Involvement of miR-142 and miR-155 in Non-Infectious Complications of CVID
Abstract
1. Introduction
2. Materials and Methods
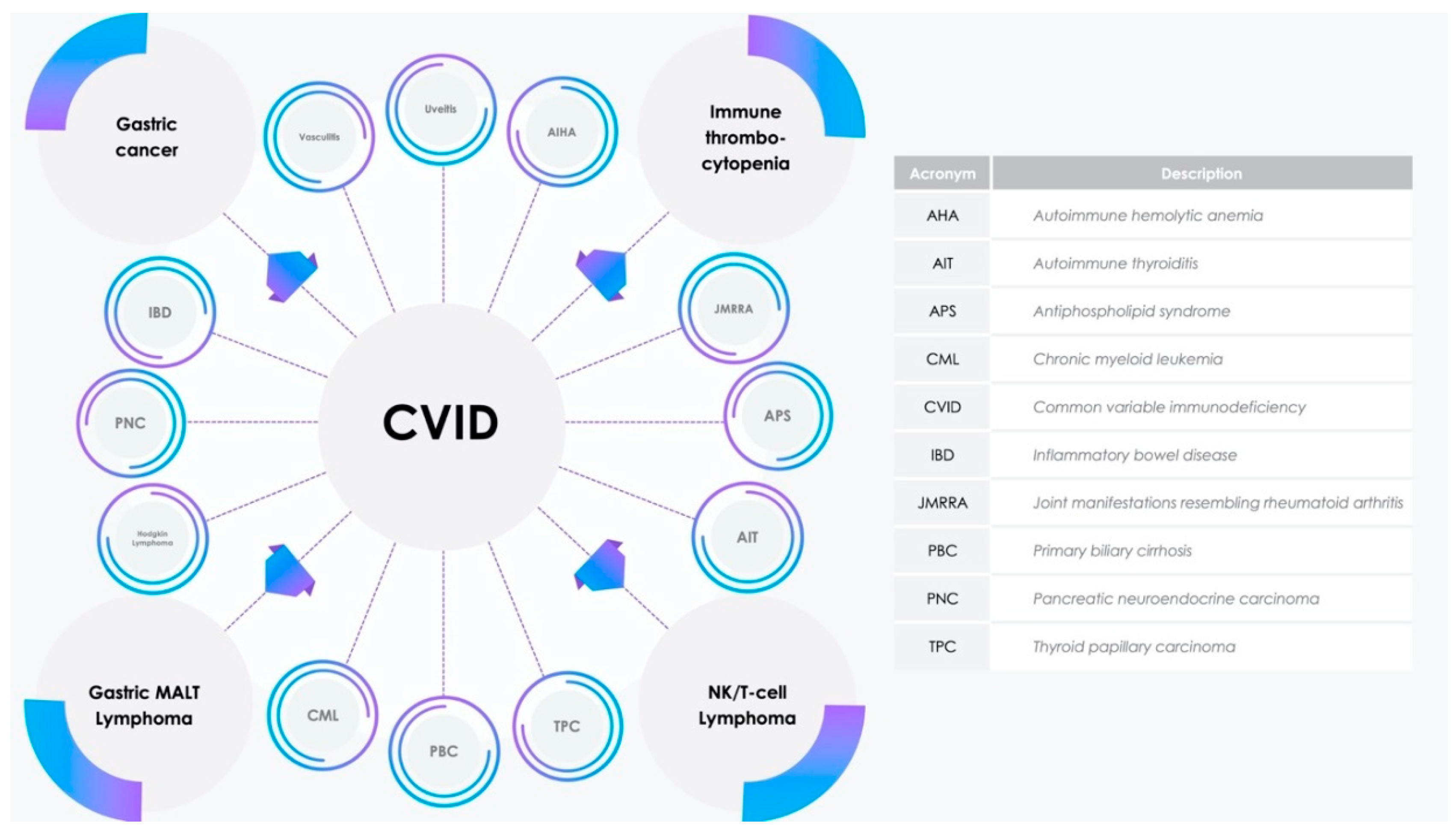
3. Results
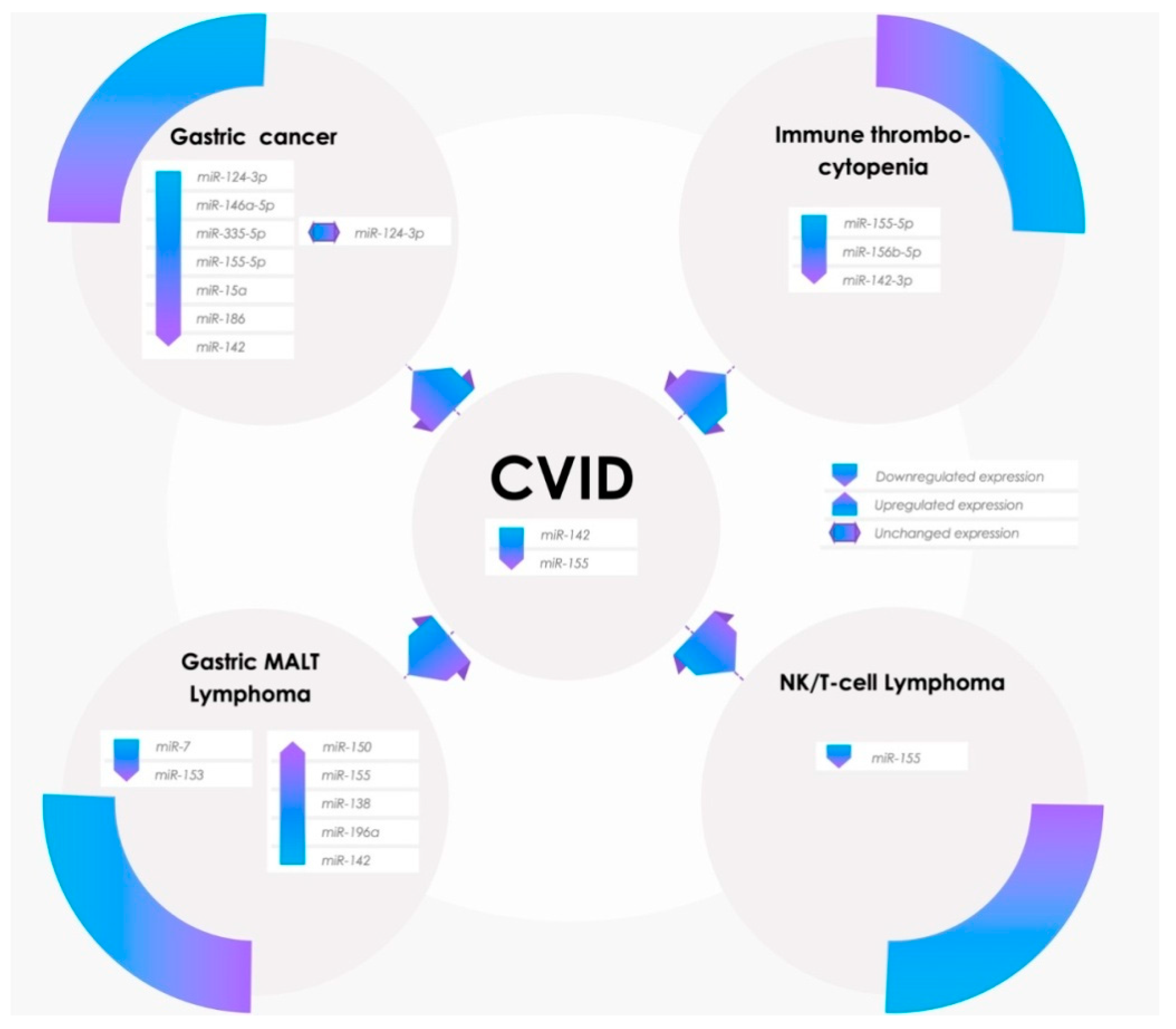
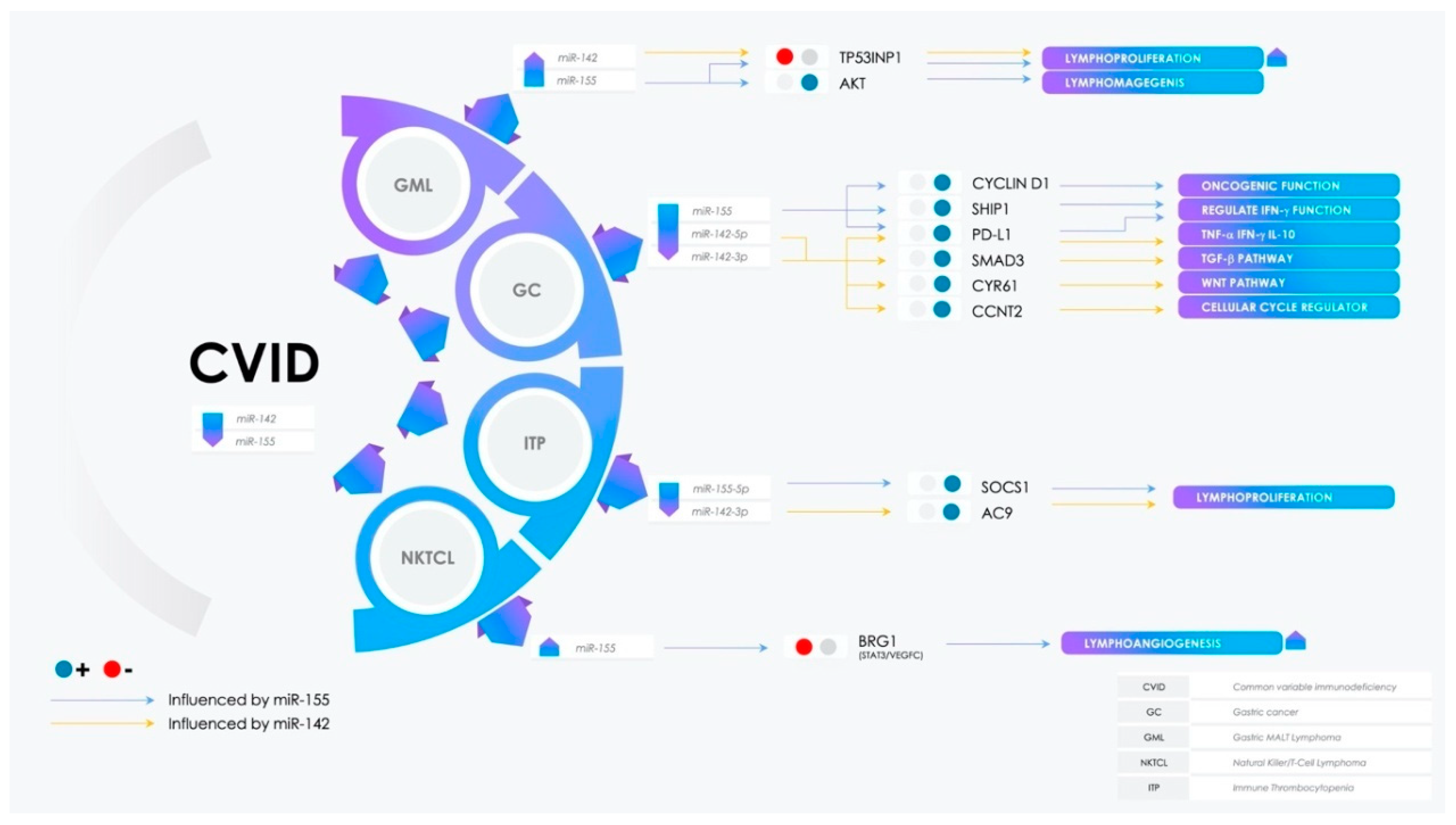
3.1. miR-155 Expression in Pathophysiological Processes
3.2. Potential Involvement of miR-142 in CVID Pathogenesis and Comorbidities
3.3. Dual Role of miR-155 and miR-142 in Gastric Cancer
3.4. Immunotolerance and Antitumor Immunity: The Involvement of the PD-L1/PD-1 Axis in Malignancies
3.5. Over the Chronic Gastritis to Overt Gastric MALT Lymphoma
3.6. Lymphangiogenesis Arrangement: The Role of miR-155
3.7. Dysregulation of Peripheric Immunotolerance and Immune Thrombocytopenia
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gangemi, S.; Allegra, A.; Musolino, C. Lymphoproliferative disease and cancer among patients with common variable immunodeficiency. Leuk. Res. 2015, 39, 389–396. [Google Scholar] [CrossRef]
- Saikia, B.; Gupta, S. Common variable immunodeficiency. Indian J. Pediatr. 2016, 83, 338–344. [Google Scholar] [CrossRef]
- Elton, T.S.; Selemon, H.; Elton, S.M.; Parinandi, N.L. Regulation of the MIR155 host gene in physiological and pathological processes. Gene 2013, 532, 1–12. [Google Scholar] [CrossRef]
- Kramer, N.J.; Wang, W.L.; Reyes, E.Y.; Kumar, B.; Chen, C.C.; Ramakrishna, C.; Cantin, E.M.; Vonderfecht, S.L.; Taganov, K.D.; Chau, N.; et al. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood, J. Am. Soc. Hematol. 2015, 125, 3720–3730. [Google Scholar] [CrossRef]
- Rodriguez, A.; Vigorito, E.; Clare, S.; Warren, M.V.; Couttet, P.; Soond, D.R.; Dongen, S.V.; Grocock, R.J.; Das, P.P.; Miska, E.A. Requirement of bic/microRNA-155 for normal immune function. Science 2007, 416, 608–611. [Google Scholar] [CrossRef]
