Two-Step One-Pot Reductive Amination of Furanic Aldehydes Using CuAlOx Catalyst in a Flow Reactor
Abstract
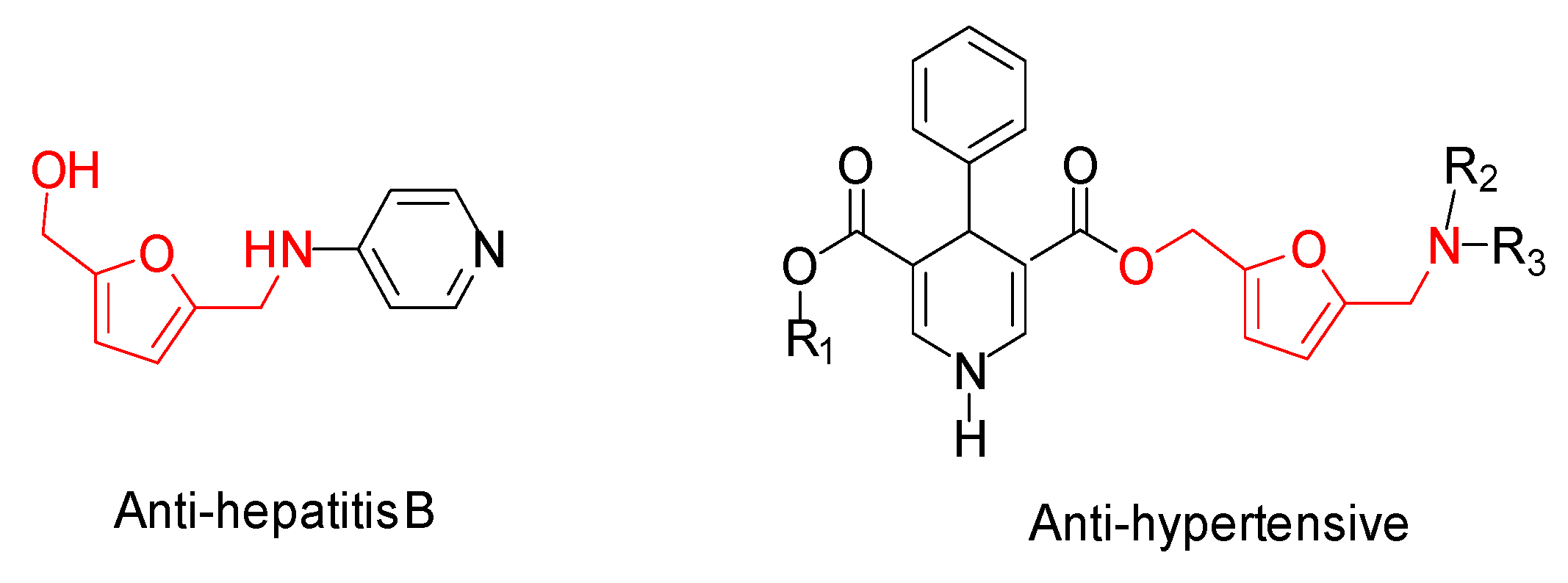
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Catalyst Preparation
3.3. Characterization Techniques
3.4. General Procedure for Reductive Amination of Furanic Aldehydes with Primary Amines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feriani, A.; Gaviraghi, G.; Toson, G.; Mor, M.; Barbieri, A.; Grana, E.; Boselli, C.; Guarneri, M.; Simoni, D.; Manfredini, S. Cholinergic Agents Structurally Related to Furtrethonium. 2. Synthesis and Antimuscarinic Activity of a Series of N-[5-[(1′-Substituted-acetoxy)methyl]-2-furfuryl]dialkylamines. J. Med. Chem. 1994, 37, 4278–4287. [Google Scholar] [CrossRef] [PubMed]
- Caetano, J.A.T.; Fernandes, A.C.; Catano, J.A.T. One-pot synthesis of amines from biomass resources catalyzed by HReO4. Green Chem. 2018, 20, 2494–2498. [Google Scholar] [CrossRef]
- Zhu, M.-M.; Tao, L.; Zhang, Q.; Dong, J.; Liu, Y.-M.; He, H.-Y.; Cao, Y. Versatile CO-assisted direct reductive amination of 5-hydroxymethylfurfural catalyzed by a supported gold catalyst. Green Chem. 2017, 19, 3880–3887. [Google Scholar] [CrossRef]
- Li, X.; Xu, R.; Yang, J.; Nie, S.; Liu, D.; Liu, Y.; Si, C. Production of 5-hydroxymethylfurfural and levulinic acid from lignocellulosic biomass and catalytic upgradation. Ind. Crop. Prod. 2019, 130, 184–197. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent advances in catalytic transformation of biomass-derived 5-hydroxymethylfurfural into the innovative fuels and chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Froidevaux, V.; Negrell, C.; Caillol, S.; Pascault, J.-P.; Boutevin, B. Biobased Amines: From Synthesis to Polymers; Present and Future. Chem. Rev. 2016, 116, 14181–14224. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Yan, P.; Xu, W.; Jia, S.; Xia, Z.; Chung, B.; Hou, G. Direct reductive amination of 5-hydroxymethylfurfural with primary/secondary amines via Ru-complex catalyzed hydrogenation. RSC Adv. 2014, 4, 59083–59087. [Google Scholar] [CrossRef]
