Modeling of the Production of Lipid Microparticles Using PGSS® Technique
Abstract
1. Introduction
2. Results and Discussion
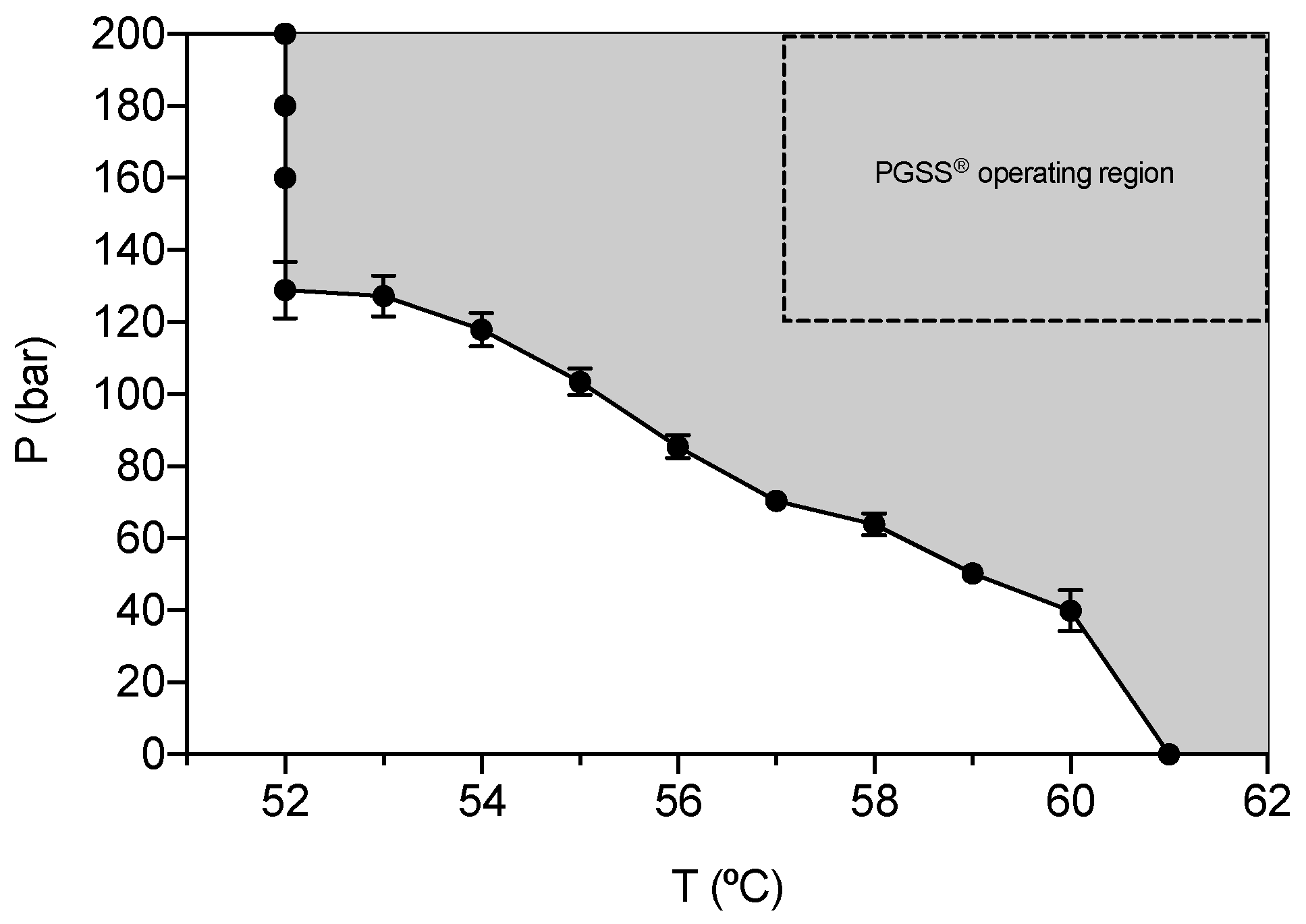
2.1. Melting Point Depression of GMS in the Presence of CO2
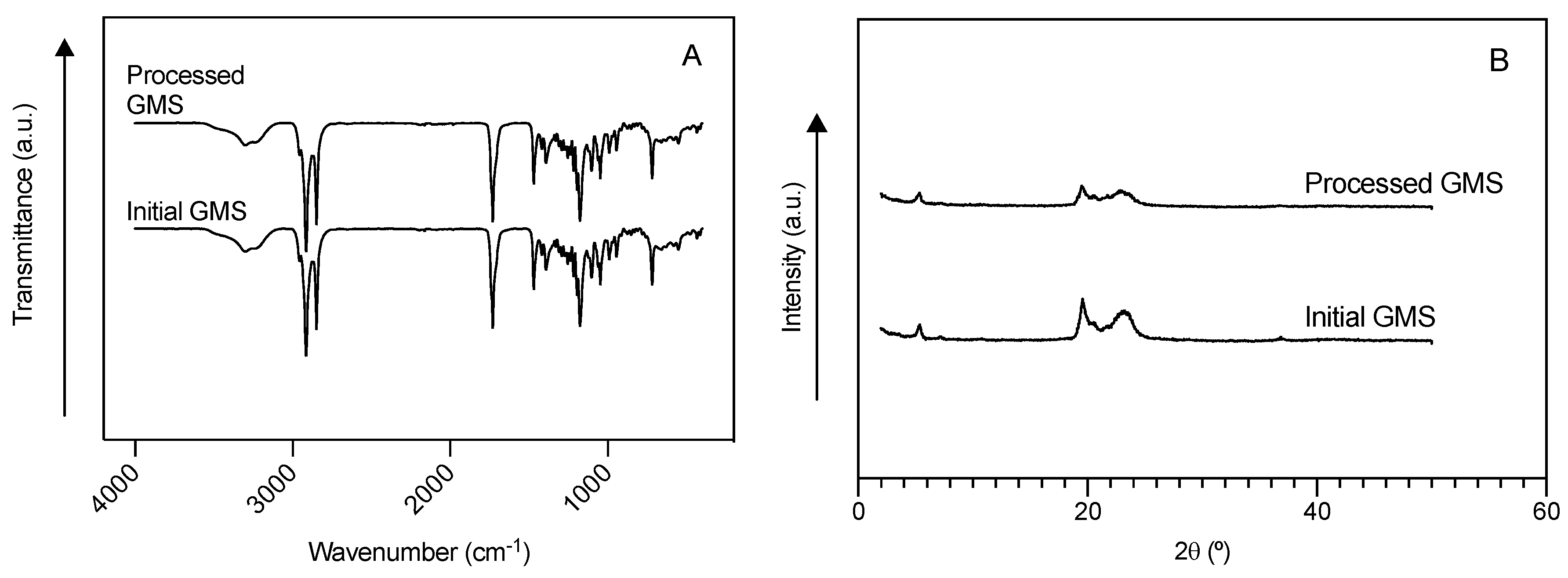
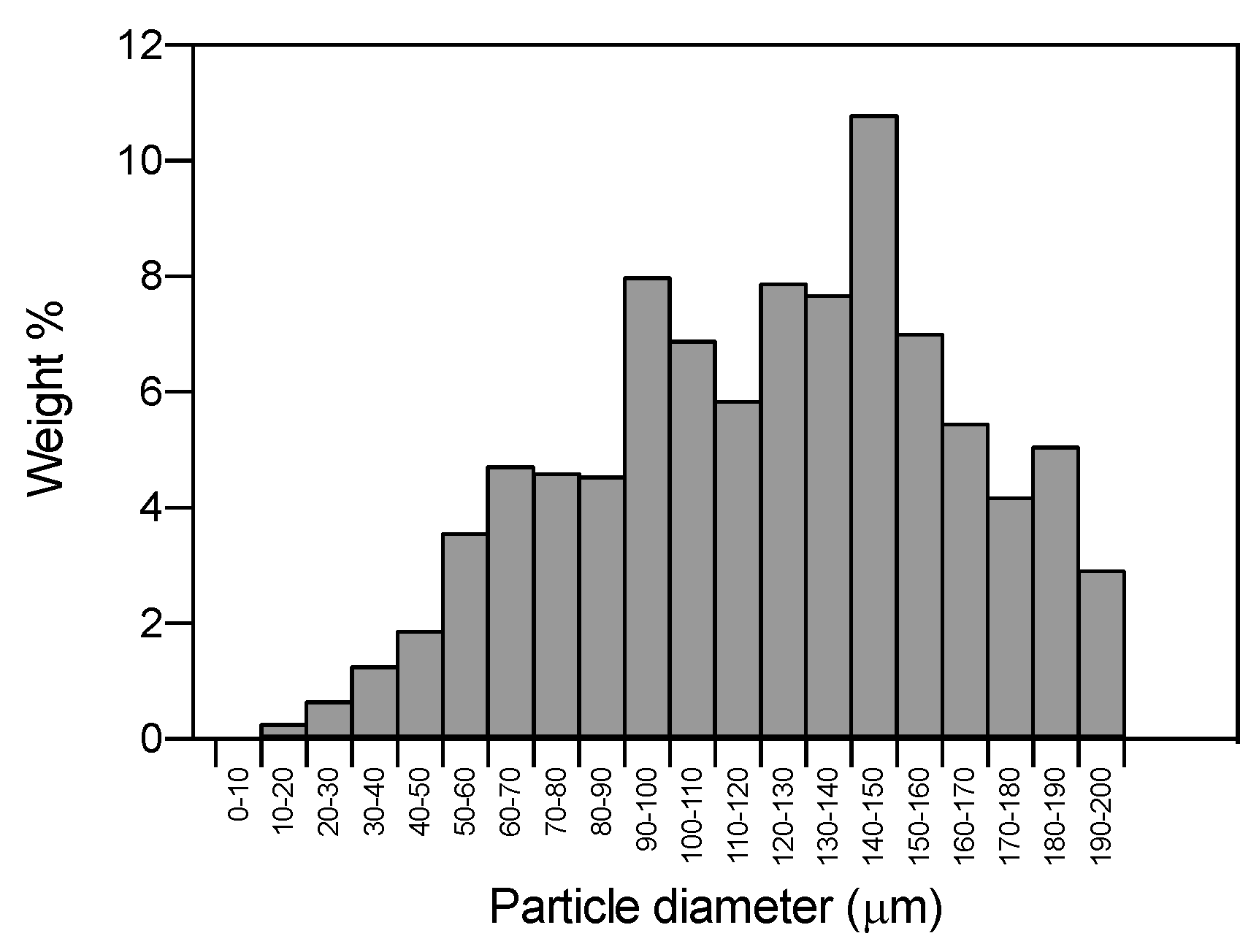
2.2. Particle Size Distribution (PSD), Morphological and Physichochemical Characterization of GMS Particles
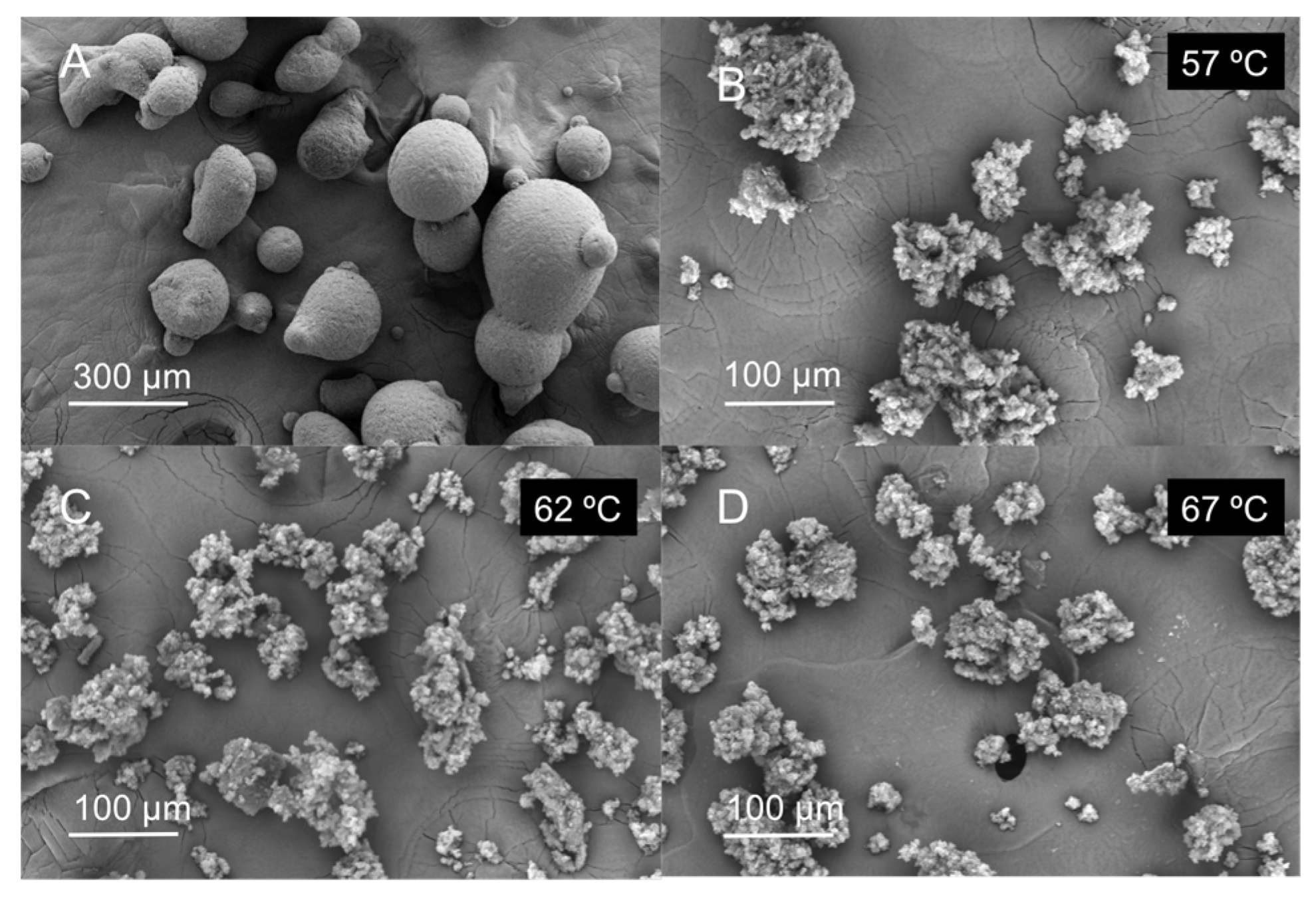
2.3. Morphological Characterization and Modeling of GMS Particle Production Using Neurofuzzy Tool
3. Materials and Methods
3.1. Materials
3.2. Determination of the Melting Point of GMS in the Presence of Compressed CO2 at Different Pressures
3.3. SLMPs Production by the PGSS Technique
3.4. Morphological Analysis, Physicochemical Characterization and Particle Size Distribution (PSD)
3.5. Modeling
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Parameter | Submodel | Rule |
|---|---|---|
| Mean diameter | 1 | IF T is low THEN mean diameter is high (1.0) |
| IF T is high THEN mean diameter is low (0.79) | ||
| 2 | IF P is low and nozzle is large THEN mean diameter is low (1.0) | |
| IF P is low and nozzle is small THEN mean diameter is high (0.69) | ||
| IF P is high and nozzle is large THEN mean diameter is high (0.69) | ||
| IF P is high and nozzle is small THEN mean diameter is high (0.53) | ||
| Standard deviation | 1 | IF P is low and nozzle is large THEN SD is low (0.63) |
| IF P is low and nozzle is small THEN SD is high (0.85) | ||
| IF P is high and nozzle is large THEN SD is high (0.85) | ||
| IF P is high and nozzle is small THEN SD is high (0.78) | ||
| % fine particles | 1 | IF nozzle is large and P is low THEN % particles is low (1.0) |
| IF nozzle is large and P is high THEN % particles is high (0.67) | ||
| IF nozzle is small and P is low THEN % particles is high (0.58) | ||
| IF nozzle is small and P is high THEN % particles is high (0.50) | ||
| 2 | IF T is low THEN % particles is high (0.90) | |
