Exopolysaccharide II Is Relevant for the Survival of Sinorhizobium meliloti under Water Deficiency and Salinity Stress
Abstract
1. Introduction
2. Results
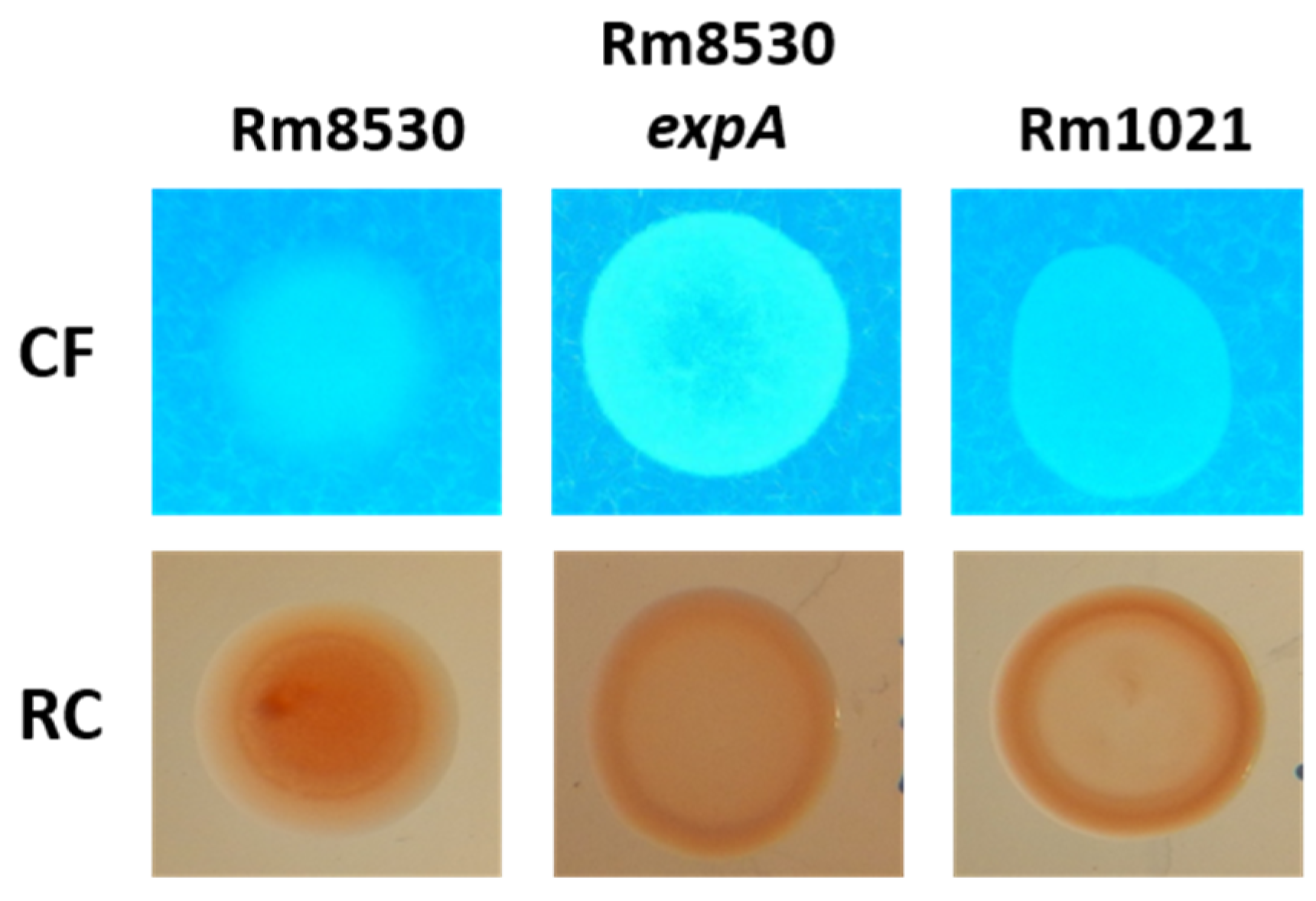
2.1. EPS Production in S. meliloti
2.2. Test of Viability in Sand: Water and Saline Stress
2.2.1. Water Stress (Desiccation Assay)
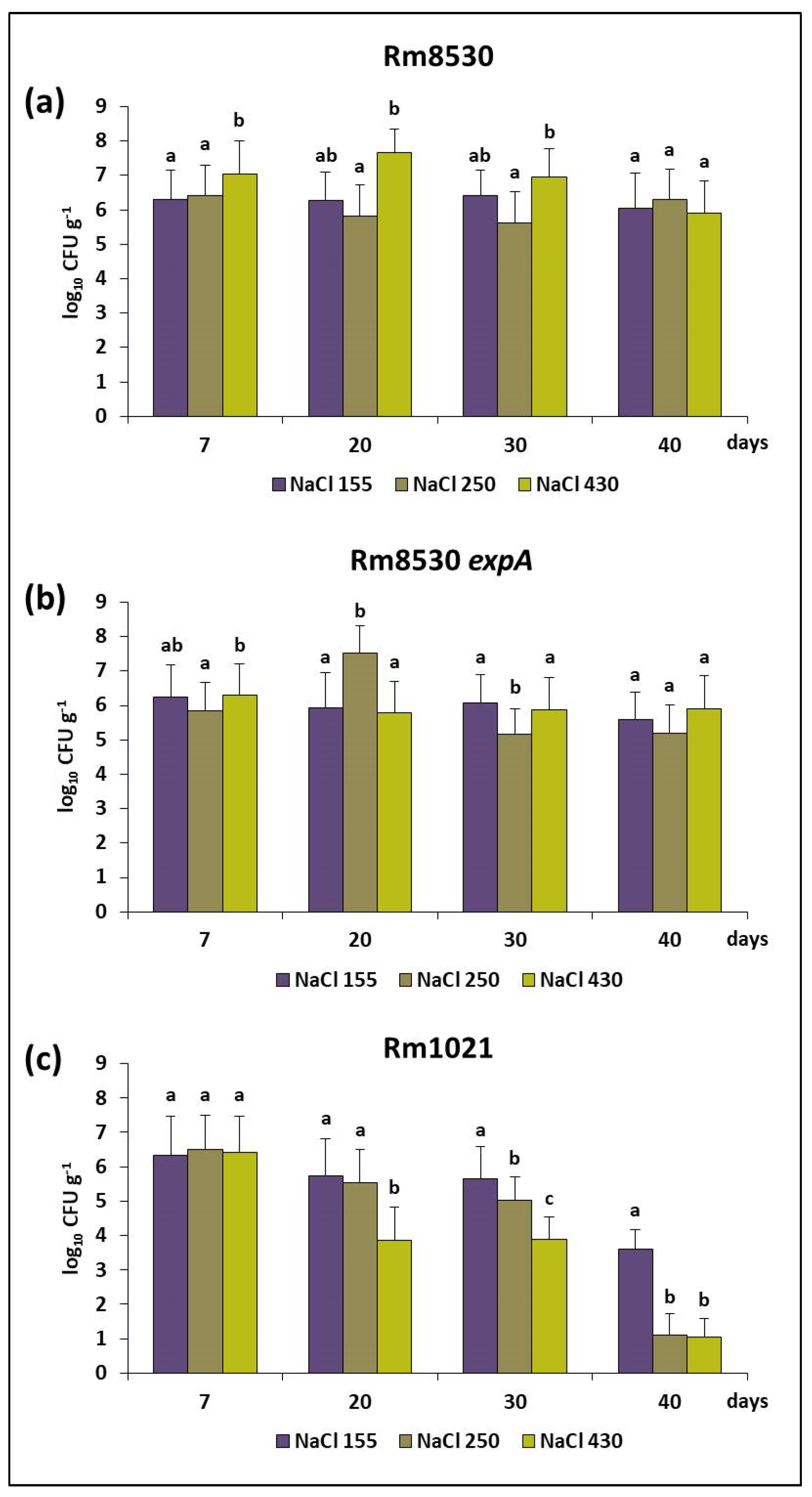
2.2.2. Saline Stress
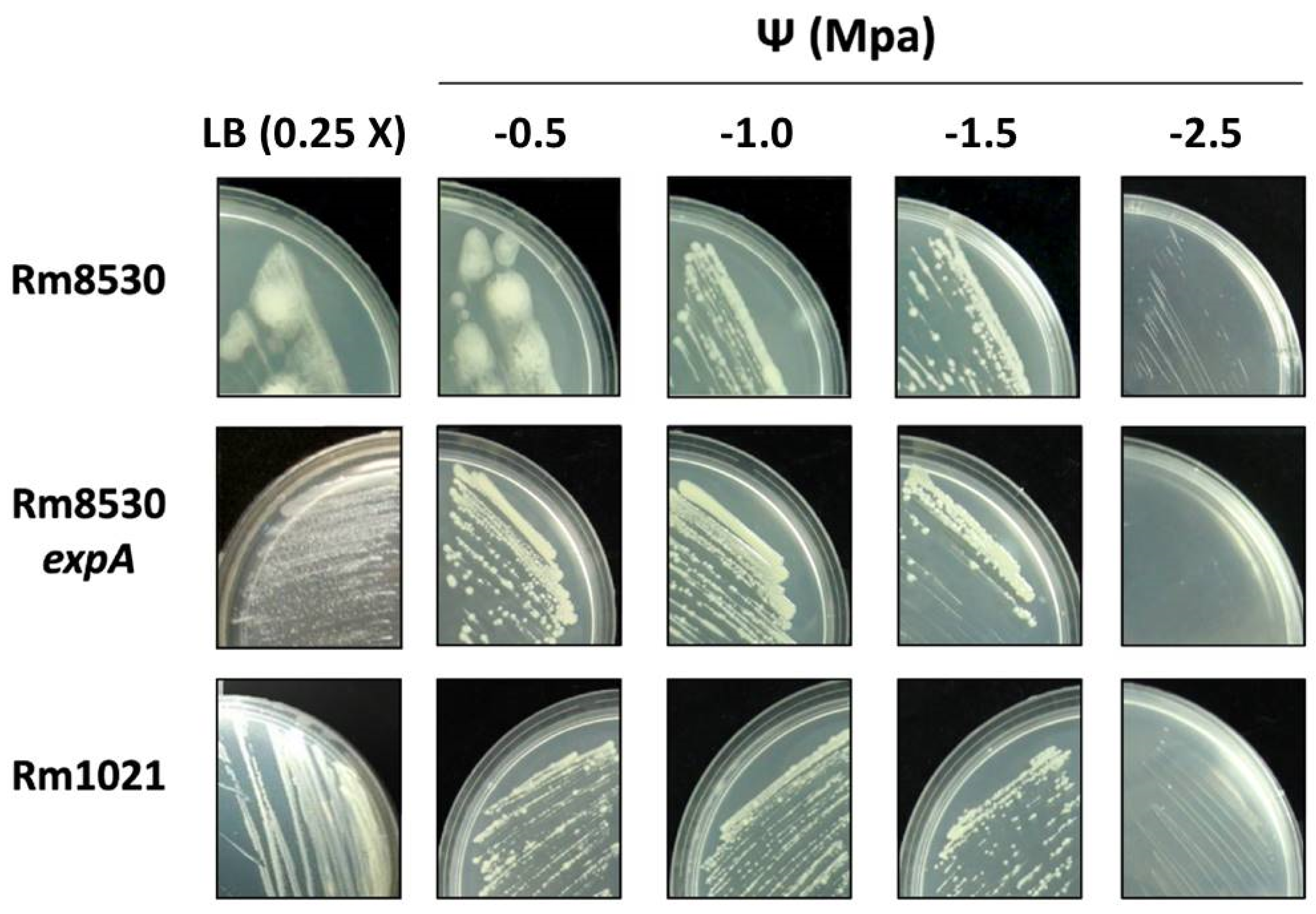
2.3. Growth and Colony Phenotype of S. meliloti Strains
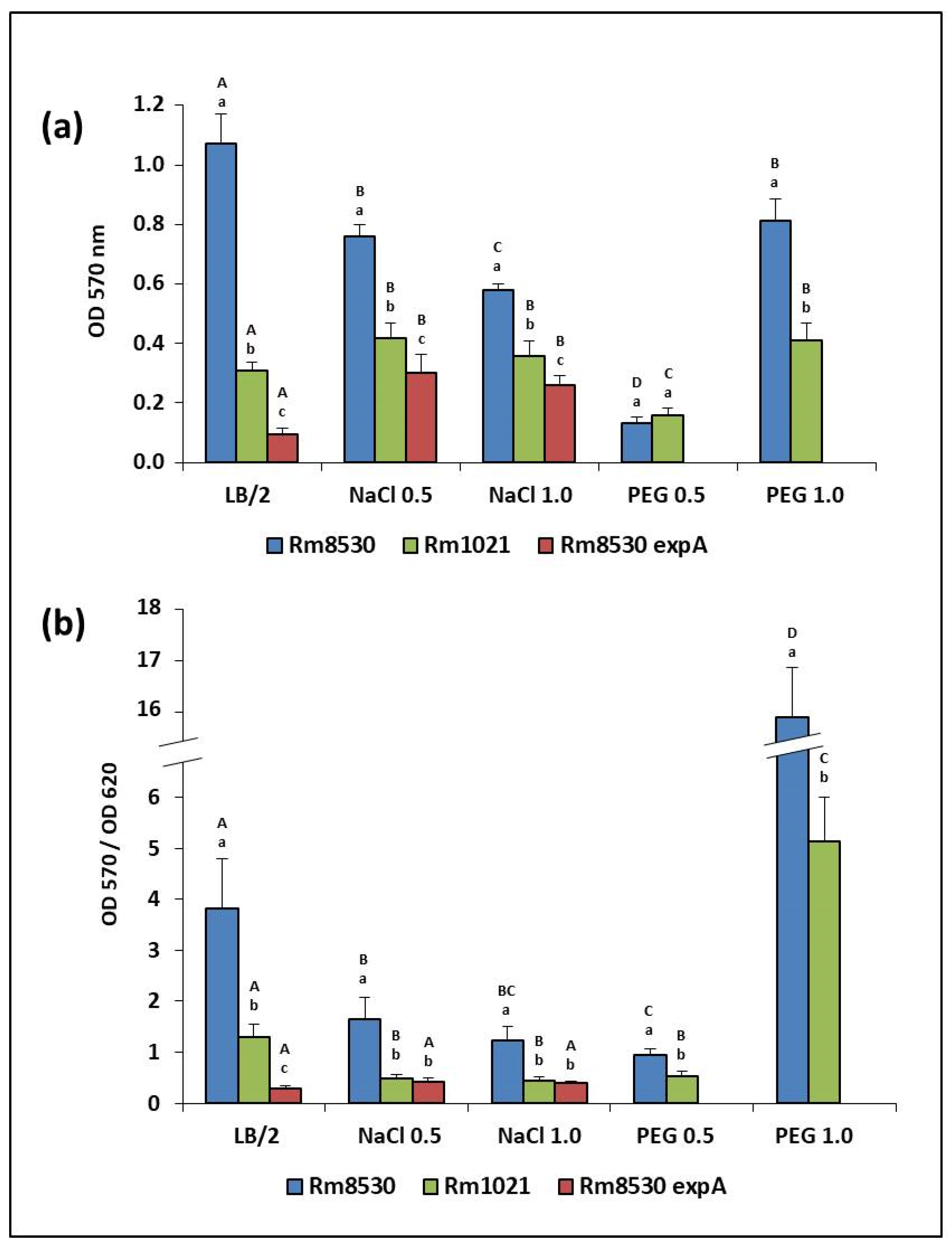
2.4. Biofilm-Forming Capacity (BFC)
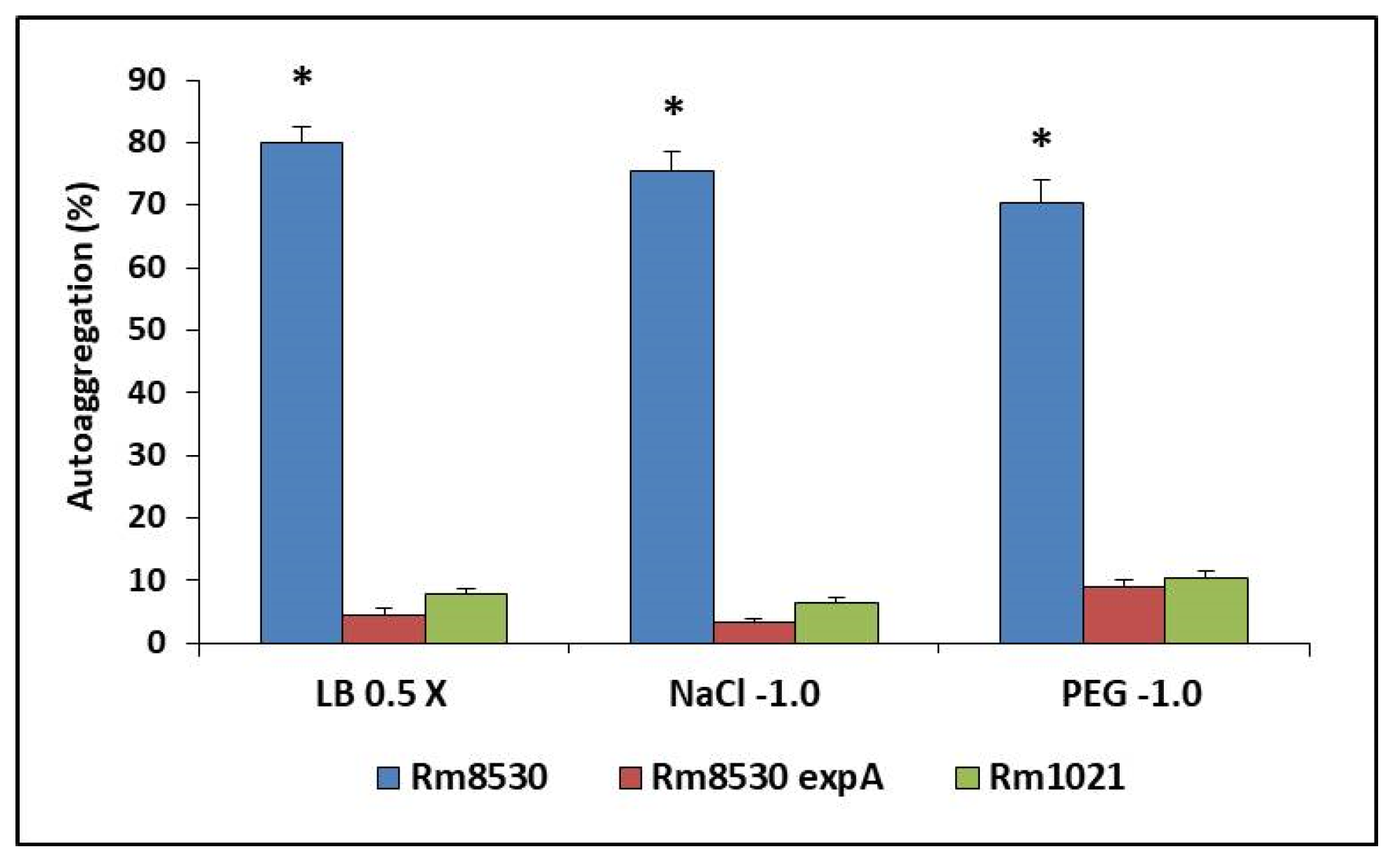
2.5. Autoaggregation Assay
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Growth Conditions
4.2. Bacterial Cell Viability Assays under Specific Stress Conditions
4.2.1. Water Stress
4.2.2. Saline Stress
4.2.3. Strain Growth and Colony Morphology on Saline Stress Plates
4.3. BFC Test
BFC Test under Stress Conditions
4.4. Autoaggregation Assay
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ates, O. Systems biology of microbial exopolysaccharides production. Front. Bioeng. Biotechnol. 2015, 3, 200. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.B.; Sousa, A.; Santos, M.; Araújo, M.; Serôdio, F.; Granja, P.; Tamagnini, P. Strategies to obtain designer polymers based on cyanobacterial extracellular polymeric substances (EPS). Int. J. Mol. Sci. 2019, 20, 5693. [Google Scholar] [CrossRef] [PubMed]
- Fraysse, N.; Couderc, F.; Poinsot, V. Surface polysaccharide involvement in establishing the rhizobium-legume symbiosis. Eur. J. Biochem. 2003, 270, 1365–1380. [Google Scholar] [CrossRef]
