Application of Ionic Liquids for Chemical Demulsification: A Review
Abstract
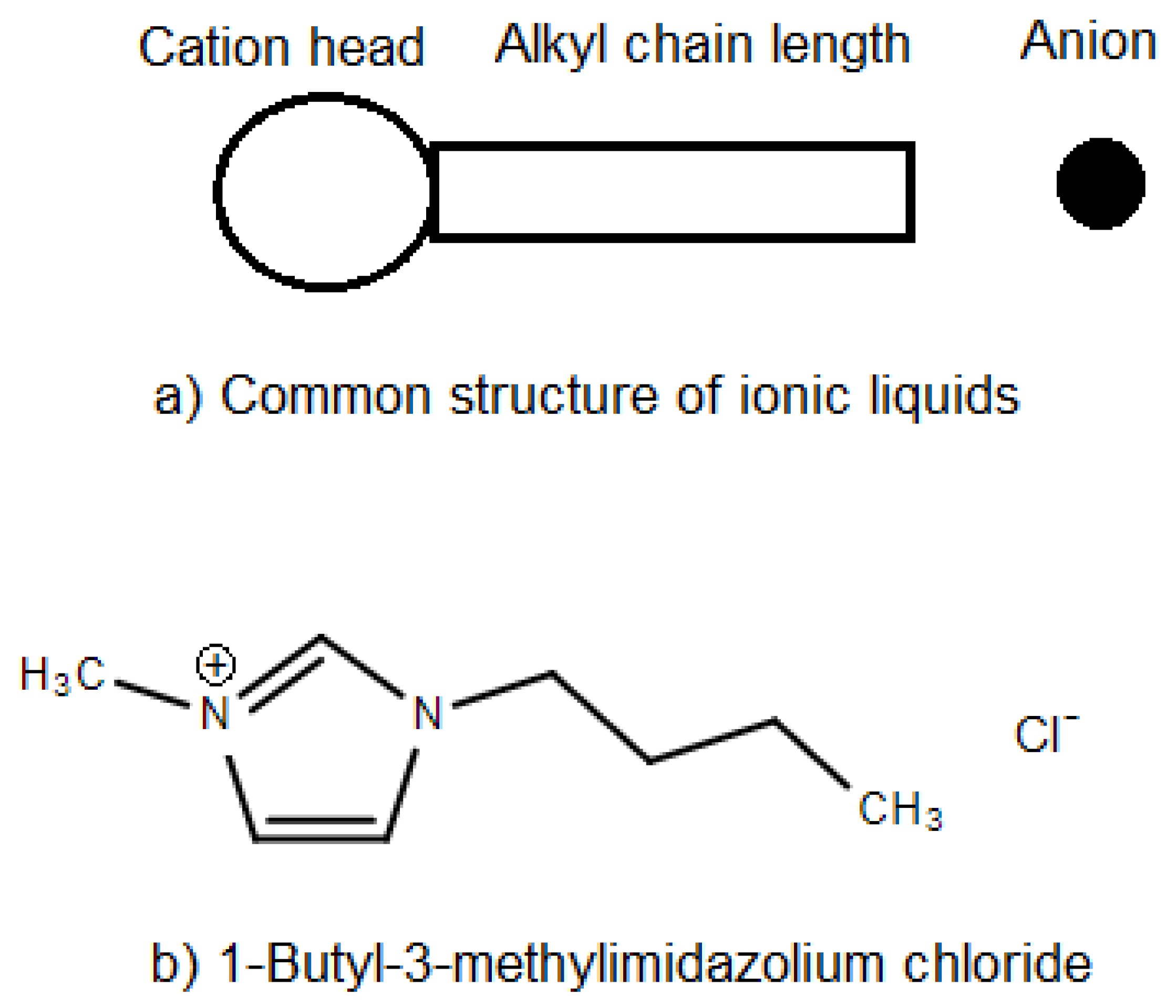
1. Introduction
2. Emulsions
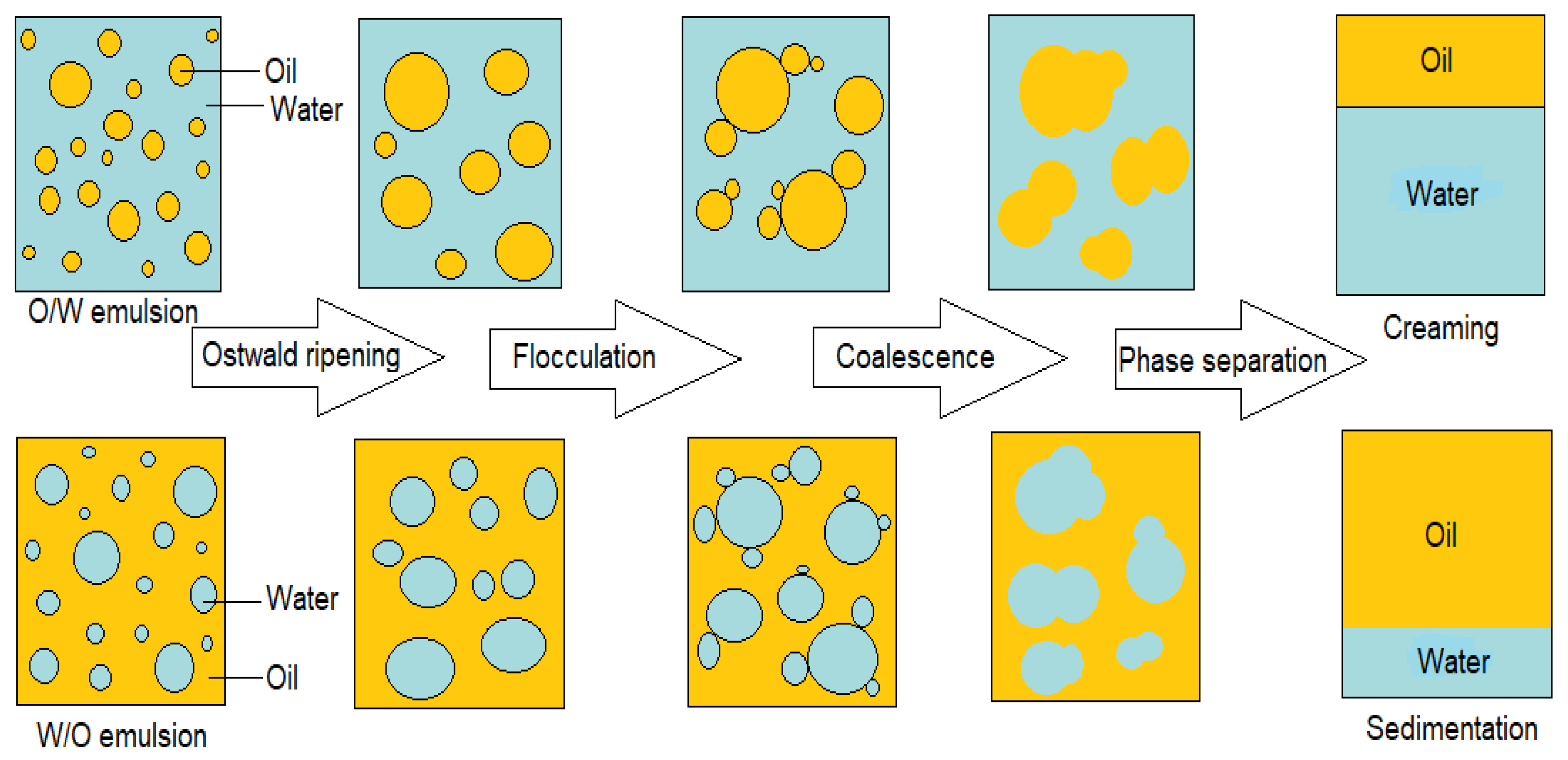
2.1. Emulsion Formation
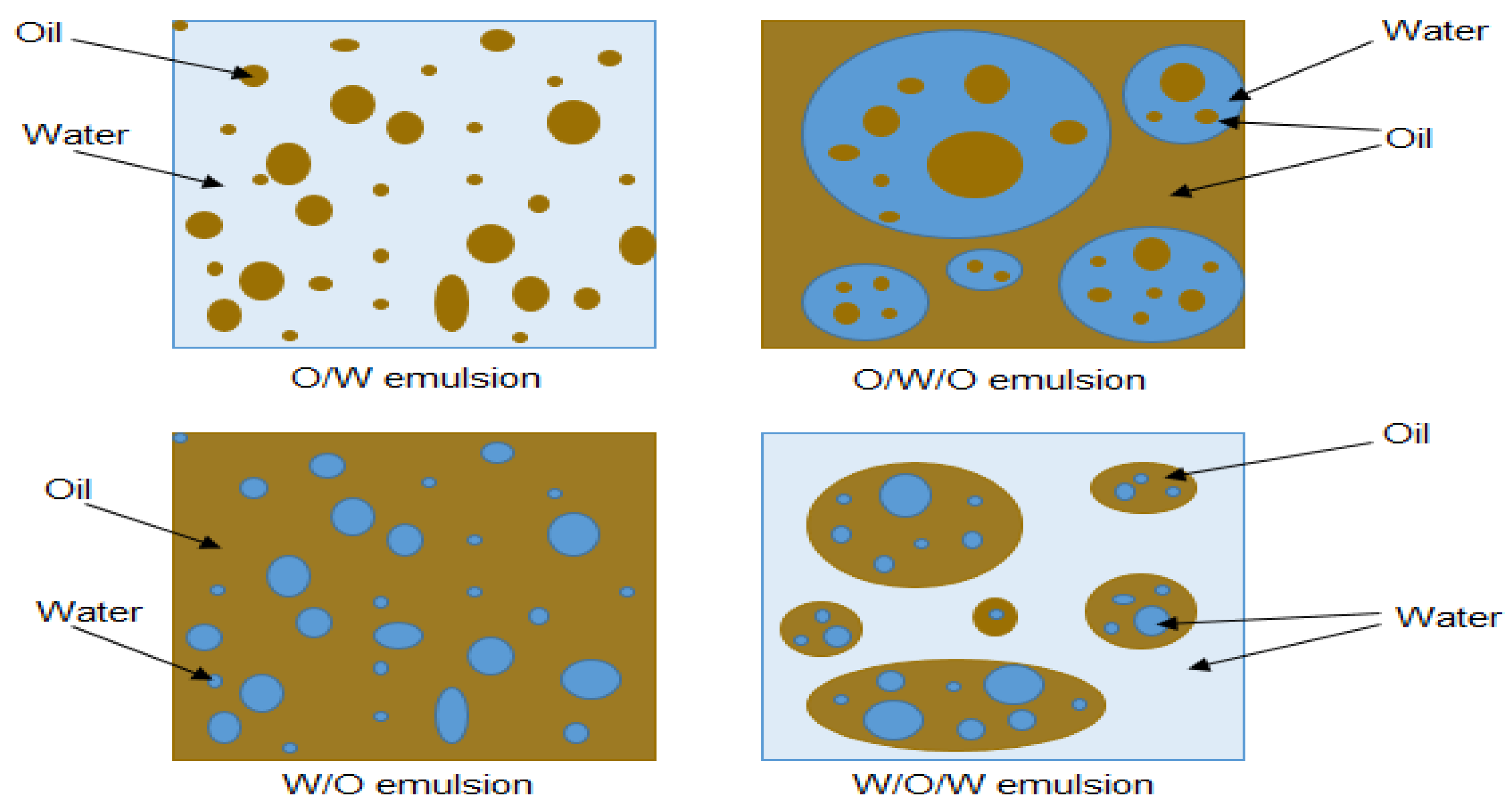
2.2. Emulsion Types
3. Ionic Liquid Demulsification
3.1. Chemical Demulsification System
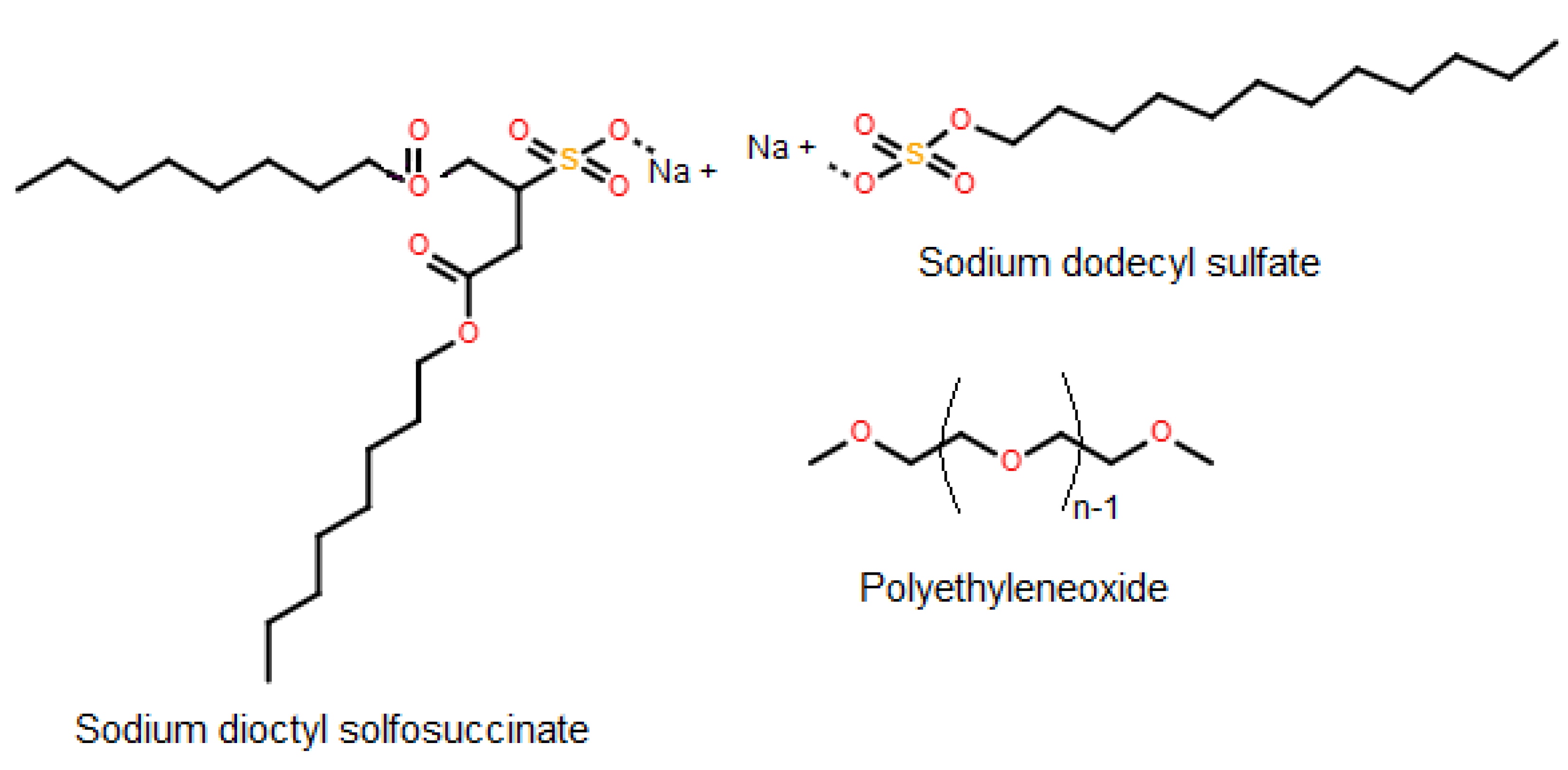
3.2. Applicaion of Ionic Liquids and Their Characteristics
3.3. Demulsification Mechanism of Ionic Liquids
4. Factors Affecting Ionic Liquids Demulsification
4.1. Concentration
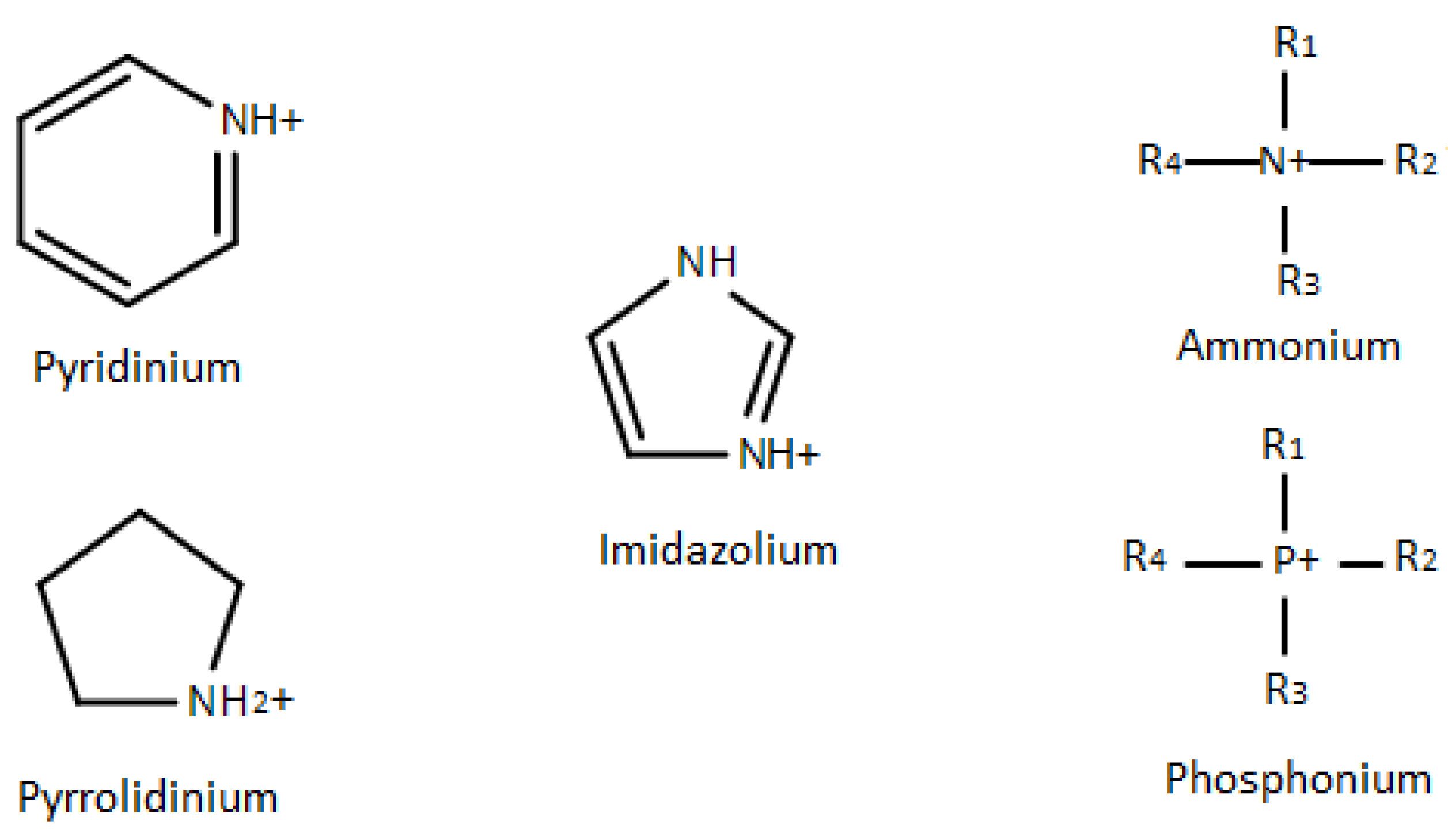
4.2. Cation Type and Structure of Ionic Liquids
4.3. Anion Type of Ionic Liquids
4.4. Molecular Weight
4.5. Salinity
4.6. Temperature
4.7. Oil Types
5. Challenges and Opportunities
5.1. Toxicity of Ionic Liquids
5.2. Viscosity of Ionic Liquids
5.3. Recovery of Ionic Liquids
5.4. Combination of Ionic Liquids with Nanoparticles
5.5. Poly Ionic Liquids
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ionic liquids Acronyms | |
| C4mim CF3CO2 | 1-butyl-3-methylimidazolium trifluoroacetate |
| C6mim Cl | 1-hexyl-3-methylimidazolium chloride |
| C8mim Cl | 1-octyl-3-methylimidazolium chloride |
| C10mim Cl | 1-decyl-3-methylimidazolium chloride |
| C12mim Cl | 1-dodecyl-3-methylimidazolium chloride |
| C14mim Cl | 1-tetradecyl-3-methylimidazolium chloride |
| C16mim Cl | 1-hexadecyl-3-methylimidazolium chloride |
| C16mim I | 1-hexadecyl-3-methylimidazolium iodide |
| C16mim Br | 1-hexadecyl-3-methylimidazolium bromide |
| C2mim BF4 | 1-ethyl-3-methyl-imidazolium tetrafluoroborate |
| C4mim BF4 | 1-butyl-3-methylimidazolium tetrafluoroborate |
| C8mim BF4 | 1-octyl-3-methylimidazolium tetrafluoroborate |
| C10mim BF4 | 1-decyl-3-methylimidazolium tetrafluoroborate |
| C12mim BF4 | 1-dodecyl-3-methylimidazolium tetrafluoroborate |
| C2mim TfO | 1-ethyl-3-methyl imidazolium trifluoromethanesulfonates |
| C8mim OTf | 1-methyl-3-octylimidazolium triflate bis(trifluoromethylsulfonil) |
| C4mim NTf2 | 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| C8mim NTf2 | 1-methyl-3-octylimidazolium bis(trifluoromethylsulfonyl)imide |
| C10mim NTf2 | 1-decyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| C12mim NTf2 | 1-dodecyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| C14mim NTf2 | 1-tetradecyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide |
| C4mim PF6 | -butyl-3-methylimidazolium hexafluorophosphate |
| C6mim PF6 | 1-hexyl-3-methylimidazolium hexafluorophosphate |
| C8mim PF6 | 1-octyl-3-methylimidazolium hexafluorophosphate |
| C10mim PF6 | 1-decyl-3-methylimidazolium hexafluorophosphate |
| C12mim PF6 | 1-dodecyl-3-methylimidazolium hexafluorophosphate |
| C14mim PF6 | 1-tetradecyl-3-methylimidazolium hexafluorophosphate |
| C4py NTf2 | 1-butylpyridinium bis(trifluoromethylsulfonyl)imide |
| C8Py Cl | 1-octylpyridinium chloride |
| C12Py Cl | 1-dodecylpyridinium chloride |
| HEOD-TS | N,N-bis-hexaoxyethlene octadecylamine tosylate |
| P666,14 N(CN)2 | Trihexyltetradecylphosphonium dicyanamide |
| P666,14 Phos | Trihexyltetradecylphosphonium bis(2,4,4-trimethylpentyl)phosphinate |
| P666,14 NTf2 | Trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)imide |
| P666,14 Cl | Trihexyltetradecylphosphonium chloride |
| P666,14 Br | Trihexyltetradecylphosphonium bromide |
| N2224 N(CN)2 | Triethylbutylammonium dicyanamide |
| N6222 NTf2 | Triethylhexyaammonium bis(trifluoromethylsulfonyl)imides |
| TOMAC | Trioctylmethylammonium chloride |
| TOMAB | Trioctylmethylammonium bromide |
| CTAB | 1-Hexadecyltrimethylammonium bromide |
| AMPS/AA-TE | The quaternized octadecyl amine diethoxylate with tetraethylene glycol ith copolymers of 2-acrylamido-2-methylpropane sulfonic acid (AMPS) nd acrylic acid (AA) |
| AMPS/AA-OA | The quaternized octadecylamine with copolymers of 2-acrylamido-2-ethylpropane sulfonic acid (AMPS) and acrylic acid (AA) |
| GEB | Reaction of the epoxy ring of glycidyl 4-nonylphenyl ether using ethanol mine, followed by quaternization using bis(2-chloroethyl) ether resulted n corresponding ionic liquid; GEB |
| EDHI | Etherified di-heptyl imidazolium acetate |
| EPHIB | Polymer of EDHI |
| EDDI | Etherified di-dodecyl imidazolium acetate |
| EPDIB | Polymer of EDDI |
| CP C6H13COO | Caprolactam hexanoate |
References
- Langevin, D.; Poteau, S.; Hénaut, I.; Argillier, J.F. Crude oil emulsion properties and their application to heavy oil transportation. Oil Gas Sci. Technol. 2004, 59, 511–521. [Google Scholar] [CrossRef]
- Thompson, D.G.; Taylor, A.S.; Graham, D.E. Emulsification and demulsification related to crude oil production. Colloids Surf. 1985, 15, 175–189. [Google Scholar] [CrossRef]
- Prabowo, A.R.; Bae, D.M. Environmental risk of maritime territory subjected to accidental phenomena: Correlation of oil spill and ship grounding in the Exxon Valdez’s case. Results Eng. 2019, 4, 100035. [Google Scholar] [CrossRef]
- Beland, L.P.; Oloomi, S. Environmental disaster, pollution and infant health: Evidence from the Deepwater Horizon oil spill. J. Environ. Econ. Manag. 2019, 98, 102265. [Google Scholar] [CrossRef]
- Adzigbli, L.; Yuewen, D. Assessing the impact of oil spills on marine organisms. J. Oceanogr Mar. Res. 2018, 6, 2. [Google Scholar]
- Kuppusamy, S.; Maddela, N.R.; Megharaj, M.; Venkateswarlu, K. Impact of Total Petroleum Hydrocarbons on Human Health. In Total Petroleum Hydrocarbons; Springer: Cham, Switzerland, 2020; pp. 139–165. [Google Scholar]
- Taleghani, N.D.; Tyagi, M. Impacts of major offshore oil spill incidents on petroleum industry and regional economy. J. Energy Resour. Technol. 2017, 139. [Google Scholar] [CrossRef]
- Rawlins, C.H. Flotation of fine oil droplets in petroleum production circuits. Recent Adv. Miner. Process. Plant. Des. 2009, 232. [Google Scholar]
- Mohayeji, M.; Farsi, M.; Rahimpour, M.R.; Shariati, A. Modeling and operability analysis of water separation from crude oil in an industrial gravitational coalescer. J. Taiwan Inst. Chem. Eng. 2016, 60, 76–82. [Google Scholar] [CrossRef]
