A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment
Abstract
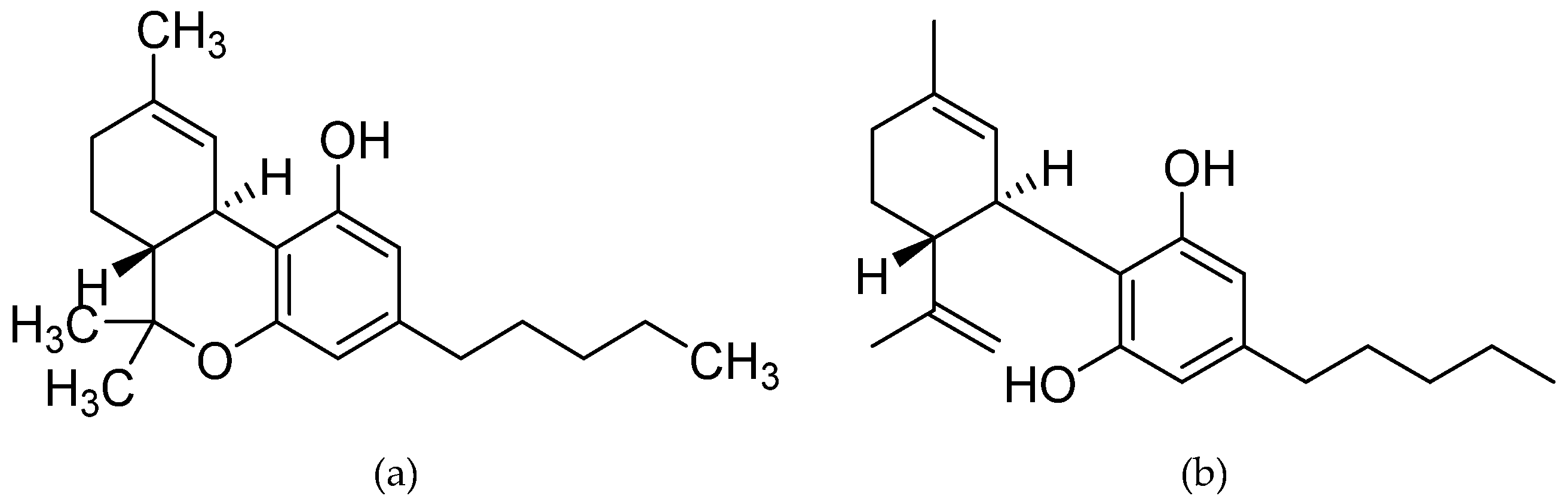
1. Introduction
1.1. Multiple Sclerosis, Neuropathology, Treatment Avenues and Outcomes
1.2. The Endocannabinoid System and Multiple Sclerosis
2. Animal Studies
2.1. Animal Models of Multiple Sclerosis
2.1.1. Virally-Induced Demyelination
2.1.2. Toxin-Induced Demyelination
2.1.3. Experimental Autoimmune Encephalomyelitis (EAE)
2.2. Cannabidiol Slows Symptom Onset in EAE Mice
2.3. Combinations of Δ9-THC and CBD Are More Effective in Symptom Relief and Neuroinflammation Reduction
3. Human Studies
3.1. Cannabinoids Are Only Moderately Effective in Reducing MS Related Spasticity
3.2. Cannabinoids May Be Effective in Reducing Neuropathic Pain in pwMS
3.3. Cognitive Effects of Cannabinoid Treatment
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, H.-L. An archaeological and historical account of cannabis in China. Econ. Bot. 1973, 28, 437–448. [Google Scholar] [CrossRef]
- Luginbuhl, A.M. Industrial Hemp (Cannabis savita L): The Geography of a Controversial Plant. Calif. Geogr. 2001, 41, 1–14. [Google Scholar]
- Degenhardt, L.; Hall, W. Extent of illicit drug use and dependence, and their contribution to the global burden of disease. Lancet 2012, 379, 55–70. [Google Scholar] [CrossRef]
- Panagis, G.; Vlachou, S.; Nomikos, G.G. Behavioral pharmacology of cannabinoids with a focus on preclinical models for studying reinforcing and dependence-producing properties. Curr. Drug Abus. Rev. 2008, 1, 350–374. [Google Scholar] [CrossRef] [PubMed]
- Panagis, G.; Mackey, B.; Vlachou, S. Cannabinoid Regulation of Brain Reward Processing with an Emphasis on the Role of CB1 Receptors: A Step Back into the Future. Front. Psychiatry 2014, 5, 92. [Google Scholar] [CrossRef]
- Vlachou, S.; Panagis, G. Regulation of brain reward by the endocannabinoid system: A critical review of behavioral studies in animals. Curr. Pharm. Des. 2014, 20, 2072–2088. [Google Scholar] [CrossRef][Green Version]
- Hathaway, A.D. Cannabis users’ informal rules for managing stigma and risk. Deviant Behav. 2004, 25, 559–577. [Google Scholar] [CrossRef]
- Bruni, N.; Della Pepa, C.; Oliaro-Bosso, S.; Pessione, E.; Gastaldi, D.; Dosio, F. Cannabinoid Delivery Systems for Pain and Inflammation Treatment. Molecules 2018, 23, 2478. [Google Scholar] [CrossRef]
- Vlachou, S.; Nomikos, G.G.; Stephens, D.N.; Panagis, G. Lack of evidence for appetitive effects of Δ9-tetrahydrocannabinol in the intracranial self-stimulation and conditioned place preference procedures in rodents. Behav. Pharmacol. 2007, 18, 311–319. [Google Scholar] [CrossRef]
- A Losseff, N.; Webb, S.L.; I O’Riordan, J.; Page, R.; Wang, L.; Barker, G.J.; Tofts, P.S.; I McDonald, W.; Miller, D.H.; Thompson, A.J. Spinal cord atrophy and disability in multiple sclerosis. A new reproducible and sensitive MRI method with potential to monitor disease progression. Brain 1996, 119, 701–708. [Google Scholar]