- García-Ortiz, A.; Vidal, J.D.; Climent, M.J.; Concepción, P.; Corma, A.; Iborra, S. Chemicals from Biomass: Selective Synthesis of N-Substituted Furfuryl Amines by the One-Pot Direct Reductive Amination of Furanic Aldehydes. ACS Sustain. Chem. Eng. 2019, 7, 6243–6250. [Google Scholar] [CrossRef]
- Karve, V.V.; Sun, D.T.; Trukhina, O.; Yang, S.; Oveisi, E.; Luterbacher, J.S.; Queen, W.L. Efficient reductive amination of HMF with well dispersed Pd nanoparticles immobilized in a porous MOF/polymer composite. Green Chem. 2020, 22, 368–378. [Google Scholar] [CrossRef]
- Cukalovic, A.; Stevens, C.V. Production of biobased HMF derivatives by reductive amination. Green Chem. 2010, 12, 1201–1206. [Google Scholar] [CrossRef]
- Chieffi, G.; Braun, M.; Esposito, D. Continuous Reductive Amination of Biomass-Derived Molecules over Carbonized Filter Paper-Supported FeNi Alloy. ChemSusChem 2015, 8, 3590–3594. [Google Scholar] [CrossRef] [PubMed]
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bukhtiyarova, G.A. Cu-Al mixed oxide derived from layered double hydroxide as an efficient catalyst for continuous-flow reductive amination of aromatic aldehydes. J. Chem. Technol. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Hizartzidis, L.; Cossar, P.J.; Robertson, M.J.; Simone, M.I.; Young, K.A.; McCluskey, A.; Gordon, C.P. Expanding the utility of flow hydrogenation—A robust protocol restricting hydrodehalogenation. RSC Adv. 2014, 4, 56743–56748. [Google Scholar] [CrossRef]
- Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bulavchenko, O.A.; Bukhtiyarova, G.A. Flow hydrogenation of 5-acetoxymethylfurfural over Cu-based catalysts. Mol. Catal. 2020, 494, 111132. [Google Scholar] [CrossRef]