| IF T is medium THEN % particles is low (0.90) | ||
| IF T is high THEN % particles is low (0.56) |
References
- Rashid, M.; Kaur, V.; Hallan, S.S.; Sharma, S.; Mishra, N.K. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: A brief review. Saudi Pharm. J. 2016, 24, 458–472. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug delivery systems and materials for wound healing applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef] [PubMed]
- Lengyel, M.; Kállai-Szabó, N.; Antal, V.; Laki, A.J.; Antal, I. Microparticles, Microspheres, and Microcapsules for Advanced Drug Delivery. Sci. Pharm. 2019, 87, 20. [Google Scholar] [CrossRef]
- Galogahi, F.M.; Zhu, Y.; An, H.; Nguyen, N.-T. Core-shell microparticles: Generation approaches and applications. J. Sci. Adv. Mater. Devices 2020. [Google Scholar] [CrossRef]
- Sagis, L.M.C. Microencapsulation and Microspheres for Food Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Kohane, D.S. Microparticles and nanoparticles for drug delivery. Biotechnol. Bioeng. 2006, 96, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Perry, S.L.; McClements, D.J. Recent Advances in Encapsulation, Protection, and Oral Delivery of Bioactive Proteins and Peptides using Colloidal Systems. Molecules 2020, 25, 1161. [Google Scholar] [CrossRef]
- Dalpiaz, A.; Cacciari, B.; Mezzena, M.; Strada, M.; Scalia, S. Solid Lipid Microparticles for the Stability Enhancement of a Dopamine Prodrug. J. Pharm. Sci. 2010, 99, 4730–4737. [Google Scholar] [CrossRef]
- Willerth, S. Engineering Neural Tissue from Stem Cells; Academic Press: Cambridge, MA, USA, 2017; pp. 159–180. [Google Scholar]
- El-Sherbiny, I.M.; El-Baz, N.M.; Yacoub, M.H. Inhaled nano- and microparticles for drug delivery. Glob. Cardiol. Sci. Pr. 2015, 2015, 2. [Google Scholar] [CrossRef]
- Li, J.; Ghatak, S.; El Masry, M.S.; Das, A.; Liu, Y.; Roy, S.; Lee, R.J.; Sen, C.K. Topical Lyophilized Targeted Lipid Nanoparticles in the Restoration of Skin Barrier Function following Burn Wound. Mol. Ther. 2018, 26, 2178–2188. [Google Scholar] [CrossRef]
