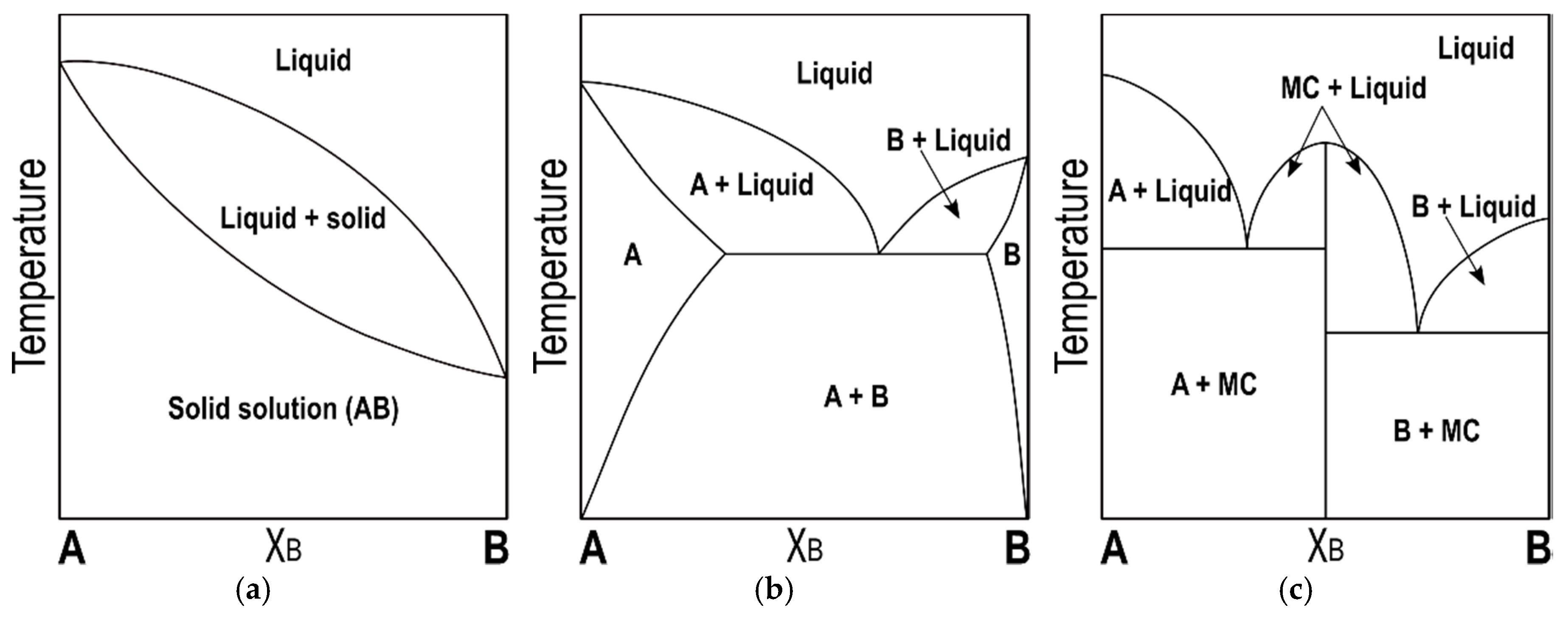
3.1. Mixtures of Saturated-cis-Unsaturated Mixed-Acid TAGs
Table 4 [
24,
25,
42,
46,
64,
115,
116,
117,
118,
119,
120] summarizes the polymorphic forms, including transient phases, such as liquid crystalline structures (LC), encountered for the main saturated-
cis-unsaturated mixed-acid TAGs that are included in the mixtures under review. Along with the characteristic methyl-end stacking and glycerol conformation, their polymorphism (subcell packing, number of forms and subforms, chain-length structure) becomes remarkably complicated by the steric hindrance between saturated and unsaturated acid moieties, with the latter usually being oleic acid (O). Likewise, intermolecular Sat-Sat, Unsat-Unsat, and Sat-Unsat chain interactions influence the lateral packing in mixed TAG systems and, hence, their tendency to form either solid solutions, eutectic mixtures, or molecular compounds.
The formation of molecular compounds between TAGs at a 1:1 concentration ratio was first suggested by Moran for the POP/OPO system. The diffraction patterns of thermodynamically stabilized mixtures showed the increasing presence of a novel β-2L structure towards equivalent mass ratio of TAGs, in contrast with the typical β-3L stability of pure POP and OPO component TAGs [
121]. Rossell suggested similar behavior for SOS/SSO mixtures on the basis of melting behavior and infrared spectroscopy data provided by Freeman [
122] and Chapman [
123], which was subsequently confirmed by Engstrom in a systematic study of TAG mixtures based on S, P, and O fatty acids [
124]. Moreover, the formation of crystal compounds suggested in SOS/PSO, SOS/SPO, SOS/PPO, and POP/PPO systems indicated that this type of mixing behavior seemed to be restricted to some mixtures with a specific Sat-O-Sat/O-Sat-O and Sat-O-Sat/Sat-Sat-O configuration.
Years later, Sato’s group carried out a more detailed study of the molecular compound-forming systems SOS/SSO, SOS/OSO, POP/PPO, and POP/OPO, with special emphasis on the polymorphism and mixing phase behavior under dynamic thermal conditions [
57,
64,
113,
115]. In addition, the role of chain-chain interactions, glycerol conformation, and methyl-end stacking in the structural stabilization was deeply investigated by implementing SR-XRD and FT-IR techniques. [
65,
125,
126].
