Structural Study on Fat Crystallization Process Heterogeneously Induced by Graphite Surfaces
Abstract
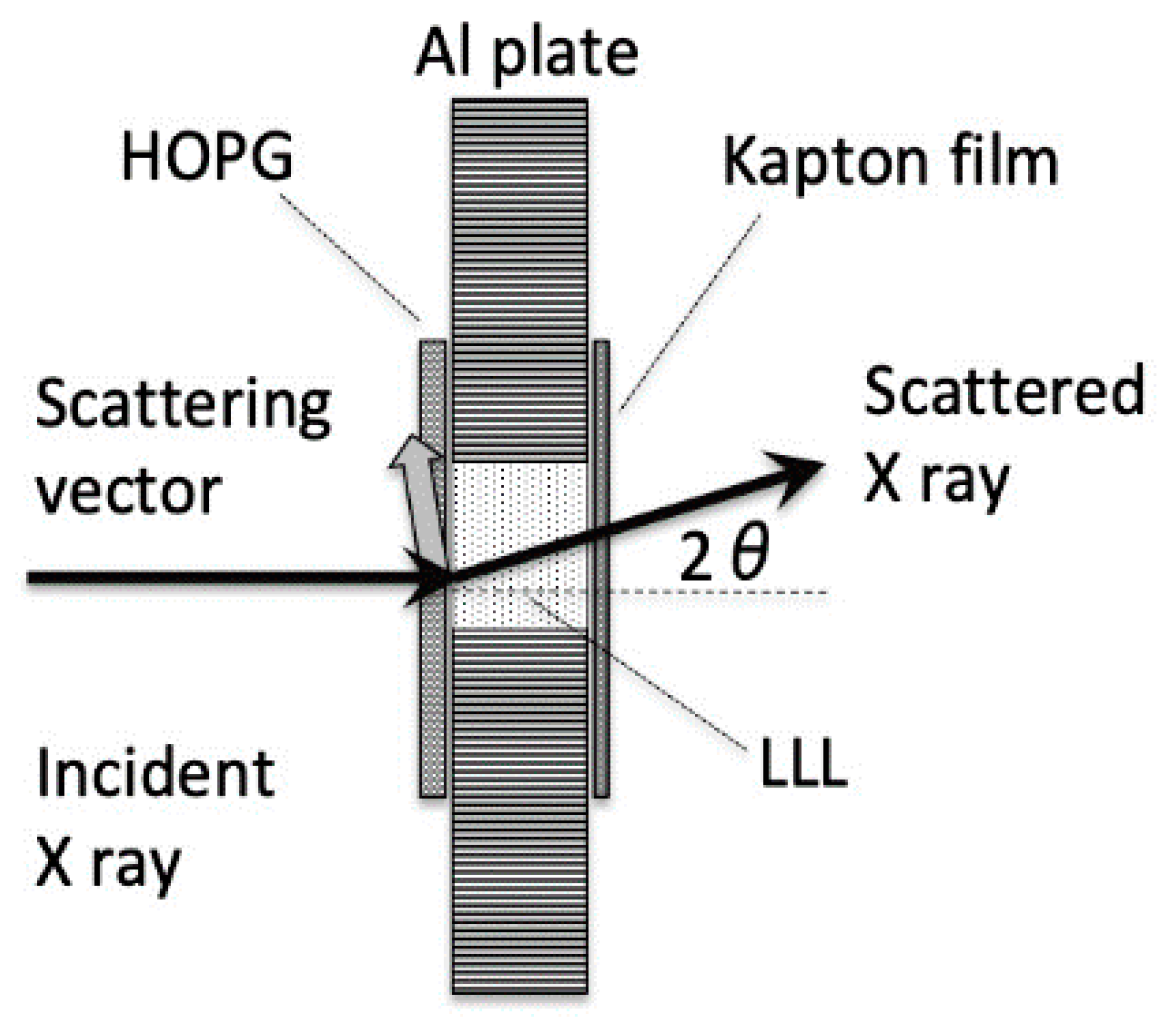
1. Introduction
2. Results and Discussion
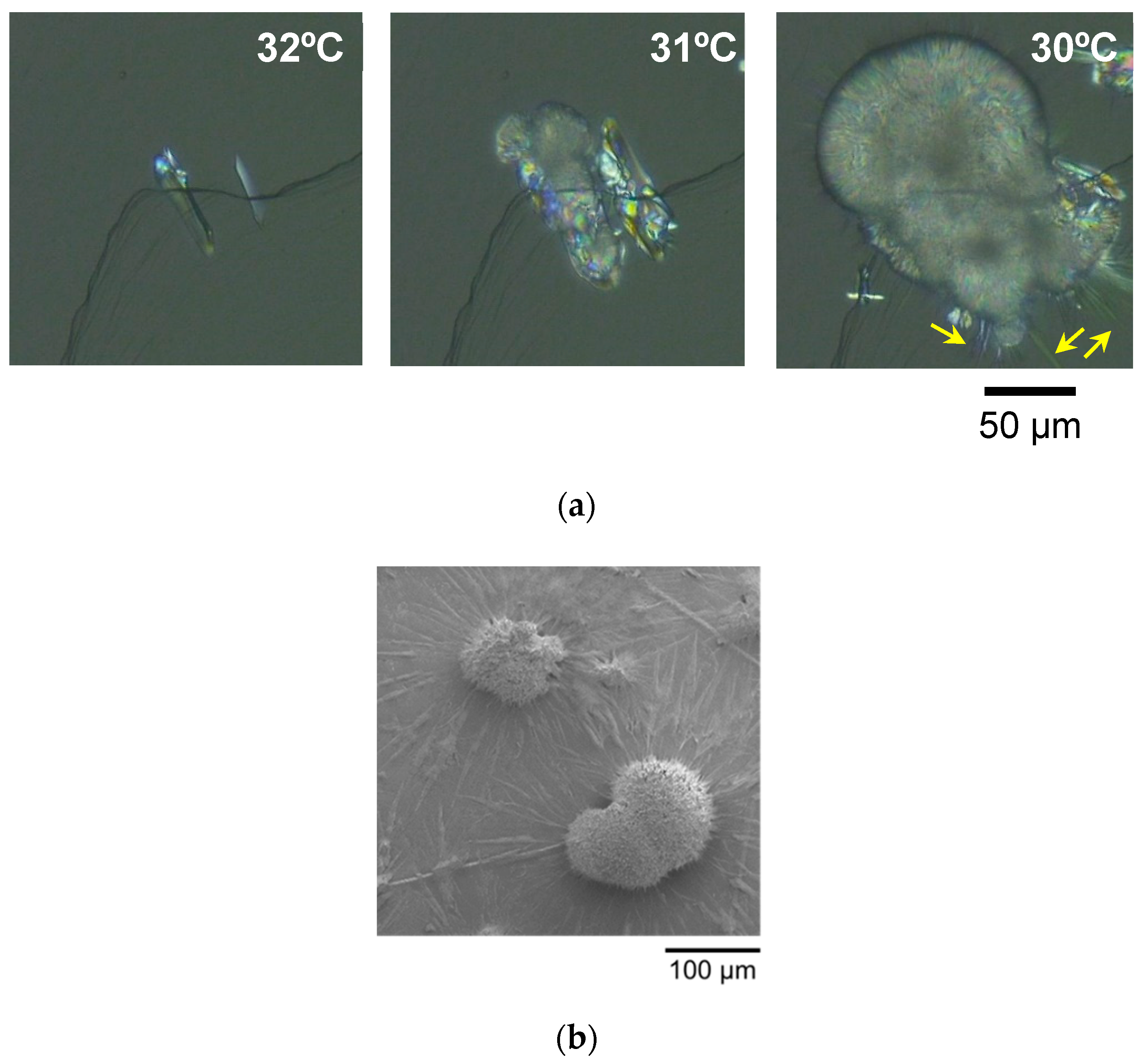
2.1. Microscopic Observation of LLL Crystals on HOPG Sheets