- Lassmann, H. Neuropathology in multiple sclerosis: New concepts. Mult. Scler. J. 1998, 4, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.; Cameron, M.H. Cannabinoids for Treatment of MS Symptoms: State of the Evidence. Curr. Neurol. Neurosci. Rep. 2018, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Rudick, R.A. Disease-modifying drugs for relapsing-remitting multiple sclerosis and future directions for multiple sclerosis therapeutics. Arch. Neurol. 1999, 56, 1079–1084. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fischer, J.S.; Priore, R.L.; Jacobs, L.D.; Cookfair, D.L.; Rudick, R.A.; Herndon, R.M.; Richert, J.R.; Salazar, A.M.; Goodkin, D.E.; Granger, C.V.; et al. Neuropsychological effects of interferon β-1a in relapsing multiple sclerosis. Ann. Neurol. 2000, 48, 885–892. [Google Scholar] [CrossRef]
- Kasper, L.H.; Reder, A.T. Immunomodulatory activity of interferon-beta. Ann. Clin. Transl. Neurol. 2014, 1, 622–631. [Google Scholar] [CrossRef]
- Milo, R.; Miller, A. Revised diagnostic criteria of multiple sclerosis. Autoimmun. Rev. 2014, 13, 518–524. [Google Scholar] [CrossRef]
- Pinel, J.P.J.; Barnes, S.J. Biopsychology; Pearson Education: London, UK, 2009. [Google Scholar]
- Pertwee, R.G. Targeting the endocannabinoid system with cannabinoid receptor agonists: Pharmacological strategies and therapeutic possibilities. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 3353–3363. [Google Scholar] [CrossRef]
- Guerrero-Alba, R.; Barragan-Iglesias, P.; Gonzalez-Hernandez, A.; Valdez-Moráles, E.E.; Granados-Soto, V.; Condés-Lara, M.; Rodríguez, M.G.; Marichal-Cancino, B.A. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 2019, 9, 1496. [Google Scholar] [CrossRef]
- Vemuri, V.K.; Makriyannis, A. Medicinal chemistry of cannabinoids. Clin. Pharmacol. Ther. 2015, 97, 553–558. [Google Scholar] [CrossRef] [PubMed]
- DeVane, W.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- De Petrocellis, L.; Di Marzo, V. An introduction to the endocannabinoid system: From the early to the latest concepts. Best Pr. Res. Clin. Endocrinol. Metab. 2009, 23, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Bisogno, T.; Di Marzo, V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nat. Rev. Neurol. 2019, 16, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Maurya, N.; Velmurugan, B.K. Therapeutic applications of cannabinoids. Chem. Interact. 2018, 293, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Gaoni, Y.; Mechoulam, R. Isolation, Structure, and Partial Synthesis of an Active Constituent of Hashish. J. Am. Chem. Soc. 1964, 86, 1646–1647. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shvo, Y. Hashish-I: The structure of cannabidiol. Tetrahedron 1963, 19, 2073–2078. [Google Scholar] [CrossRef]
- Bloemendal, V.R.L.J.; Van Hest, J.C.M.; Rutjes, F.P.J.T. Synthetic pathways to tetrahydrocannabinol (THC): An overview. Org. Biomol. Chem. 2020, 18, 3203–3215. [Google Scholar] [CrossRef]
- Eisenreich, W.; Schwarz, M.; Cartayrade, A.; Arigoni, D.; Zenk, M.H.; Bacher, A. The deoxyxylulose phosphate pathway of terpenoid biosynthesis in plants and microorganisms. Chem. Biol. 1998, 5, R221–R233. [Google Scholar] [CrossRef]
- Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and Medicinal Chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef]
