Assessment of Soybean Oil Oxidative Stability from Rapid Analysis of its Minor Component Profile
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Commercial Virgin and Refined Soybean Oils
2.1.1. Composition in Main Components Determined by 1H-NMR
2.1.2. Composition in Minor Components Determined by DI-SPME-GC/MS
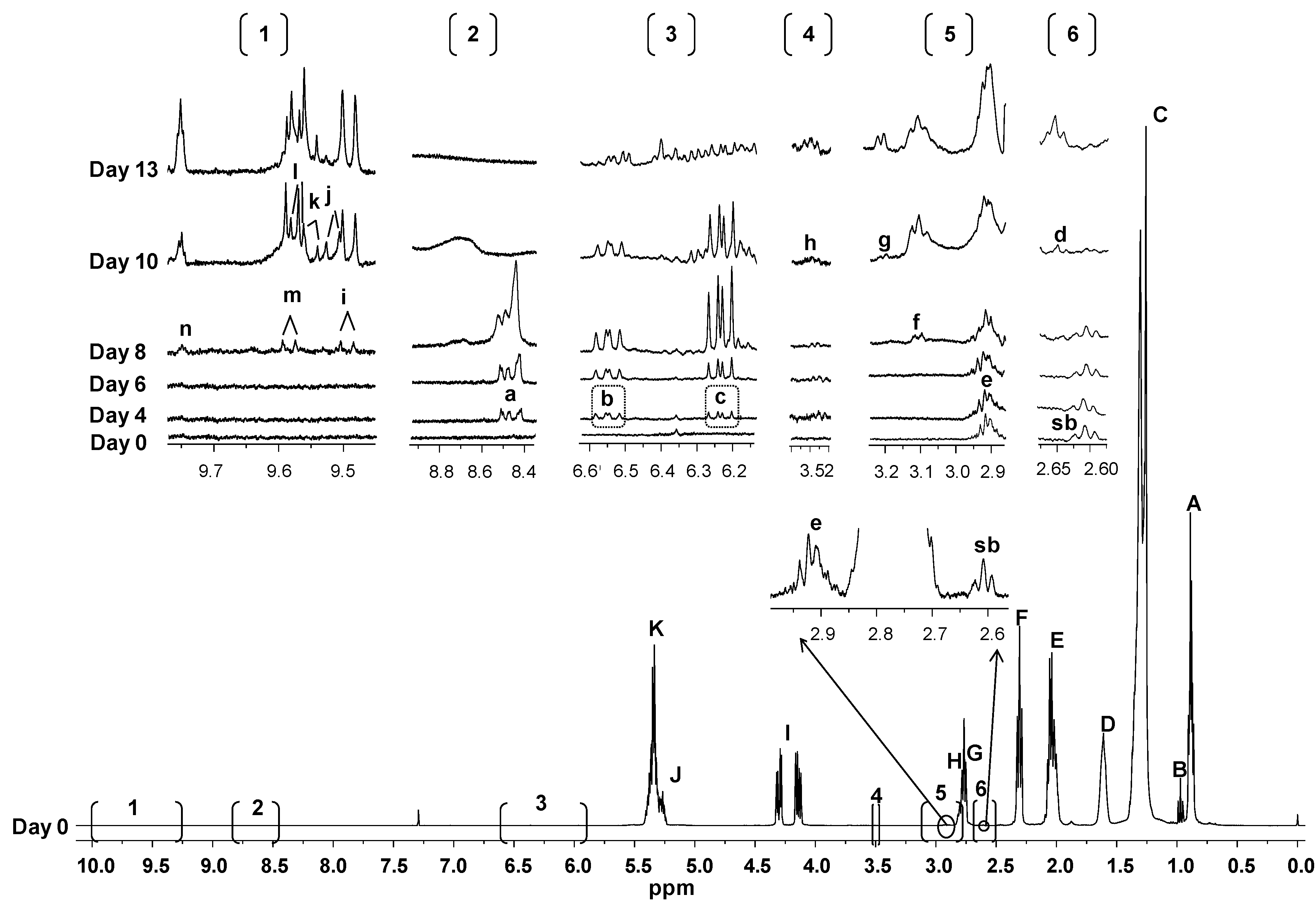
2.2. Study by 1H-NMR of the Evolution Under AS Conditions of VSO and RSO
2.2.1. Evolution of the Different Types of Oil Acyl Groups
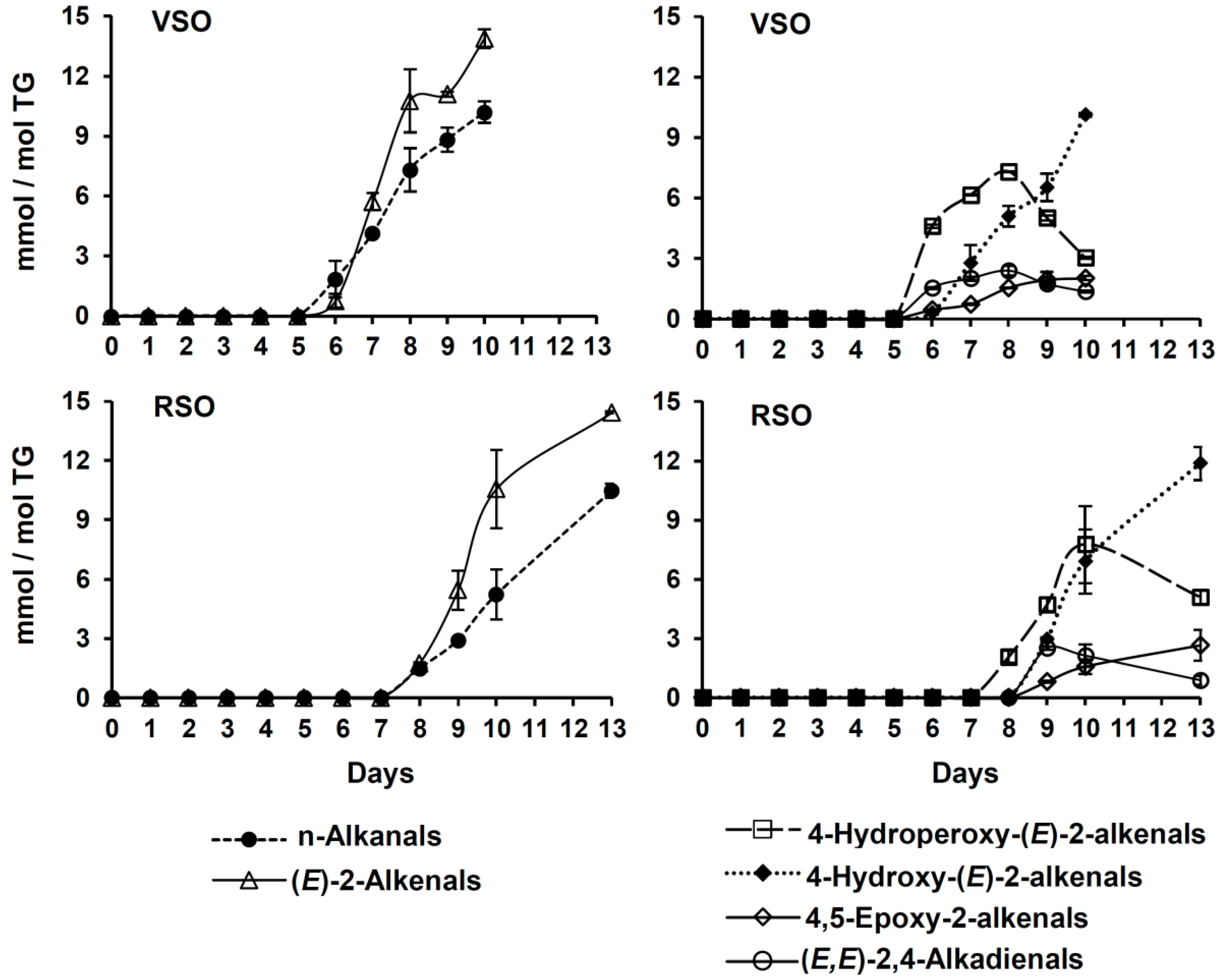
2.2.2. Formation and Evolution of Oxidation Products
- Hydroperoxides giving signal between 8.3 and 9 ppm, and their associated conjugated (Z,E)- and (E,E)-dienes
- Epoxides
- Aldehydes
2.3. Final Remarks
3. Materials and Methods
3.1. Characterization of the Oils Subject of Study
3.1.1. Analysis of the Main Oil Components (Acyl Groups)
3.1.2. Analysis of the Minor Oil Components
3.2. Accelerated Storage (AS) Process
3.3. Monitoring by 1H-NMR of the Evolution of VSO and RSO Throughout the AS Process
3.3.1. Operating Conditions
3.3.2. Identification of Some Components
3.3.3. Quantitative Data Derived from 1H-NMR Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Martínez-Yusta, A.; Goicoechea, E.; Guillén, M.D. A review of thermo-oxidative degradation of food lipids studied by 1H-NMR spectroscopy: Influence of degradative conditions and food lipid nature. Compr. Rev. Food Sci. Food Saf. 2014, 13, 838–859. [Google Scholar] [CrossRef]
- Choe, E.; Min, D.B. Mechanisms and factors for edible oil oxidation. Compr. Rev. Food Sci. Saf. 2006, 5, 169–186. [Google Scholar] [CrossRef]
- Kamal-Eldin, A. Effect of fatty acids and tocopherols on the oxidative stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2006, 108, 1051–1061. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Bioactive compounds detected for the first time in corn oil: Cyclic dipeptides and other nitrogenated compounds. J. Food Compost. Anal. 2017, 62, 197–204. [Google Scholar] [CrossRef]