- Rae, W. Indications to epigenetic dysfunction in the pathogenesis of common variable immunodeficiency. Arch. Immunol. Ther. Exp. 2017, 65, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Yim, R.L.; Wong, K.Y.; Kwong, Y.L.; Loong, F.; Leung, C.Y.; Chu, R.; Lam, W.W.; Hui, P.K.; Lai, R.; Chim, C.S. Methylation of miR-155-3p in mantle cell lymphoma and other non-Hodgkin’s lymphomas. Oncotarget 2014, 5, 9770. [Google Scholar] [CrossRef][Green Version]
- Sharma, S. Immunomodulation: A definitive role of microRNA-142. Dev. Comp. Immunol. 2017, 77, 150–156. [Google Scholar] [CrossRef]
- Chen, C.-Z.; Li, L.; Lodish, H.F.; Bartel, D.P. MicroRNAs modulate hematopoietic lineage differentiation. Science 2004, 303, 83–86. [Google Scholar] [CrossRef]
- Shrestha, A.; Mukhametshina, R.T.; Taghizadeh, S.; Vásquez-Pacheco, E.; Cabrera-Fuentes, H.; Rizvanov, A.; Mari, B.; Carraro, G.; Bellusci, S. MicroRNA-142 is a multifaceted regulator in organogenesis, homeostasis, and disease. Dev. Dyn. 2017, 246, 285–290. [Google Scholar] [CrossRef]
- Wang, X.-S.; Gong, J.N.; Yu, J.; Wang, F.; Zhang, X.H.; Yin, X.L.; Tan, Z.Q.; Luo, Z.M.; Yang, G.H.; Shen, C.; et al. MicroRNA-29a and microRNA-142-3p are regulators of myeloid differentiation and acute myeloid leukemia. Blood 2012, 119, 4992–5004. [Google Scholar] [CrossRef]
- Xu, S.; Wei, J.; Wang, F.; Kong, L.Y.; Ling, X.Y.; Nduom, E.; Gabrusiewicz, K.; Doucette, T.; Yang, Y.; Yaghi, N.K.; et al. Effect of miR-142-3p on the M2 macrophage and therapeutic efficacy against murine glioblastoma. J. Natl. Cancer Inst. 2014, 106, 1–11. [Google Scholar] [CrossRef]