- Dybowski, M.P.; Dawidowicz, A.L.; Typek, R.; Rombel, M. Conversion of cannabidiol (CBD) to Δ9-tetrahydrocannabinol (Δ9-THC) during protein precipitations prior to plasma samples analysis by chromatography—Troubles with reliable CBD quantitation when acidic precipitation agents are applied. Talanta 2020, 220, 121390. [Google Scholar] [CrossRef]
- Banister, S.D.; Arnold, J.C.; Connor, M.; Glass, M.; McGregor, I.S. Dark Classics in Chemical Neuroscience: Δ9-Tetrahydrocannabinol. ACS Chem. Neurosci. 2019, 10, 2160–2175. [Google Scholar] [CrossRef]
- Taura, F.; Morimoto, S.; Shoyama, Y.; Mechoulam, R. First Direct Evidence for the Mechanism of Δ1-Tetrahydrocannabinolie Acid Biosynthesis. J. Am. Chem. Soc. 1995, 117, 9766–9767. [Google Scholar] [CrossRef]
- Luo, X.; Reiter, M.A.; D’Espaux, L.; Wong, J.; Denby, C.M.; Lechner, A.; Zhang, Y.; Grzybowski, A.T.; Harth, S.; Lin, W.; et al. Complete biosynthesis of cannabinoids and their unnatural analogues in yeast. Nat. Cell Biol. 2019, 567, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular targets of the phytocannabinoids: A complex picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [PubMed]
- Gupta, R.C. Nutraceuticals: Efficacy, Safety and Toxicity; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ujváry, I.; Hanuš, L. Human Metabolites of Cannabidiol: A Review on Their Formation, Biological Activity, and Relevance in Therapy. Cannabis Cannabinoid Res. 2016, 1, 90–101. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Vellani, V.; Schiano-Moriello, A.; Marini, P.; Magherini, P.C.; Orlando, P.; Di Marzo, V. Plant-Derived Cannabinoids Modulate the Activity of Transient Receptor Potential Channels of Ankyrin Type-1 and Melastatin Type-8. J. Pharmacol. Exp. Ther. 2008, 325, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Watkins, A.R. Cannabinoid interactions with ion channels and receptors. Channels 2019, 13, 162–167. [Google Scholar] [CrossRef]
- Ghovanloo, M.-R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef]
- Bisogno, T.; Hanuš, L.; De Petrocellis, L.; Tchilibon, S.; E Ponde, D.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Gülck, T.; Moller, B.L. Phytocannabinoids: Origins and Biosynthesis. Trends Plant. Sci. 2020, 25, 985. [Google Scholar] [CrossRef]
- Yang, Y.; Vyawahare, R.; Lewis-Bakker, M.; Clarke, H.; Wong, A.H.C.; Kotra, L.P. Bioactive Chemical Composition of Cannabis Extracts and Cannabinoid Receptors. Molecules 2020, 25, 3466. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef] [PubMed]
- Gruber, S.A.; Sagar, K.A. Marijuana on the Mind? The Impact of Marijuana on Cognition, Brain Structure, and Brain Function, and Related Public Policy Implications. Policy Insights Behav. Brain Sci. 2017, 4, 104–111. [Google Scholar] [CrossRef]
- Becker, B.; Wagner, D.; Gouzoulis-Mayfrank, E.; Spuentrup, E.; Edaumann, J. The impact of early-onset cannabis use on functional brain correlates of working memory. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2010, 34, 837–845. [Google Scholar] [CrossRef]