- Dessi, M.A.; Deiana, M.; Day, B.W.; Rosa, A.; Banni, S.; Corongiu, F.P. Oxidative stability of polyunsaturated fatty acids: Effect of squalene. Eur. J. Lipid Sci. Technol. 2002, 104, 506–512. [Google Scholar] [CrossRef]
- Seppanen, C.M.; Song, Q.; Csallany, A.S. The antioxidant functions of tocopherol and tocotrienol homologues in oils, fats, and food systems. J. Am. Oil Chem. Soc. 2010, 87, 469–481. [Google Scholar] [CrossRef]
- Yoshida, Y.; Niki, E. Antioxidant effects of phytosterol and its components. J. Nutr. Sci. Vitaminol. 2003, 49, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.Y.; Yoon, S.H.; Min, D.B. Effects of processing steps on the contents of minor compounds and oxidation of soybean oil. J. Am. Oil. Chem. Soc. 1989, 66, 118–120. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Topkafa, M.; Kara, H.; Sherazi, S.T.H. Evaluation of fatty acid composition, tocols profile, and oxidative stability of some fully refined edible oils. Int. J. Food Prop. 2015, 18, 2064–2076. [Google Scholar] [CrossRef]
- Bozan, B.; Temelli, F. Chemical composition and oxidative stability of flax, safflower and poppy seed and seed oils. Bioresour. Technol. 2008, 99, 6354–6359. [Google Scholar] [CrossRef]
- Castelo-Branco, V.N.; Santana, I.; Di-Sarli, V.O.; Freitas, S.P.; Torres, A.G. Antioxidant capacity is a surrogate measure of the quality and stability of vegetable oils. Eur. J. Lipid Sci. Technol. 2016, 118, 224–235. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the relationship between vegetable oil composition and oxidative stability: A multifactorial approach. J. Food Compost. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Yang, M.; Zheng, C.; Zhou, Q.; Huang, F.; Liu, C.; Wang, H. Minor components and oxidative stability of cold-pressed oil from rapeseed cultivars in China. J. Food Compost. Anal. 2013, 29, 1–9. [Google Scholar] [CrossRef]
- Zheng, C.; Yang, M.; Zhou, Q.; Huang, F.; Li, W.; Liu, C. Bioactive compounds and antioxidant activities of cold-pressed seed oils. Oil Crop. Sci. 2018, 3, 189–200. [Google Scholar]
- Farhoosh, R.; Moosavi, S.M.R. Rancimat test for the assessment of used frying oils quality. J. Food Lipids 2007, 14, 263–271. [Google Scholar] [CrossRef]