2.1.1. Melt-Crystallization with Constant-Rate Cooling
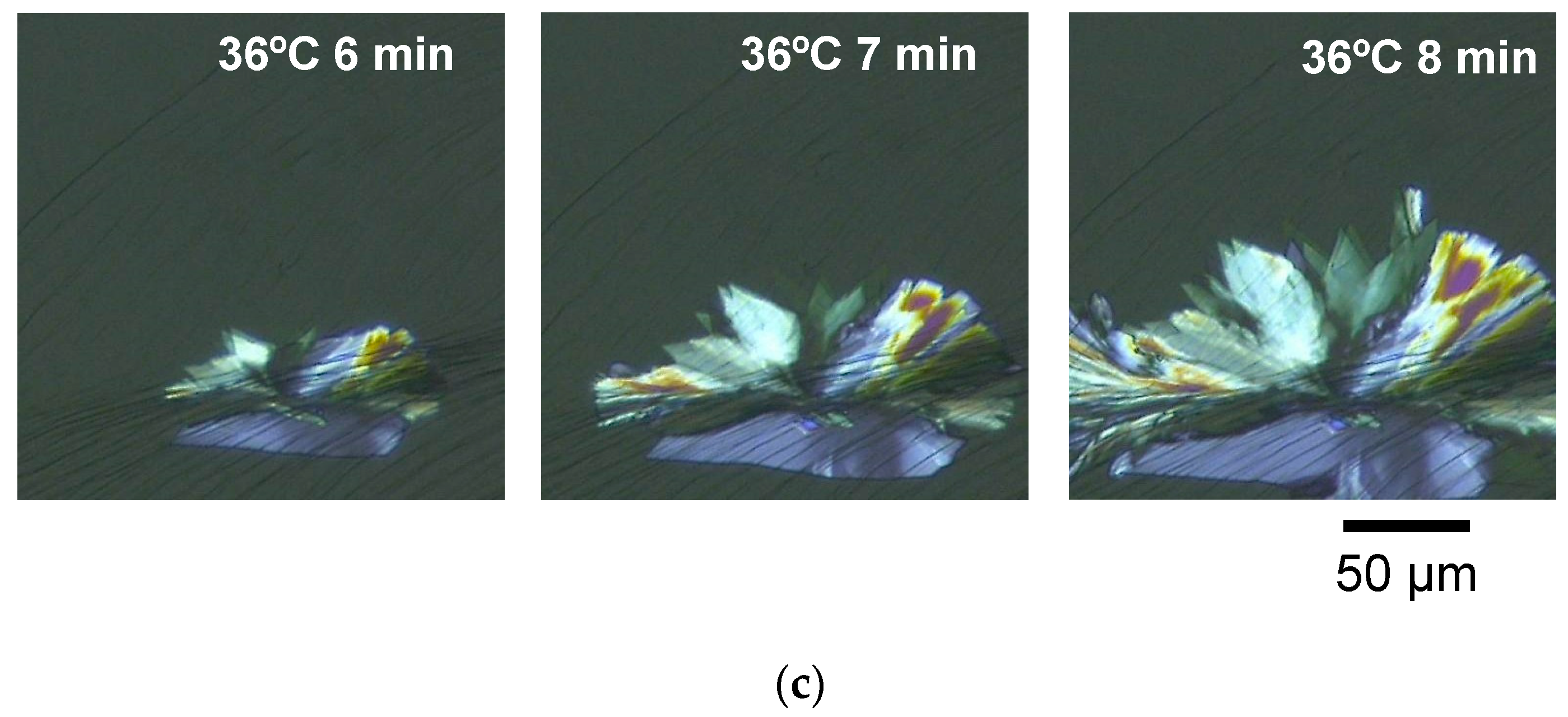
2.1.2. Melt-Crystallization with Isothermal Cooling
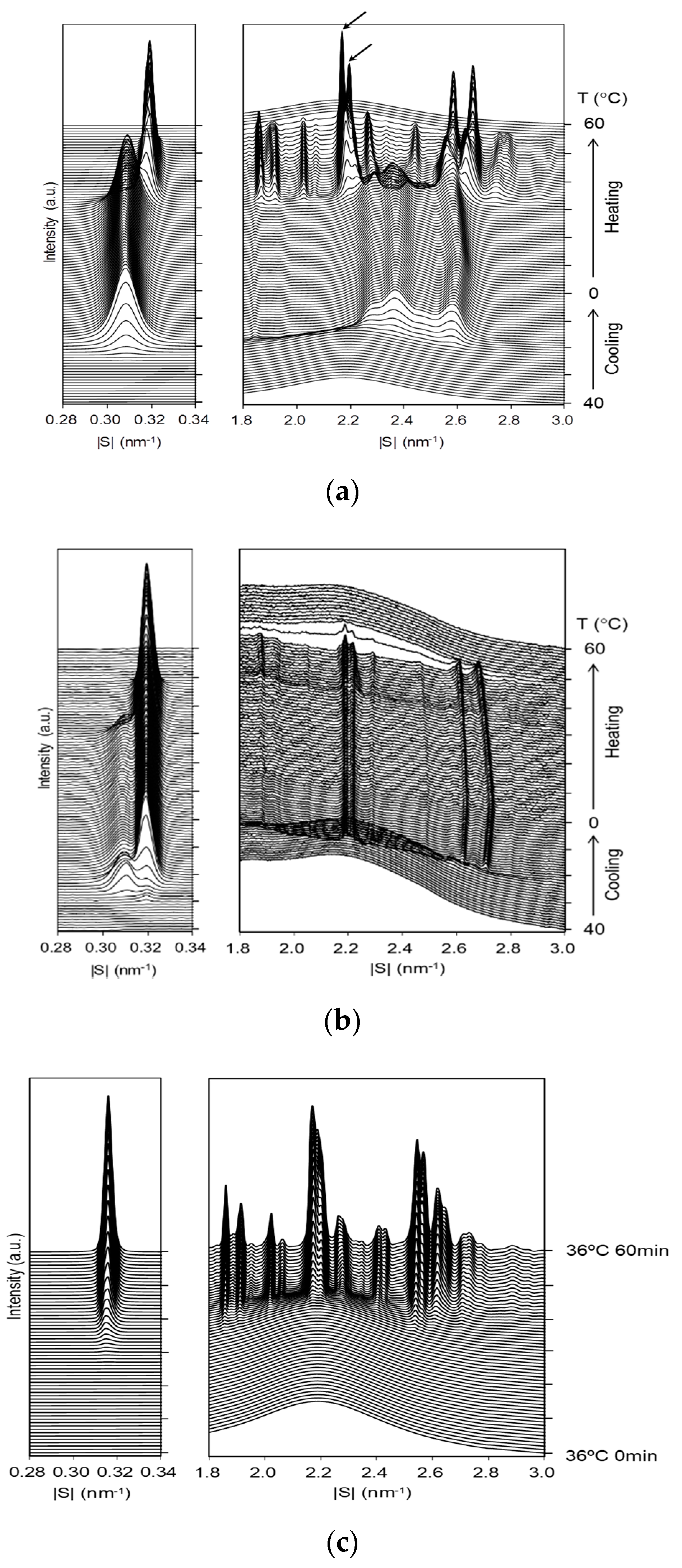
2.2. Structural Changes during Crystallization
2.2.1. Crystallization of Neat LLL with Constant-Rate Cooling and Heating