- Theiler, M. Spontaneous encephalomyelitis of mice—A new virus disease. J. Exp. Med. 1937, 65, 705–719. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Johnson, A.J.; Bieber, A.J.; Warrington, A.E.; Rodriguez, M.; Pirko, I. The relevance of animal models in multiple sclerosis research. Pathophysiology 2011, 18, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Pirko, I.; Gamez, J.; Johnson, A.J.; Macura, S.; Rodriguez, M. Dynamics of MRI lesion development in an animal model of viral-induced acute progressive CNS demyelination. NeuroImage 2004, 21, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Pirko, I.; Suidan, G.L.; Rodriguez, M.; Johnson, A.J. Acute hemorrhagic demyelination in a murine model of multiple sclerosis. J. Neuroinflamm. 2008, 5, 31. [Google Scholar] [CrossRef]
- Kipp, M.; Clarner, T.; Dang, J.; Copray, S.; Beyer, C. The cuprizone animal model: New insights into an old story. Acta Neuropathol. 2009, 118, 723–736. [Google Scholar] [CrossRef]
- Zhan, J.; Mann, T.; Joost, S.; Behrangi, N.; Frank, M.; Kipp, M. The Cuprizone Model: Dos and Do Nots. Cells 2020, 9, 843. [Google Scholar] [CrossRef]
- Rivers, T.M.; Sprunt, D.H.; Berry, G.P. Observations on attempts to produce acute disseminated encephalomyelitis in monkeys. J. Exp. Med. 1933, 58, 39–53. [Google Scholar] [CrossRef]
- Gerhauser, I.; Hansmann, F.; Ciurkiewicz, M.; Löscher, W.; Beineke, A. Facets of Theiler’s Murine Encephalomyelitis Virus-Induced Diseases: An Update. Int. J. Mol. Sci. 2019, 20, 448. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.; Kabat, E.A.; Bezer, A.E. The pathology of acute disseminated encephalomyelitis produced experimentally in the rhesus monkey and its resemblance to human demyelinating disease. J. Neuropathol. Exp. Neurol. 1947, 6, 333–357. [Google Scholar] [CrossRef] [PubMed]
- Sriram, S.; Steiner, I. Experimental allergic encephalomyelitis: A misleading model of multiple sclerosis. Ann. Neurol. 2005, 58, 939–945. [Google Scholar] [CrossRef]
- Elliott, D.M.; Singh, N.; Nagarkatti, M.; Nagarkatti, P.S. Cannabidiol attenuates experimental autoimmune encephalomyelitis model of multiple sclerosis through induction of myeloid-derived suppressor cells. Front. Immunol. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- González-García, C.; Moreno-Torres, I.; García-Hernández, R.; Campos-Ruíz, L.; Esparragoza, L.R.; Coronado, M.J.; Grande, A.G.; García-Merino, A.; Sánchez-López, A.J. Mechanisms of action of cannabidiol in adoptively transferred experimental autoimmune encephalomyelitis. Exp. Neurol. 2017, 298, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.M.; Kummari, E.; Sherman, J.; Yang, E.-J.; Dhital, S.; Gilfeather, C.; Yray, G.; Morgan, T.; Kaplan, B.L.F. CBD Suppression of EAE Is Correlated with Early Inhibition of Splenic IFN-γ + CD8+ T Cells and Modest Inhibition of Neuroinflammation. J. Neuroimmune Pharmacol. 2020, 15, 1–17. [Google Scholar] [CrossRef]
- Kozela, E.; Lev, N.; Kaushansky, N.; Eilam, R.; Rimmerman, N.; Levy, R.; Ben-Nun, A.; Juknat, A.; Vogel, Z. Cannabidiol inhibits pathogenic T cells, decreases spinal microglial activation and ameliorates multiple sclerosis-like disease in C57BL/6 mice. Br. J. Pharmacol. 2011, 163, 1507–1519. [Google Scholar] [CrossRef]