- Pellock, B.J.; Teplitski, M.; Boinay, R.P.; Bauer, W.D.; Walker, G.C. A LuxR homolog controls production of symbiotically active extracellular polysaccharide II by Sinorhizobium meliloti. J. Bacteriol. 2002, 184, 5067–5076. [Google Scholar] [CrossRef]
- Zhan, H.; Lee, C.C.; Leigh, J.A. Induction of the second exopolysaccharide (EPSb) in Rhizobium meliloti SU47 by low phosphate concentrations. J. Bacteriol. 1991, 173, 7391–7394. [Google Scholar] [CrossRef] [PubMed]
- Fuqua, C.; Parsek, M.R.; Greenberg, E.P. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001, 35, 439–468. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, N.A.; Barnard, A.M.L.; Slater, H.; Simpson, N.J.L.; Salmond, G.P.C. Quorum-sensing in Gram-negative bacteria. FEMS Microbiol. Rev. 2001, 25, 365–404. [Google Scholar] [CrossRef]
- Hoang, H.H.; Becker, A.; González, J.E. The LuxR homolog ExpR, in combination with the sin quorum sensing system, plays a central role in Sinorhizobium meliloti gene expression. J. Bacteriol. 2004, 186, 5460–5472. [Google Scholar] [CrossRef]
- Glenn, S.A.; Gurich, N.; Feeney, M.A.; González, J.E. The ExpR/Sin quorum-sensing system controls succinoglycan production in Sinorhizobium meliloti. J. Bacteriol. 2007, 189, 7077–7088. [Google Scholar] [CrossRef]
- Bogino, P.C.; Oliva, M.; de las, M.; Sorroche, F.G.; Giordano, W. The role of bacterial biofilms and surface components in plant-bacterial associations. Int. J. Mol. Sci. 2013, 14, 15838–15859. [Google Scholar] [CrossRef]
- Castiblanco, L.F.; Sundin, G.W. New insights on molecular regulation of biofilm formation in plant-associated bacteria. J. Integr. Plant Biol. 2016, 58, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Limoli, D.H.; Jones, C.J.; Wozniak, D.J. Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol. Spectr. 2015, 3, 223–247. [Google Scholar] [CrossRef] [PubMed]