2.2.2. Crystallization of LLL/HOPG with Cooling and Heating at Constant Rates
2.2.3. Crystallization of LLL/HOPG with Isothermal Cooling
2.2.4. Structural Changes of LLL Crystals on a HOPG Sheet
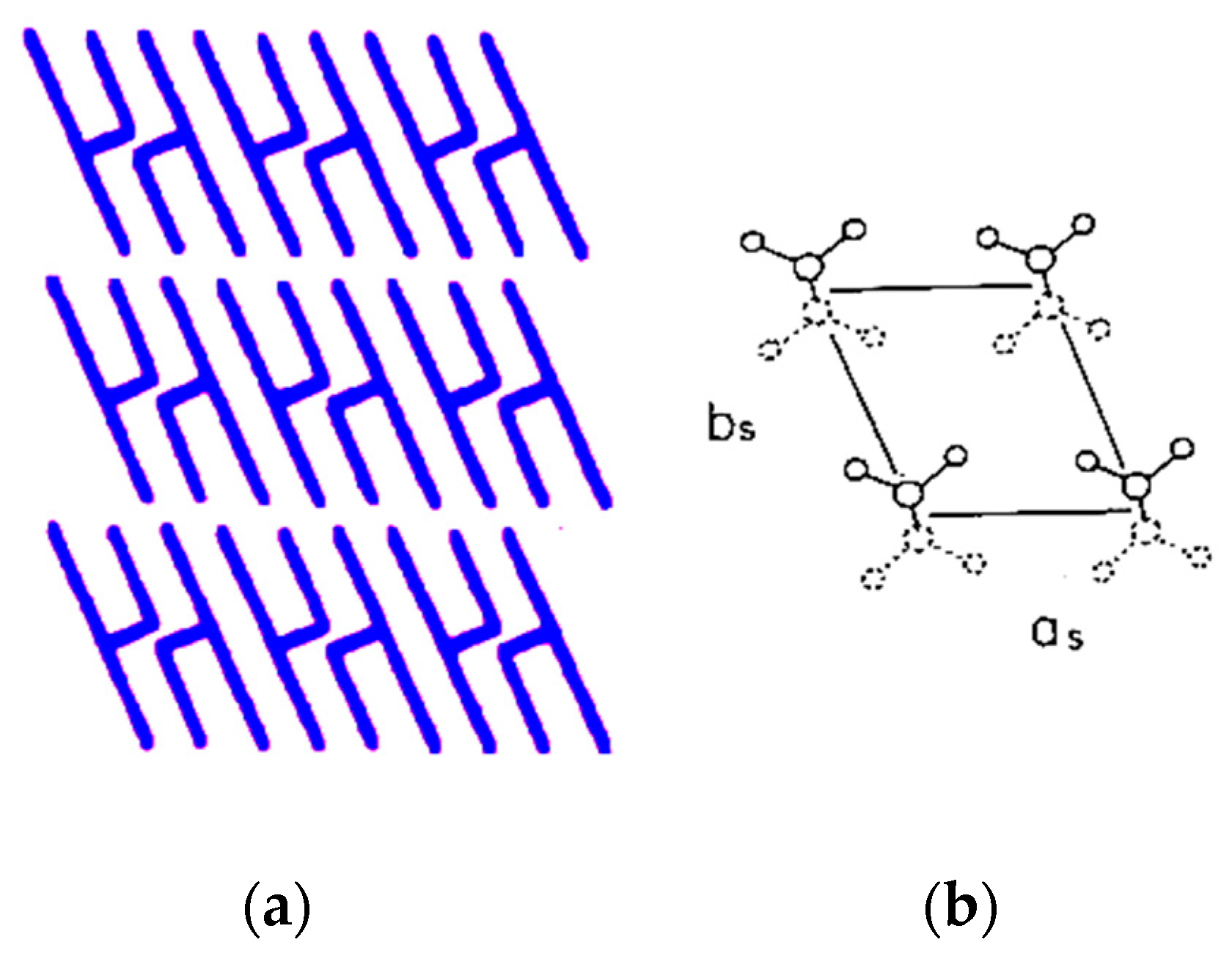
2.3. Polymorph and Orientation of LLL Crystal on a Graphite Sheet
3. Discussion
3.1. Roles of Graphite Surfaces
3.2. Occurrence of the β′ Phase
3.3. Later Stages of Crystallization
- In the cooling at a rate of 1 °C/min, the occurrence of the β phase was observed by SAXS at 30 °C, while at 32 °C by microscopic observation.
- As for the isothermal crystallization at 36 °C, the occurrence of the β phase was confirmed by microscopic observation after holding only for several minutes, while the reflections due to the β phase finally appeared after about half an hour in the SR-XRD measurements.
- In the continuous cooling crystallization, the reflections of the β phase were weak compared with those of the neat LLL sample.