- Ismail, N.H.; Salleh, W.N.W.; Ismail, A.F.; Hasbullah, H.; Yusof, N.; Aziz, F.; Jaafar, J. Hydrophilic polymer-based membrane for oily wastewater treatment: A review. Sep. Purif. Technol. 2020, 233, 116007. [Google Scholar] [CrossRef]
- Saththasivam, J.; Loganathan, K.; Sarp, S. An overview of oil–water separation using gas flotation systems. Chemosphere 2016, 144, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahian, M.; Emtiazi, G.; Caruso, G.; Cappello, S. Bioremediation (bioaugmentation/biostimulation) trials of oil polluted seawater: A mesocosm simulation study. Mar. Environ. Res. 2014, 95, 28–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Gong, H.; Cao, J.; Yin, H.; Yan, Y.; He, L. Enhanced separation of water-in-oil emulsions using ultrasonic standing waves. Chem. Eng. Sci. 2019, 203, 285–292. [Google Scholar] [CrossRef]
- Santos, D.; da Rocha, E.C.; Santos, R.L.; Cancelas, A.J.; Franceschi, E.; Santos, A.F.; Dariva, C. Demulsification of water-in-crude oil emulsions using single mode and multimode microwave irradiation. Sep. Purif. Technol. 2017, 189, 347–356. [Google Scholar] [CrossRef]
- Martínez-Palou, R.; Aburto, J. Ionic liquids as surfactants–applications as demulsifiers of petroleum emulsions. Ion. Liq. State Art 2015, 305–326. [Google Scholar] [CrossRef]
- Doshi, B.; Sillanpää, M.; Kalliola, S. A review of bio-based materials for oil spill treatment. Water Res. 2018, 135, 262–277. [Google Scholar] [CrossRef]
- Motta, F.L.; Stoyanov, S.R.; Soares, J.B. Application of solidifiers for oil spill containment: A review. Chemosphere 2018, 194, 837–846. [Google Scholar] [CrossRef]
- Shehzad, F.; Hussein, I.A.; Kamal, M.S.; Ahmad, W.; Sultan, A.S.; Nasser, M.S. Polymeric surfactants and emerging alternatives used in the demulsification of produced water: A review. Polym. Rev. 2018, 58, 63–101. [Google Scholar] [CrossRef]
- Balsamo, M.; Erto, A.; Lancia, A. Chemical demulsification of model water-in-oil emulsions with low water content by means of ionic liquids. Braz. J. Chem. Eng. 2017, 34, 273–282. [Google Scholar] [CrossRef]
- Alves, D.; Lourenço, E.; Franceschi, E.; Santos, A.F.; Santana, C.C.; Borges, G.; Dariva, C. Influence of ionic liquids on the viscoelastic properties of crude oil emulsions. Energy Fuels 2017, 31, 9132–9139. [Google Scholar] [CrossRef]
- Sun, P.; Armstrong, D.W. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kunz, W.; Häckl, K. The hype with ionic liquids as solvents. Chem. Phys. Lett. 2016, 661, 6–12. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 5, 2405–2426. [Google Scholar] [CrossRef] [PubMed]
- Atta, A.M.; Al-Lohedan, H.A.; Abdullah, M.M.; ElSaeed, S.M. Application of new amphiphilic ionic liquid based on ethoxylated octadecylammonium tosylate as demulsifier and petroleum crude oil spill dispersant. J. Ind. Eng. Chem. 2016, 33, 122–130. [Google Scholar] [CrossRef]
- Saad, M.A.; Kamil, M.; Abdurahman, N.H.; Yunus, R.M.; Awad, O.I. An Overview of Recent Advances in State-of-the-Art Techniques in the Demulsification of Crude Oil Emulsions. Processes 2019, 7, 470. [Google Scholar] [CrossRef]
- Raya, S.A.; Saaid, I.M.; Ahmed, A.A.; Umar, A.A. A critical review of development and demulsification mechanisms of crude oil emulsion in the petroleum industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1711–1728. [Google Scholar] [CrossRef]
- Kokal, S.L. Crude oil emulsions: A state-of-the-art review. SPE Prod. Facil. 2005, 20, 5–13. [Google Scholar] [CrossRef]
- Goodarzi, F.; Zendehboudi, S. A comprehensive review on emulsions and emulsion stability in chemical and energy industries. Can. J. Chem. Eng. 2019, 97, 281–309. [Google Scholar] [CrossRef]
- Bancroft, W.D. The theory of emulsification, V. J. Phys. Chem. 2002, 17, 501–519. [Google Scholar] [CrossRef]
- Wong, S.F.; Lim, J.S.; Dol, S.S. Crude oil emulsion: A review on formation, classification and stability of water-in-oil emulsions. J. Pet. Sci. Eng. 2015, 135, 498–504. [Google Scholar] [CrossRef]
- Abdulredha, M.M.; Aslina, H.S.; Luqman, C.A. Overview on petroleum emulsions, formation, influence and demulsification treatment techniques. Arab. J. Chem. 2020, 13, 3403–3428. [Google Scholar] [CrossRef]
- Lee, R.F. Agents which promote and stabilize water-in-oil emulsions. Spill Sci. Technol. Bull. 1999, 5, 117–126. [Google Scholar] [CrossRef]
- Canevari, G.P. The formulation of an effective demulsifier for oil spill emulsions. Mar. Pollut. Bull. 1982, 13, 49–54. [Google Scholar] [CrossRef]
- Grenoble, Z.; Trabelsi, S. Mechanisms, performance optimization and new developments in demulsification processes for oil and gas applications. Adv. Colloid Interface Sci. 2018, 260, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Mandal, A. Surfactant stabilized oil-in-water nanoemulsion: Stability, interfacial tension, and rheology study for enhanced oil recovery application. Energy Fuels 2018, 32, 6452–6466. [Google Scholar] [CrossRef]
- Abullah, M.M.; Al-Lohedan, H.A.; Attah, A.M. Synthesis and application of amphiphilic ionic liquid based on acrylate copolymers as demulsifier and oil spill dispersant. J. Mol. Liq. 2016, 219, 54–62. [Google Scholar] [CrossRef]
- Muschiolik, G.; Dickinson, E. Double emulsions relevant to food systems: Preparation, stability, and applications. Compr. Rev. Food Sci. Food Saf. 2017, 16, 532–555. [Google Scholar] [CrossRef]
- Kovács, A.; Erős, I.; Csóka, I. Optimization and development of stable w/o/w cosmetic multiple emulsions by means of the Quality by Design approach. Int. J. Cosmet. Sci. 2016, 38, 128–138. [Google Scholar] [CrossRef]
- Iqbal, M.; Zafar, N.; Fessi, H.; Elaissari, A. Double emulsion solvent evaporation techniques used for drug encapsulation. Int. J. Pharm. 2015, 496, 173–190. [Google Scholar] [CrossRef]