- Nikitina, V.E.; Ponomareva, E.G.; Alen’kina, S.A.; Konnova, S.A. The role of cell-surface lectins in the aggregation of azospirilla. Microbiology 2001, 70, 408–412. [Google Scholar] [CrossRef]
- Zaini, P.A.; Burdman, S.; Igo, M.M.; Parker, J.K.; De La Fuente, L. Chapter 5: Fimbrial and afimbrial adhesins involved in bacterial attachment to surfaces. In Virulence Mechanisms of Plant-Pathogenic Bacteria; De La Fuente, L., Igo, M.M., Parker, J.K., Burdman, S., Zaini, P.A., Eds.; Amer Phytopathological Society: St. Paul, MN, USA, 2016; pp. 73–106. [Google Scholar] [CrossRef]
- Anderson, G.G.; O’Toole, G.A. Innate and induced resistance mechanisms of bacterial biofilms. Curr. Top. Microbiol. Immunol. 2008, 322, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Rinaudi, L.; Fujishige, N.A.; Hirsch, A.M.; Banchio, E.; Zorreguieta, A.; Giordano, W. Effects of nutri-tional and environmental conditions on Sinorhizobium meliloti biofilm formation. Res. Microbiol. 2006, 157, 867–875. [Google Scholar] [CrossRef]
- Rinaudi, L.; Giordano, W. An integrated view of biofilm formation in rhizobia. FEMS Microbiol. Lett. 2010, 304, 1–11. [Google Scholar] [CrossRef]
- Sorroche, F.G.; Rinaudi, L.V.; Zorreguieta, Á.; Giordano, W. EPS II-dependent autoaggregation of Sinorhizobium meliloti planktonic cells. Curr. Microbiol. 2010, 61, 465–470. [Google Scholar] [CrossRef]
- Sorroche, F.G.; Spesia, M.B.; Zorreguieta, Á.; Giordano, W. A positive correlation between bacterial autoaggregation and biofilm formation in native Sinorhizobium meliloti isolates from Argentina. Appl. Environ. Microbiol. 2012, 78, 4092–4101. [Google Scholar] [CrossRef]
