Reduction and Oxidation of Cu Species in Cu-Faujasites Studied by IR Spectroscopy
Abstract
1. Introduction
2. Results and Discussion
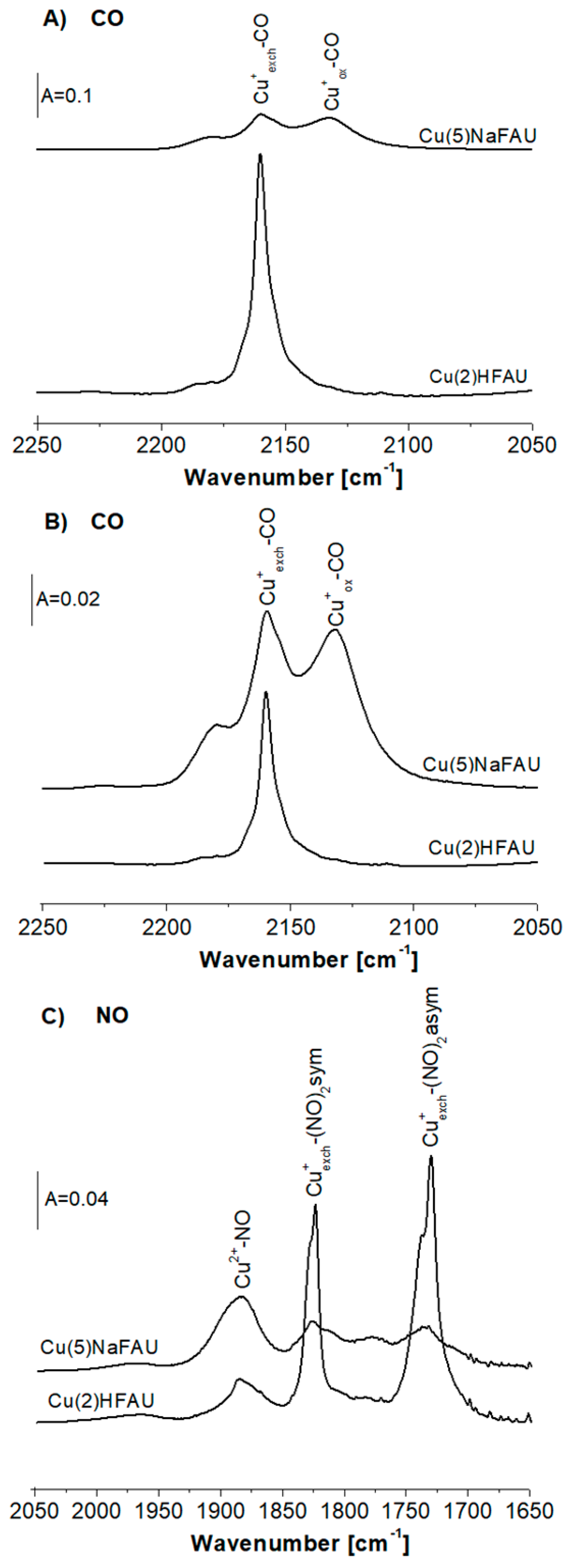
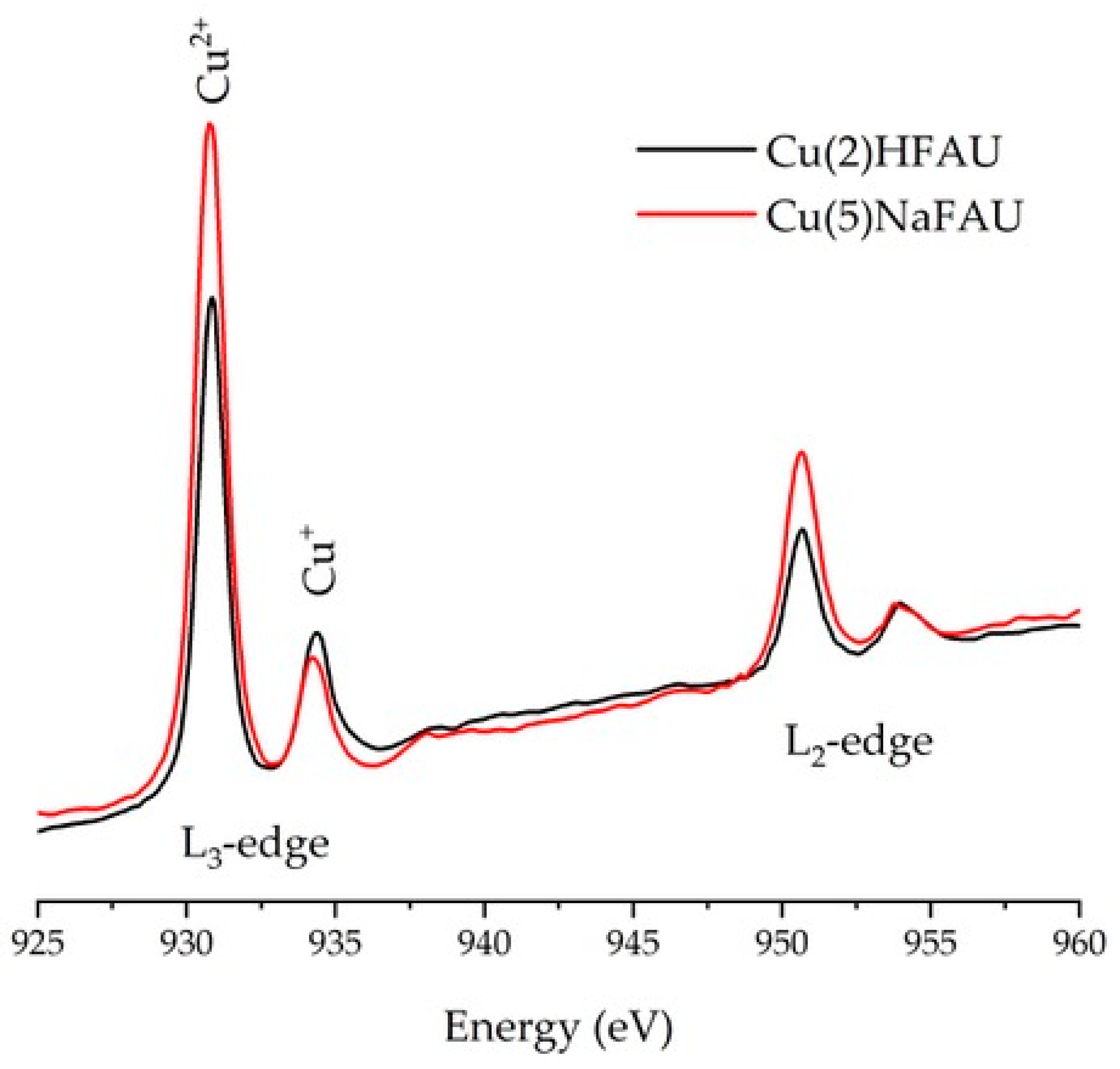
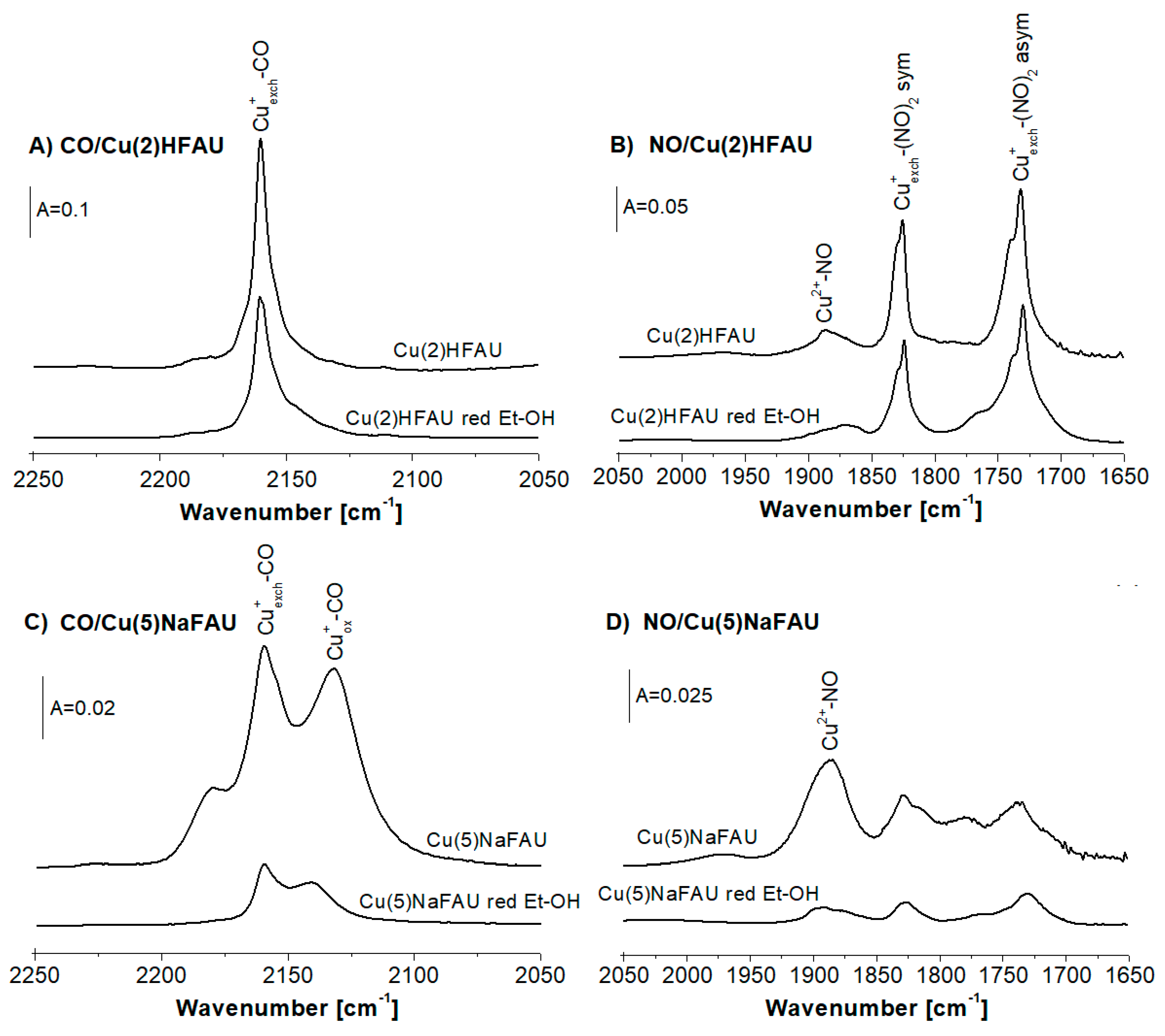
2.1. Cu Species in Cu(2)HFAU and Cu(5)NaFAU