- Capek, I. Preparation of metal nanoparticles in water-in-oil (w/o) microemulsions. Adv. Colloid Interface Sci. 2004, 110, 49–74. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’l-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.; Pendashteh, A. Demulsification techniques of water-in-oil and oil-in-water emulsions in petroleum industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Fingas, M.; Fieldhouse, B. Formation of water-in-oil emulsions and application to oil spill modelling. J. Hazard. Mater. 2004, 107, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Fingas, M.; Fieldhouse, B.; Mullin, J. Studies of water-in-oil emulsions and techniques to measure emulsion treating agents. In Arctic and Marine Oilspill Program Technical Seminar; Ministry of Supply and Services: Ottawa, ON, Canada, 1994; p. 213. [Google Scholar]
- Griffin, W.C. Classification of surface-active agents by “HLB”. J. Soc. Cosmet. Chem. 1949, 1, 311–326. [Google Scholar]
- Salager, J.L.; Morgan, J.C.; Schechter, R.S.; Wade, W.H.; Vasquez, E. Optimum formulation of surfactant/water/oil systems for minimum interfacial tension or phase behavior. Soc. Pet. Eng. J. 1979, 19, 107–115. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.; Dabros, T.; Hamza, H. Development of a method for measurement of relative solubility of nonionic surfactants. Colloids Surf. A Physicochem. Eng. Asp. 2004, 232, 229–237. [Google Scholar] [CrossRef]
- Winsor, P.A. Solvent Properties of Amphiphilic Compounds; Butterworths Scientific Publications: London, UK, 1954. [Google Scholar]
- Borges, B.; Rondón, M.; Sereno, O.; Asuaje, J. Breaking of water-in-crude-oil emulsions. 3. Influence of salinity and water− oil ratio on demulsifier action. Energy Fuels 2009, 23, 1568–1574. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Kamal, M.S. Demulsification of water-in-oil emulsions using ionic liquids: Effects of counterion and water type. J. Mol. Liq. 2019, 279, 411–419. [Google Scholar] [CrossRef]
- Sastry, N.V.; Vaghela, N.M.; Aswal, V.K. Effect of alkyl chain length and head group on surface active and aggregation behavior of ionic liquids in water. Fluid Phase Equilibria 2012, 327, 22–29. [Google Scholar] [CrossRef]
- Moradi, M.; Alvarado, V.; Huzurbazar, S. Effect of salinity on water-in-crude oil emulsion: Evaluation through drop-size distribution proxy. Energy Fuels 2011, 25, 260–268. [Google Scholar] [CrossRef]
- Walden, P. Molecular weights and electrical conductivity of several fused salts. Bull. Acad. Imper. Sci. 1914, 1800. [Google Scholar]
- Huang, W.; Wu, X.; Qi, J.; Zhu, Q.; Wu, W.; Lu, Y.; Chen, Z. Ionic liquids: Green and tailor-made solvents in drug delivery. Drug Discov. Today 2019, 25, 901–908. [Google Scholar] [CrossRef]
- Dharaskar Swapnil, A. Ionic liquids (a review): The green solvents for petroleum and hydrocarbon industries. Res. J. Chem. Sci. ISSN 2012, 2231, 606X. [Google Scholar]
- Bera, A.; Agarwal, J.; Shah, M.; Shah, S.; Vij, R.K. Recent advances in ionic liquids as alternative to surfactants/chemicals for application in upstream oil industry. J. Ind. Eng. Chem. 2020, 82, 17–30. [Google Scholar] [CrossRef]
- Pernak, J.; Rzemieniecki, T.; Klejdysz, T.; Qu, F.; Rogers, R.D. Conversion of quinine derivatives into biologically active ionic liquids: Advantages, multifunctionality and perspectives. ACS Sustain. Chem. Eng. 2020, 8, 9263–9267. [Google Scholar] [CrossRef]
- Berton, P.; Manouchehr, S.; Wong, K.; Ahmadi, Z.; Abdelfatah, E.; Rogers, R.D.; Bryant, S.L. Ionic Liquids-Based Bitumen Extraction: Enabling Recovery with Environmental Footprint Comparable to Conventional Oil. ACS Sustain. Chem. Eng. 2019, 8, 632–641. [Google Scholar] [CrossRef]
- Kore, R.; Uppara, P.V.; Rogers, R.D. Replacing HF or AlCl3 in the Acylation of Isobutylbenzene with Chloroaluminate Ionic Liquids. ACS Sustain. Chem. Eng. 2020, 8, 10330–10334. [Google Scholar] [CrossRef]
- Moshikur, R.M.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Ionic liquids with N-methyl-2-pyrrolidonium cation as an enhancer for topical drug delivery: Synthesis, characterization, and skin-penetration evaluation. J. Mol. Liq. 2020, 299, 112166. [Google Scholar] [CrossRef]
- Chowdhury, M.R.; Moshikur, R.M.; Wakabayashi, R.; Tahara, Y.; Kamiya, N.; Moniruzzaman, M.; Goto, M. Development of a novel ionic liquid–curcumin complex to enhance its solubility, stability, and activity. Chem. Commun. 2019, 55, 7737–7740. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.M.; Veiga, M.; Freire, M.G. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Santos, M.M.; Branco, L.C. Ionic Liquids and Deep Eutectic Solvents for Application in Pharmaceutics. Pharmaceutics 2020, 12, 909. [Google Scholar] [CrossRef]
- Subramanian, D.; Wu, K.; Firoozabadi, A. Ionic liquids as viscosity modifiers for heavy and extra-heavy crude oils. Fuel 2015, 143, 519–526. [Google Scholar] [CrossRef]
- Zhao, H.; Baker, G.A. Oxidative desulfurization of fuels using ionic liquids: A review. Front. Chem. Sci. Eng. 2015, 9, 262–279. [Google Scholar] [CrossRef]
- Chen, D.X.; OuYang, X.K.; Wang, Y.G.; Yang, L.Y.; He, C.H. Liquid–liquid extraction of caprolactam from water using room temperature ionic liquids. Sep. Purif. Technol. 2013, 104, 263–267. [Google Scholar] [CrossRef]
- Florindo, C.; Monteiro, N.V.; Ribeiro, B.D.; Branco, L.C.; Marrucho, I.M. Hydrophobic deep eutectic solvents for purification of water contaminated with Bisphenol-A. J. Mol. Liq. 2020, 297, 111841. [Google Scholar] [CrossRef]
- Dimitrijević, A.; Tavares, A.P.; Almeida, M.R.; Vraneš, M.; Sousa, A.C.; Cristóvão, A.C.; Freire, M.G. Valorization of Expired Energy Drinks by Designed and Integrated Ionic Liquid-Based Aqueous Biphasic Systems. ACS Sustain. Chem. Eng. 2020, 8, 5683–5692. [Google Scholar] [CrossRef]
- Ardakani, E.K.; Kowsari, E.; Ehsani, A. Imidazolium-derived polymeric ionic liquid as a green inhibitor for corrosion inhibition of mild steel in 1.0 M HCl: Experimental and computational study. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124195. [Google Scholar] [CrossRef]
- Kammakakam, I.; Bara, J.E.; Jackson, E.M.; Lertxundi, J.; Mecerreyes, D.; Tomé, L.C. Tailored CO2-Philic Anionic Poly (ionic liquid) Composite Membranes: Synthesis, Characterization, and Gas Transport Properties. ACS Sustain. Chem. Eng. 2020, 8, 5954–5965. [Google Scholar] [CrossRef]
- Patinha, D.J.; Wang, H.; Yuan, J.; Rocha, S.M.; Silvestre, A.J.; Marrucho, I.M. Thin Porous Poly (ionic liquid) Coatings for Enhanced Headspace Solid Phase Microextraction. Polymers 2020, 12, 1909. [Google Scholar] [CrossRef]
