.D.; Craft, D.W.; Shiromoto, R.S.; Yan, P.O. Alternative cell line for virus isolation. J. Clin. Microbiol. 1986, 24, 265–268. [Google Scholar] [CrossRef]
- Chen, J.H.; Lin, H.H.; Chiang, T.A.; Hsu, J.D.; Ho, H.H.; Lee, Y.C.; Wang, C.J. Gaseous nitrogen oxide promotes human lung cancer cell line A549 migration, invasion, and metastasis via iNOS-mediated MMP-2 production. Toxicol. Sci. Off. J. Soc. Toxicol. 2008, 106, 364–375. [Google Scholar] [CrossRef]
Sample Availability: The samples of RPE are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, W.-C.; Choi, H.-K.; Kim, K.-T.; Lim, T.-G. Rose (Rosa gallica) Petal Extract Suppress Proliferation, Migration, and Invasion of Human Lung Adenocarcinoma A549 Cells through via the EGFR Signaling Pathway. Molecules 2020, 25, 5119. https://doi.org/10.3390/molecules25215119
Lim W-C, Choi H-K, Kim K-T, Lim T-G. Rose (Rosa gallica) Petal Extract Suppress Proliferation, Migration, and Invasion of Human Lung Adenocarcinoma A549 Cells through via the EGFR Signaling Pathway. Molecules. 2020; 25(21):5119. https://doi.org/10.3390/molecules25215119
Chicago/Turabian StyleLim, Won-Chul, Hyo-Kyung Choi, Kyung-Tack Kim, and Tae-Gyu Lim. 2020. "Rose (Rosa gallica) Petal Extract Suppress Proliferation, Migration, and Invasion of Human Lung Adenocarcinoma A549 Cells through via the EGFR Signaling Pathway" Molecules 25, no. 21: 5119. https://doi.org/10.3390/molecules25215119
APA StyleLim, W.-C., Choi, H.-K., Kim, K.-T., & Lim, T.-G. (2020). Rose (Rosa gallica) Petal Extract Suppress Proliferation, Migration, and Invasion of Human Lung Adenocarcinoma A549 Cells through via the EGFR Signaling Pathway. Molecules, 25(21), 5119. https://doi.org/10.3390/molecules25215119