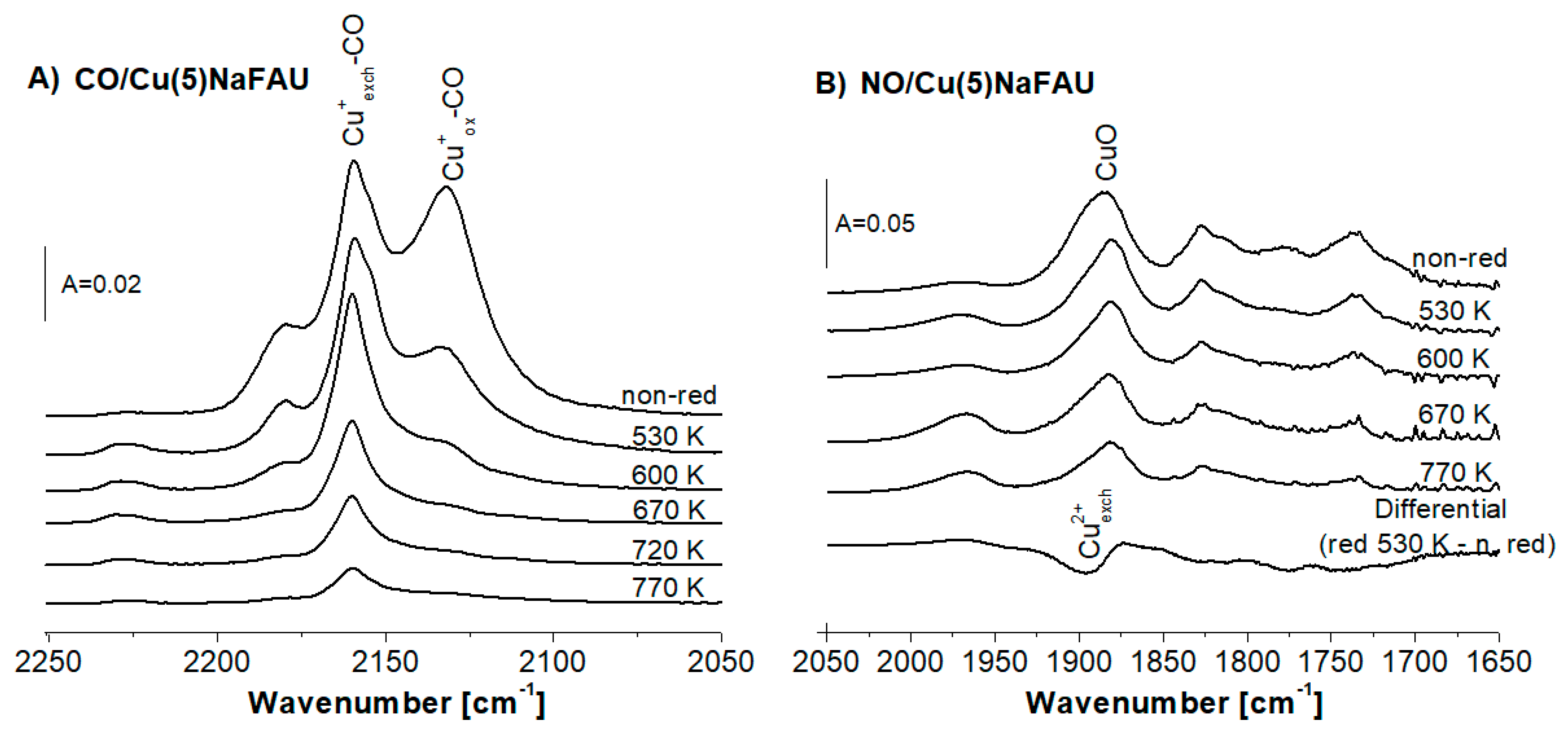
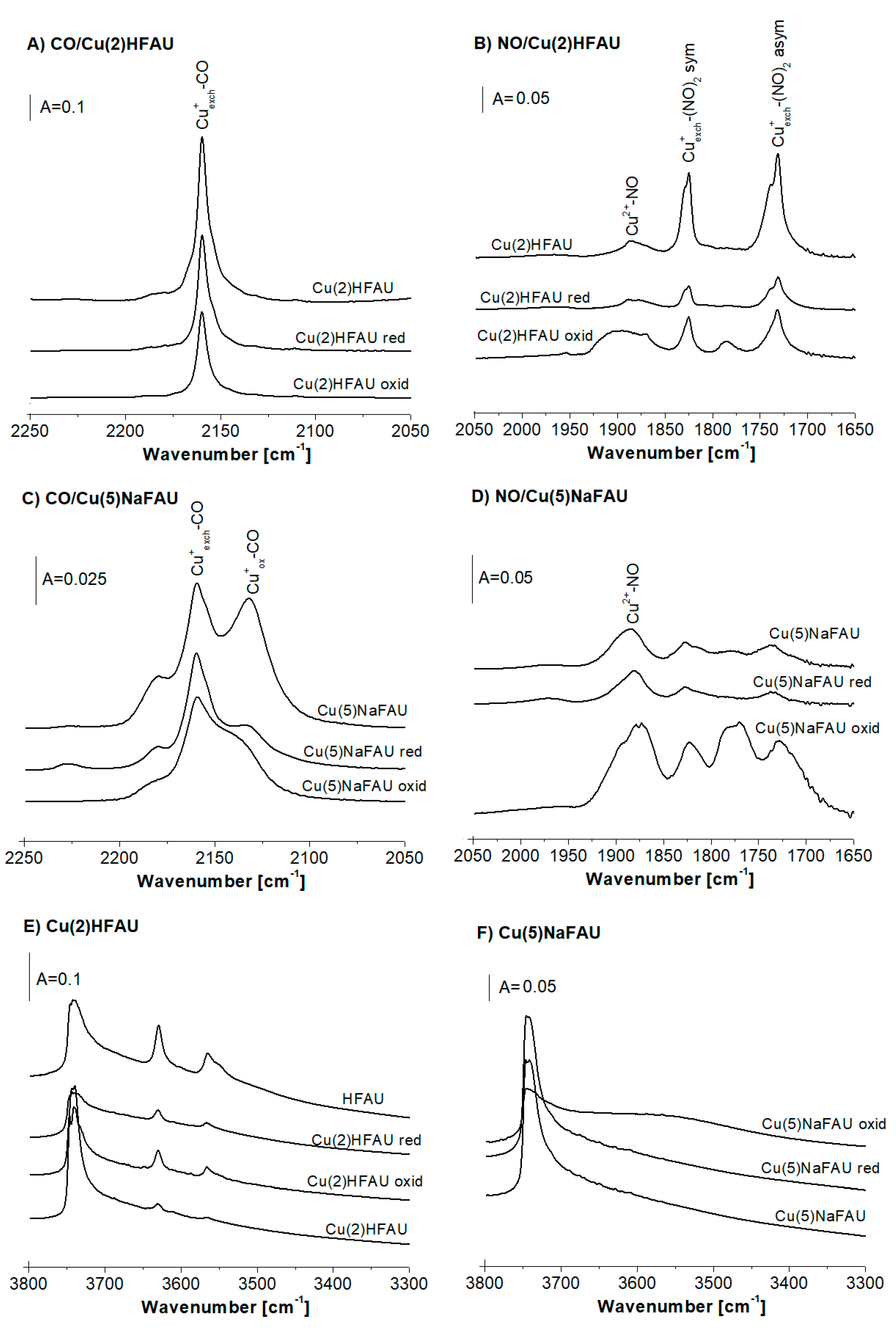
2.2. Reduction of Cu Species by Hydrogen
2.3. Reduction of Cu Sites by Ethanol
2.4. Oxidation of Cu Sites in Zeolites by Oxygen
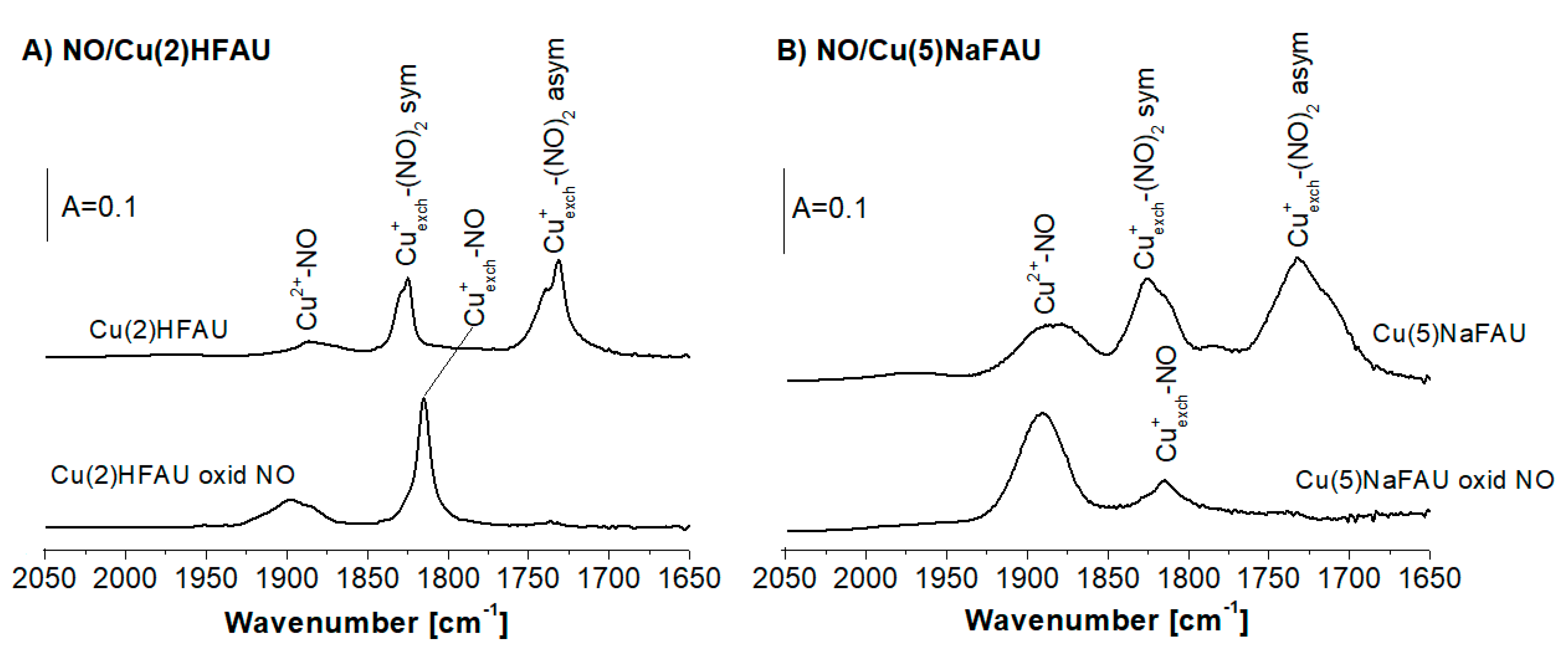
2.5. Oxidation of Cu Sites in Zeolites by NO
3. Materials and Methods
3.1. Catalyst Preparation
3.2. IR Studies
3.3. XAS Studies
3.4. XRD Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kaushik, V.; Ravindranathan, M. X.p.s. Study of Copper-Containing Y Zeolites for the Hydration of Acrylonitrile to Acrylamide. Zeolites 1992, 12, 415–419. [Google Scholar] [CrossRef]
- Maxwell, I.; De Boer, J.; Downing, R. Copper-Exchanged Zeolite Catalysts for the Cyclodimerization of Butadiene II. Catalyst Structure. J. Catal. 1980, 61, 493–502. [Google Scholar] [CrossRef]
- Pestryakov, A.; Lunin, V.V. Physicochemical Study of Active Sites of Metal Catalysts for Alcohol Partial Oxidation. J. Mol. Catal. A: Chem. 2000, 158, 325–329. [Google Scholar] [CrossRef]
- Parthly, S.A. Zeolite-Encapsulated Cu(II)-Salen Complex as a Catalyst for Oxidation of Cyclohexanol. Indian J. Chem. 1996, 35A, 1–3. Available online: http://nopr.niscair.res.in/bitstream/123456789/41239/1/IJCA%2035A(1)%201-3.pdf (accessed on 16 October 2020).