- Davis, S.S. Coming of age of lipid-based drug delivery systems. Adv. Drug Deliv. Rev. 2004, 56, 1241–1242. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Jaspart, S.; Piel, G.; Delattre, L.; Evrard, B. Solid lipid microparticles: Formulation, preparation, characterisation, drug release and applications. Expert Opin. Drug Deliv. 2005, 2, 75–87. [Google Scholar] [CrossRef] [PubMed]
- López-Iglesias, C.; Quílez, C.; Barros, J.; Velasco, D.; Alvarez-Lorenzo, C.; Jorcano, J.L.; Monteiro, F.J.; García-González, C. Lidocaine-Loaded Solid Lipid Microparticles (SLMPs) Produced from Gas-Saturated Solutions for Wound Applications. Pharmaceutics 2020, 12, 870. [Google Scholar] [CrossRef]
- Esfandiari, N. Production of micro and nano particles of pharmaceutical by supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 129–141. [Google Scholar] [CrossRef]
- Melgosa, R.; Benito-Román, Ó.; Sanz, M.T.; De Paz, E.; Beltrán, S. Omega-3 encapsulation by PGSS-drying and conventional drying methods. Particle characterization and oxidative stability. Food Chem. 2019, 270, 138–148. [Google Scholar] [CrossRef]
- Weidner, E.; Steiner, R.; Knez, Ž. Powder generation from polyethyleneglycols with compressible fluids. In High Pressure Chemical Engineering, Proceedings of the 3rd International Symposium on High Pressure Chemical Engineering; Rohr, R.V., Trepp, C., Eds.; Elsevier BV: Zürich, Switzerland, 1996; Volume 12, pp. 223–228. [Google Scholar]
- Weidner, E.; Knez, Z.; Novak, Z. A process and equipment for production and fractionation of fine particles from gas saturated solutions. World Patent WO 1994, 95, 21688. [Google Scholar]
- Santos-Rosales, V.; Gallo, M.; Jaeger, P.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. New insights in the morphological characterization and modelling of poly(ε-caprolactone) bone scaffolds obtained by supercritical CO2 foaming. J. Supercrit. Fluids 2020, 166, 105012. [Google Scholar] [CrossRef]