- Pereira, J.F.; Barber, P.S.; Kelley, S.P.; Berton, P.; Rogers, R.D. Double salt ionic liquids based on 1-ethyl-3-methylimidazolium acetate and hydroxyl-functionalized ammonium acetates: Strong effects of weak interactions. Phys. Chem. Chem. Phys. 2017, 19, 26934–26943. [Google Scholar] [CrossRef]
- Yao, C.; Hou, Y.; Sun, Y.; Wu, W.; Ren, S.; Liu, H. Extraction of aromatics from aliphatics using a hydrophobic dicationic ionic liquid adjusted with small-content water. Sep. Purif. Technol. 2020, 236, 116287. [Google Scholar] [CrossRef]
- Guglielmero, L.; Mezzetta, A.; Pomelli, C.S.; Chiappe, C.; Guazzelli, L. Evaluation of the effect of the dicationic ionic liquid structure on the cycloaddition of CO2 to epoxides. J. Co2 Util. 2019, 34, 437–445. [Google Scholar] [CrossRef]
- Kuhn, B.L.; Osmari, B.F.; Heinen, T.M.; Bonacorso, H.G.; Zanatta, N.; Nielsen, S.O.; Frizzo, C.P. Dicationic imidazolium-based dicarboxylate ionic liquids: Thermophysical properties and solubility. J. Mol. Liq. 2020, 308, 112983. [Google Scholar] [CrossRef]
- Clarke, C.J.; Bui-Le, L.; Hallett, J.P.; Licence, P. Thermally Stable Imidazolium Dicationic Ionic Liquids with Pyridine Functional Groups. ACS Sustain. Chem. Eng. 2020, 8, 8762–8772. [Google Scholar] [CrossRef]
- Khezeli, T.; Ghaedi, M.; Bahrani, S.; Daneshfar, A.; Soylak, M. Deep eutectic solvent in separation and preconcentration of organic and inorganic species. In New Generation Green Solvents for Separation and Preconcentration of Organic and Inorganic Species; Elsevier: Amsterdam, The Netherlands, 2020; pp. 381–423. [Google Scholar]
- Abranches, D.O.; Silva, L.P.; Martins, M.A.; Pinho, S.P.; Coutinho, J.A. Understanding the Formation of Deep Eutectic Solvents: Betaine as a Universal Hydrogen Bond Acceptor. ChemSusChem 2020, 13, 4916–4921. [Google Scholar] [CrossRef]
- Schaeffer, N.; Conceição, J.H.; Martins, M.A.; Neves, M.C.; Pérez-Sánchez, G.; Gomes, J.R.; Coutinho, J.A. Non-ionic hydrophobic eutectics–versatile solvents for tailored metal separation and valorisation. Green Chem. 2020, 22, 2810–2820. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef]
- Gondal, H.Y.; Mumtaz, S.; Abbaskhan, A.; Mumtaz, N.; Cano, I. New alkoxymethyl-functionalized pyridinium-based chiral ionic liquids: Synthesis, characterization and properties. Chem. Pap. 2020, 74, 2951–2963. [Google Scholar] [CrossRef]
- Schmidt, F.; Schönhoff, M. Solvate Cation Migration and Ion Correlations in Solvate Ionic Liquids. J. Phys. Chem. B 2020, 124, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Mandai, T.; Yoshida, K.; Ueno, K.; Dokko, K.; Watanabe, M. Criteria for solvate ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 8761–8772. [Google Scholar] [CrossRef] [PubMed]
- Ratti, R. Ionic liquids: Synthesis and applications in catalysis. Adv. Chem. 2014, 2014, 1–16. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Angel, M.J.; Carda-Broch, S. Ionic liquids in separation techniques. J. Chromatogr. A 2008, 1184, 6–18. [Google Scholar] [CrossRef]
- Zhang, S.; Sun, N.; He, X.; Lu, X.; Zhang, X. Physical properties of ionic liquids: Database and evaluation. J. Phys. Chem. Ref. Data 2006, 35, 1475–1517. [Google Scholar] [CrossRef]
- Patel, D.D.; Lee, J.M. Applications of ionic liquids. Chem. Rec. 2012, 12, 329–355. [Google Scholar] [CrossRef]
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef]
- Pillai, P.; Kumar, A.; Mandal, A. Mechanistic studies of enhanced oil recovery by imidazolium-based ionic liquids as novel surfactants. J. Ind. Eng. Chem. 2018, 63, 262–274. [Google Scholar] [CrossRef]
- Bin Dahbag, M.S.; Hossain, M.E.; AlQuraishi, A.A. Efficiency of ionic liquids as an enhanced oil recovery chemical: Simulation approach. Energy Fuels 2016, 30, 9260–9265. [Google Scholar] [CrossRef]
- Irge, D.D. Ionic liquids: A review on greener chemistry applications, quality ionic liquid synthesis and economical viability in a chemical processes. Am. J. Phys. Chem. 2016, 5, 74–79. [Google Scholar] [CrossRef]
- Usuki, T.; Onda, S.; Yoshizawa-Fujita, M.; Rikukawa, M. Use of [C 4 mim] Cl for efficient extraction of caffeoylquinic acids from sweet potato leaves. Sci. Rep. 2017, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hanamertani, A.S.; Pilus, R.M.; Irawan, S.A.S.; Pilus, R.M.; Irawan, S. A Review on the Application of Ionic Liquids for Enhanced Oil Recovery. In ICIPEG 2016; Springer: Singapore, 2017; pp. 133–147. [Google Scholar]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic liquids—An overview. Aust. J. Chem. 2004, 57, 113–119. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive investigation on the thermal stability of 66 ionic liquids by thermogravimetric analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Maton, C.; De Vos, N.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Zhao, H. Current studies on some physical properties of ionic liquids. Phys. Chem. Liq. 2003, 41, 545–557. [Google Scholar] [CrossRef]
- Yahya, M.S.; Sangapalaarachchi, D.T.; Lau, E.V. Effects of carbon chain length of imidazolium-based ionic liquid in the interactions between heavy crude oil and sand particles for enhanced oil recovery. J. Mol. Liq. 2019, 274, 285–292. [Google Scholar] [CrossRef]
- Hezave, A.Z.; Dorostkar, S.; Ayatollahi, S.; Nabipour, M.; Hemmateenejad, B. Investigating the effect of ionic liquid (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl])) on the water/oil interfacial tension as a novel surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2013, 421, 63–71. [Google Scholar] [CrossRef]
- Hezave, A.Z.; Dorostkar, S.; Ayatollahi, S.; Nabipour, M.; Hemmateenejad, B. Effect of different families (imidazolium and pyridinium) of ionic liquids-based surfactants on interfacial tension of water/crude oil system. Fluid Phase Equilibria 2013, 360, 139–145. [Google Scholar] [CrossRef]
- Hezave, A.Z.; Dorostkar, S.; Ayatollahi, S.; Nabipour, M.; Hemmateenejad, B. Dynamic interfacial tension behavior between heavy crude oil and ionic liquid solution (1-dodecyl-3-methylimidazolium chloride ([C12mim][Cl]+ distilled or saline water/heavy crude oil)) as a new surfactant. J. Mol. Liq. 2013, 187, 83–89. [Google Scholar] [CrossRef]