- Anandagoda, N.; Willis, J.C.; Hertweck, A.; Roberts, L.B.; Jackson, I.; Gökmen, M.R.; Jenner, R.G.; Howard, J.K.; Lord, G.M. microRNA-142–mediated repression of phosphodiesterase 3B critically regulates peripheral immune tolerance. J. Clin. Invest. 2019, 129, 1257–1271. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xie, S.; Liu, M.; Chen, Z.; Liu, X.; Wang, L.; Li, D.; Zhou, Y. The clinical significance of downregulation of mir-124-3p, mir-146a-5p, mir-155-5p and mir-335-5p in gastric cancer tumorigenesis. Int. J. Oncol. 2014, 45, 197–208. [Google Scholar] [CrossRef]
- Zare, A.; Alipoor, B.; Omrani, M.D.; Zali, M.R.; Alamdari, N.M.; Ghaedi, H. Decreased miR-155-5p, miR-15a, and miR-186 expression in gastric cancer is associated with advanced tumor grade and metastasis. Iran. Biomed. J. 2019, 23, 338. [Google Scholar] [CrossRef]
- Ma, Z.; Ma, Y.; Xia, Q.; Li, Y.; Li, R.; Chang, W.; Chen, J.; Leng, Z.; Tao, K. MicroRNA-155 expression inversely correlates with pathologic stage of gastric cancer and it inhibits gastric cancer cell growth by targeting cyclin D1. J. Cancer Res. Clin. Oncol. 2016, 142, 1201–1212. [Google Scholar] [CrossRef]
- Li, S.; Zhang, T.; Zhou, X.; Du, Z.; Chen, F.; Luo, J.; Liu, Q. The tumor suppressor role of miR-155-5p in gastric cancer. Oncol. Lett. 2018, 16, 2709–2714. [Google Scholar] [CrossRef]
- Michaille, J.; Awad, N.; Fortman, E.C.; Efanov, A.A.; Tili, E. miR-155 expression in antitumor immunity: The higher the better? Genes Chromosom. Cancer 2019, 58, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Fioravanti, J.; Zhu, W.; Wang, H.; Wu, T.; Hu, J.; Lacey, N.E.; Gautam, S.; Le Gall, J.B.; Yang, X.; et al. miR-155 harnesses Phf19 to potentiate cancer immunotherapy through epigenetic reprogramming of CD8+ T cell fate. Nat. Commun. 2019, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Rafiei, H.; Mohammadinejad, R.; Farkhondeh, T.; Samarghandian, S. Wnt-regulating microRNAs role in gastric cancer malignancy. Life Sci. 2020, 250, 117547. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Z.; Wang, L.; Liu, S.; Cai, J. Downregulation of microRNA-142-3p and its tumor suppressor role in gastric cancer. Oncol. Lett. 2018, 15, 8172–8180. [Google Scholar] [CrossRef]