- García-González, C.; Argemí, A.; De Sousa, A.S.; Duarte, C.; Saurina, J.; Domingo, C. Encapsulation efficiency of solid lipid hybrid particles prepared using the PGSS® technique and loaded with different polarity active agents. J. Supercrit. Fluids 2010, 54, 342–347. [Google Scholar] [CrossRef]
- Fraile, M.; Martínez, Á.M.; Deodato, D.; Rodriguez-Rojo, S.; Nogueira, I.; Simplício, A.; Cocero, M.; Duarte, C. Production of new hybrid systems for drug delivery by PGSS (Particles from Gas Saturated Solutions) process. J. Supercrit. Fluids 2013, 81, 226–235. [Google Scholar] [CrossRef]
- Ciftci, O.N.; Temelli, F. Formation of solid lipid microparticles from fully hydrogenated canola oil using supercritical carbon dioxide. J. Food Eng. 2016, 178, 137–144. [Google Scholar] [CrossRef]
- Shekunov, B.Y.; Chattopadhyay, P.; Seitzinger, J.; Huff, R. Nanoparticles of Poorly Water-Soluble Drugs Prepared by Supercritical Fluid Extraction of Emulsions. Pharm. Res. 2006, 23, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Sodeifian, G.; Sajadian, S.A.; Ardestani, N.S.; Razmimanesh, F. Production of Loratadine drug nanoparticles using ultrasonic-assisted Rapid expansion of supercritical solution into aqueous solution (US-RESSAS). J. Supercrit. Fluids 2019, 147, 241–253. [Google Scholar] [CrossRef]
- Akolade, J.O.; Nasir-Naeem, K.O.; Swanepoel, A.; Yusuf, A.A.; Balogun, M.; Labuschagne, P. CO2-assisted production of polyethylene glycol/lauric acid microparticles for extended release of Citrus aurantifolia essential oil. J. CO2 Util. 2020, 38, 375–384. [Google Scholar] [CrossRef]
- Pascual, C.D.; Subra-Paternault, P. Supercritical Fluid Nanotechnology; Pan Stanford Publishing: Singapore, 2015. [Google Scholar]
- Tokunaga, S.; Ono, K.; Ito, S.; Sharmin, T.; Kato, T.; Irie, K.; Mishima, K.; Satho, T.; Harada, T.; Aida, T.M.; et al. Microencapsulation of drug with enteric polymer Eudragit L100 for controlled release using the particles from gas saturated solutions (PGSS) process. J. Supercrit. Fluids 2021, 167, 105044. [Google Scholar] [CrossRef]