4. Materials and Methods
4.1. Samples
4.2. Polarized FTIR Spectroscopy with ATR Sampling Technique
4.3. SR-XRD Analysis
4.4. SEM Observation
4.5. POM Observation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Smith, K.W.; Sato, K. Effects of Foreign and Indigenous Minor Components. In Crystallization of Lipids; Wiley: Hoboken, NJ, USA, 2018; pp. 263–281. [Google Scholar]
- Smith, K.W.; Bhaggan, K.; Talbot, G.; Van Malssen, K.F. Crystallization of Fats: Influence of Minor Components and Additives. J. Am. Oil Chem. Soc. 2011, 88, 1085–1101. [Google Scholar] [CrossRef]
- Ishibashi, C.; Hondoh, H.; Ueno, S. Epitaxial Growth of Fat Crystals on Emulsifier Crystals with Different Fatty Acid Moieties. Cryst. Growth Des. 2017, 17, 6363–6371. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Kida, H.; Sato, K. Promotional Effects of New Types of Additives on Fat Crystallization. J. Oleo Sci. 2014, 63, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Kida, H.; Sato, K. Fat crystallization with talc particles is influenced by particle size, concentration, and cooling rate. Eur. J. Lipid Sci. Technol. 2015, 117, 858–868. [Google Scholar] [CrossRef]
- Sato, K. Crystallization behavior of fats and lipids—A review. Chem. Eng. Sci. 2001, 56, 2255–2265. [Google Scholar] [CrossRef]
- Sato, K.; Ueno, S. Physical properties of fats in food. In Fats in Food Technology, 2nd ed.; Rajah, K.K., Ed.; John Wiley & Sons Ltd.: Chichester, UK, 2014; pp. 1–38. [Google Scholar]
- Takeuchi, M.; Ueno, S.; Sato, K. Synchrotron Radiation SAXS/WAXS Study of Polymorph-Dependent Phase Behavior of Binary Mixtures of Saturated Monoacid Triacylglycerols. Cryst. Growth Des. 2003, 3, 369–374. [Google Scholar] [CrossRef]
- Chung, D.D.L. Review Graphite. J. Mater. Sci. 2002, 37, 1475–1489. [Google Scholar] [CrossRef]
- Kellens, M.; Meeussen, W.; Gehrke, R.; Reynaers, H. Synchrotron radiation investigations of the polymorphic transitions of saturated monoacid triglycerides. Part 1: Tripalmitin and tristearin. Chem. Phys. Lipids 1991, 58, 131–144. [Google Scholar] [CrossRef]
- Kellens, M.; Meeussen, W.; Gehrke, R.; Reynaers, H. Synchrotron radiation investigations of the polymorphic transitions in saturated monoacid triglycerides. Part 2: polymorphism study of a 50: 50 mixture of tripalmitin and tristearin during crystallization and melting. Chem. Phys. Lipids 1991, 58, 145–158. [Google Scholar] [CrossRef]
- Sato, K.; Ueno, S.; Yano, J. Molecular interactions and kinetic properties of fats. Prog. Lipid Res. 1999, 38, 91–116. [Google Scholar] [CrossRef]
- Milosevic, M. Internal Reflection and ATR Spectroscopy. Appl. Spectrosc. Rev. 2004, 39, 365–384. [Google Scholar] [CrossRef]
- Chapman, D. The 720 cm.–1 band in the infrared spectra of crystalline long-chain compounds. J. Chem. Soc. 1957, 4489–4491. [Google Scholar] [CrossRef]
- Chapman, D. Infrared spectroscopic characterization of glyceriddes. J. Am. Oil Chem. Soc. 1960, 37, 73–77. [Google Scholar] [CrossRef]
- Yano, J.; Kaneko, F.; Kobayashi, M.; Sato, K. Structural Analyses of Triacylglycerol Polymorphs with FT-IR Techniques. 1. Assignments of CH2Progression Bands of Saturated Monoacid Triacylglycerols. J. Phys. Chem. B 1997, 101, 8112–8119. [Google Scholar] [CrossRef]
- Yano, J.; Kaneko, F.; Kobayashi, M.; Kodali, D.R.; Small, D.M.; Sato, K. Structural Analyses and Triacylglycerol Polymorphs with FT-IR Techniques. 2. β′1-Form of 1,2-Dipalmitoyl-3-myristoyl-sn-glycerol. J. Phys. Chem. B 1997, 101, 8120–8128. [Google Scholar] [CrossRef]
- Jensen, L.H.; Mabis, A.J. Crystal Structure of β-Tricaprin. Nat. Cell Biol. 1963, 197, 681–682. [Google Scholar] [CrossRef]