- Jia, L.; Xi, Q.; Wang, H.; Zhang, Z.; Liu, H.; Cheng, Y.; Guo, X.; Zhang, J.; Zhang, Q.; Zhang, L.; et al. miR-142-5p regulates tumor cell PD-L1 expression and enhances anti-tumor immunity. Biochem. Biophys. Res. Commun. 2017, 488, 425–431. [Google Scholar] [CrossRef]
- Blosse, A.; Levy, M.; Robe, C.; Staedel, C.; Copie-Bergman, C.; Lehours, P. Deregulation of miRNA in Helicobacter pylori-Induced Gastric MALT Lymphoma: From Mice to Human. J. Clin. Med. 2019, 8, 845. [Google Scholar] [CrossRef]
- Saito, Y.; Suzuki, H.; Tsugawa, H.; Imaeda, H.; Matsuzaki, J.; Hirata, K.; Hosoe, N.; Nakamura, M.; Mukai, M.; Saito, H.; et al. Overexpression of miR-142-5p and miR-155 in gastric mucosa-associated lymphoid tissue (MALT) lymphoma resistant to Helicobacter pylori eradication. PLoS ONE 2012, 7, e47396. [Google Scholar] [CrossRef]
- Fernandez, C.; Bellosillo, B.; Ferraro, M.; Seoane, A.; Sanchez-Gonzalez, B.; Pairet, S.; Poins, A.; Barranco, L.; Vela, C.V.; Gimmeno, E.; et al. MicroRNAs 142-3p, miR-155 and miR-203 are deregulated in gastric MALT lymphomas compared to chronic gastritis. Cancer Genom. Proteom. 2017, 14, 75–82. [Google Scholar] [CrossRef][Green Version]
- Bedewy, A.M.L.; Elmaghraby, S.M.; Shehata, A.A.; Kandil, N.S. Prognostic value of miRNA-155 expression in B-cell non-Hodgkin lymphoma. Turkish J. Hematol. 2017, 34, 207. [Google Scholar]
- Cuadros, M.; Sánchez-Martín, V.; Herrera, A.; Baliñas, C.; Martín-Padrón, J.; Boyero, L.; Peinado, P.; Medina, P.P. BRG1 reèègulation by miR-155 in human leukemia and lymphoma cell lines. Clin. Transl. Oncol. 2017, 19, 1010–1017. [Google Scholar] [CrossRef]