- Haq, M.; Chun, B.-S. Microencapsulation of omega-3 polyunsaturated fatty acids and astaxanthin-rich salmon oil using particles from gas saturated solutions (PGSS) process. LWT 2018, 92, 523–530. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Naylor, A.; Whitaker, M.; Palmieri, G.; Giorgioni, G.; Casettari, L. Evaluation of P(L)LA-PEG-P(L)LA as processing aid for biodegradable particles from gas saturated solutions (PGSS) process. Int. J. Pharm. 2014, 468, 250–257. [Google Scholar] [CrossRef]
- Pedro, A.S.; Villa, S.D.; Caliceti, P.; De Melo, S.A.V.; Cabral-Albuquerque, E.C.; Bertucco, A.; Salmaso, S. Curcumin-loaded solid lipid particles by PGSS technology. J. Supercrit. Fluids 2016, 107, 534–541. [Google Scholar] [CrossRef]
- Chakravarty, P.; Famili, A.; Nagapudi, K.; Al-Sayah, M.A. Using Supercritical Fluid Technology as a Green Alternative During the Preparation of Drug Delivery Systems. Pharmaceutics 2019, 11, 629. [Google Scholar] [CrossRef] [PubMed]
- Pestieau, A.; Krier, F.; Lebrun, P.; Brouwers, A.; Streel, B.; Evrard, B. Optimization of a PGSS (particles from gas saturated solutions) process for a fenofibrate lipid-based solid dispersion formulation. Int. J. Pharm. 2015, 485, 295–305. [Google Scholar] [CrossRef]
- Strumendo, M.; Bertucco, A.; Elvassore, N. Modeling of particle formation processes using gas saturated solution atomization. J. Supercrit. Fluids 2007, 41, 115–125. [Google Scholar] [CrossRef]
- de Azevedo, E.G.; Jun, L.; Matos, H. Proceedings of 6th International Symposium on Supercritical Fluids; Institut National Polytechnique de Lorraine: Versailles, France, 2003. [Google Scholar]
- Landin, M.; Rowe, R.C. Artificial neural networks technology to model, understand, and optimize drug formulations. In Formulation Tools for Pharmaceutical Development; Aguilar, J.E., Ed.; Woodhead Publishing, Ltd.: Sawston, UK, 2013; pp. 7–37. [Google Scholar]