- Frankel, E.N.; Neff, W.E.; Rohwedder, W.K.; Khambay, B.P.; Garwood, R.F.; Weedon, B.C.L. Analysis of autoxidized fats by gas chromatography-mass spectrometry: II. Methyl linoleate. Lipids 1977, 12, 908–913. [Google Scholar] [CrossRef]
- Mancebo-Campos, V.; Salvador, M.D.; Fregapane, G. Antioxidant capacity of individual and combined virgin olive oil minor compounds evaluated at mild temperature (25 and 40 °C) as compared to accelerated and antiradical assays. Food Chem. 2014, 150, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Läubi, M.W.; Bruttel, P.A. Determination of oxidative stability of fats and oils: Comparison between the active oxygen method (AOCS Cd 12-57) and the Rancimat method. J. Am. Oil Chem. Soc. 1986, 63, 792–795. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Oxidation process of oils with high content of linoleic acyl groups and formation of toxic hydroperoxy- and hydroxyalkenals. A study by 1H nuclear magnetic resonance. A study by 1H nuclear magnetic resonance. J. Sci. Food Agric. 2005, 85, 2413–2420. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Study by proton nuclear magnetic resonance of the thermal oxidation of oils rich in oleic acyl groups. J. Am. Oil Chem. Soc. 2005, 82, 349–355. [Google Scholar] [CrossRef]
- Guillén, M.D.; Ruiz, A. Monitoring of heat-induced degradation of edible oils by proton NMR. Eur. J. Lipid Sci. Technol. 2008, 110, 52–60. [Google Scholar] [CrossRef]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Cristillo, G.; Sopelana, P.; Guillén, M.D. A new methodology capable of characterizing most volatile and less volatile minor edible oils components in a single chromatographic run without solvents or reagents. Detection of new components. Food Chem. 2017, 221, 1135–1144. [Google Scholar] [CrossRef]
- Martin-Rubio, A.S.; Ibargoitia, M.L.; Sopelana, P.; Guillén, M.D. Controversia en relación al contenido de aceite de soja virgen y refinado en componentes beneficiosos para la salud. In Proceedings of the 6th International Congress about Own-Checks and Food Safety, Vitoria-Gasteiz, Spain, 25–27 May 2016. [Google Scholar]