- Kumalaputri, A.J.; Bottari, G.; Erne, P.M.; Heeres, H.; Barta, K. Tunable and Selective Conversion of 5-HMF to 2,5-Furandimethanol and 2,5-Dimethylfuran over Copper-Doped Porous Metal Oxides. ChemSusChem 2014, 7, 2266–2275. [Google Scholar] [CrossRef] [PubMed]
- Layer, R.W. The Chemistry of Imines. Chem. Rev. 1963, 63, 489–510. [Google Scholar] [CrossRef]
- Kang, E.-S.; Hong, Y.-W.; Chae, D.W.; Kim, B.; Kim, B.; Kim, Y.J.; Cho, J.K.; Kim, Y.G. From Lignocellulosic Biomass to Furans via 5-Acetoxymethylfurfural as an Alternative to 5-Hydroxymethylfurfural. ChemSusChem 2015, 8, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Gavilà, L.; Esposito, D. Cellulose acetate as a convenient intermediate for the preparation of 5-acetoxymethylfurfural from biomass. Green Chem. 2017, 19, 2496–2500. [Google Scholar] [CrossRef]
- Shinde, S.; Deval, K.; Chikate, R.; Rode, C.V. Cascade Synthesis of 5-(Acetoxymethyl)furfural from Carbohydrates over Sn-Mont Catalyst. ChemistrySelect 2018, 3, 8770–8778. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
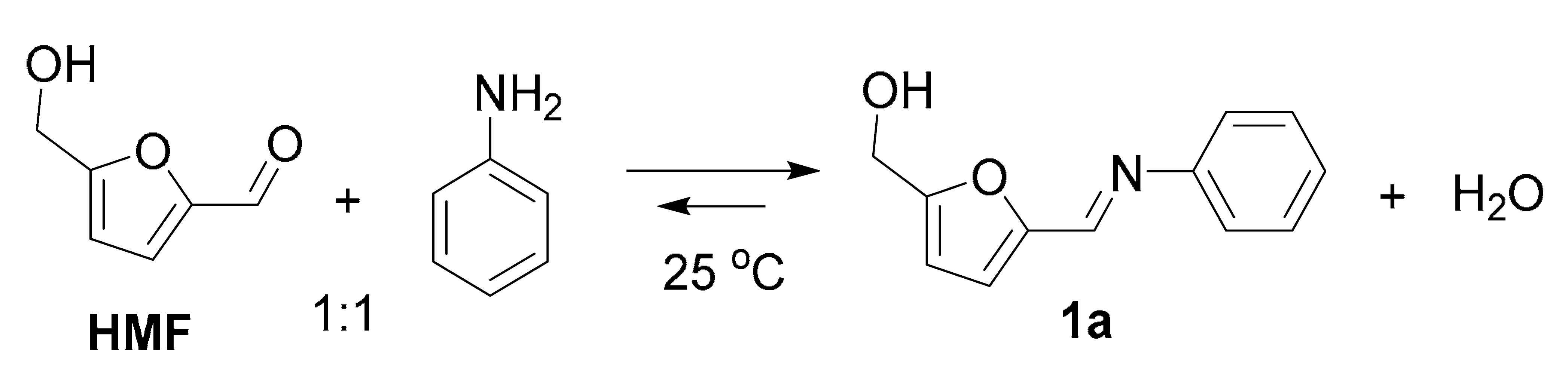
| Entry | Solvent | Yield, % 2 |
|---|---|---|
| 1 | Methanol | 98 |
| 2 | Ethanol | 70 |
| 3 | Isopropanol | 31 |
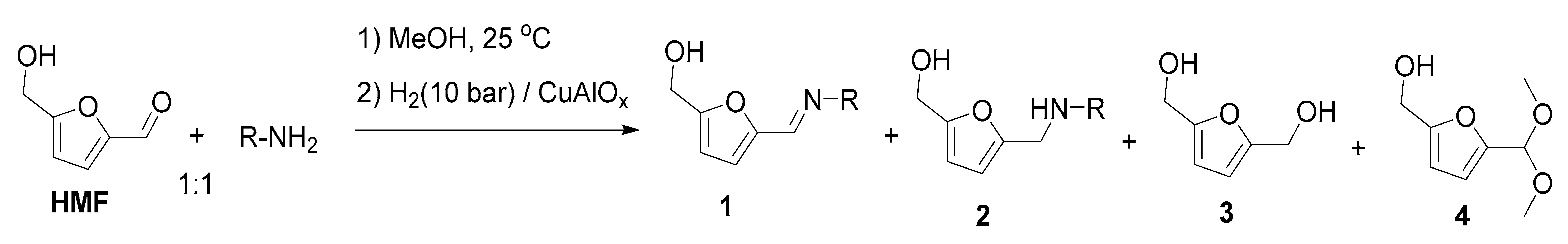
| Entry | R | Step 1, h | T, °C | 2 | Yield, % 2 | |||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||
| 1 | Ph | 3 | 100 | 2a | n.d. | 97 | 3 | n.d. |
| 2 3 | Ph | 3 | 100 | 2a | n.d. | 74 | 26 | n.d. |
| 3 4 | Ph | 3 | 100 | 2a | n.d. | 67 | 33 | n.d. |
| 4 5 | Ph | 3 | 100 | 2a | 3 | 94 | 3 | <0.5 |
| 5 5 | Ph | 3 | 120 | 2a | 1 | 98 | <1 | 0.5 |
| 6 | p-CH3C6H4 | 3 | 100 | 2b | n.d. | 97 | 3 | n.d. |
| 7 | m-CH3C6H4 | 3 | 100 | 2c | n.d. | 96 | 4 | n.d. |
| 8 | o-CH3C6H4 | 16 | 100 | 2d | n.d. | 88 | 12 | <0.5 |
| 9 | p-CH3OC6H4 | 3 | 100 | 2e | n.d. | 95 | 5 | n.d. |
| 10 | p-FC6H4 | 3 | 100 | 2f | n.d. | 94 | 6 | n.d. |
| 11 | p-ClC6H4 | 3 | 100 | 2g | n.d. | 93 | 7 | n.d. |