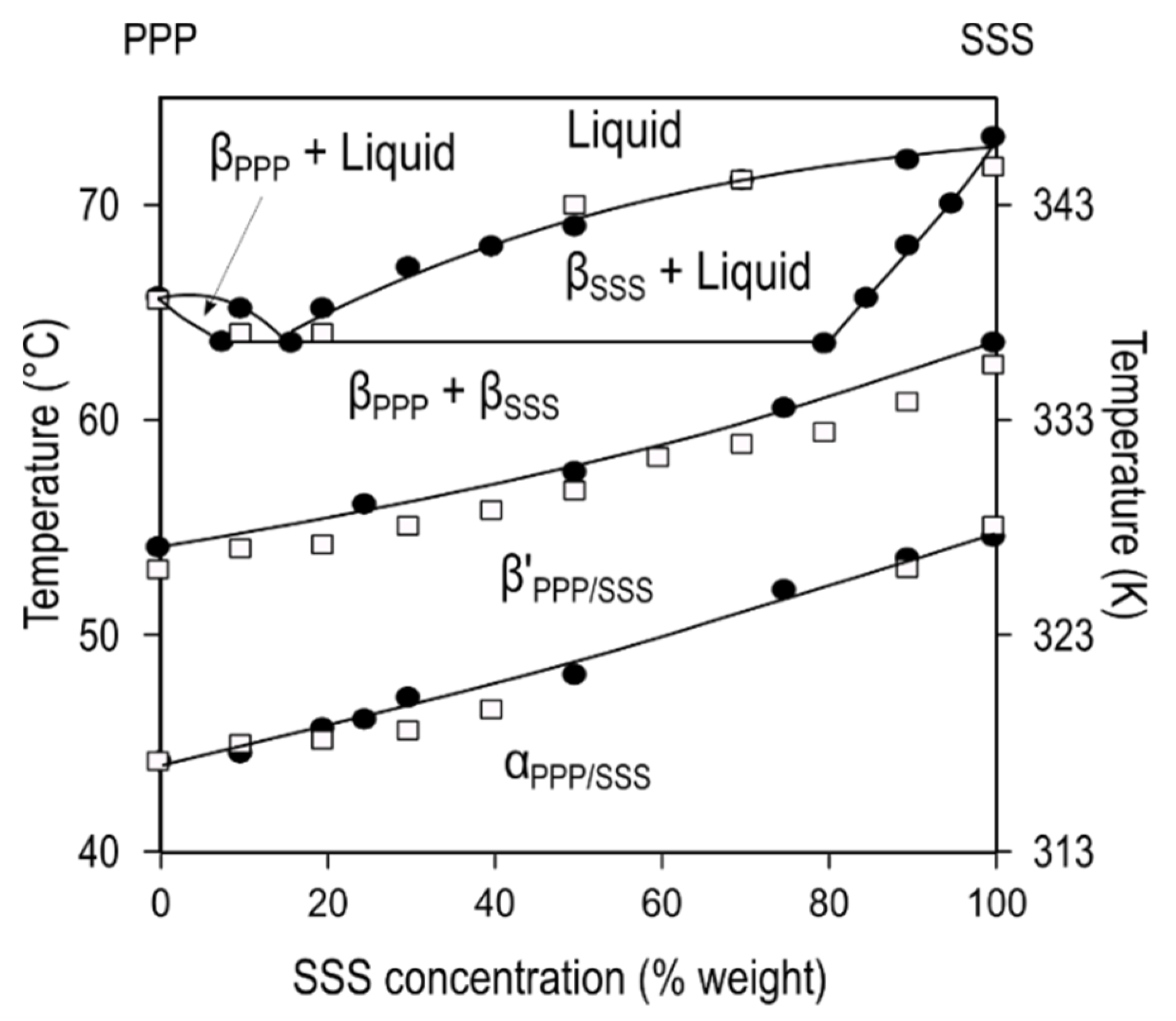
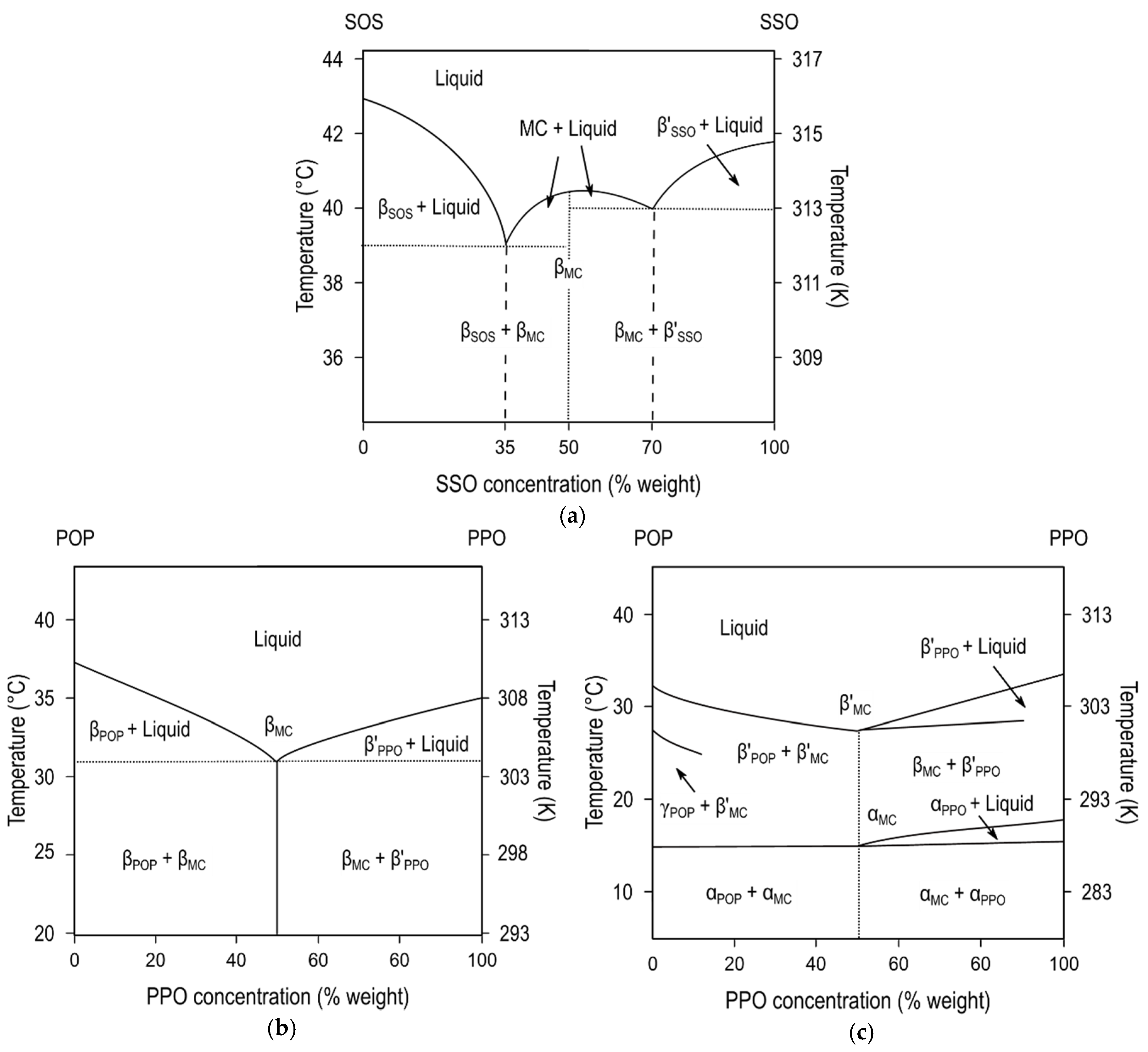
Figure 6a depicts the phase diagram of SOS/SSO mixtures in the most stable forms, obtained after thermodynamic stabilization [
115]. The formation of molecular compound crystals with a maximum
Tm was detected at the 50SOS/50SSO composition, whereas the two depressions in melting point at a Χ
SOS of 35% and 70% showed the respective β-3L (SOS)/β-2L (MC
SOS/SSO) and β-2L (MC
SOS/SSO)/β’-3L (SSO) eutectic mixtures at the SOS- and SSO-rich regions of the diagram. Conversely, no increase in the
Tm of β-2L (MC) was observed between the juxtaposed eutectic regions in POP/PPO, POP/OPO, and SOS/OSO binary mixtures [
57,
64,
113] (
Figure 6b).