- Chang, Y.; Cui, M.; Fu, X.; Zhang, L.; Li, X.; Li, L.; Wu, J.; Sun, Z.; Zhang, X.; Li, Z.; et al. MiRNA-155 regulates lymphangiogenesis in natural killer/T-cell lymphoma by targeting BRG1. Cancer Biol. Ther. 2019, 20, 31–41. [Google Scholar] [CrossRef]
- Huang, B.; Zhao, J.; Lei, Z.; Shen, S.; Li, D.; Shen, G.X.; Zhang, G.M.; Feng, Z.H. miR-142-3p restricts cAMP production in CD4+ CD25− T cells and CD4+ CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep. 2009, 10, 180–185. [Google Scholar] [CrossRef]
- Liu, L.; Hua, M.; Liu, C.; He, N.; Li, Z.; Ma, D. The aberrant expression of microRNAs and correlations with T cell subsets in patients with immune thrombocytopenia. Oncotarget 2016, 7, 76453. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, H.; Xie, X.; Zheng, Z.; Ling, Y. MicroRNA expression profile in Treg cells in the course of primary immune thrombocytopenia. J. Investig. Med. 2019, 67, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Vacca, A.; Dammacco, F.; Racanelli, V. Common variable immunodeficiency and gastric malignancies. Int. J. Mol. Sci. 2018, 19, 451. [Google Scholar] [CrossRef] [PubMed]
- Rivkin, N.; Chapnik, E.; Birger, Y.; Yanowski, E.; Curato, C.; Mildner, A.; Porat, Z.; Amir, G.; Izraeli, S.; Jung, S.; et al. Rac1 functions downstream of miR-142 in regulation of erythropoiesis. Haematologica 2017, 102, e476. [Google Scholar] [CrossRef] [PubMed]
- Gottmann, P.; Ouni, M.; Zellner, L.; Jähnert, M.; Rittig, K.; Walther, D.; Schürmann, A. Polymorphisms in miRNA binding sites involved in metabolic diseases in mice and humans. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Picascia, A.; Grimaldi, V.; Pignalosa, O.; De Pascale, M.R.; Schiano, C.; Napoli, C. Epigenetic control of autoimmune diseases: From bench to bedside. Clin. Immunol. 2015, 157, 1–15. [Google Scholar] [CrossRef]