- Rodríguez-Dorado, R.; Landin, M.; Altai, A.; Russo, P.; Aquino, R.P.; Del Gaudio, P. A novel method for the production of core-shell microparticles by inverse gelation optimized with artificial intelligent tools. Int. J. Pharm. 2018, 538, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jara, M.O.; Catalan-Figueroa, J.; Landin, M.; Morales, J. Finding key nanoprecipitation variables for achieving uniform polymeric nanoparticles using neurofuzzy logic technology. Drug Deliv. Transl. Res. 2017, 8, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Rouco, H.; Alvarez-Lorenzo, C.; Rama-Molinos, S.; Remuñán-López, C.; Landin, M. Delimiting the knowledge space and the design space of nanostructured lipid carriers through Artificial Intelligence tools. Int. J. Pharm. 2018, 553, 522–530. [Google Scholar] [CrossRef]
- Shah, M.; Agrawal, Y. Ciprofloxacin hydrochloride-loaded glyceryl monostearate nanoparticle: Factorial design of Lutrol F68 and Phospholipon 90G. J. Microencapsul. 2012, 29, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Holm, R. Solid lipid nanocarriers in drug delivery: Characterization and design. Expert Opin. Drug Deliv. 2018, 15, 771–785. [Google Scholar] [CrossRef]
- De Sousa, A.S.; Simplício, A.L.; De Sousa, H.C.; Duarte, C.M. Preparation of glyceryl monostearate-based particles by PGSS®—Application to caffeine. J. Supercrit. Fluids 2007, 43, 120–125. [Google Scholar] [CrossRef]
- García-González, C.A.; Da Sousa, A.S.; Argemí, A.; Periago, A.L.; Saurina, J.; Duarte, C.; Domingo, C. Production of hybrid lipid-based particles loaded with inorganic nanoparticles and active compounds for prolonged topical release. Int. J. Pharm. 2009, 382, 296–304. [Google Scholar] [CrossRef]
- Weidner, E. High pressure micronization for food applications. J. Supercrit. Fluids 2009, 47, 556–565. [Google Scholar] [CrossRef]
- Van Ginneken, L.; Weyten, H. Particle Formation Using Supercritical Carbon Dioxide. In Carbon Dioxide Recovery and Utilization; Aresta, M., Ed.; Springer Science and Business Media LLC: Berlin, Germany, 2003; pp. 123–136. [Google Scholar]