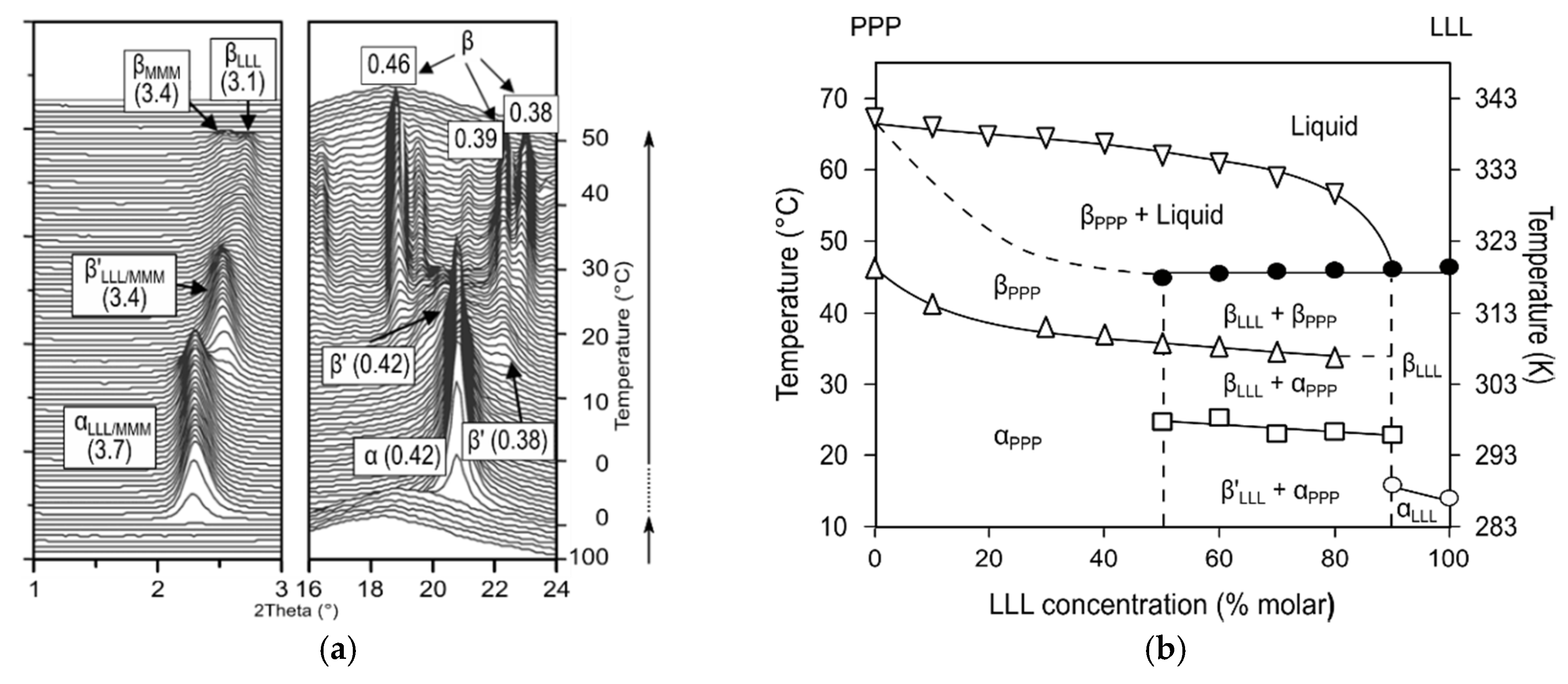
DSC and SR-XRD experiments that were carried out on these systems under kinetic conditions confirmed the occurrence of molecular compound metastable forms, also exhibiting immiscible behavior with pure component phases. During the heating of the POP/PPO mixture at a 1:1 concentration ratio, α (MC
POP/PPO) transformed into β’ (MC
POP/PPO) via melt-mediation [
57] (see phase diagram in
Figure 6c), whereas in POP/OPO α (MC
POP/OPO) directly transformed into β (MC
POP/OPO) through the solid state [
64]. In addition, the strong influence of molecular compounds on the crystallization and polymorphic transformation behavior of TAG mixtures became evident, especially in POP-rich regions of the diagrams. The α-2L→β’-2L transformation of POP through an intermediate γ-3L was hindered, and the β
2-3L→β
1-3L was retarded at low concentration levels of MC
POP/PPO in POP/PPO mixtures. As for the POP/OPO system, the stabilization of POP was remarkably accelerated through a promoted β’-2L (POP)→β-3L (POP) transition in the presence of MC
POP/OPO.
The crystallization and mixing phase behavior of molecular compounds is particularly relevant in industrial processes, as they may condition the efficient separation of TAGs during oil fractionation [
10] or the functionalities of lipidic materials obtained through fat blending. In this connection, molecular compounds have been suggested as alternative structuring agents to increase oleic acid and reduce saturated and
trans fats content in food products [
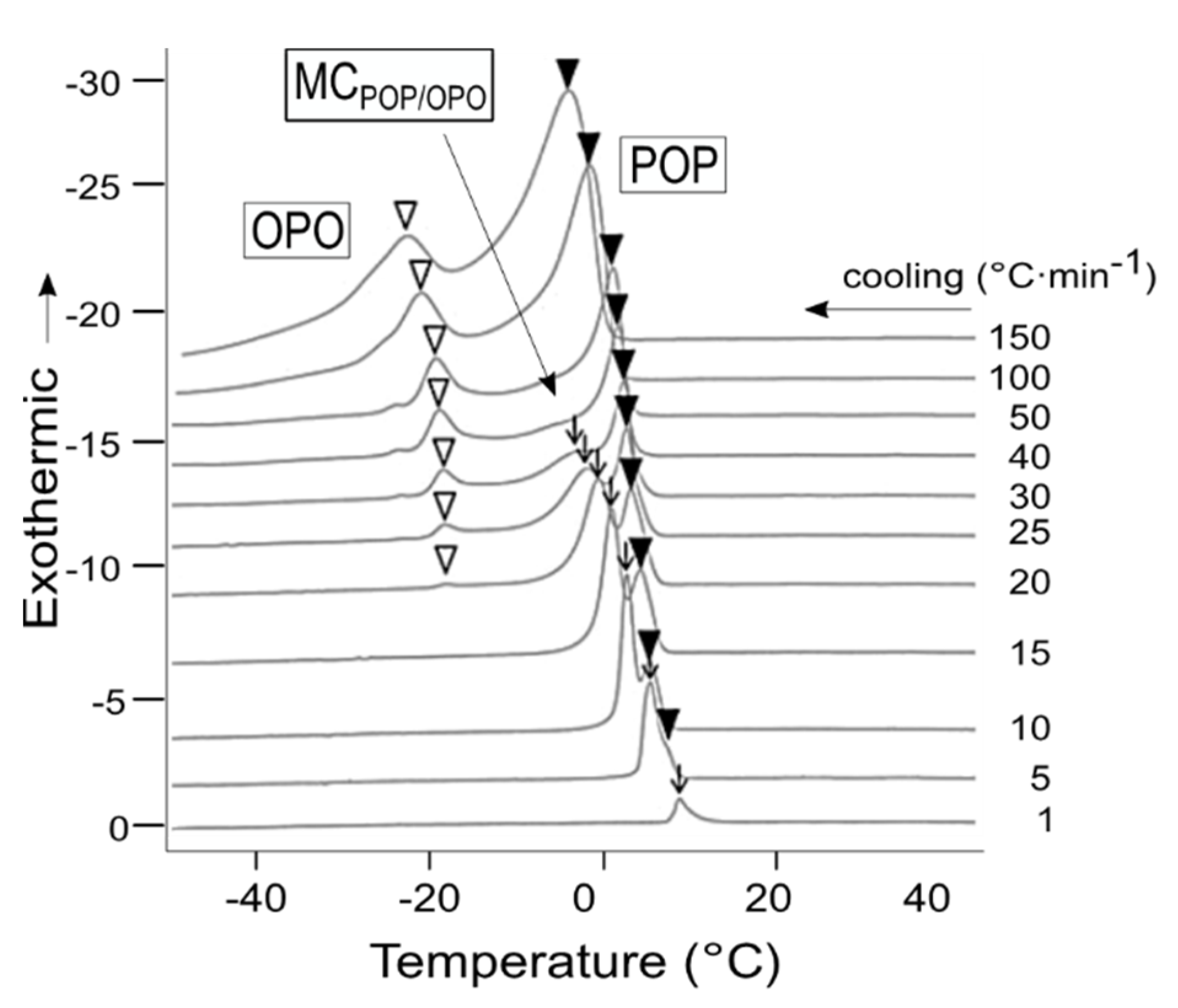
11]. Thus, valuable information for fat systems can be obtained from TAG mixtures when close to real manufacture conditions are employed. For example, the application of varying cooling rates (1 to 150 °C·min
−1) has shown to influence the crystallization behavior of POP/OPO mixtures in a way that the single crystallization of molecular compound is limited to relatively slow cooling, whereas the system tends to complete immiscibility when the rate is sufficiently increased [
47] (
Figure 7).