- Cerretani, L.; Lerma-García, M.J.; Herrero-Martínez, J.M.; Gallina-Toschi, T.; Simó-Alfons, E.F. Determination of tocopherols and tocotrienols in vegetable oils by nanoliquid chromatography with ultraviolet-visible detection using a silica monolithic column. J. Agric. Food Chem. 2009, 58, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.K.; Perkins, E.G. Identification and estimation of tocopherols and tocotrienols in vegetable oils using gas chromatography-mass spectrometry. J. Agric. Food Chem. 1972, 20, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Mastellone, C.; D’Abrosca, B.; Pacifico, S.; Scognamiglio, M.; Cefarelli, G.; Caputo, R.; Monaco, P. δ-Tocomonoenol: A new vitamin E from kiwi (Actinidia chinensis) fruits. Food Chem. 2009, 115, 187–192. [Google Scholar] [CrossRef]
- Siger, A.; Nogala-Kalucka, M.; Lampart-Szczapa, E. The content and antioxidant activity of phenolic compounds in cold-pressed plant oils. J. Food Lipids 2008, 15, 137–149. [Google Scholar] [CrossRef]
- Phillips, K.M.; Ruggio, D.M.; Toivo, J.I.; Swank, M.A.; Simpkins, A.H. Free and esterified sterol composition of edible oils and fats. J. Food Compost. Anal. 2002, 15, 123–142. [Google Scholar] [CrossRef]
- Chu, Y.H.; Lin, J.Y. Factors affecting the content of tocopherol in soybean oil. J. Am. Oil Chem. Soc. 1993, 70, 1263–1268. [Google Scholar] [CrossRef]
- Erickson, D.R. Practical Handbook of Soybean Processing and Utilization; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Alberdi-Cedeño, J.; Ibargoitia, M.L.; Guillén, M.D. Oxylipins associated to current diseases detected for the first time in the oxidation of corn oil as a model system of oils rich in omega-6 polyunsaturated groups. A global, broad and in-depth study by 1H-NMR spectroscopy. Antioxidants 2020, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Guillén, M.D.; Goicoechea, E. Toxic oxygenated α,β-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008, 48, 119–136. [Google Scholar] [CrossRef]