- Rahimi, A.; Faizi, M.; Talebi, F.; Noorbakhsh, F.; Kahrizi, F.; Naderi, N. Interaction between the protective effects of cannabidiol and palmitoylethanolamide in experimental model of multiple sclerosis in C57BL/6 mice. Neuroscience 2015, 290, 279–287. [Google Scholar] [CrossRef]
- Hilliard, A.; Stott, C.; Wright, S.; Guy, G.; Pryce, G.; Al-Izki, S.; Bolton, C.; Giovannoni, G. Evaluation of the Effects of Sativex (THC BDS: CBD BDS) on Inhibition of Spasticity in a Chronic Relapsing Experimental Allergic Autoimmune Encephalomyelitis: A Model of Multiple Sclerosis. ISRN Neurol. 2012, 2012, 1–7. [Google Scholar] [CrossRef]
- Moreno-Martet, M.; Feliú, A.; Espejo-Porras, F.; Mecha, M.; Carrillo-Salinas, F.J.; Fernández-Ruiz, J.; Guaza, C.; De Lago, E. The disease-modifying effects of a Sativex-like combination of phytocannabinoids in mice with experimental autoimmune encephalomyelitis are preferentially due to Δ9-tetrahydrocannabinol acting through CB1 receptors. Mult. Scler. Relat. Disord. 2015, 4, 505–511. [Google Scholar] [CrossRef]
- Al-Ghezi, Z.Z.; Busbee, P.B.; Alghetaa, H.; Nagarkatti, P.; Nagarkatti, M. Combination of cannabinoids, delta-9-tetrahydrocannabinol (THC) and cannabidiol (CBD), mitigates experimental autoimmune encephalomyelitis (EAE) by altering the gut microbiome. Brain Behav. Immun. 2019, 82, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Sheean, G. The pathophysiology of spasticity. Eur. J. Neurol. 2002, 9, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Ahmad, T.K.; Alrushaid, S.; Pozdirca, M.; Ethans, K.; Intrater, H.; Le, T.; Burczynski, F.J.; Kong, J.; Namaka, M. Therapeutic impact of orally administered cannabinoid oil extracts in an experimental autoimmune encephalomyelitis animal model of multiple sclerosis. Biochem. Biophys. Res. Commun. 2019, 516, 373–380. [Google Scholar] [CrossRef]
- Giacoppo, S.; Rajan, T.S.; Galuppo, M.; Pollastro, F.; Grassi, G.; Bramanti, P.; Mazzon, E. Purified Cannabidiol, the main non-psychotropic component of Cannabis sativa, alone, counteracts neuronal apoptosis in experimental multiple sclerosis. Eur. Rev. Med. Pharmacol. Sci 2015, 19, 4906–4919. [Google Scholar]
- Fitzpatrick, J.-M.K.; Downer, E.J. Toll-like receptor signalling as a cannabinoid target in Multiple Sclerosis. Neuropharmacology 2017, 113, 618–626. [Google Scholar] [CrossRef]
- Markovà, J.; Essner, U.; Akmaz, B.; Marinelli, M.; Trompke, C.; Lentschat, A.; Vila, C. Sativex® as add-on therapy vs. further optimized first-line ANTispastics (SAVANT) in resistant multiple sclerosis spasticity: A double-blind, placebo-controlled randomised clinical trial. Int. J. Neurosci. 2018, 129, 119–128. [Google Scholar] [CrossRef]
- Koehler, J.; Feneberg, W.; Meier, M.; Pöllmann, W. Clinical experience with THC:CBD oromucosal spray in patients with multiple sclerosis-related spasticity. Int. J. Neurosci. 2014, 124, 652–656. [Google Scholar] [CrossRef] [PubMed]
- Leocani, L.; Nuara, A.; Houdayer, E.; Schiavetti, I.; Del Carro, U.; Amadio, S.; Straffi, L.; Rossi, P.; Martinelli, V.; Vila, C.; et al. Sativex® and clinical–neurophysiological measures of spasticity in progressive multiple sclerosis. J. Neurol. 2015, 262, 2520–2527. [Google Scholar] [CrossRef]