In an attempt to illustrate the behavior of molecular compound-forming systems under conditions resembling those of edible fats and oils fractionation processes, the work of Minato et al. on MC
POP/OPO and MC
POP/PPO crystals grown from neat liquid [
57,
64] was further developed by a study on the mixing behavior of POP/OPO and POP/PPO mixtures in
n-dodecane (at 20% and 50% solution) [
127,
128]. Overall, crystallization and melting temperatures were affected by the amount of solvent in the mixtures due to solubility effects. However, the specific interactions between TAGs were so strong that the solvent did not affect the formation of molecular compounds, even at levels up to 98%. Moreover, the single phases of molecular compound obtained during cooling the equimolecular mixture of POP/OPO at 2 °C·min
−1 and 5 °C·min
−1 indicated its favored crystallization at the expense of pure components, which was strongly influenced by solubility properties and the rates of nucleation and crystal growth. In line with this, Bayés-García et al. used synchrotron radiation microbeam X-ray diffraction (SR-μ-XRD) to clarify the competitive crystallization of POP, OPO, and MC
POP/OPO through the microstructure of spherulites composed by 75POP/25OPO and 25POP/75OPO mixtures, which were crystallized under selected supercooling conditions [
129]. In POP-rich spherulites, the inner part was dominated by β (MC
POP/OPO) crystals, whereas β-3L (POP) form predominated towards the outer region. This indicated that the rate of nucleation of the molecular compound was higher than that of β-3L (POP) at the experimental conditions examined. By contrast, the uniform microstructure mostly composed by β-3L (OPO) in OPO-rich spherulites suggested the similar or slightly higher nucleation rate of OPO compared to that of MC
POP/OPO. Additional extensive studies by using SR-μ-XRD may shed more light on the crystallization kinetics and growth mechanisms of TAG mixtures and complex fat systems.
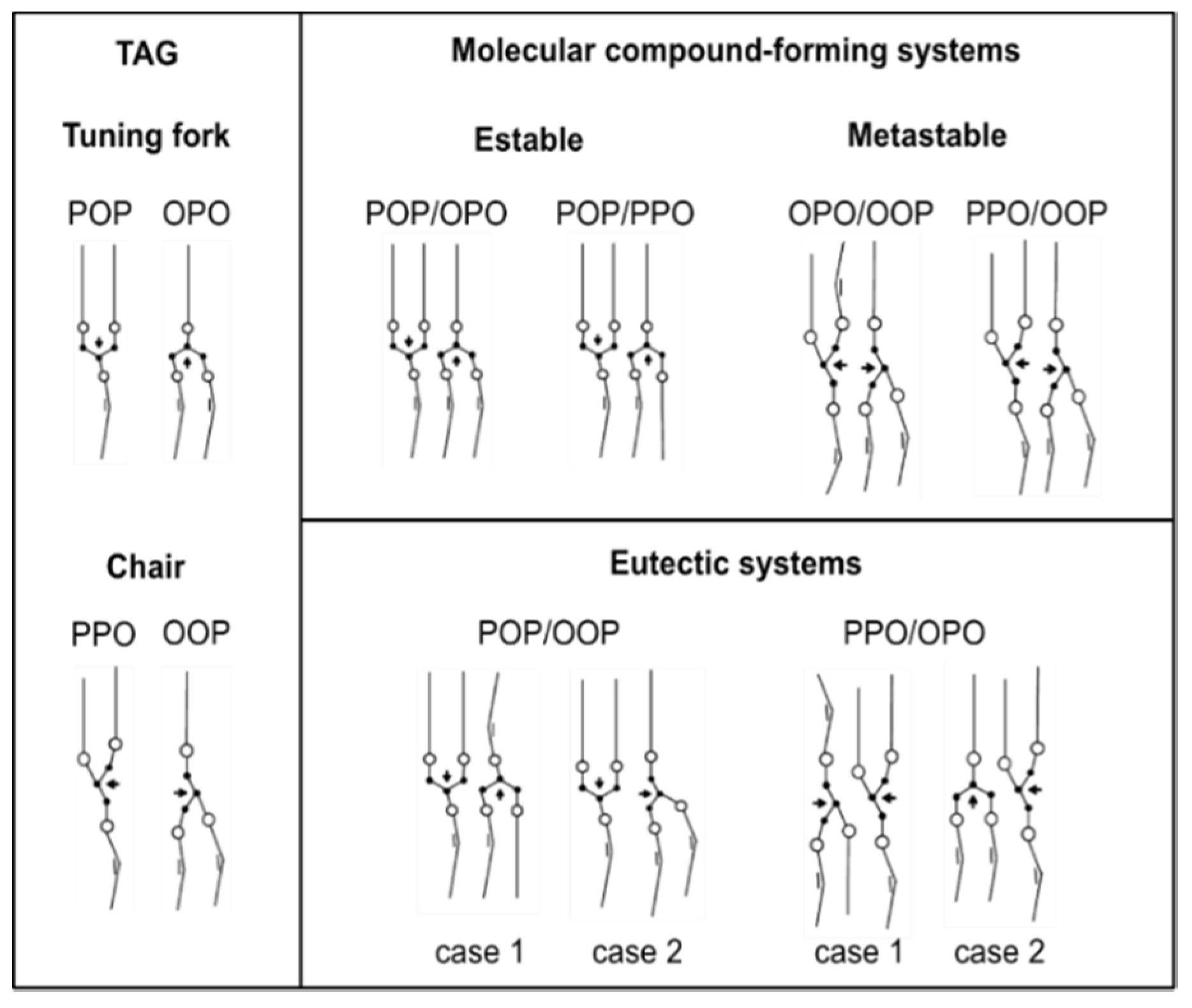
The formation of molecular compound in saturated-unsaturated mixed-acid TAGs may be more easily understood by considering the corresponding molecular structure models for pure TAGs and analyzing the stabilizing or destabilizing molecular interactions between them, as depicted in
Figure 8 [
57,
64,
118,
130]. In this regard, it is worth mentioning the close structural properties between molecular compounds ascribed by Minato et al. to the pairs SOS/SSO and POP/PPO, and SOS/OSO and POP/OPO [
65], which allow foreseeing an equivalent nature of the molecular interactions that are involved in their stabilization.