- Hazrati, N.; Beigi, A.A.M.; Abdouss, M. Demulsification of water in crude oil emulsion using long chain imidazolium ionic liquids and optimization of parameters. Fuel 2018, 229, 126–134. [Google Scholar] [CrossRef]
- Oropeza, E.A.F.; Sotelo, L.V.C.; Ortega, A.L.; Cortez, J.G.H.; Ramírez, F.A.; Moreno, F.S.V.; Cassou, M.L. Dehydrating and Desalting Median, Heavy and Extra-Heavy Oils Using Ionic Liquids and Their Formulations. U.S. Patent 9,404,052, 2 July 2016. [Google Scholar]
- Tian, Y.; McGill, W.B.; Whitcombe, T.W.; Li, J. Ionic Liquid-Enhanced Solvent Extraction for Oil Recovery from Oily Sludge. Energy Fuels 2019, 33, 3429–3438. [Google Scholar] [CrossRef]
- Slater, C.S.; Savelski, M. A method to characterize the greenness of solvents used in pharmaceutical manufacture. J. Environ. Sci. Health Part A 2007, 42, 1595–1605. [Google Scholar] [CrossRef]
- Capello, C.; Fischer, U.; Hungerbühler, K. What is a green solvent? A comprehensive framework for the environmental assessment of solvents. Green Chem. 2007, 9, 927–934. [Google Scholar] [CrossRef]
- Bin-Dahbag, M.S.; Al Quraishi, A.A.; Benzagouta, M.S.; Kinawy, M.M.; Al Nashef, I.M.; Al Mushaegeh, E. Experimental study of use of ionic liquids in enhanced oil recovery. J. Pet. Environ. Biotechnol. 2014, 4, 1–7. [Google Scholar]
- Biniaz, P.; Farsi, M.; Rahimpour, M.R. Demulsification of water in oil emulsion using ionic liquids: Statistical modeling and optimization. Fuel 2016, 184, 325–333. [Google Scholar] [CrossRef]
- Flores, C.A.; Flores, E.A.; Hernández, E.; Castro, L.V.; García, A.; Alvarez, F.; Vázquez, F.S. Anion and cation effects of ionic liquids and ammonium salts evaluated as dehydrating agents for super-heavy crude oil: Experimental and theoretical points of view. J. Mol. Liq. 2014, 196, 249–257. [Google Scholar] [CrossRef]
- Sakthivel, S.; Velusamy, S.; Nair, V.C.; Sharma, T.; Sangwai, J.S. Interfacial tension of crude oil-water system with imidazolium and lactam-based ionic liquids and their evaluation for enhanced oil recovery under high saline environment. Fuel 2017, 191, 239–250. [Google Scholar] [CrossRef]
- Saien, J.; Kharazi, M. A comparative study on the interface behavior of different counter anion long chain imidazolium ionic liquids. J. Mol. Liq. 2016, 220, 136–141. [Google Scholar] [CrossRef]
- Saien, J.; Kharazi, M.; Asadabadi, S. Adsorption Behavior of Short Alkyl Chain Imidazolium Ionic Liquidsat N-Butyl Acetate+ Water Interface: Experiments and Modeling. Iran. J. Chem. Eng. 2015, 12, 59–74. [Google Scholar]
- Saien, J.; Kharazi, M.; Asadabadi, S. Adsorption behavior of long alkyl chain imidazolium ionic liquids at the n-butyl acetate+ water interface. J. Mol. Liq. 2015, 212, 58–62. [Google Scholar] [CrossRef]
- Guzman-Lucero, D.; Flores, P.; Rojo, T.; Martínez-Palou, R. Ionic liquids as demulsifiers of water-in-crude oil emulsions: Study of the microwave effect. Energy Fuels 2010, 24, 3610–3615. [Google Scholar] [CrossRef]
- Lemos, R.C.; da Silva, E.B.; dos Santos, A.; Guimaraes, R.C.; Ferreira, B.M.; Guarnieri, R.A.; Fortuny, M. Demulsification of water-in-crude oil emulsions using ionic liquids and microwave irradiation. Energy Fuels 2010, 24, 4439–4444. [Google Scholar] [CrossRef]
- Silva, E.B.; Santos, D.; Alves, D.R.; Barbosa, M.S.; Guimarães, R.C.; Ferreira, B.M.; Fortuny, M. Demulsification of heavy crude oil emulsions using ionic liquids. Energy Fuels 2013, 27, 6311–6315. [Google Scholar] [CrossRef]
- Abdullah, M.M.; Al-Lohedan, H.A. Demulsification of Arabian Heavy Crude Oil Emulsions Using Novel Amphiphilic Ionic Liquids Based on Glycidyl 4-Nonylphenyl Ether. Energy Fuels 2019, 33, 12916–12923. [Google Scholar] [CrossRef]
- Ezzat, A.O.; Atta, A.M.; Al-Lohedan, H.A.; Hashem, A.I. Synthesis and application of new surface active poly (ionic liquids) based on 1, 3-dialkylimidazolium as demulsifiers for heavy petroleum crude oil emulsions. J. Mol. Liq. 2018, 251, 201–211. [Google Scholar] [CrossRef]
- Li, X.; Kersten, S.R.; Schuur, B. Efficiency and mechanism of demulsification of oil-in-water emulsions using ionic liquids. Energy Fuels 2016, 30, 7622–7628. [Google Scholar] [CrossRef]
- Peña, A.A.; Hirasaki, G.J.; Miller, C.A. Chemically induced destabilization of water-in-crude oil emulsions. Ind. Eng. Chem. Res. 2005, 44, 1139–1149. [Google Scholar] [CrossRef]
- Hao, L.; Jiang, B.; Zhang, L.; Yang, H.; Sun, Y.; Wang, B.; Yang, N. Efficient demulsification of diesel-in-water emulsions by different structural dendrimer-based demulsifiers. Ind. Eng. Chem. Res. 2016, 55, 1748–1759. [Google Scholar] [CrossRef]
- Wu, J.; Xu, Y.; Dabros, T.; Hamza, H. Effect of demulsifier properties on destabilization of water-in-oil emulsion. Energy Fuels 2003, 17, 1554–1559. [Google Scholar] [CrossRef]
- Abdullah, M.M.; AlQuraishi, A.A.; Allohedan, H.A.; AlMansour, A.O.; Atta, A.M. Synthesis of novel water soluble poly (ionic liquids) based on quaternary ammonium acrylamidomethyl propane sulfonate for enhanced oil recovery. J. Mol. Liq. 2017, 233, 508–516. [Google Scholar] [CrossRef]
- Shinoda, K. The correlation between the dissolution state of nonionic surfactant and the type of dispersion stabilized with the surfactant. J. Colloid Interface Sci. 1967, 24, 4–9. [Google Scholar] [CrossRef]
- Adilbekova, A.O.; Omarova, K.I.; Karakulova, A.; Musabekov, K.B. Nonionic surfactants based on polyoxyalkylated copolymers used as demulsifying agents. Colloids Surf. A Physicochem. Eng. Asp. 2015, 480, 433–438. [Google Scholar] [CrossRef]
- Gathergood, N.; Garcia, M.T.; Scammells, P.J. Biodegradable ionic liquids: Part I. Concept, preliminary targets and evaluation. Green Chem. 2004, 6, 166–175. [Google Scholar] [CrossRef]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef]
- Gathergood, N.; Scammells, P.J.; Garcia, M.T. Biodegradable ionic liquids Part III. The first readily biodegradable ionic liquids. Green Chem. 2006, 8, 156–160. [Google Scholar] [CrossRef]