- Lepore, A.W.; Li, Z.; Davison, B.H.; Foo, G.-S.; Wu, Z.; Narula, C.K. Catalytic Dehydration of Biomass Derived 1-Propanol to Propene over M-ZSM-5 (M = H, V, Cu, or Zn). Ind. Eng. Chem. Res. 2017, 56, 4302–4308. [Google Scholar] [CrossRef]
- Kyriienko, P.I.; Larina, O.V.; Soloviev, S.O.; Orlyk, S.M.; Calers, C.; Dzwigaj, S. Ethanol Conversion into 1,3-Butadiene by the Lebedev Method over MTaSiBEA Zeolites (M = Ag, Cu, Zn). ACS Sustain. Chem. Eng. 2017, 5, 2075–2083. [Google Scholar] [CrossRef]
- De Oliveira, T.K.R.; Rosset, M.; Perez-Lopez, O.W. Ethanol Dehydration to Diethyl Ether over Cu-Fe/ZSM-5 Catalysts. Catal. Commun. 2018, 104, 32–36. [Google Scholar] [CrossRef]
- Klein, A.; Keisers, K.; Palkovits, R. Formation of 1,3- Butadiene from Ethanol in a Two-Step Process Using Modified Zeolite-β Catalysts. Appl. Catal. A Gen. 2016, 514, 192–202. [Google Scholar] [CrossRef]
- Kristiani, A.; Sudiyarmanto, S.; Aulia, F.; Hidayati, L.N.; Abimanyu, H. Metal Supported on Natural Zeolite as Catalysts for Conversion of Ethanol to Gasoline. MATEC Web Conf. 2017, 101, 1001. [Google Scholar] [CrossRef]
- Nash, C.P.; Ramanathan, A.; Ruddy, D.A.; Behl, M.; Gjersing, E.; Griffin, M.; Zhu, H.; Subramaniam, B.; Schaidle, J.A.; Hensley, J.E. Mixed Alcohol Dehydration Over BRønsted and Lewis Acidic Catalysts. Appl. Catal. A Gen. 2016, 510, 110–124. [Google Scholar] [CrossRef]
- Song, Z.; Takahashi, A.; Mimura, N.; Fujitani, T. Production of Propylene from Ethanol Over ZSM-5 Zeolites. Catal. Lett. 2009, 131, 364–369. [Google Scholar] [CrossRef]
- Matsumoto, H.; Tanabe, S. Catalytic Behavior and Structure of Active Species on CU-Y Zeolite in Oxidation of Carbon Monoxide. J. Phys. Chem. 1990, 94, 4207–4212. [Google Scholar] [CrossRef]
- Jirka, I.; Bosacek, V. ESCA Study of Cu2+-Y and Cu2+-ZSM-5. Zeolites 1991, 11, 77. [Google Scholar] [CrossRef]
- Huang, Y. Ethylene Complexes in Copper(I) and Silver (I) Y Zeolites. J. Catal. 1980, 61, 461–476. [Google Scholar] [CrossRef]
- Márquez-Álvarez, C.; McDougall, G.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Study of the Surface Species Formed from the Interaction of NO and Co with Copper Ions in ZSM-5 and Y Zeolites. Appl. Surf. Sci. 1994, 78, 477–484. [Google Scholar] [CrossRef]
- Howard, J.; Nicol, J. FTi.r. Studies of Copper-Containing Y Zeolites: Part 1. Location of Copper (I)-Carbonyl Complexes. Zeolites 1988, 8, 142–150. [Google Scholar] [CrossRef]
- Weckhuysen, B.M.; Verberckmoes, A.A.; Fu, L.; Schoonheydt, R.A. Zeolite-Encapsulated Copper(II) Amino Acid Complexes: Synthesis, Spectroscopy, and Catalysis. J. Phys. Chem. 1996, 100, 9456–9461. [Google Scholar] [CrossRef][Green Version]
- Gil, B.; Datka, J.; Witkowski, S.; Sojka, Z.; Brocŀawik, E. Copper Redox Chemistry in Cu/ZSM-5 Zeolites: EPR, IR and DFT Investigations. Stud. Surf. Sci. Catal. 2000, 130, 3249–3254. [Google Scholar] [CrossRef]
- Pietrzyk, P.; Sojka, Z. EPR Spectroscopy and DFT Calculations of the g Tensors of {VO}1/ZSM-5, {CuNO}11/ZSM-5 and {NaNO}1/ZSM-5 Intrazeolitic Complexes. Stud. Surf. Sci. Catal. 2005, 158, 617–624. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Guerrero-Ruiz, A.; Fierro, J.L.G. Changes of Copper Location in CuY Zeolites Induced by Preparation Methods. Catal. Lett. 1996, 41, 55–61. [Google Scholar] [CrossRef]