It is assumed that glycerol groups in symmetric (achiral) TAGs tend to adopt a “tuning fork” configuration, whereas asymmetric (chiral) TAGs exhibit the “chair” configuration [
131]. Subsequently, fatty acid chains at the
sn-1 and
sn-3 positions are set in an opposite direction to that of the
sn-2 position in POP and OPO, whereas fatty acids located at the
sn-1 and
sn-2 positions are directed in opposite turn to that located in the
sn-3 position in PPO and OOP. This arrangement of the fatty acid chains contributes to the molecular stability by avoiding steric hindrance between palmitoyl and oleoyl chains. Thus, the predicted structure for MC
POP/OPO with an opposite turn of the neighboring glycerol groups and separated saturated and unsaturated leaflets seems plausible, and steric hindrance emerges as the driving force for crystal packing in a double chain-length structure. In addition, the close contact between glycerol and methyl-end groups assumed in stable β-3L forms of POP and OPO and the subsequent excess energy for molecular packing would be prevented in the molecular compound. This is expected to contribute to the reported higher crystallization rate of MC
POP/OPO before pure POP [
128].
The stability of MC
POP/PPO might be more difficult to explain, since the unbalanced content in palmitic and oleic fatty acids may inevitably lead to the presence of adjacent palmitoyl and oleoyl chains in one of the leaflets. According to FT-IR data on MC
POP/PPO, the resulting steric effect seems to be responsible for the deviation in the olefinic conformation from the typical skew-
cis-skew’ (s-
c-s’) and skew-
cis-skew (s-
c-s) types [
65]. However, the destabilizing effect of palmitic-oleic interactions could be compensated if a structure with both TAGs in a “tuning fork” configuration is assumed. The more favorable disposition of glycerol groups in parallel arrangement and with an opposite turn, together with affinity interactions in the palmitoyl leaflet, might be decisive in the stable nature of MC
POP/PPO.
Similarly, chain–chain interactions and glycerol conformation seem to play a major role on the singular mixing phase behavior reported for the PPO/OOP and OPO/OOP systems. Monitoring the mixtures during thermodynamic stabilization over a 17-month period confirmed that both of the systems were able to form metastable molecular compounds, but displayed eutectic behavior once stability was reached. In PPO/OOP mixtures, the decomposition of the molecular compound into constituent TAGs over time was confirmed by the decrease of intensity of diffraction peaks corresponding to a molecular compound β’-2L structure at the expense of those of β’-3L (PPO) and β’-3L (OOP) [
130]. As for the OPO/OOP system, a preceding β’ (MC
OPO/OOP)→β (MC
OPO/OOP) transformation could be identified before phase demixing took place. The occurrence of molecular compound crystals in this system may be favored by the stable oleoyl leaflet and the parallel disposition of neighboring glycerol groups assuming a “chair” configuration of OPO (
Figure 8). However, a transformation to a more stable “tuning fork” glycerol configuration during thermodynamic stabilization may increase the steric hindrance, thus explaining the metastable nature of the molecular compound.
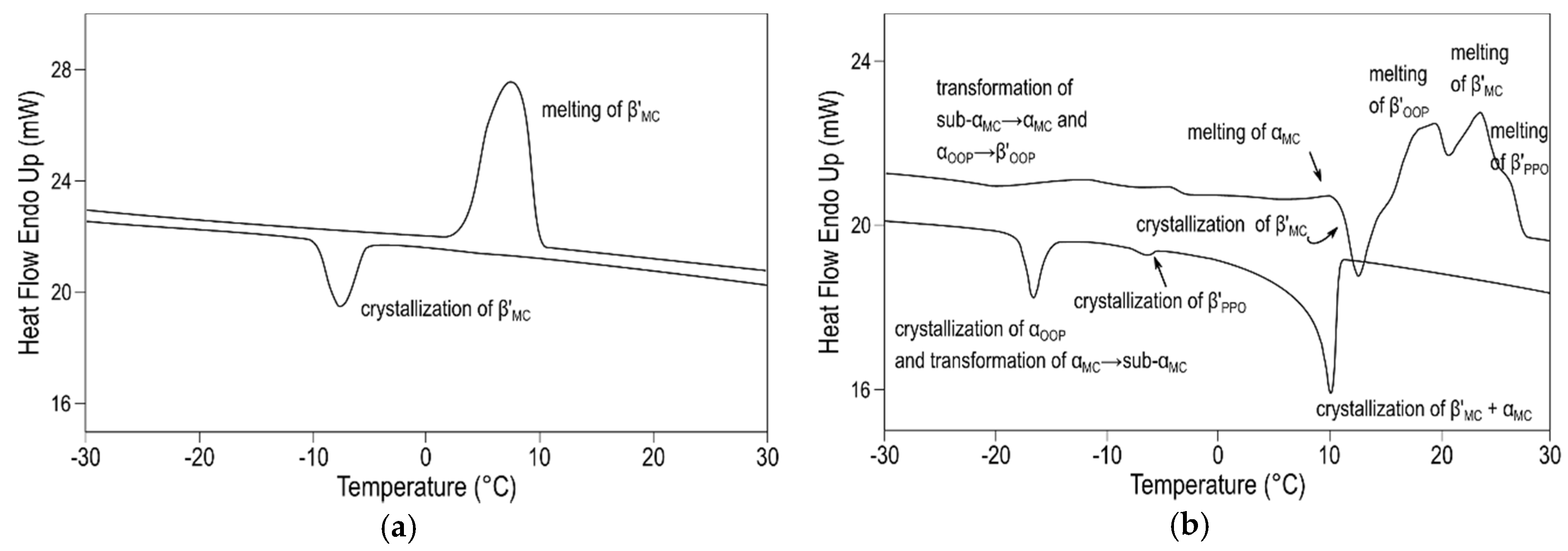
Additional experiments under varying cooling and heating conditions allowed for confirming the further occurrence of a sub-α form of MC
OPO/OOP and sub-α and α forms of MC
PPO/OOP. As examples,
Figure 9a and b depict the DSC thermograms that were obtained for these two molecular compounds under different thermal protocols. During cooling at 0.5 and 2 °C·min
−1, the single peak displayed in 50OPO/50OOP cooling thermograms indicated that the system readily crystallized in a β’ (MC
OPO/OOP) form, thus leading to quite simple behavior upon melting. By contrast, the 50PPO/50OOP mixture exhibited the crystallization of α (MC
PPO/OOP), β’ (MC
PPO/OOP), and pure TAGs during the cooling process at 2 °C·min
−1, which was followed by complex polymorphic transformation processes during the subsequent heating step. Such a complicated polymorphic behavior might be indicative of competitive S-OPP/R-PPO, S-OOP/R-POO, and (R-)S-OPP/(R-)S-OOP interactions during the molecular arrangement of TAGs. Therefore, the racemicity exhibited by both TAGs could be the underlying cause of the metastability of MC
PPO/OOP, hardly explained just by means of the proposed molecular structure [
130] (
Figure 8).