- Sorroche, F.; Bogino, P.; Russo, D.M.; Zorreguieta, A.; Nievas, F.; Morales, G.M.; Hirsch, A.M.; Giordano, W. Cell autoaggregation, biofilm formation, and plant attachment in a Sinorhizobium meliloti lpsB mutant. Mol. Plant-Microbe Interact. 2018, 31, 1075–1082. [Google Scholar] [CrossRef]
- Primo, E.D.; Cossovich, S.; Nievas, F.; Bogino, P.; Humm, E.A.; Hirsch, A.M.; Giordano, W. Exopolysaccharide production in Ensifer meliloti laboratory and native strains and their effects on alfalfa inoculation. Arch. Microbiol. 2020, 202, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Hidalgo, P.; Hirsch, A.M. The nodule microbiome: N2-fixing rhizobia do not live alone. Phytobiomes J. 2017, 1, 70–82. [Google Scholar] [CrossRef]
- Bogino, P.; Abod, A.; Nievas, F.; Giordano, W. Water-limiting conditions alter the structure and biofilm-forming ability of bacterial multispecies communities in the alfalfa rhizosphere. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- Spiers, A.J.; Kahn, S.G.; Bohannon, J.; Travisano, M.; Rainey, P.B. Adaptive divergence in experimental populations of Pseudomonas fluorescens. I. Genetic and phenotypic bases of wrinkly spreader fitness. Genetics. 2002, 161, 33–46. [Google Scholar]
- Kneen, B.E.; LaRue, T.A. Congo red absorption by Rhizobium leguminosarum. Appl. Environ. Microbiol. 1983, 45, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.M. Increased production of the exopolysaccharide succinoglycan enhances Sinorhizobium meliloti 1021 symbiosis with the host plant Medicago truncatula. J. Bacteriol. 2012, 194, 4322–4331. [Google Scholar] [CrossRef]
- Miller, K.J.; Wood, J.M. Osmoadaptation by rhizosphere bacteria. Annu. Rev. Microbiol. 1996, 50, 101–136. [Google Scholar] [CrossRef]
