Neurite Outgrowth-Promoting Activity of Compounds in PC12 Cells from Sunflower Seeds
Abstract
1. Introduction
2. Results and Discussion
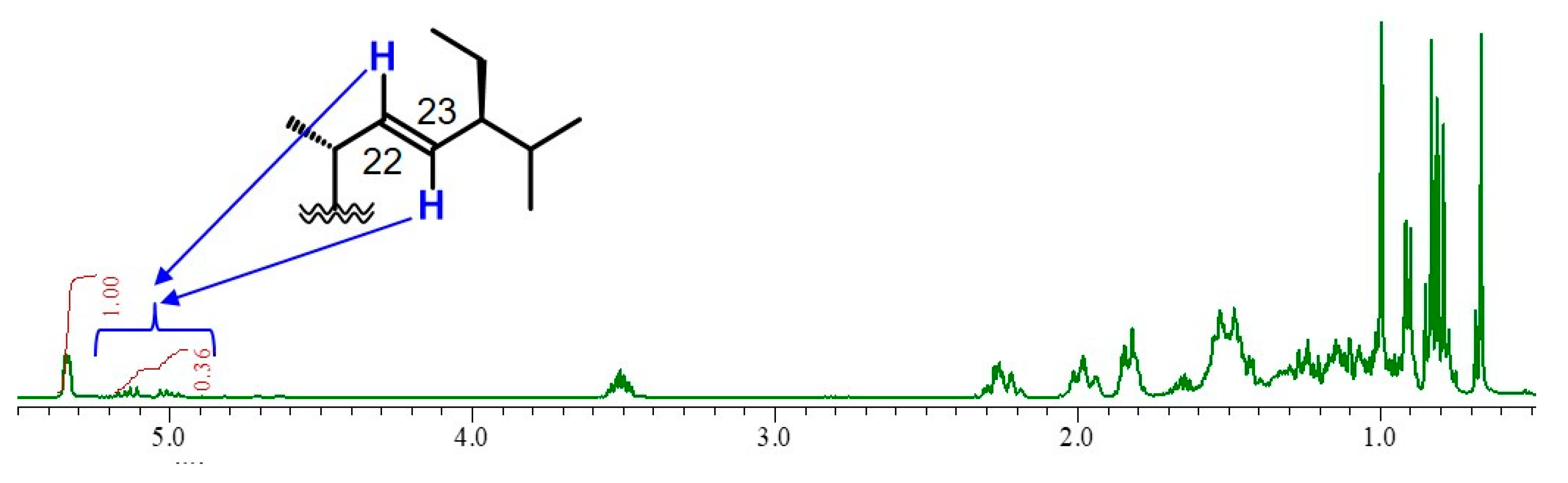
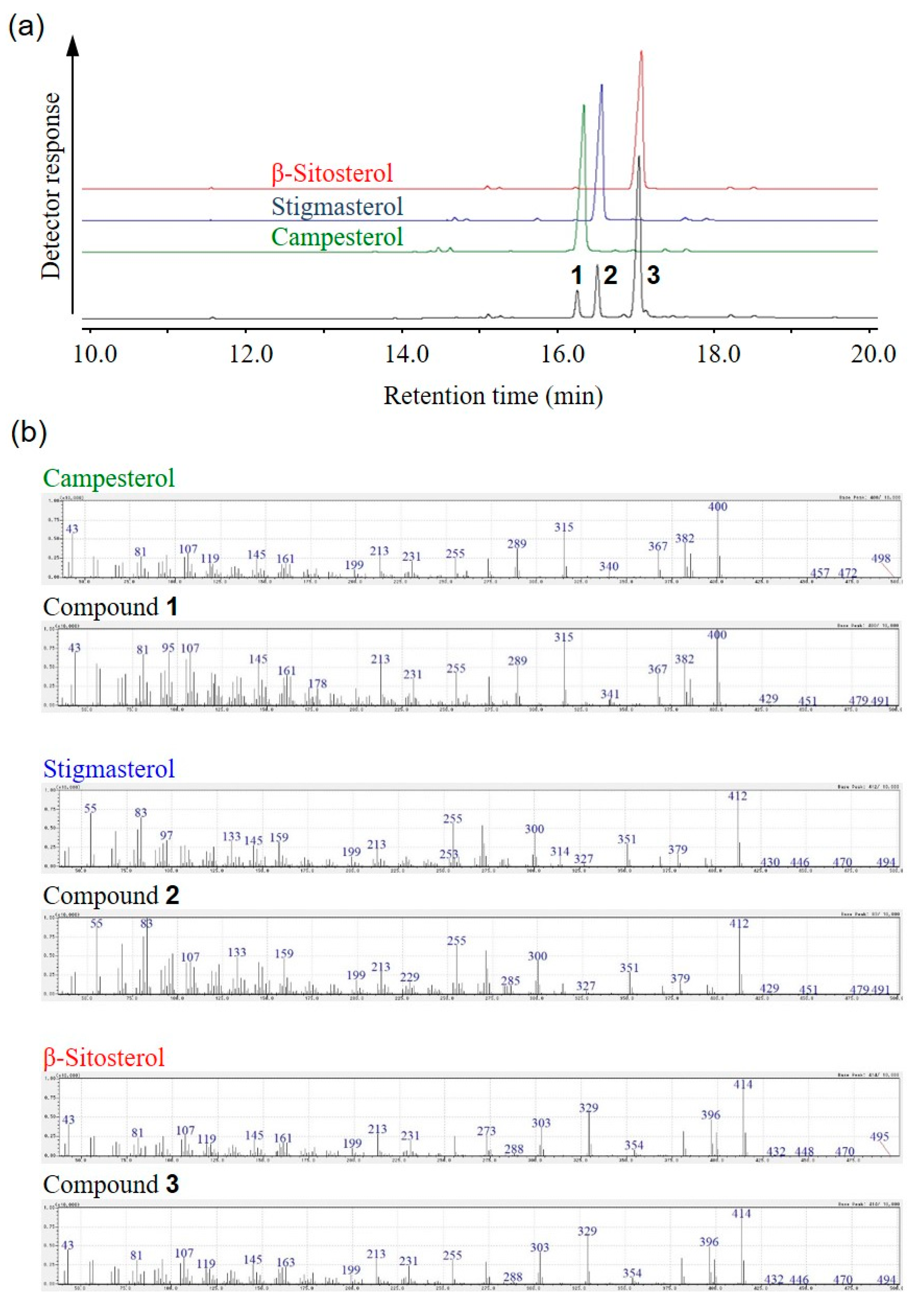
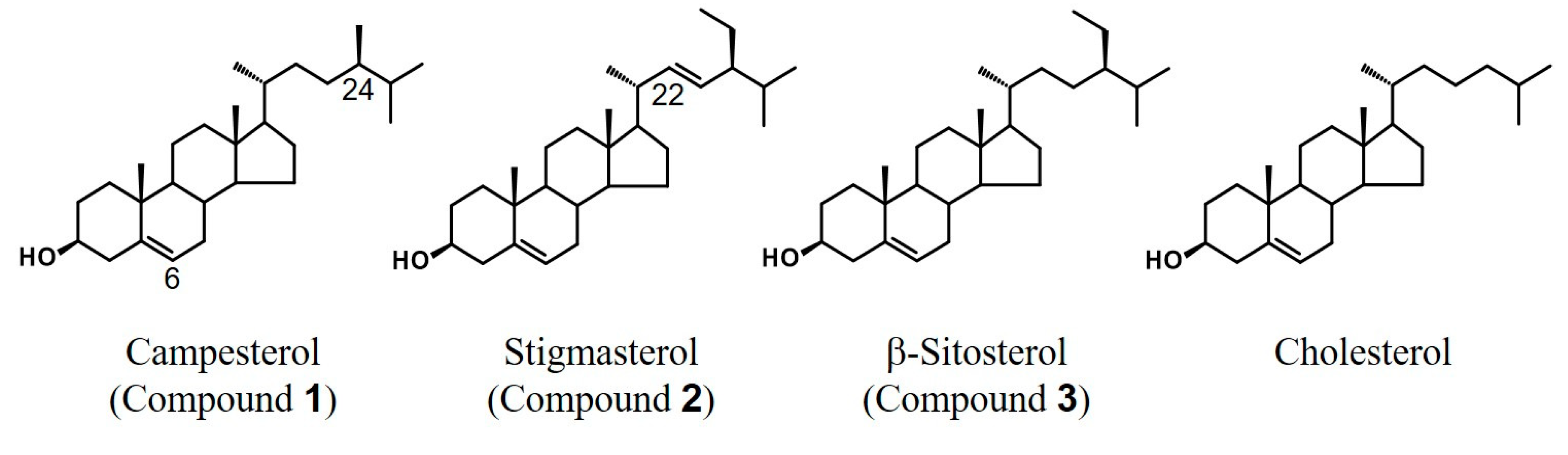
2.1. Isolation of β-Sitosterol from Sunflower Seeds
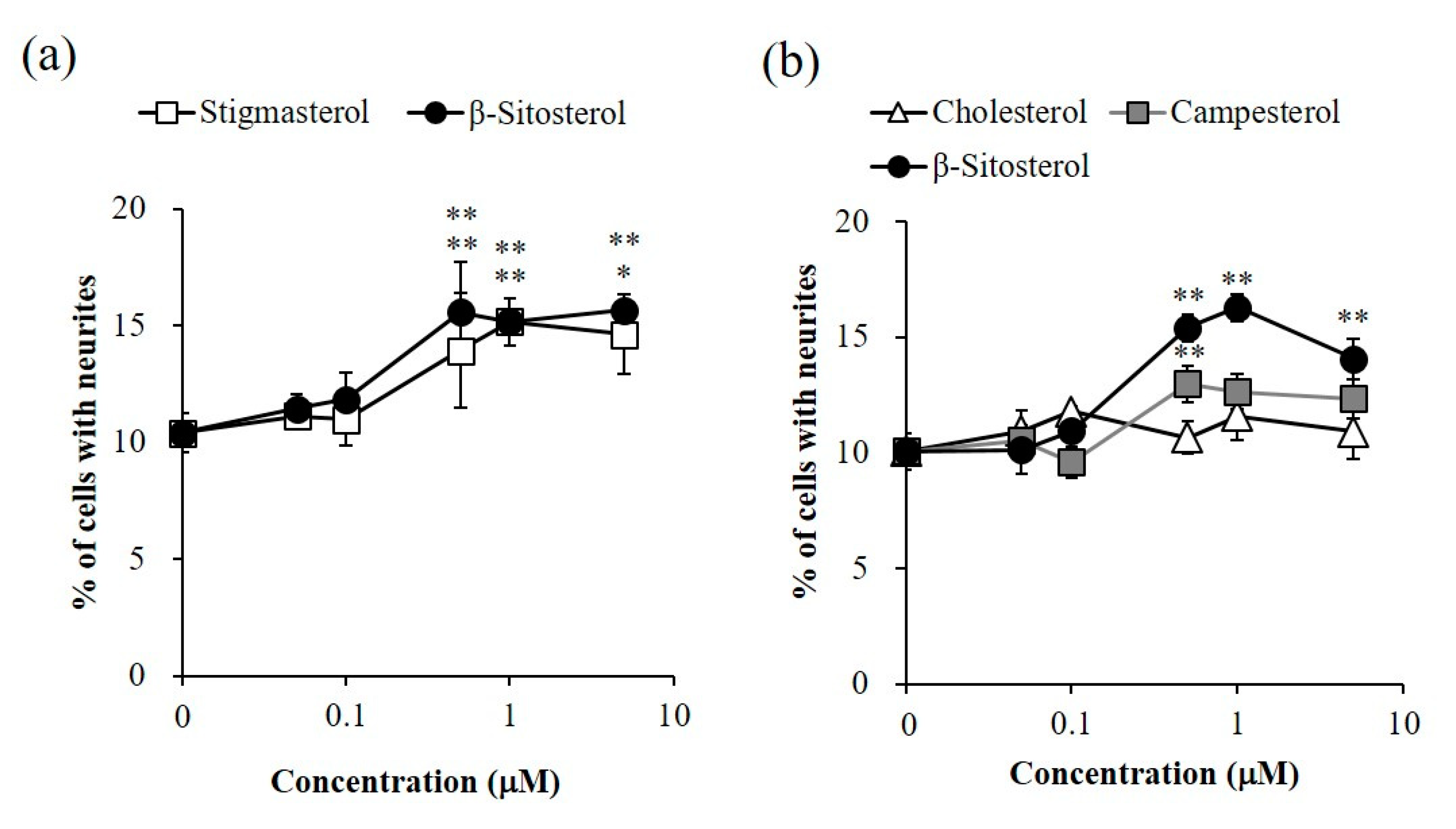