- Romero, A.; Santos, A.; Tojo, J.; Rodriguez, A. Toxicity and biodegradability of imidazolium ionic liquids. J. Hazard. Mater. 2008, 151, 268–273. [Google Scholar] [CrossRef]
- Quijano, G.; Couvert, A.; Amrane, A.; Darracq, G.; Couriol, C.; Le Cloirec, P.; Carrié, D. Toxicity and biodegradability of ionic liquids: New perspectives towards whole-cell biotechnological applications. Chem. Eng. J. 2011, 174, 27–32. [Google Scholar] [CrossRef]
- Gundolf, T.; Weyhing-Zerrer, N.; Sommer, J.; Kalb, R.; Schoder, D.; Rossmanith, P.; Mester, P. Biological Impact of Ionic Liquids Based on Sustainable Fatty Acid Anions Examined with a Tripartite Test System. ACS Sustain. Chem. Eng. 2019, 7, 15865–15873. [Google Scholar] [CrossRef]
- Hijo, A.A.T.; Barros, H.D.; Maximo, G.J.; Cazarin, C.B.; da Costa, L.B.; Pereira, J.F.; Meirelles, A.J. Subacute Toxicity Assessment of Biobased Ionic Liquids in Rats. Food Res. Int. 2020, 134, 109125. [Google Scholar] [CrossRef] [PubMed]
- Mezzetta, A.; Łuczak, J.; Woch, J.; Chiappe, C.; Nowicki, J.; Guazzelli, L. Surface active fatty acid ILs: Influence of the hydrophobic tail and/or the imidazolium hydroxyl functionalization on aggregates formation. J. Mol. Liq. 2019, 289, 111155. [Google Scholar] [CrossRef]
- Sernaglia, M.; Blanco, D.; Battez, A.H.; Viesca, J.L.; González, R.; Bartolomé, M. Two fatty acid anion-based ionic liquids-part I: Physicochemical properties and tribological behavior as neat lubricants. J. Mol. Liq. 2020, 305, 112827. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Lu, B.; Li, Y.; Liang, Y.; Yuan, J.; Zhang, J. Novel bio-renewable matrinium-based ionic liquids derived from Chinese herb medicine: Synthesis, physicochemical properties and biological activity. J. Mol. Liq. 2019, 296, 111822. [Google Scholar] [CrossRef]
- Oulego, P.; Faes, J.; González, R.; Viesca, J.L.; Blanco, D.; Battez, A.H. Relationships between the physical properties and biodegradability and bacteria toxicity of fatty acid-based ionic liquids. J. Mol. Liq. 2019, 292, 111451. [Google Scholar] [CrossRef]
- Mondal, D.; Sharma, M.; Quental, M.V.; Tavares, A.P.; Prasad, K.; Freire, M.G. Suitability of bio-based ionic liquids for the extraction and purification of IgG antibodies. Green Chem. 2016, 18, 6071–6081. [Google Scholar] [CrossRef]
- Huet, G.; Araya-Farias, M.; Alayoubi, R.; Laclef, S.; Bouvier, B.; Gosselin, I.; Husson, E. New biobased-zwitterionic ionic liquids: Efficiency and biocompatibility for the development of sustainable biorefinery processes. Green Chem. 2020, 22, 2935–2946. [Google Scholar] [CrossRef]
- Hulsbosch, J.; De Vos, D.E.; Binnemans, K.; Ameloot, R. Biobased ionic liquids: Solvents for a green processing industry? ACS Sustain. Chem. Eng. 2016, 4, 2917–2931. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, N.; Zhou, J.; Li, X.; He, L.; Sui, H. Novel Synthesis of Choline-Based Amino Acid Ionic Liquids and Their Applications for Separating Asphalt from Carbonate Rocks. Nanomaterials 2019, 9, 504. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Jiang, Y.; Geng, T.; Ju, H.; Wang, Y. Wetting, foaming, and emulsification properties of novel methyltriphenylphosphonium carboxylate ionic liquid surfactants. J. Dispers. Sci. Technol. 2020, 41, 47–53. [Google Scholar] [CrossRef]
- Becherini, S.; Mezzetta, A.; Chiappe, C.; Guazzelli, L. Levulinate amidinium protic ionic liquids (PILs) as suitable media for the dissolution and levulination of cellulose. New J. Chem. 2019, 43, 4554–4561. [Google Scholar] [CrossRef]
- Pereira, M.M.; Pedro, S.N.; Gomes, J.; Sintra, T.E.; Ventura, S.P.; Coutinho, J.A.; Mohamadou, A. Synthesis and characterization of analogues of glycine-betaine ionic liquids and their use in the formation of aqueous biphasic systems. Fluid Phase Equilibria 2019, 494, 239–245. [Google Scholar] [CrossRef]
- Silva, L.P.; Moya, C.; Sousa, M.; Santiago, R.; Sintra, T.E.; Carreira, A.R.; Carvalho, P.J. Encapsulated Amino-Acid-Based Ionic Liquids for CO2 Capture. Eur. J. Inorg. Chem. 2020, 2020, 3158–3166. [Google Scholar] [CrossRef]
- Kirchhecker, S.; Esposito, D. Amino acid based ionic liquids: A green and sustainable perspective. Curr. Opin. Green Sustain. Chem. 2016, 2, 28–33. [Google Scholar] [CrossRef]
- Szepiński, E.; Smolarek, P.; Milewska, M.J.; Łuczak, J. Application of surface active amino acid ionic liquids as phase-transfer catalyst. J. Mol. Liq. 2020, 303, 112607. [Google Scholar] [CrossRef]
- Khan, A.; Gusain, R.; Sahai, M.; Khatri, O.P. Fatty acids-derived protic ionic liquids as lubricant additive to synthetic lube base oil for enhancement of tribological properties. J. Mol. Liq. 2019, 293, 111444. [Google Scholar] [CrossRef]
- Turguła, A.; Stęsik, K.; Materna, K.; Klejdysz, T.; Praczyk, T.; Pernak, J. Third-generation ionic liquids with N-alkylated 1, 4-diazabicyclo [2.2. 2] octane cations and pelargonate anions. RSC Adv. 2020, 10, 8653–8663. [Google Scholar]
- Patsos, N.; Lewis, K.; Picchioni, F.; Kobrak, M.N. Extraction of acids and bases from aqueous phase to a pseudoprotic ionic liquid. Molecules 2019, 24, 894. [Google Scholar] [CrossRef] [PubMed]
- Shirota, H.; Castner, E.W. Why are viscosities lower for ionic liquids with− CH2Si (CH3) 3 vs− CH2C (CH3) 3 substitutions on the imidazolium cations? J. Phys. Chem. B 2005, 109, 21576–21585. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Han, B.; Hu, S.; Xie, Y.; Yang, G. Effect of water and organic solvents on the ionic dissociation of ionic liquids. J. Phys. Chem. B 2007, 111, 6452–6456. [Google Scholar] [CrossRef] [PubMed]
- Abu-Eishah, S.I. Ionic liquids recycling for reuse. Ion. Liq. Cl. Prop. 2011, 239–272. [Google Scholar] [CrossRef]
- Lemus, J.; Palomar, J.; Heras, F.; Gilarranz, M.A.; Rodriguez, J.J. Developing criteria for the recovery of ionic liquids from aqueous phase by adsorption with activated carbon. Sep. Purif. Technol. 2012, 97, 11–19. [Google Scholar] [CrossRef]
- Saien, J.; Hashemi, S. Long chain imidazolium ionic liquid and magnetite nanoparticle interactions at the oil/water interface. J. Pet. Sci. Eng. 2018, 160, 363–371. [Google Scholar] [CrossRef]