- Yun, J.-H.; Lee, H.-Y.; Asaduzzaman, A.; Chun, B.-S. Micronization and characterization of squid lecithin/polyethylene glycol composite using particles from gas saturated solutions (PGSS) process. J. Ind. Eng. Chem. 2013, 19, 686–691. [Google Scholar] [CrossRef]
- Roebuck, J.R.; Murrell, T.A.; Miller, E.E. The Joule-Thomson Effect in Carbon Dioxide. J. Am. Chem. Soc. 1942, 64, 400–411. [Google Scholar] [CrossRef]
- Sampaio de Sousa, A.R. Development of Functional Particles Using Supercritical Fluid Technology. Ph.D Thesis, Universidade Nova de Lisboa, Oeiras, Portugal, 2007. [Google Scholar]
- Montes, A.; Litwinowicz, A.A.; Gradl, U.; Gordillo, M.D.; Pereyra, C.; De La Ossa, E.J.M.; Fernández-Ponce, M.T. Exploring High Operating Conditions in the Ibuprofen Precipitation by Rapid Expansion of Supercritical Solutions Process. Ind. Eng. Chem. Res. 2013, 53, 474–480. [Google Scholar] [CrossRef]
- Colbourn, E.; Rowe, R. Neural Computing and Pharmaceutical Formulation. In Encyclopedia of Pharmaceutical Technology; Swarbrick, J., Ed.; Marcel Dekker: New York, NY, USA, 2005; pp. 145–157. [Google Scholar]
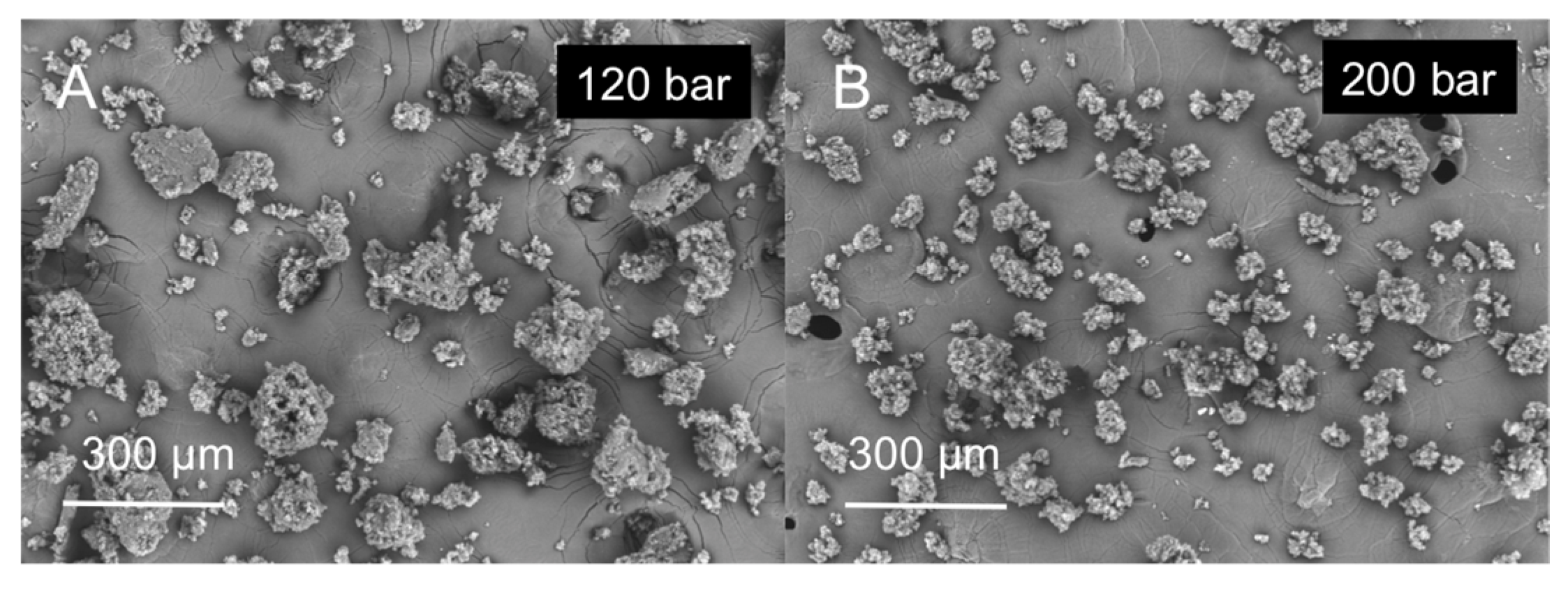
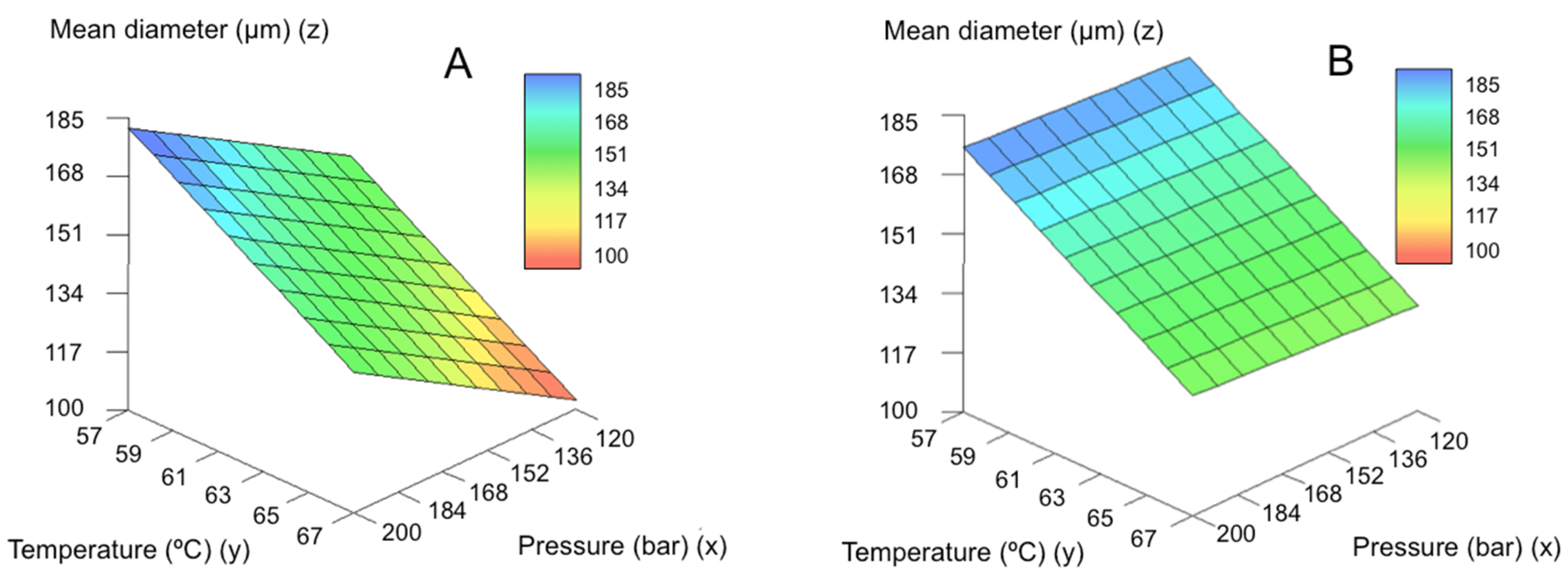
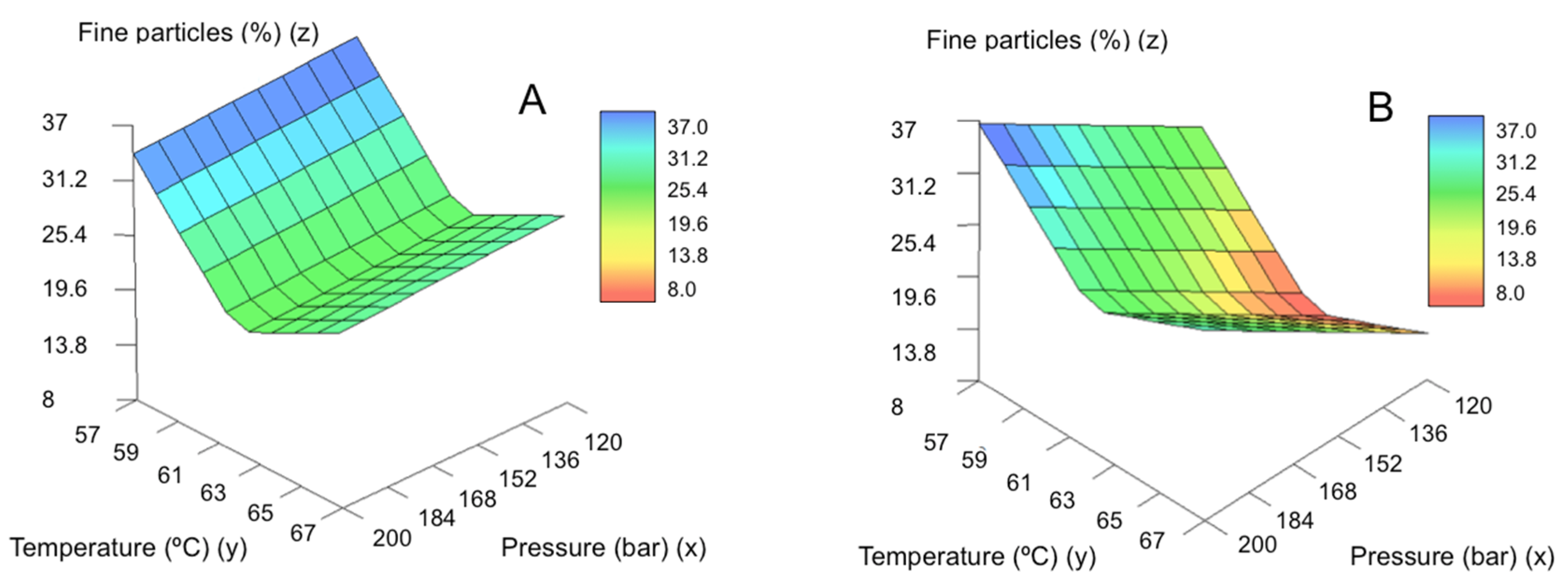
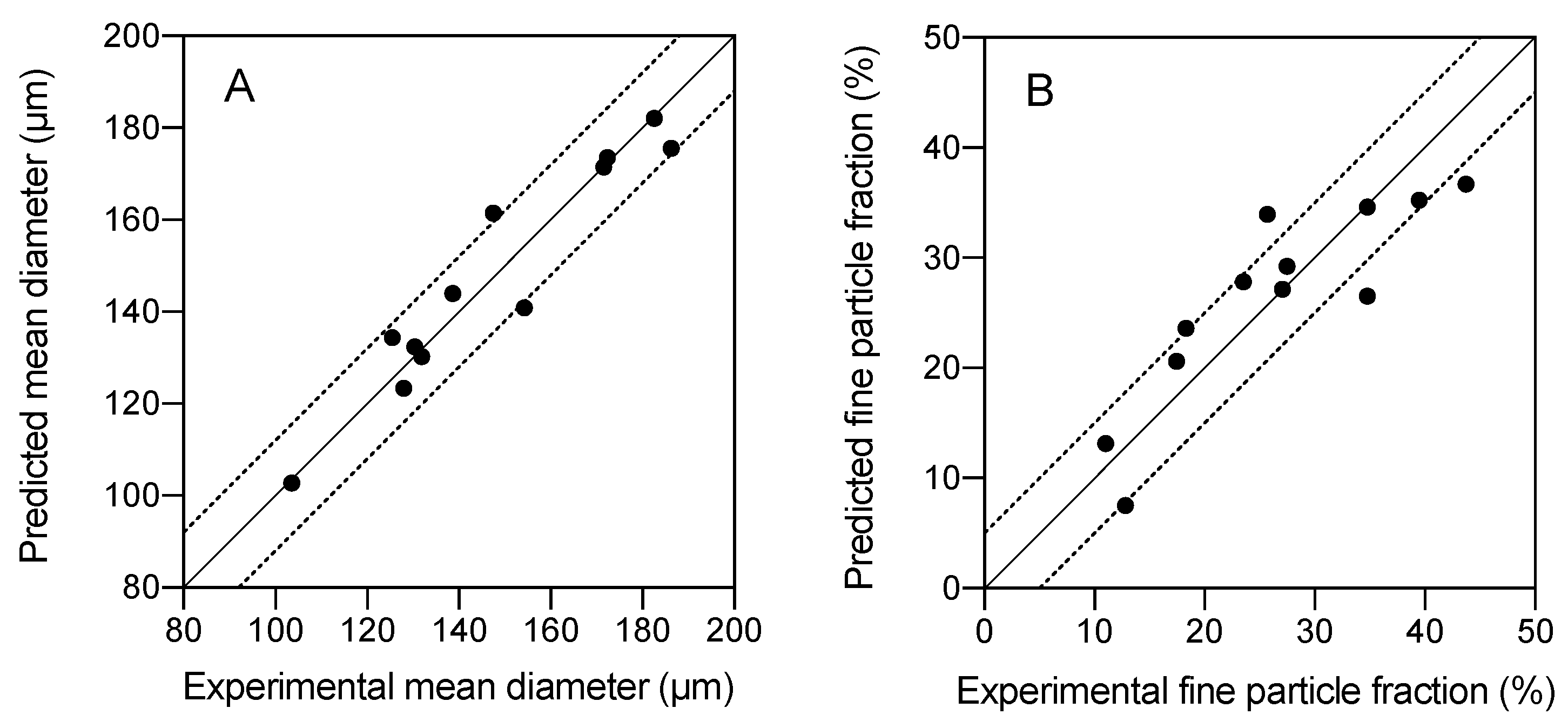
| SLMPs | Mean Diameter (μm) | Standard Deviation (μm) | % Fine Particles |
|---|---|---|---|
| GMS-4-57-120 | 138.7 | 47.0 | 17.4 |
| GMS-4-57-200 | 182.6 | 63.3 | 43.7 |
| GMS-4-62-120 | 128.0 | 41.8 | 12.8 |
| GMS-4-62-200 | 147.4 | 48.3 | 18.3 |
| GMS-4-67-120 | 103.5 | 33.1 | 11.0 |
| GMS-4-67-200 | 154.3 | 52.1 | 27.5 |
| GMS-1-57-120 | 171.6 | 56.8 | 39.5 |
| GMS-1-57-160 | 172.3 | 51.6 | 34.8 |
| GMS-1-57-200 | 186.2 | 57.5 | 25.7 |
| GMS-1-67-120 | 131.9 | 44.4 | 23.5 |
| GMS-1-67-160 | 130.3 | 50.0 | 27.1 |
| GMS-1-67-200 | 125.4 | 43.1 | 34.8 |
| Output | Submodel | Inputs Selected | R2 | Degrees of Freedom | f Value | Critical f Value |
|---|---|---|---|---|---|---|
| Mean diameter | 1 | T | 91.5012 | 5 and 6 | 12.92 | 4.39 |