- Van Amerongen, G.; Kanhai, K.; Baakman, A.C.; Heuberger, J.; Klaassen, E.; Beumer, T.L.; Strijers, R.L.M.; Killestein, J.; van Gerven, J.; Cohen, A.; et al. Effects on spasticity and neuropathic pain of an oral formulation of Δ9-tetrahydrocannabinol in patients with progressive multiple sclerosis. Clin. Ther. 2018, 40, 1467–1482. [Google Scholar] [CrossRef]
- Ferrè, L.; Nuara, A.; Pavan, G.; Radaelli, M.; Moiola, L.; Rodegher, M.; Colombo, B.; Sarmiento, I.J.K.; Martinelli, V.; Leocani, L.; et al. Efficacy and safety of nabiximols (Sativex®) on multiple sclerosis spasticity in a real-life Italian monocentric study. Neurol. Sci. 2015, 37, 235–242. [Google Scholar] [CrossRef]
- Turri, M.; Teatini, F.; Donato, F.; Zanette, G.; Tugnoli, V.; Deotto, L.; Bonetti, B.; Squintani, G. Pain Modulation after Oromucosal Cannabinoid Spray (SATIVEX®) in Patients with Multiple Sclerosis: A Study with Quantitative Sensory Testing and Laser-Evoked Potentials. Medicine 2018, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Langford, R.M.; Mares, J.; Novotná, A.; Váchová, M.; Nováková, I.; Notcutt, W.; Ratcliffe, S. A double-blind, randomized, placebo-controlled, parallel-group study of THC/CBD oromucosal spray in combination with the existing treatment regimen, in the relief of central neuropathic pain in patients with multiple sclerosis. J. Neurol. 2012, 260, 984–997. [Google Scholar] [CrossRef]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol Is a Safe Long-Term Treatment Option for Neuropathic Pain Patients. Eur. Neurol. 2017, 78, 320–329. [Google Scholar] [CrossRef]
- Aragona, M.; Onesti, E.; Tomassini, V.; Conte, A.; Gupta, S.; Gilio, F.; Pantano, P.; Pozzilli, C.; Inghilleri, M. Psychopathological and cognitive effects of therapeutic cannabinoids in multiple sclerosis: A double-blind, placebo controlled, crossover study. Clin. Neuropharmacol. 2009, 32, 41–47. [Google Scholar] [CrossRef]
- Brady, C.M.; Dasgupta, R.; Dalton, C.; Wiseman, O.J.; Berkley, K.J.; Fowler, C.J. An open-label pilot study of cannabis-based extracts for bladder dysfunction in advanced multiple sclerosis. Mult. Scler. J. 2004, 10, 425–433. [Google Scholar] [CrossRef]
- Garter, R.; Seefried, M.; Volberding, P. Dronabinol effects on weight in patients with HIV infection. AIDS 1992, 6, 127. [Google Scholar] [CrossRef]
- Russo, M.; Naro, A.; Leo, N.A.; Sessa, E.; D’Aleo, G.; Bramanti, P.; Calabrò, R.S. Evaluating Sativex® in Neuropathic Pain Management: A Clinical and Neurophysiological Assessment in Multiple Sclerosis. Pain Med. 2016, 17, 1145–1154. [Google Scholar] [CrossRef]
- Rog, D.J.; Nurmikko, T.J.; Friede, T.; Young, C.A. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005, 65, 812–819. [Google Scholar] [CrossRef]
- Feinstein, A.; Meza, C.; Stefan, C.; Staines, R.W. Coming off cannabis: A cognitive and magnetic resonance imaging study in patients with multiple sclerosis. Brain 2019, 142, 2800–2812. [Google Scholar] [CrossRef] [PubMed]
- Honarmand, K.; Tierney, M.C.; O’Connor, P.; Feinstein, A. Effects of cannabis on cognitive function in patients with multiple sclerosis. Neurology 2011, 76, 1153–1160. [Google Scholar] [CrossRef]