- Hassan, S.A.; Abdalla, B.K.; Mustafa, M.A. Addition of silica nano-particles for the enhancement of crude oil demulsification process. Pet. Sci. Technol. 2019, 37, 1603–1611. [Google Scholar] [CrossRef]
- Atta, A.M.; Ezzat, A.O.; Hashem, A.I. Synthesis and application of monodisperse hydrophobic magnetite nanoparticles as an oil spill collector using an ionic liquid. RSC Adv. 2017, 7, 16524–16530. [Google Scholar] [CrossRef]
- Mi, T.; Cai, Y.; Wang, Q.; Habibul, N.; Ma, X.; Su, Z.; Wu, W. Synthesis of Fe3O4 nanocomposites for efficient separation of ultra-small oil droplets from hexadecane–water emulsions. RSC Adv. 2020, 10, 10309–10314. [Google Scholar] [CrossRef]
| Ionic Liquid | Melting Point (°C) |
|---|---|
| C2mim BF4 | 15 |
| C2mim TfO | −10.15 |
| C6mim PF6 | −61 |
| C8mim BF4 | −80 |
| N6222 NTf2 | 20 |
| Anion | Abbreviation | Types (Organic/Inorganic) |
|---|---|---|
| Alkyl sulfate | R-O-SO3− | Organic |
| Methane sulfonate | R3C-S-O3− | Organic |
| Tosylate | C7H7O3S− | Organic |
| Trifluoroacetate | CF3CO2− | Organic |
| Chloride | Cl− | Inorganic |
| Fluoride | F− | Inorganic |
| Bromide | Br− | Inorganic |
| Iodide | I− | Inorganic |
| Tetrachloroaluminate | AlCl4− | Inorganic |
| Hexafluorophosphate | PF6− | Inorganic |
| Tetrafluoroborate | BF4− | Inorganic |
| Bis(trifluoromethylsulfonyl) imide | [(CF3SO2)2N]− | Inorganic |
| Preferred | Usable | Undesirable |
|---|---|---|
| Acetone | Cyclohexane | Pentane |
| Ethyl acetate | Heptane | Hexane(s) |
| Water | Toluene | Di isopropyl ether |
| Ethanol | Methyl cyclohexane | Diethyl ether |
| Methanol | Isooctane | Dichloromethane |
| 2-propanol | Acetonitrile | Dichloromethane |
| 1-propanol | 2-Methyltetrahydrofuran | Chloroform |
| Isopropylacetate | Tetrahydrofuran | Pyridine |
| 1-butanol | Xylenes | Dioxane |
| Tert-butyl alcohol | Dimethyl sulfoxide | Dimethoxyethane |
| Acetic acid | Benzene | |
| Ethylene glycol | Carbon tetrachloride | |
| Methyl Ethyl Ketone |
| Ionic Liquid | Cation Type | Anion Type | Emulsion Type | Dose (ppm) | CMC (ppm) | DE (%) | IFT Reduction (%) | Key Findings | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Cnmim NTf2 n = 10, 12, 14 | Imidazolium | bis(trifluoromethylsulfonyl)imide | SW/O | 100–3500 | N.A. | 93.6–100 | 77–95 | Demulsification experiments were conducted at the temperature of 60 °C. Increasing the dose and alkyl cation chain of hydrophobic ionic liquids improved the demulsification process (100% demulsification) as well as reduced the IFT (95% reduction). Higher hydrophobicity of NTf2 results in improving demulsification efficiency even with shorter alkyl chain length (e.g., demulsification efficiency was in the range of 93.6–100%). By contrast, for hydrophilic ionic liquid, increasing the dose and alkyl cation chain length of ionic liquid led to aggregation and caused poor demulsification as well as increased IFT. | [101] |
| Cnmim PF6 n = 10, 12, 14 | Hexafluoro phosphate | 500–3500 | 71.25–86.25 | 54–81 | |||||
| Cnmim Cl n = 10, 12, 14 | Chloride | 500–3500 | 76.25–93.75 | 64–80 | |||||
| TOMAC | Ammonium | Chloride | W/O | 1000–2000 | N.A. | 100 | N.A. | The efficiency of three ionic liquids (TOMAC, TOMAB, CTAB) with different hydrophobicity and hydrophilicity were evaluated for demulsification of W/O emulsions. Response surface methodology was applied to investigate the effect of temperature (50 °C–80 °C), pH (5–9), and water of aqueous phase (3–10%) on the demulsification efficiency. They observed that increasing ionic liquids concentration to 1039.2 ppm, 1480 ppm, 332.09 ppm for TOMAC, TOMAB, and CTAB, respectively led to the maximum demulsification efficiency (100, 90.29, and 64.9% for TOMAC, TOMAB, and CTAB, respectively). Demulsification efficiency increased at the pH of 7 and the temperature of 80 °C. Increasing water of emulsion (up to 10%) increased the demulsification efficiency of system to 64.88% and 90.29% using hydrophilic TOMAB and CTAB ionic liquids, respectively. Among three ionic liquids, TOMAC had the highest efficiency (100%) because it was more hydrophobic than other ionic liquids. | [107] |
| TOMAB | Bromide | 1000–2000 | N.A. | 64.9 | |||||
| CTAB | Bromide | 300–700 | N.A. | 90.29 | |||||
| Trihexyltetra decylphosphonium [Y] | Phosphonium | Chloride | W/O | 50–4000 | N.A. | 71.42–99 | N.A. | Experiments were conducted at different temperatures (60 °C and 80 °C) to investigate the efficiency of phosphonium based ionic liquids at different concentration of 50 to 4000 ppm on demulsifying W/O emulsions. Different hydrophobicity of anions led to different demulsification efficiency (e.g., varying from 14.29 to 99%). | [49] |
| Decanoate | 14.29–92.86 | ||||||||
| Dicyanamide | 50–99 | ||||||||
| AMPS/AA-TE | Oxyethylene ammonium | Sulfonate and carboxylate | W/O | 100–500 | 0.00027 * | 8–100 | N.A. | Experiments were conducted at the temperature of 65 °C and different water content of emulsions (10, 20, 30, 50%). AMPS/AA-TE poly ionic liquid has oxyethylene in its structure which increased the polarity of AMPS/AA-TE. This led to AMPS/AA-TE having lower CMC than AMPS/AA-OA. | [36] |
| AMPS/AA-OA | Ammonium | 0.00053 * | 10–100 | ||||||
| TOMAC | Ammonium | Chloride | W/O | 1500 | N.A. | 90 | N.A. | Experiments were conducted at the temperature of 80 °C. TOMAC removed water from an extra heavy crude oil in less than an hour while two hours were required for trioctylmethyl ammonium ethyl sulfate and trioctylmethyl ammonium methyl sulfate to remove the same amount of water. | [102] |
| Trioctyl methyl ammonium [Y] | Ethyl sulfate | N.A. | |||||||
| Methyl sulfate | N.A. | ||||||||
| C12 mim NTf2 | Imidazolium | bis(trifluoromethylsulfonyl)imide | W/O | 5–125 | 100 | N.A. | 33.3 | Applying ionic liquids with long alkyl chain length (12 carbon atoms) was more capable to displace the natural emulsifying agents of the crude oil which resulted in enhancing the IFT reduction (33%). Increasing ionic liquid concentration to CMC (100 ppm) reduced IFT, while no significant change was observed with concentration more than CMC. | [20] |