| 2 | P × Nozzle | |||||
| Standard deviation | 1 | P × Nozzle | 58.3925 | 4 and 7 | 2.46 | 4.12 |
| % fine particles | 1 | P × Nozzle | 75.1098 | 6 and 5 | 2.51 | 4.93 |
| 2 | T |
| SLMPs | Nozzle (mm) | T (°C) | P (bar) |
|---|---|---|---|
| GMS-4-57-120 | 4 | 57 | 120 |
| GMS-4-57-200 | 4 | 57 | 200 |
| GMS-4-62-120 | 4 | 62 | 120 |
| GMS-4-62-200 | 4 | 62 | 200 |
| GMS-4-67-120 | 4 | 67 | 120 |
| GMS-4-67-200 | 4 | 67 | 200 |
| GMS-1-57-120 | 1 | 57 | 120 |
| GMS-1-57-160 | 1 | 57 | 160 |
| GMS-1-57-200 | 1 | 57 | 200 |
| GMS-1-67-120 | 1 | 67 | 120 |
| GMS-1-67-160 | 1 | 67 | 160 |
| GMS-1-67-200 | 1 | 67 | 200 |
| Minimization parameters |
| Ridge Regression Factor: 10−6 |
| Model Selection Criteria |
| Minimum Description Length |
| Number of Set Densities: 2 |
| Set Densities: 2.3 |
| Adapt Nodes: TRUE |
| Max. Inputs Per SubModel: 2 |
| Max. Nodes Per Input: 10 |
Sample Availability: Samples of the compounds before and after processing are available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Iglesias, C.; López, E.R.; Fernández, J.; Landin, M.; García-González, C.A. Modeling of the Production of Lipid Microparticles Using PGSS® Technique. Molecules 2020, 25, 4927. https://doi.org/10.3390/molecules25214927
López-Iglesias C, López ER, Fernández J, Landin M, García-González CA. Modeling of the Production of Lipid Microparticles Using PGSS® Technique. Molecules. 2020; 25(21):4927. https://doi.org/10.3390/molecules25214927
Chicago/Turabian StyleLópez-Iglesias, Clara, Enriqueta R. López, Josefa Fernández, Mariana Landin, and Carlos A. García-González. 2020. "Modeling of the Production of Lipid Microparticles Using PGSS® Technique" Molecules 25, no. 21: 4927. https://doi.org/10.3390/molecules25214927
APA StyleLópez-Iglesias, C., López, E. R., Fernández, J., Landin, M., & García-González, C. A. (2020). Modeling of the Production of Lipid Microparticles Using PGSS® Technique. Molecules, 25(21), 4927. https://doi.org/10.3390/molecules25214927