- Smith-Kielland, A.; Skuterud, B.; Mørland, J. Urinary Excretion of 11-nor-9-Carboxy-9-Tetrahydrocannabinol and Cannabinoids in Frequent and Infrequent Drug Users. J. Anal. Toxicol. 1999, 23, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.P.; Feinstein, A. Cannabis and cognitive functioning in multiple sclerosis: The role of gender. Mult. Scler. J.-Exp. Transl. Clin. 2017, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pavisian, B.; MacIntosh, B.J.; Szilagyi, G.; Staines, R.W.; O’Connor, P.; Feinstein, A. Effects of cannabis on cognition in patients with MS: A psychometric and MRI study. Neurology 2014, 82, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Romero, K.; Pavisian, B.; Staines, R.W.; Feinstein, A. Multiple sclerosis, cannabis, and cognition: A structural MRI study. NeuroImage Clin. 2015, 8, 140–147. [Google Scholar] [CrossRef]
- Al-Ghezi, Z.Z.; Miranda, K.; Nagarkatti, M.; Nagarkatti, P. Combination of Cannabinoids, Δ9- Tetrahydrocannabinol and Cannabidiol, Ameliorates Experimental Multiple Sclerosis by Suppressing Neuroinflammation Through Regulation of miRNA-Mediated Signaling Pathways. Front. Immunol. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Caprariello, A.V.; Rogers, J.A.; Morgan, M.L.; Hoghooghi, V.; Plemel, J.R.; Koebel, A.; Tsutsui, S.; Dunn, J.F.; Kotra, L.P.; Ousman, S.S.; et al. Biochemically altered myelin triggers autoimmune demyelination. Proc. Natl. Acad. Sci. USA 2018, 115, 5528–5533. [Google Scholar] [CrossRef]
- Manterola, A.; Bernal-Chico, A.; Cipriani, R.; Canedo-Antelo, M.; Moreno-García, Á.; Martin-Fontecha, M.; Pérez-Cerdá, F.; Sánchez-Gómez, M.V.; Ortega-Gutiérrez, S.; Brown, J.M.; et al. Deregulation of the endocannabinoid system and therapeutic potential of ABHD6 blockade in the cuprizone model of demyelination. Biochem. Pharmacol. 2018, 157, 189–201. [Google Scholar] [CrossRef]
| Ligand (Route) | Concentration (Time of Administration in Days Post Disease Induction) | Species (Sex) | Effect | Reference |
|---|---|---|---|---|
| CBD (IP) | 20 mg/kg (9–25) | C57BL/6 (f) | ↑ | [56] |
| CBD (IP) | 5–10 mg/kg (0) | C57BL/6 (f) | ↑ | [57] |
| 10 mg/kg (0) | ||||
| 50 mg/kg (0) | ||||
| CBD (OG) | 75 mg/kg (1) | C57BL/6 (f) | ↑ | [58] |
| CBD (IP) | 5 mg/kg (19–21) | C57BL/6 (f) | ↑ | [59] |
| CBD (IP) | 5 mg/kg (11–13) | C57BL/6 (f) | ↑ | [60] |
| CBD:Δ9-THC (IV) | 5 mg/kg (210) | Biozzi ABH (m/f) | ↑ | [61] |
| 10 mg/kg (210) | ||||
| CBD:Δ9-THC (SC) | 10 mg/kg (11) | C57BL/6 (f) | ↑ | [62] |
| Δ9-THC (SC) | 20 mg/kg (11) | C57BL/6 (f) | ↑ | [62] |
| CBD (SC) | 20 mg/kg (11) | C57BL/6 (f) | — | [62] |
| CBD:Δ9-THC (SC) | 10 mg/kg (10–15) | C57BL/6 (f) | ↑ | [63] |
| CBD (SC) | 20 mg/kg (10–15) | C57BL/6 (f) | — | [63] |
| Δ9-THC (SC) | 20 mg/kg (10–15) | C57BL/6 (f) | ↑ | [63] |
| CBD:Δ9-THC (IP) | 10 mg/kg (10–15) | C57BL/6 (f) | ↑ | [64] |
| CBD:Δ9-THC oil extract (OG) | 215 mg/kg (6–18) | Lewis (f) | ↑ | [65] |
| CBD oil extract (OG) | 215 mg/kg (6–18) | Lewis (f) | — | [65] |
| Δ9-THC oil extract (OG) | 215 mg/kg (6–18) | Lewis (f) | — | [65] |
| Adverse Event | [69] | [70] | [71] | [72] | [73] | [74] | [75] | [76] | Total |
|---|---|---|---|---|---|---|---|---|---|