- Mhamdi, R.; Nouairi, I.; ben Hammouda, T.; Mhamdi, R.; Mhadhbi, H. Growth capacity and biochemical mechanisms involved in rhizobia tolerance to salinity and water deficit. J. Basic Microbiol. 2015, 55, 451–461. [Google Scholar] [CrossRef]
- Cardoso, P.; Freitas, R.; Figueira, E. Salt tolerance of rhizobial populations from contrasting environmental conditions: Understanding the implications of climate change. Ecotoxicology 2015, 24, 143–152. [Google Scholar] [CrossRef]
- Lloret, J.; Wulff, B.B.H.; Rubio, J.M.; Downie, J.A.; Bonilla, I.; Rivilla, R. Exopolysaccharide II production is regulated by salt in the halotolerant strain Rhizobium meliloti EFB1. Appl. Environ. Microbiol. 1998, 64, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Nocelli, N.; Bogino, P.C.; Banchio, E.; Giordano, W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of rhizobia. Materials 2016, 9, 418. [Google Scholar] [CrossRef] [PubMed]
- Breedveld, M.W.; Zevenhuizen, L.P.T.M.; Zehnder, A.J.B. Osmotically induced oligo- and polysaccharide synthesis by Rhizobium meliloti Su-47. J. Gen. Microbiol. 1990, 136, 2511–2519. [Google Scholar] [CrossRef]
- Vriezen, J.A.C.; De Bruijn, F.J.; Nüsslein, K. Responses of rhizobia to desiccation in relation to osmotic stress, oxygen, and temperature. Appl. Environ. Microbiol. 2007, 73, 3451–3459. [Google Scholar] [CrossRef]
- Bui, E.N. Soil salinity: A neglected factor in plant ecology and biogeography. J. Arid. Environ. 2013, 92, 14–25. [Google Scholar] [CrossRef]
- Djordjevic, M.A.; Chen, H.C.; Natera, S.; Van Noorden, G.; Menzel, C.; Taylor, S.; Renard, C.; Geiger, O.; Weiller, G.F. A global analysis of protein expression profiles in Sinorhizobium meliloti: Discovery of new genes for nodule occupancy and stress adaptation. Mol. Plant–Microbe Interact. 2003, 16, 508–524. [Google Scholar] [CrossRef]