In view of the above, the stereochemistry of asymmetric TAGs becomes an additional factor to be considered when it comes to solid-state miscibility of multicomponent TAG systems. It seems relevant to gain a clear picture about the polymorphic behavior of chiral TAGs in both their pure enantiomeric forms and in racemic mixtures. Different studies on asymmetric TAGs with diverse chain-length mismatch and degree of unsaturation, such as LLM, PPM, or POS [
99,
132,
133], concluded that enantiomers tended to β’ stability, whereas racemates showed stable β forms. This was explained by Craven & Lencki through a model of the relative stereochemistry in unit cells, based on the available data on crystalline tendency and space group determination in asymmetric TAGs. Accordingly, unit cells of β’ polymorphs may be enantiomerically pure, whereas those of β forms may be stereochemically mixed with the formation of a racemic compound [
131,
134].
However, mixtures of S-OPP and R-PPO were shown to come into conflict with the above mentioned, as no stable β forms were obtained at a 1:1 concentration ratio after thermodynamic stabilization [
57]. Moreover, most stable form for both the enantiomeric forms and the racemic mixture was β’-3L [
117]. The concurrent polymorphic crystallization and transformation of pure enantiomers and racemic compound when cooling and heating S-OPP/R-PPO mixtures (10% concentration intervals) at 2 °C·min
−1 stated the eutectic nature of the system. In addition, the R and S enantiomers exhibited similar thermophysical properties, and these differed from those of the racemic mixture, which showed lower crystallization and melting temperatures. During cooling, S- and R-PPO crystallized in β’
1-3L form, and complementary experiments at varying cooling conditions confirmed that the least stable α
2-2L form only occurred when rates above 10 °C·min
−1 were applied. Furthermore, the SR-XRD patterns of the equimolecular composition agreed with the reported tendency of racemic mixtures to crystallize in α forms [
135]. The occurrence of the two distinct α
2-2L and α
1-3L forms at the end of the cooling process, and their respective α
2-2L→β’
1-3L polymorphic transformation, for S and R enantiomers, and the sequence α
1-3L→β’
2-2L→β’
1-3L, for the racemic mixture; during heating proved that, even at equal ratio of enantiomers in the mixture, not all of the molecules contributed to the formation of the racemic compound [
117]. Although a few steps have already been taken to assess the effect of stereochemistry in mixed TAGs systems [
116,
118], there is still a considerable gap in our knowledge regarding the influence of chiral TAGs in either the enantiomeric form or racemic mixture on the structure-function properties of lipid blends.
As previously stated, in the absence of specific interactions that allow for a close molecular packing, the presence of kink sites arising from cis double bonds may contribute to the immiscibility of TAGs systems by causing a disruption during crystal packing. The most glaring example can be found in mixtures of tri-saturated and saturated-unsaturated mixed-acid TAGs, typically showing a very pronounced eutectic behavior with the ΧE near pure composition of the lower-melting TAG.
Figure 10a displays the phase diagram of PPP/POP binary mixtures in the most stable polymorphs. The continuous liquidus line evidenced a eutectic composition that was probably well below a Χ
PPP of 10%, while the solidus boundary at the PPP-rich region showed that around 40–50% of solid POP integrated in the crystalline phase of PPP. On the whole, the behavior of the system is likely due to the great ∆
Tm between TAGs (∼30 °C), with the contribution of steric hindrance between palmitoyl and oleoyl chains, and the different chain-length structure of PPP (β-2L) and POP (β-3L) in the low intersolubility reported [
60]. Interestingly, the kinetic phase diagram obtained after cooling at 15 °C·min
−1 showed lower miscibility of the system in the less stable α form, which agreed with previous results obtained by Gibon et al. after melting and quenching at 25 °C·min
−1 [
59]. However, this behavior seems to be ascribed to kinetic effects, since a more recent study carried out at a lower cooling rate suggested that only α forms of PPP might be present in mixtures at a Χ
PPP of 60% and above [
58], which is more in accordance with the favored solid-state miscibility attributed to metastable phases.
Similar results, which consisted of a eutectic behavior with a very asymmetric Χ
E, were reported for PPP/OOP mixtures, as expected from the higher ∆
Tm caused by the additional oleoyl chain in OOP [
58,
68]. Furthermore, the integration of OOP in the crystal lattice of PPP was noticeably lower (∼15%) than that of POP, which suggested a greater disturbance during crystallization at increasing number of olefinic groups. Understandably, equivalent behavior of immiscible nature was displayed by mixtures of monoacid saturated and unsaturated TAGs, such as PPP/OOO and SSS/OOO [
67,
74,
136].
As for binary systems in which both TAGs present at least one unsaturated fatty acid moiety, the occurrence and complexity of the eutectic behavior is especially sensitive to small changes in the fatty acid distribution at the
sn-positions. Contrary to the stable molecular compounds that were reported for POP/OPO and POP/PPO, and the metastable ones of PPO/OOP and OPO/OOP, the fatty acid disposition in PPO/OPO and POP/OOP systems led to immiscibility between component TAGs under stable and metastable conditions [
118,
130]. Moreover, while the PPO/OPO system exhibited very simple eutectic behavior with a Χ
E near pure OPO, POP/OOP mixtures resulted in a peritectic-type phase diagram with each component TAG being able to solubilize around 20% of the other in its solid phase (
Figure 10b). Likewise, kinetic measurements on POP/OOP pointed out that crystallization and polymorphic transformation behavior of the mixtures located at the ends of the phase diagram was ruled by pure TAGs, whereas the influence of each TAG over the other became stronger towards equimolecular composition [
58,
118]. Very similar results were obtained in the study of SOS/
sn-OOS, with only slight differences being ascribed to the distinct polymorphism of TAGs containing palmitic and stearic fatty acids. Therefore, the immiscibility of these systems was presumably driven by incompatible interactions involving glycerol groups and hydrocarbon chains (
Figure 8) with no apparent influence of whether the chiral TAG is present in its optically active or racemic form [
116].