| Dizziness | 5 | 7 | 13 | 35 | 4 | 34 | 50 | 4 | 152 |
| Headache | 0 | 0 | 9 | 0 | 0 | 7 | 9 | 3 | 208 |
| Somnolence | 0 | 0 | 9 | 11 | 0 | 16 | 0 | 0 | 36 |
| Muscle Weakness | 3 | 2 | 5 | 0 | 0 | 1 | 0 | 3 | 14 |
| Spasticity | 0 | 0 | 3 | 4 | 0 | 0 | 0 | 0 | 7 |
| Paraesthesia | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Tremor | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 1 | 4 |
| Vertigo | 2 | 1 | 0 | 0 | 0 | 16 | 34 | 4 | 57 |
| Tinnitus | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 |
| Mood Disruption | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 1 | 3 |
| Euphoria | 0 | 0 | 9 | 0 | 0 | 0 | 0 | 1 | 10 |
| Attention | 0 | 0 | 1 | 0 | 2 | 6 | 0 | 11 | 20 |
| Insomnia | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 3 |
| Fatigue | 5 | 0 | 5 | 29 | 2 | 16 | 25 | 6 | 88 |
| Feeling abnormal | 0 | 0 | 5 | 50 | 0 | 5 | 0 | 0 | 60 |
| Feeling hot | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 3 |
| Oral Discomfort | 4 | 0 | 3 | 0 | 0 | 19 | 13 | 5 | 44 |
| Nausea | 2 | 0 | 1 | 0 | 0 | 12 | 17 | 2 | 34 |
| Appetite | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 5 |
| Stomatitis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Incontinence | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Hypertension | 0 | 1 | 0 | 0 | 0 | 8 | 0 | 0 | 9 |
| Pharyngodynia | 0 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 3 |
| Vision Blurred | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 0 | 4 |
| Diarrhoea | 0 | 0 | 0 | 0 | 0 | 7 | 13 | 0 | 20 |
| Vomiting | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 |
| Memory Impairment | 0 | 0 | 0 | 0 | 0 | 6 | 0 | 0 | 6 |
| Psychomotor Impairment | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 5 |
| Total Adverse Events | 806 | ||||||||
| Total Participants | 166 | 22 | 24 | 144 | 28 | 312 | 333 | 17 |
| Ligand | Chemical Name | Reference |
|---|---|---|
| Nabiximols (Sativex) | (6aR,10aR)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydrobenzo[c]chromen-1-ol;2-[(6R)-3-methyl-6-prop-1-en-2-ylcyclohex-2-en-1-yl]-5-pentylbenzene-1,3-diol | [77] |
| Δ9-tetrahydrocannabinol (Dronabinol) | (−)-(6aR,10aR)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol | [78] |
| ECP002A | (−)-(6aR,10aR)-6,6,9-trimethyl-3-pentyl-6a,7,8,10a-tetrahydro-6H-benzo[c]chromen-1-ol | [77] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, É.; Vlachou, S. A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment. Molecules 2020, 25, 4930. https://doi.org/10.3390/molecules25214930
Jones É, Vlachou S. A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment. Molecules. 2020; 25(21):4930. https://doi.org/10.3390/molecules25214930
Chicago/Turabian StyleJones, Éamon, and Styliani Vlachou. 2020. "A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment" Molecules 25, no. 21: 4930. https://doi.org/10.3390/molecules25214930
APA StyleJones, É., & Vlachou, S. (2020). A Critical Review of the Role of the Cannabinoid Compounds Δ9-Tetrahydrocannabinol (Δ9-THC) and Cannabidiol (CBD) and their Combination in Multiple Sclerosis Treatment. Molecules, 25(21), 4930. https://doi.org/10.3390/molecules25214930