- Lloret, J.; Bolanos, L.; Lucas, M.M.; Peart, J.M.; Brewin, N.J.; Bonilla, I.; Rivilla, R. Ionic stress and osmotic pressure induce different alterations in the lipopolysaccharide of a Rhizobium meliloti strain. Appl. Environ. Microbiol. 1995, 61, 3701–3704. [Google Scholar] [CrossRef]
- Domínguez-Ferreras, A.; Pérez-Arnedo, R.; Becker, A.; Olivares, J.; Soto, M.J.; Sanjuán, J. Transcriptome profiling reveals the importance of plasmid pSymB for osmoadaptation of Sinorhizobium meliloti. J. Bacteriol. 2006, 188, 7617–7625. [Google Scholar] [CrossRef]
- Dupuy, P.; Gourion, B.; Sauviac, L.; Bruand, C. DNA double-strand break repair is involved in desiccation resistance of Sinorhizobium meliloti, but is not essential for its symbiotic interaction with Medicago truncatula. Microbiology 2017, 163, 333–342. [Google Scholar] [CrossRef]
- Rinaudi, L.V.; González, J.E. The low-molecular-weight fraction of exopolysaccharide II from Sinorhizobium meliloti is a crucial determinant of biofilm formation. J. Bacteriol. 2009, 191, 7216–7224. [Google Scholar] [CrossRef] [PubMed]
- Calatrava-Morales, N.; McIntosh, M.; Soto, M.J. Regulation mediated by N-acyl homoserine lactone quorum sensing signals in the Rhizobium-Legume Symbiosis. Genes 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.H.; Gurich, N.; González, J.E. Regulation of motility by the ExpR/Sin quorum-sensing system in Sinorhizobium meliloti. J. Bacteriol. 2008, 190, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Nogales, J.; Bernabéu-Roda, L.; Cuéllar, V.; Soto, M.J. ExpR is not required for swarming but promotes sliding in Sinorhizobium meliloti. J. Bacteriol. 