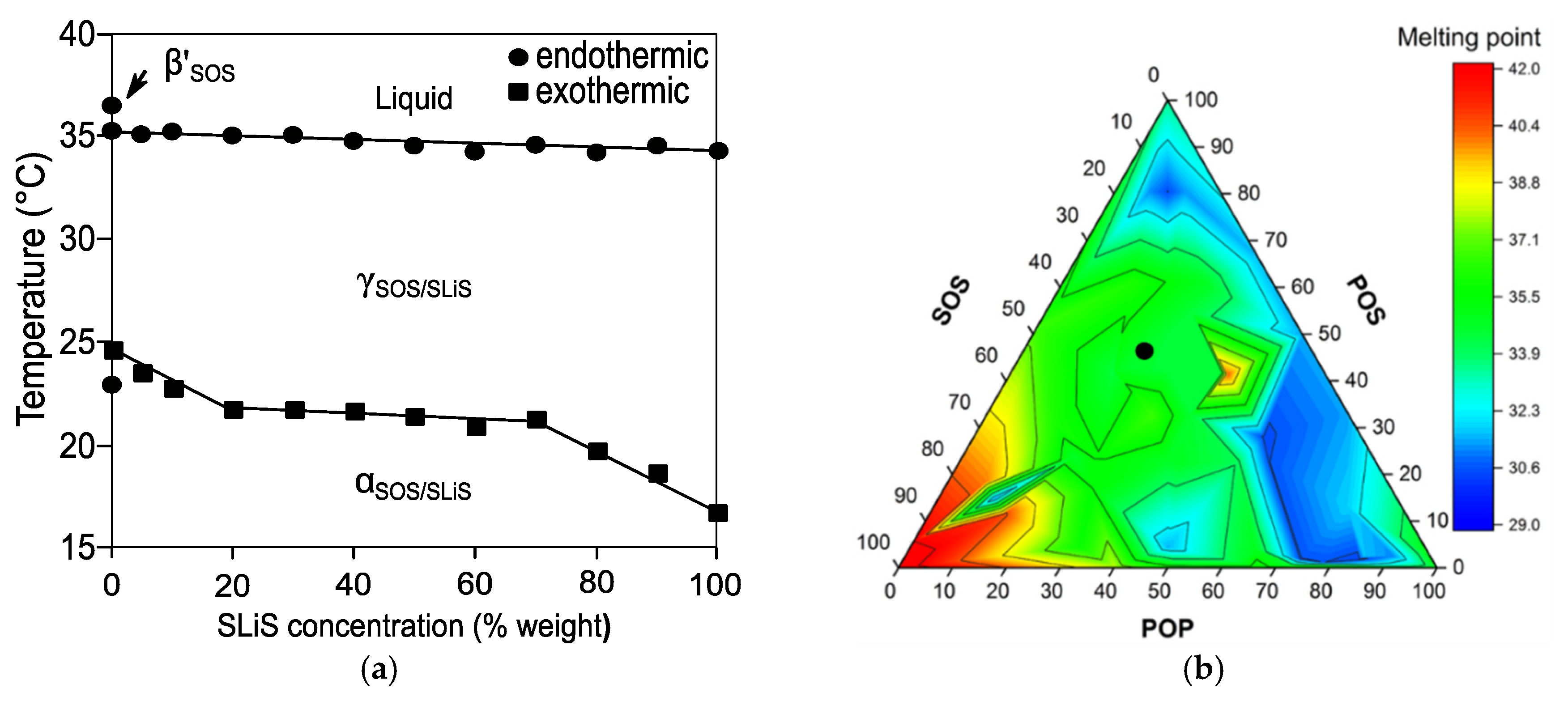
The formation of complete solid solution in mixtures of saturated-unsaturated mixed-acid TAGs was revealed in the SOS/SLiS (with Li being linoleic acid) binary system. Kinetic SR-XRD experiments confirmed the formation of an α-2L miscible phase during fast cooling and its subsequent solid-state polymorphic transformation into a more stable γ-3L form when heating at 2 °C·min
−1 [
137] (
Figure 11a). As pointed out for the α-2L→γ-3L transformation in pure SLiS [
42], the rapid stabilization of SOS/SLiS phases in the γ-3L form was sterically favored through the separation of stearoyl and unsaturated (oleoyl and linoleoyl) leaflets by chain sorting. The absence of further transformation events at the specified conditions indicated that the typical γ-3L→β’-3L transformation of SOS [
25] was prevented in the mixtures, probably due to strong chain–chain interactions hindering the separation of mixed components. However, additional experiments of cooling and rapid heating showed to affect miscibility properties of the system during the α-2L (SOS/SLS) melt-mediated transformation to more stable forms. The former became clear in mixtures at a Χ
SLiS below 30%, in which the concurrent recrystallization of γ-3L (SOS/SLiS) with either β’-2L or β-3L forms of SOS were indicative of phase separation. Although the specific structure-interaction relationships were left open for future research, the miscibility of the system was primarily attributed to olefinic interactions. More concretely, the disordered molecular configuration of di-unsaturated linoleoyl chains seemed to play a crucial role on the strength of molecular packing and, thus, in the phase demixing observed at low concentration levels of SLiS [
137].
The implications of solid-sate miscibility of TAGs in real systems might be cleared up taking a relatively simple fat, such as cocoa butter, as a case example. With POS, SOS, and POP accounting for more than 80% of its total TAG content, thermophysical properties are dictated by their mixing phase behavior at a specific composition around 22POP/46POS/32SOS [
138]. Thus, research carried out on binary and ternary mixtures of the former TAGs turned out to be useful not only for cocoa butter characterization, but also as a guide for the development of suitable lipid blends with potential use as cocoa butter alternatives.
For the POS/SOS binary system, Rousset et al. reported complete miscibility between β-3L (POS) and β
2-3L (SOS) after several weeks of thermal incubation at room temperature [
139]. By contrast, the pseudo phase diagram constructed from iso-solid lines and melting point data reported by Smith et al. suggested a possible eutectic at a Χ
POS around 80–90% [
140]. Likewise, POP/POS and POP/SOS systems exhibited a binary eutectic diagram with respective solid-solid-liquid equilibrium points situated at a Χ
POP of 50% and 35%, which were very close to those that were predicted by applying the Hildebrand model. Nevertheless, more complex behavior was observed by Sasaki et al. for POP/SOS mixtures, consisting of a solid solution at a Χ
POP of 50% and above, eutectic at a Χ
POP between 45% and 20%, and a crystalline phase rich in SOS below the latter concentration [
54] (see
Table 5). The successfulness of the stabilization processes and the distinct experimental approaches are the likely cause of the main discrepancies that were observed among the mentioned studies.