- Moretti, G. The Contribution of X-ray Photoelectron and X-ray Excited Auger Spectroscopies in the Characterization of Zeolites and of Metal Clusters Entrapped in Zeolites. Zeolites 1994, 14, 469–475. [Google Scholar] [CrossRef]
- Sharma, M.; Das, B.; Sharma, M.; Deka, B.K.; Park, Y.-B.; Bhargava, S.K.; Bania, K.K. Pd/Cu-Oxide Nanoconjugate at Zeolite-Y Crystallite Crafting the Mesoporous Channels for Selective Oxidation of Benzyl-Alcohols. ACS Appl. Mater. Interfaces 2017, 9, 35453–35462. [Google Scholar] [CrossRef]
- Jacobs, P.A.; Tielen, M.; Linart, J.-P.; Uytterhoeven, J.B.; Beyer, H. Redox Behaviour of Transition Metal Ions in Zeolites. Part 4. Kinetic Study of the Reduction and Reoxidation of Copper–Y Zeolites. J. Chem. Soc. Faraday Trans. 1976, 72, 2793–2804. [Google Scholar] [CrossRef]
- Boyce, A.L.; Graville, S.R.; Sermon, P.A.; Vong, M.S.W. Reduction of CuO-containing catalysts, CuO: II, XRD and XPS. React. Kinet. Catal. Lett. 1991, 44, 13–18. [Google Scholar] [CrossRef]
- Vong, M.S.W.; Sermon, P.A.; Grant, K. In-Situ Study of Reduction of Copper Catalysts. Catal. Lett. 1990, 4, 15–24. [Google Scholar] [CrossRef]
- Campos-Martin, J.M.; Guerrero-Ruiz, A.; Fierro, J. Structural and Surface Properties of CuO-ZnO-Cr2O3 Catalysts and Their Relationship with Selectivity to Higher Alcohol Synthesis. J. Catal. 1995, 156, 208–218. [Google Scholar] [CrossRef]
- Kuterasiński, Ł.; Podobiński, J.; Rutkowska-Zbik, D.; Datka, J. IR Studies of the Cu Ions in Cu-Faujasites. Molecules 2019, 24, 4250. [Google Scholar] [CrossRef]
- Palomino, G.T.; Bordiga, S.; Zecchina, A.; Marra, G.L.; Lamberti, C. XRD, XAS, and IR Characterization of Copper-Exchanged Y Zeolite. J. Phys. Chem. B 2000, 104, 8641–8651. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Palomares, A.E.; Glanowska, A.; Sadowska, K.; Datka, J. Copper Sites in Zeolites—Quantitative IR Studies. Microporous Mesoporous Mater. 2012, 162, 175–180. [Google Scholar] [CrossRef]
- Ziolek, M.; Sobczak, I.; Nowak, I.; Daturi, M.; LaValley, J. Effect of Sulfur Dioxide on Nitric Oxide Adsorption and Decomposition on Cu-Containing Micro-and Mesoporous Molecular Sieves. Top. Catal. 2000, 11, 343–350. [Google Scholar] [CrossRef]
- Wichterlová, B.; Dědeček, J.; Sobalík, Z.; Vondrová, A.; Klier, K. On the Cu Site in ZSM-5 Active in Decomposition of NO: Luminescence, FTIR Study, and Redox Properties. J. Catal. 1997, 169, 194–202. [Google Scholar] [CrossRef]
- Henriques, C.; Ribeiro, M.F.; Abreu, C.; Murphy, D.M.; Poignant, F.; Saussey, J.; LaValley, J. An FT-IR Study of NO Adsorption Over Cu-Exchanged MFI Catalysts: Effect of Si/Al Ratio, Copper Loading and Catalyst Pre-Treatment. Appl. Catal. B 1998, 16, 79–95. [Google Scholar] [CrossRef]
- Davydov, A.A.; Budneva, A.A. IR Spectra of CO and NO Adsorbed on CuO. React. Kinet. Catal. Lett. 1984, 25, 121–124. [Google Scholar] [CrossRef]
- Beaumont, S. K Soft XAS as an In Situ Technique for the Study of Heterogeneous Catalysts. Phys. Chem. Chem. Phys. 2020, 22, 18747. [Google Scholar] [CrossRef]
- Sarangi, R.; Aboelella, N.; Fujisawa, K.; Tolman, W.B.; Hedman, B.; Hodgson, K.O.; Solomon, E.I. X-ray Absorption Edge Spectroscopy and Computational Studies on LCuO2 Species: Superoxide−CuII Versus Peroxide−CuIII Bonding. J. Am. Chem. Soc. 2006, 128, 8286–8296. [Google Scholar] [CrossRef]