2012, 194, 2027–2035. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Vik, Å.; Friedman, L.; Kolter, R. Biofilms: The matrix revisited. Trends Microbiol. 2005, 13, 20–26. [Google Scholar] [CrossRef]
- Davey, M.E.; O’toole, G.A. Microbial biofilms: From ecology to molecular genetics. Microbiol. Mol. Biol. Rev. 2000, 64, 847–867. [Google Scholar] [CrossRef]
- Beringer, J.E. R factor transfer in Rhizobium leguminosarum. J. Gen. Microbiol. 1974, 84, 188–198. [Google Scholar] [CrossRef]
- Meade, H.M.; Long, S.R.; Ruvkun, G.B.; Brown, S.E.; Ausubel, F.M. Physical and genetic characterization of symbiotic and auxotrophic mutants of Rhizobium meliloti induced by transposon Tn5 mutagenesis. J. Bacteriol. 1982, 149, 114–122. [Google Scholar] [CrossRef]
- Glazebrook, J.; Walker, G.C. A novel exopolysaccharide can function in place of the calcofluor-binding exopolysaccharide in nodulation of alfalfa by Rhizobium meliloti. Cell 1989, 56, 661–672. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef]
Sample Availability: Samples of the crude extracts are available from the authors. |

| Strains | ||||
|---|---|---|---|---|
| LB Medium (0.25X) | Rm1021 | Rm8530 | ||
| Addition of NaCl (g L−1) | Ψ (MPa) * | wt | wt | expA |
| 1.25 | −0.15 | CG (D) | CG (M) | CG (D) |
| 6.4 | −0.65 (−0.5) | CG (D) | CG (M) | CG (D) |
| 12.8 | −1.15 (−1.0) | CG (D) | CG (D) | CG (D) |
| 19.2 | −1.65 (−1.5) | LG (D) | LG (D) | LG (D) |
| 32.0 | −2.65 (−2.5) | SG (D) | SG (D) | NG |
| S. meliloti Strain | Relevant Properties | EPS I/EPS II Phenotype | Reference |
|---|---|---|---|
| Rm1021 | SU47 str21 expR102::ISRm2011-1 (expR-) | EPS I | [48] |