- Barriuso, B.; Astiasarán, I.; Ansorena, D. A review of analytical methods measuring lipid oxidation status in foods: A challenging task. Eur. Food Res. Technol. 2013, 236, 1–15. [Google Scholar] [CrossRef]
- Dolde, D.; Wang, T. Oxidation of corn oils with spiked tocols. J. Am. Oil Chem. Soc. 2011, 88, 1759–1765. [Google Scholar] [CrossRef]
- Zaunschirm, M.; Pignitter, M.; Kienesberger, J.; Hernler, N.; Riegger, C.; Eggersdorfer, M.; Somoza, V. Contribution of the ratio of tocopherol homologs to the oxidative stability of commercial vegetable oils. Molecules 2018, 23, 206. [Google Scholar] [CrossRef]
- Guillén, M.D.; Uriarte, P.S. Study by 1H-NMR spectroscopy of the evolution of extra virgin olive oil composition submitted to frying temperature in an industrial fryer for a prolonged period of time. Food Chem. 2012, 134, 162–172. [Google Scholar] [CrossRef]



| Sample | Linolenic | Linoleic | Oleic | Saturated |
|---|---|---|---|---|
| VSO | 5.3 ± 0.7 | 43.7 ± 0.5 | 31.9 ± 0.7 | 19.1 ± 0.5 |
| RSO | 4.8 ± 0.1 | 45.8 ± 0.8 | 32.2 ± 0.5 | 17.2 ± 1.2 |
Sample Availability: Oil samples are not available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Rubio, A.S.; Sopelana, P.; Guillén, M.D. Assessment of Soybean Oil Oxidative Stability from Rapid Analysis of its Minor Component Profile. Molecules 2020, 25, 4860. https://doi.org/10.3390/molecules25204860
Martin-Rubio AS, Sopelana P, Guillén MD. Assessment of Soybean Oil Oxidative Stability from Rapid Analysis of its Minor Component Profile. Molecules. 2020; 25(20):4860. https://doi.org/10.3390/molecules25204860
Chicago/Turabian StyleMartin-Rubio, Ana S., Patricia Sopelana, and María D. Guillén. 2020. "Assessment of Soybean Oil Oxidative Stability from Rapid Analysis of its Minor Component Profile" Molecules 25, no. 20: 4860. https://doi.org/10.3390/molecules25204860
APA StyleMartin-Rubio, A. S., Sopelana, P., & Guillén, M. D. (2020). Assessment of Soybean Oil Oxidative Stability from Rapid Analysis of its Minor Component Profile. Molecules, 25(20), 4860. https://doi.org/10.3390/molecules25204860