As expected from the varying mixing phase behavior displayed by the binary systems, the study carried out on POP/POS/SOS ternary mixtures under stable conditions evidenced that the formation of either eutectic or miscible phases was strongly influenced by the balance of TAGs in the mixture. Thus, eutectic behavior predominated at Χ
POP and Χ
SOS above 15% and 35%, respectively, whereas, in mixtures at high POS concentration (Χ
POS ≥ 50%) and a Χ
SOS below 35%, miscible phases occurred [
54]. Higher miscibility was revealed in POP/POS/SOS ternary mixtures in the β
2 polymorph obtained by both solvent and melt crystallization [
55] (green area in
Figure 11b). The depression in melting points depicted by dark blue proved that the eutectic behavior of ternary mixtures was mainly governed by the POP/POS binary system, with the effect being particularly noticeable in the POP-rich region of the diagram. This agreed with a previous statement by Koyano et al., who pointed out the detrimental effect that POP concentration levels above those typically found in cocoa butter (denoted by a black circle in the miscible area) may have on the physical properties of lipid blends intended for being used as cocoa butter alternatives [
141]. Unexpectedly, a reduced area of increased
Tm was observed around the 40POP/40POS/20SOS composition, far from the SOS-rich high melting region. This exemplifies the importance of an in-depth understanding of multicomponent TAG systems in the design of versatile lipidic materials that allow for covering a wider range of applications. In this connection, a recent report by Watanabe et al. clarified the complex mixing phase behavior of SOS/SSO/OSO ternary mixtures. After 10 days of thermodynamic stabilization at 28 °C, mixtures with a 50SOS/50(SSO/OSO) composition showed to be exclusively formed by molecular compound and, more specifically, by a miscible β-2L phase of MC
SOS/SSO and MC
SOS/OSO. By contrast, eutectic mixtures of MC
SOS/SSO/OSO and either β’-3L (SSO) or β-3L (OSO) crystals were formed when the mentioned concentration ratio was not present in the mixtures [
66]. According to the SR-XRD data gathered during the cooling and subsequent heating of the mixtures at 2 °C·min
−1, MC
SOS/SSO/OSO was not readily obtained from the melt, and α (MC
SOS/SSO) and β (MC
SOS/OSO) crystallized instead. Eventually, the stability of the SOS/SSO/OSO system in a single mixed phase was achieved once the α (MC
SOS/SSO)→β (MC
SOS/SSO) polymorphic transformation took place. These findings at a molecular level were further explored during the study of fat blends and dark chocolate preparations with a lipidic base composed by cocoa butter mixed with Sat-Sat-O and O-Sat-O fat fractions rich in SSO and OSO, respectively [
142]. In fat blends with a constant Sat-Unsat-Sat TAG content around 50%, higher amounts of Sat-Sat-O favored the occurrence of metastable β’ (MC), whereas direct formation of stable β (MC) took place in blends rich in O-Sat-O without need of further tempering. As to dark chocolate preparations, pure cocoa butter-based chocolate-like hardness shown by samples composed by 50CB/20-30Sat-Sat-O/20-30O-Sat-O mixtures, together with the absence of fat bloom formation after one-year storage at 15–30 °C, pointed out the suitability of these specific compositions as cocoa butter equivalents.
Despite the mentioned studies, there is a great lack of recent experimental reports dealing with the solid-state miscibility properties of ternary mixtures of TAGs. A few preceding ternary phase diagrams have been reported [
61,
67], but the reliability of the data has been called into question and further investigation, paying special attention to the purity of the samples, stabilization procedures, and measurement techniques employed, might provide a more clear picture of the miscibility properties in multicomponent TAG systems. Therefore, basic and applied research on the mixing phase behavior of lipid mixtures with more than two components, and the interconnection with physical properties of complex fat systems, remain as an open field worthy of further exploration.
3.2. Mixtures of Saturated-trans-Unsaturated Mixed-Acid TAGs
Almost all the trans-fats present in food goods arise from industrial processes through the partial hydrogenation of vegetable oils, meant to confer them higher shelf life, oxidative resistance, and desired organoleptic properties, such as plasticity, pleasant mouthfeel, and high melting point. Although widely spread in the past, its correlation with a higher incidence of cardiovascular disease over the last decades has led to the pursuit of triglyceride (interesterification, use of tropical oils) and non-triglyceride (oleogelation, structured emulsions) alternatives that provide solid-like properties to edible oils in low- or zero-
trans food products [
143].
As to some examples, the structural differences between stearic (C18:0), oleic (9-
cis-C18:1) and elaidic (9-
trans-C18:1, E) acids primarily rely on the presence and configuration of the double bond. The kink in the acyl chain of oleic fatty acid due to the
cis double bond becomes much less pronounced in the
trans-isomer, according to the straighter saturated-like elaidic acyl chain. Thus, a less sterically hindered and more dense crystalline packing results in the characteristic low solubility, high density, and thermal stability of
trans-fats [
144,
145]. When considering that they may account for up to 15% in some end products, understanding the structure-interaction-function properties of
trans-TAG systems through polymorphic and mixing phase behavior studies might be useful for alternative fat structuring.
Table 6 [
86,
146,
147,
148] shows the polymorphic forms encountered for elaidic acid-based TAGs, which become slightly different to those of their saturated and
cis-unsaturated counterparts. As an example, the
trans-monoacid EEE exhibited crystalline α-2L and β-2L, but, contrary to SSS and OOO, no intermediate β’ forms were observed during melt crystallization or polymorphic transformation [
24,
149,
150]. The substitution of S by E in the tri-saturated PSP and PPS did not significantly influence the molecular packing and crystal structure in the resulting TAGs PEP and PPE, which showed the respective β’
1-2L and β-2L stable polymorphs [
86,
148]. As to stearo-elaidic TAGs, their greater disposition to β stability was revealed in SES, ESS, and SEE [
146]. For SES and PEP, changes at the methyl-end plane as a function of the angle formed between aliphatic chains and the step plane were suggested by Van Mechelen et al. as the cause of their different polymorphic stability [
147].
In stearo-elaidic binary systems, the close structural similarity between S and E seems to be determinant in the resulting mixing phase behavior. Grootscholten reported on the formation of solid solutions in SSS/SES, SSS/SSE and SSS/SEE mixtures [
61,
68], in contrast with the eutectic SSS/SOS, SSS/SSO and SSS/OOO systems (see
Table 7 [
68,
148]). Moreover, mixing phase behavior near ideal miscibility was also reported for the stable SES/SSE binary system, whereas its
cis-unsaturated equivalents SOS and SSO showed the formation of a molecular compound ascribed to strong specific interactions.
Very recently, Zhang et al. reported on the phase behavior of PEP/EPE mixtures under stable and metastable conditions, and the results were highly similar to those of the
cis-unsaturated POP/OPO system. Thermodynamically stabilized mixtures showed the formation of a β-2L molecular compound at a 1:1 ratio, and additional SR-XRD experiments of cooling and subsequent heating at 5 °C·min
−1 and 2 °C·min
−1, respectively, showed the α (MC
PEP/EPE)→β’ (MC
PEP/EPE)→β (MC
PEP/EPE) sequence of solid-state transformations. In the same manner, chain-chain interactions, glycerol group conformation, and methyl-end stacking were also suggested as the main stabilizers of the molecular structure [
148], equivalent to that of its
cis-unsaturated counterpart: a bilayer structure with separate saturated and unsaturated leaflets, and “tuning fork” configuration of glycerol groups arranged in parallel along the chain axis in opposite directions. Furthermore, the authors pointed out that the formation of MC
PEP/EPE might be energetically favored by reducing the void at PEP methyl-end plane when laterally packed with EPE. Based on these findings, the formation of molecular compounds in the structurally close PSP/SPS system was also suggested. However, this would be in contrast with a previous work based on melting point determination, which ascribed immiscible properties to mixtures of PSP and SPS [
114]. Additional studies including precise structural analysis may shed some light into this matter.