- Jensen, L.H.; Mabis, A.J. Refinement of the structure of β-tricaprin. Acta Crystallogr. 1966, 21, 770–781. [Google Scholar] [CrossRef]
- Vand, V.; Bell, I.P. A direct determination of the crystal structure of the β form of trilaurin. Acta Crystallogr. 1951, 4, 465–469. [Google Scholar] [CrossRef]
- Van Langevelde, A.; Van Malssen, K.; Hollander, F.; Peschar, R.; Schenk, H. Structure of mono-acid even-numbered β-triacylglycerols. Acta Crystallogr. Sect. B Struct. Sci. 1999, 55, 114–122. [Google Scholar] [CrossRef]
- Boistelle, R.; Astier, J. Crystallization mechanisms in solution. J. Cryst. Growth 1988, 90, 14–30. [Google Scholar] [CrossRef]
- Aquilano, D.; Sgualdino, G. Fundamental Aspects of Equilibrium and Crystallization Kinetics. In Crystallization Processes in Fats and Lipid Systems, 1st ed.; Garti, N., Kiyotaka, S., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 15–66. [Google Scholar]
- Chernov, A.A. Modern Crystallography III; Springer: Berlin, Germany, 1984. [Google Scholar]
- Hondoh, H.; Ueno, S.; Sato, K. Fundamental Aspects of Crystallization of Lipids. In Crystallization of Lipids; Sato, K., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 105–141. [Google Scholar]
- Shimizu, N.; Mori, T.; Nagatani, Y.; Ohta, H.; Saijo, S.; Takagi, H.; Takahashi, M.; Yatabe, K.; Kosuge, T.; Igarashi, N. BL-10C, the small-angle x-ray scattering beamline at the photon factory. In Proceedings of the 13th International Conference on Synchrotron Radiation Instrumentation – SRI2018 AIP Conf. Proc. 2054, Taipei, Taiwan, 11–15 June 2018; AIP Publishing: Melville, NY, USA, 2019; Volume 2054, p. 060041. [Google Scholar]
- Harrick, N.J. Internal Reflection Spectroscopy; Interscience Publishers Inc.: New York, NY, USA, 1967. [Google Scholar]
- Flournoy, P.; Schaffers, W. Attenuated total reflection spectra from surfaces of anisotropic, absorbing films. Spectrochim. Acta 1966, 22, 5–13. [Google Scholar] [CrossRef]
Sample Availability: Samples of neat LLL and LLL/HOPG are available from the authors. |


| Previous Study [8] | Present Study 1 | |||||
|---|---|---|---|---|---|---|
| Polymorphs | α | β′ | β | β′ (0 °C) | β (36 °C) | β (50 °C) |
| Sub-cell Structure | H | O⊥ | T// | O⊥ | T// | T// |
| Melting point/°C | 15.0 | 35.0 | 46.5 | — | — | — |
| Long spacing/nm | 3.5 | 3.2 | 3.1 | 3.25 | 3.15 | 3.13 |
| Short spacings/nm | 0.42 | 0.42 | 0.46 | 0.421 (s) | 0.536 (m) | 0.538 (m) |
| 0.38 | 0.39 | 0.384 (s) | 0.522 (w) | 0.525 (w) | ||
| 0.38 | 0.493 (w) | 0.522 (w) | ||||
| 0.458 (s) | 0.494 (w) | |||||
| 0.437 (w) | 0.462 (s) | |||||
| 0.413 (w) | 0.456 (s) | |||||
| 0.391 (s) | 0.441 (m) | |||||
| 0.380 (s) | 0.409 (w) | |||||
| 0.364 (w) | 0.386 (s) | |||||
| 0.376 (s) | ||||||
| 0.362 (w) | ||||||
| 0.359 (w) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaneko, F.; Yamamoto, Y.; Yoshikawa, S. Structural Study on Fat Crystallization Process Heterogeneously Induced by Graphite Surfaces. Molecules 2020, 25, 4786. https://doi.org/10.3390/molecules25204786
Kaneko F, Yamamoto Y, Yoshikawa S. Structural Study on Fat Crystallization Process Heterogeneously Induced by Graphite Surfaces. Molecules. 2020; 25(20):4786. https://doi.org/10.3390/molecules25204786
Chicago/Turabian StyleKaneko, Fumitoshi, Yoshinori Yamamoto, and Shinichi Yoshikawa. 2020. "Structural Study on Fat Crystallization Process Heterogeneously Induced by Graphite Surfaces" Molecules 25, no. 20: 4786. https://doi.org/10.3390/molecules25204786
APA StyleKaneko, F., Yamamoto, Y., & Yoshikawa, S. (2020). Structural Study on Fat Crystallization Process Heterogeneously Induced by Graphite Surfaces. Molecules, 25(20), 4786. https://doi.org/10.3390/molecules25204786