- Baker, M.L.; Mara, M.W.; Yan, J.J.; Hodgson, K.O.; Hedman, B.; Solomon, E.I. K- and L-edge X-ray Absorption Spectroscopy (XAS) and Resonant Inelastic X-ray Scattering (RIXS) Determination of Differential Orbital Covalency (DOC) of Transition Metal Sites. Coord. Chem. Rev. 2017, 345, 182–208. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Prendergast, D.; Borondics, F.; Porsgaard, S.; Giovanetti, L.; Pach, E.; Newberg, J.; Bluhm, H.; Besenbacher, F.; Salmeron, M. Experimental and Theoretical Investigation of the Electronic Structure of Cu2O and CuO Thin Films on Cu(110) Using X-ray Photoelectron and Absorption Spectroscopy. J. Chem. Phys. 2013, 138, 024704. [Google Scholar] [CrossRef]
- Grioni, M.; Goedkoop, J.B.; Schoorl, R.; De Groot, F.M.F.; Fuggle, J.C.; Schäfers, F.; Koch, E.E.; Rossi, G.; Esteva, J.-M.; Karnatak, R.C. Studies of Copper Valence States with Cu L3 X-ray-Absorption Spectroscopy. Phys. Rev. B 1989, 39, 1541–1545. [Google Scholar] [CrossRef]
- Grioni, M.; Van Acker, J.F.; Czyžyk, M.T.; Fuggle, J.C. Unoccupied Electronic Structure and Core-Hole Effects in the X-ray-Absorption Spectra of Cu2O. Phys. Rev. B 1992, 45, 3309–3318. [Google Scholar] [CrossRef]
- Davó-Quiñonero, A.; Bailón-García, E.; López-Rodríguez, S.; Juan-Juan, J.; Lozano-Castello, D.; Garcia-Melchor, M.; Herrera, F.C.; Pellegrin, E.; Escudero, C.; Bueno-López, A. Insights into the Oxygen Vacancy Filling Mechanism in CuO/CeO2 Catalysts: A Key Step Toward High Selectivity in Preferential CO Oxidation. ACS Catal. 2020, 10, 6532–6545. [Google Scholar] [CrossRef]
- Gackowski, M.; Podobiński, J.; Rutkowska-Zbik, D.; Datka, J. IR Studies of the Cu Ions in Cu-Faujasites of low Si/Al ratio. Molecules. To be published.
- Zając, M.; Giela, T.; Freindl, K.; Korecki, J.; Madej, E.; Sikora, M.; Spiridis, N.; Stankiewicz, M.; Stępień, J.; Szade, J.; et al. The Soft X-rays Spectroscopy Beamline at the National Synchrotron Radiation Centre Solaris. Synchrotron Radiat. Nat. Sci. 2020, 19, 1–4. Available online: http://biuletyn.synchrotron.org.pl/wp-content/uploads/2020/06/bull_2020_19_001_Zajac.pdf (accessed on 16 October 2020).
Sample Availability: Samples of the copper-containing faujasites are available from the authors. | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuterasiński, Ł.; Podobiński, J.; Madej, E.; Smoliło-Utrata, M.; Rutkowska-Zbik, D.; Datka, J. Reduction and Oxidation of Cu Species in Cu-Faujasites Studied by IR Spectroscopy. Molecules 2020, 25, 4765. https://doi.org/10.3390/molecules25204765
Kuterasiński Ł, Podobiński J, Madej E, Smoliło-Utrata M, Rutkowska-Zbik D, Datka J. Reduction and Oxidation of Cu Species in Cu-Faujasites Studied by IR Spectroscopy. Molecules. 2020; 25(20):4765. https://doi.org/10.3390/molecules25204765
Chicago/Turabian StyleKuterasiński, Łukasz, Jerzy Podobiński, Ewa Madej, Małgorzata Smoliło-Utrata, Dorota Rutkowska-Zbik, and Jerzy Datka. 2020. "Reduction and Oxidation of Cu Species in Cu-Faujasites Studied by IR Spectroscopy" Molecules 25, no. 20: 4765. https://doi.org/10.3390/molecules25204765
APA StyleKuterasiński, Ł., Podobiński, J., Madej, E., Smoliło-Utrata, M., Rutkowska-Zbik, D., & Datka, J. (2020). Reduction and Oxidation of Cu Species in Cu-Faujasites Studied by IR Spectroscopy. Molecules, 25(20), 4765. https://doi.org/10.3390/molecules25204765