| 12 | p-BrC6H4 | 3 | 110 | 2h | 22 | 60 | 16 | 1 |
| 13 | m-ClC6H4 | 3 | 100 | 2i | n.d. | 55 | 45 | n.d. |
| 14 | m-ClC6H4 | 16 | 100 | 2i | n.d. | 95 | 4 | 1 |
| 15 | o-ClC6H4 | 16 | 100 | 2j | n.d. | 52 | 42 | 6 |
| 16 | n-hexyl | 3 | 100 | 2k | n.d. | 93 | 7 | n.d. |
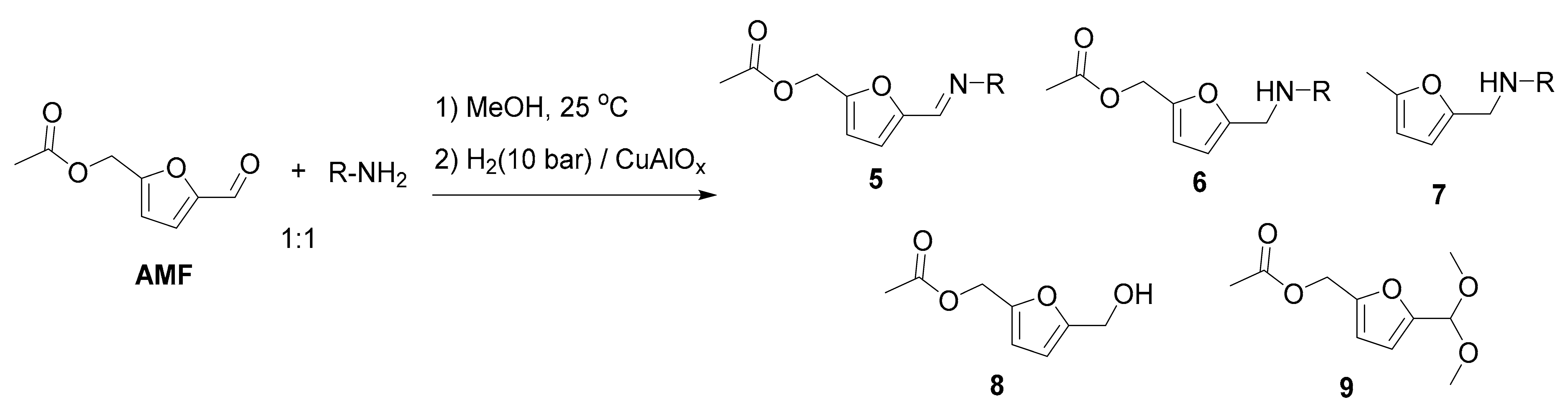
| Entry | R | Step 1, h | 6 | Yield, % 2 | ||||
|---|---|---|---|---|---|---|---|---|
| 5 | 6 | 7 | 8 | 9 | ||||
| 1 | Ph | 3 | 6a | n.d. | 99 | <0.5 | <1 | n.d. |
| 2 | p-CH3C6H4 | 3 | 6b | n.d. | >99 | n.d. | <1 | n.d. |
| 3 | m-CH3C6H4 | 3 | 6c | <0.5 | >98 | n.d. | 1 | n.d. |
| 4 3 | o-CH3C6H4 | 16 | 6d | n.d. | 96 | 1 | 3 | n.d. |
| 5 | p-CH3OC6H4 | 3 | 6e | n.d. | 99 | 0.5 | <0.5 | <0.5 |
| 6 | p-FC6H4 | 3 | 6f | <0.5 | >98 | <0.5 | 1 | n.d. |
| 7 | p-ClC6H4 | 3 | 6g | <0.5 | 97 | 0.5 | 2 | <0.5 |
| 8 | m-ClC6H4 | 16 | 6h | n.d. | 96 | <0.5 | 2 | 2 |
| 9 | n-hexyl | 3 | 6i | n.d. | 82 4 | n.d. | n.d. | n.d. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuzhdin, A.L.; Bukhtiyarova, M.V.; Bukhtiyarov, V.I. Two-Step One-Pot Reductive Amination of Furanic Aldehydes Using CuAlOx Catalyst in a Flow Reactor. Molecules 2020, 25, 4771. https://doi.org/10.3390/molecules25204771
Nuzhdin AL, Bukhtiyarova MV, Bukhtiyarov VI. Two-Step One-Pot Reductive Amination of Furanic Aldehydes Using CuAlOx Catalyst in a Flow Reactor. Molecules. 2020; 25(20):4771. https://doi.org/10.3390/molecules25204771
Chicago/Turabian StyleNuzhdin, Alexey L., Marina V. Bukhtiyarova, and Valerii I. Bukhtiyarov. 2020. "Two-Step One-Pot Reductive Amination of Furanic Aldehydes Using CuAlOx Catalyst in a Flow Reactor" Molecules 25, no. 20: 4771. https://doi.org/10.3390/molecules25204771
APA StyleNuzhdin, A. L., Bukhtiyarova, M. V., & Bukhtiyarov, V. I. (2020). Two-Step One-Pot Reductive Amination of Furanic Aldehydes Using CuAlOx Catalyst in a Flow Reactor. Molecules, 25(20), 4771. https://doi.org/10.3390/molecules25204771