| HEOD-TS | Ammonium | Tosylate | SW/O | 100–500 | N.A. | 30–100 | 95–99.5 | Demulsification experiments were conducted at 65 °C. Increasing the concentration of hydrophobic HEOD-TS ionic liquid (e.g., from 100 to 500 ppm) for demulsifying SW/O emulsions at different water contents (10, 30, 50%) resulted in demulsifying emulsions completely (100%) as well as decreasing the IFT. | [24] |
| TOMAC | Ammonium | Chloride | W/O | 1000 and 1500 | N.A. | 100 | N.A. | Demulsification experiments were conducted at 80 °C using a water bath to remove water of two extra-heavy crude oils (with the water content of 56 and 60%). Increasing the concentration of ionic liquids from 1000 to 1500 ppm resulted in 100% demulsification efficiency. Ionic liquids with smaller anion size have lower anion polarizability which result in dehydration of extra-heavy crude oils effectively. | [108] |
| Trioctylmethylammonium [Y] | Bisulfate | ||||||||
| Dihydrogenphosphate | |||||||||
| C8mim PF6 | Imidazolium | Hexafluorophosphate | W/O | 600–6200 | N.A. | 54.7–95.6 | 92 | High dosage of ionic liquids resulted in 95.6% and 87.4% of demulsifying W/O emulsions using C8mim PF6 and C8mim BF4, respectively that were implemented under microwave heating (90 °C) and different water content of emulsions (~ 30 to 50%). C8mim PF6 decreased the IFT and separated water from oil more effective than C8mim BF4 (95.6 and 87.4% for C8mim PF6 and C8mim BF4, respectively). The reason is that C8mim PF6 has bigger anion size and lower solubility in water which prevents the aggregation of ionic liquid in the medium in comparison with C8mim BF4. | [114] |
| C8mim BF4 | Tetrafluoroborate | 1000–7200 | 0–87.4 | 85 | |||||
| C4mim NTf2 | Imidazolium | bis(trifluoromethylsulfonyl)imide | SW/O | 0.74–8.9 ** | N.A. | 10 | 1 | Imidazolium and pyridinium based ionic liquids were used to demulsify W/O emulsion (water content of 40 wt%) at the temperature of 120 °C. There was no significant difference in the demulsification efficiency between the imidazolium and the pyridinium ionic liquids with the same alkyl chain length and anion type. Ionic liquids with longer alkyl chain (e.g., 8 and 12 carbon atoms) were more capable to displace the natural emulsifying agents of the crude oil which resulted in enhancing the demulsification process (74% and 90% for C8mim NTf2 and C12mim NTf2, respectively) as well as IFT reduction. Higher hydrophobicity of NTf2 results in improving demulsification efficiency (e.g., 74% and 40% for C8mim NTf2 and C8mim OTf, respectively). | [115] |
| C8mim NTf2 | 74 | 4 | |||||||
| C12mim NTf2 | 90 | 34 | |||||||
| C8mim OTf | triflate | ~40 | N.A. | ||||||
| C4py NTf2 | Pyridinium | bis(trifluoromethylsulfonyl)imide | 10> | N.A. | |||||
| EDHI | Imidazolium | Acetate | W/O | 50–250 | N.A. | 0–70 | N.A. | Demulsification process using imidazolium-based ionic liquids were conducted at 60 °C and different water contents (10, 20, 30%). Increasing ionic liquids concentration from 50 to 250 ppm increased the demulsification efficiency to 70, 85, 100, and 100 for EDHI, EPHIB, EDDI, and EDPIB, respectively at different experimental conditions. Using 4-(trifluoromethoxy)phenylborate anion increased the hydrophobicity of EPHIB compared to EDHI which resulted in enhancing the demulsification process (e.g., demulsification efficiency increased from 70 to 85%). Based on the results, the efficiency of polymeric ionic liquids was better than that of their monomeric ionic liquids. | [117] |
| EPHIB | 4-(trifluoromethoxy) phenylborate | 10–85 | |||||||
| EDDI | Acetate | 70–100 | |||||||
| EPDIB | 4-(trifluoromethoxy) phenylborate | 85–100 | |||||||
| P666,14 (CN)2 | Phosphonium | Dicyanamide | O/W | *** | N.A. | 100 | N.A. | In this research, different ionic liquids with hydrophobic cation and hydrophilic anions were used to demulsify O/W emulsions at room temperature. P666,14[N(CN)2] had high surface active area and removed oil from water completely. However, stable emulsions still existed in the systems that P666,14[Phos], P666,14[NTf2] and N2224[N(CN)2] were used because surface active area would not achieve in too hydrophobic (P666,14[Phos], P666,14[NTf2]) and too hydrophilic (N2224[N(CN)2]) ionic liquids.Halogenide ionic liquids, P666,14[Cl] and P666,14[Br] separated oil from water in a very short time (20 min) compared to non-halogenide ionic liquid P666,14[N(CN)2] (24 h). | [118] |
| P666,14 Phos | bis(2,4,4-trimethylpentyl) phosphinate | 0 | |||||||
| P666,14 NTf2 | bis(trifluoromethylsulfonyl)imide | 0 | |||||||
| P666,14 Cl | Chloride | >90 | |||||||
| P666,14 Br | Bromide | ||||||||
| N2224 N(CN)2 | Ammonium | Dicyanamide | 0 | ||||||
| C8mim Cl | Imidazolium | Chloride | W/O | 100–10000 | 1000 | N.A. | 3–73 | In this research, imidazolium and pyridinium based ionic liquids were used at different concentration (100–1000 ppm) and different temperatures (20 °C to 60 °C) to evaluate their efficiency on IFT reduction. Pyridinium cation is more hydrophobic than imidazolium cation, therefore pyridinium based ionic liquids can remain at the O-W interface better than imidazolium based ionic liquids, which enable them to reduce the IFT of crude oil-distilled water effectively at lower CMC. | [99] |
| C12mim Cl | 2000 | 6–84 | |||||||
| C8Py Cl | Pyridinium | N.A. | 4–45 | ||||||
| C12Py Cl | 500 | 7–93 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassanshahi, N.; Hu, G.; Li, J. Application of Ionic Liquids for Chemical Demulsification: A Review. Molecules 2020, 25, 4915. https://doi.org/10.3390/molecules25214915
Hassanshahi N, Hu G, Li J. Application of Ionic Liquids for Chemical Demulsification: A Review. Molecules. 2020; 25(21):4915. https://doi.org/10.3390/molecules25214915
Chicago/Turabian StyleHassanshahi, Nahid, Guangji Hu, and Jianbing Li. 2020. "Application of Ionic Liquids for Chemical Demulsification: A Review" Molecules 25, no. 21: 4915. https://doi.org/10.3390/molecules25214915
APA StyleHassanshahi, N., Hu, G., & Li, J. (2020). Application of Ionic Liquids for Chemical Demulsification: A Review. Molecules, 25(21), 4915. https://doi.org/10.3390/molecules25214915