| Rm8530 | SU47 str21 expR101 (expR+) | EPS I/EPS II | [49] |
| Rm8530 expA | expA3::Tn5-233 (expR+) | EPS I | [20] |
| Strains | ||||||
|---|---|---|---|---|---|---|
| Rm1021 | Rm8530 | Rm8530 expA | ||||
| Time (Days) | CT (H%) | ST (H%) | CT (H%) | ST (H%) | CT (H%) | ST (H%) |
| 0 | 22.1 | 22.1 | 23.1 | 23.1 | 19.7 | 19.7 |
| 7 | 15.0 | 8.2 | 15.3 | 8.6 | 17.7 | 8.6 |
| 20 | 15.0 | 4.0 | 15.5 | 4.0 | 16.0 | 6.0 |
| 30 | 15.0 | 3.0 | 16.0 | 3.5 | 15.0 | 5.0 |
| 40 | 16.0 | 0.4 | 16.5 | 0.3 | 16.0 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Primo, E.; Bogino, P.; Cossovich, S.; Foresto, E.; Nievas, F.; Giordano, W. Exopolysaccharide II Is Relevant for the Survival of Sinorhizobium meliloti under Water Deficiency and Salinity Stress. Molecules 2020, 25, 4876. https://doi.org/10.3390/molecules25214876
Primo E, Bogino P, Cossovich S, Foresto E, Nievas F, Giordano W. Exopolysaccharide II Is Relevant for the Survival of Sinorhizobium meliloti under Water Deficiency and Salinity Stress. Molecules. 2020; 25(21):4876. https://doi.org/10.3390/molecules25214876
Chicago/Turabian StylePrimo, Emiliano, Pablo Bogino, Sacha Cossovich, Emiliano Foresto, Fiorela Nievas, and Walter Giordano. 2020. "Exopolysaccharide II Is Relevant for the Survival of Sinorhizobium meliloti under Water Deficiency and Salinity Stress" Molecules 25, no. 21: 4876. https://doi.org/10.3390/molecules25214876
APA StylePrimo, E., Bogino, P., Cossovich, S., Foresto, E., Nievas, F., & Giordano, W. (2020). Exopolysaccharide II Is Relevant for the Survival of Sinorhizobium meliloti under Water Deficiency and Salinity Stress. Molecules, 25(21), 4876. https://doi.org/10.3390/molecules25214876