- Su, L.C.; Huang, A.F.; Jia, H.; Liu, Y.; Xu, W.D. Role of microRNA-155 in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 1631–1637. [Google Scholar] [CrossRef]
- Arbore, G.; Henley, T.; Biggins, L.; Andrews, S.; Vigorito, E.; Turner, M.; Leyland, R. MicroRNA-155 is essential for the optimal proliferation and survival of plasmablast B cells. Life Sci. Alliance 2019, 2, e201800244. [Google Scholar] [CrossRef]
- Desjardins, M.; Béland, M.; Dembele, M.; Lejtenyi, D.; Drolet, J.P.; Lemire, M.; Tsoukas, C.; Ben-Shoshan, M.; Noya, F.J.D.; Alizadehfar, R.; et al. Modulation of the interleukin-21 pathway with interleukin-4 distinguishes common variable immunodeficiency patients with more non-infectious clinical complications. J. Clin. Immunol. 2018, 38, 45–55. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amato, G.; Vita, F.; Quattrocchi, P.; Minciullo, P.L.; Pioggia, G.; Gangemi, S. Involvement of miR-142 and miR-155 in Non-Infectious Complications of CVID. Molecules 2020, 25, 4760. https://doi.org/10.3390/molecules25204760
Amato G, Vita F, Quattrocchi P, Minciullo PL, Pioggia G, Gangemi S. Involvement of miR-142 and miR-155 in Non-Infectious Complications of CVID. Molecules. 2020; 25(20):4760. https://doi.org/10.3390/molecules25204760
Chicago/Turabian StyleAmato, Giuliana, Federica Vita, Paolina Quattrocchi, Paola Lucia Minciullo, Giovanni Pioggia, and Sebastiano Gangemi. 2020. "Involvement of miR-142 and miR-155 in Non-Infectious Complications of CVID" Molecules 25, no. 20: 4760. https://doi.org/10.3390/molecules25204760
APA StyleAmato, G., Vita, F., Quattrocchi, P., Minciullo, P. L., Pioggia, G., & Gangemi, S. (2020). Involvement of miR-142 and miR-155 in Non-Infectious Complications of CVID. Molecules, 25(20), 4760. https://doi.org/10.3390/molecules25204760