2.2. Promoting Activity of Sterols in Neurite Formation Induced by Bt2cAMP in PC12 Cells
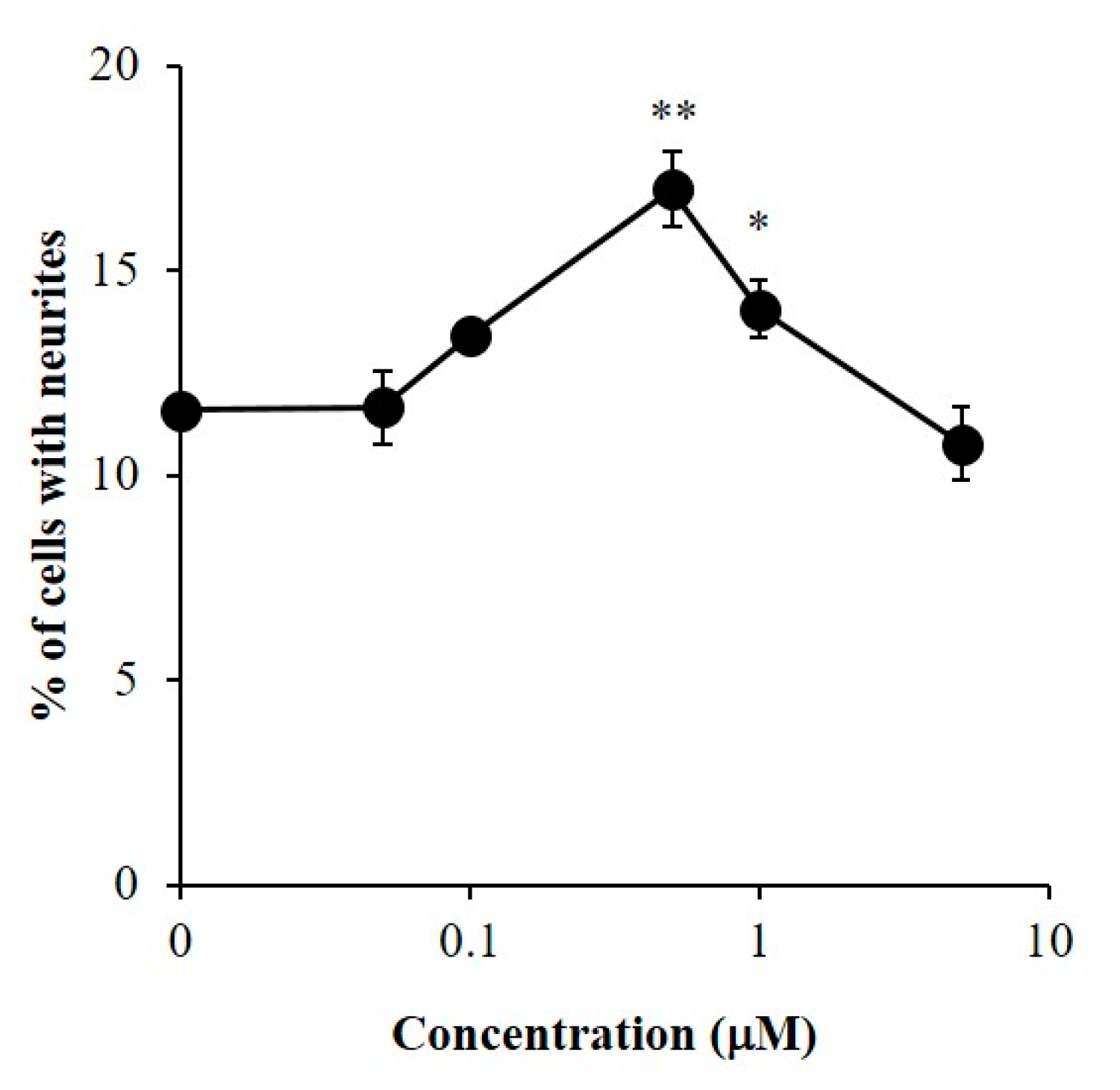
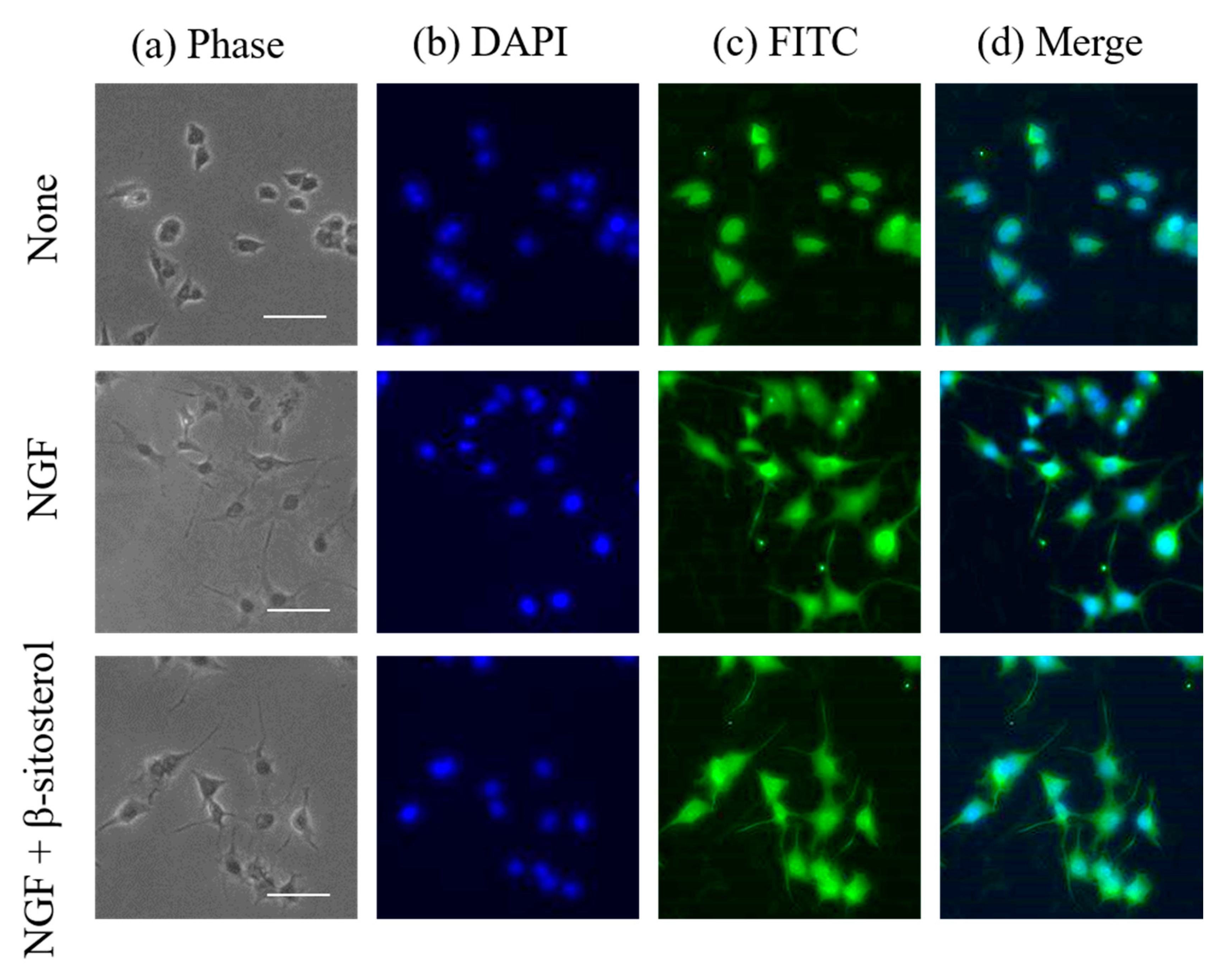
2.3. Promoting Activity of β-Sitosterol on Neurite Formation Induced by NGF in PC12 Cells
3. Materials and Methods
3.1. Materials
3.2. Isolation of β-Sitosterol from Sunflower Seed Extract
3.3. Neurite Outgrowth-Promoting Activity
3.4. Immunocytochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wortmann, M. Dementia: A global health priority—Highlights from an ADI and World Health Organization report. Alzheimer’s Res. Ther. 2012, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Vasic, V.; Barth, K.; Schmidt, M.H.H. Neurodegeneration and neuro-regeneration-Alzheimer’s disease and stem cell therapy. Int. J. Mol. Sci. 2019, 20, 4272. [Google Scholar] [CrossRef] [PubMed]
- Douchamps, V.; Mathis, C. A second wind for the cholinergic system in Alzheimer’s therapy. Behav. Pharmacol. 2017, 28, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Behbahani, H.; Eriksdotter, M. Innovative therapy for Alzheimer’s disease-with focus on biodelivery of NGF. Flont. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Lärkfors, L.; Ebendal, T.; Whittemore, S.R.; Persson, H.; Hoffer, B.; Olson, L. Decreased level of nerve growth factor (NGF) and its messenger RNA in the aged rat brain. Brain Res. 1987, 427, 55–60. [Google Scholar] [CrossRef]
- Fischer, W.; Wictorin, K.; Björklund, A.; Williams, L.R.; Varon, S.; Gage, F.H. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 1987, 329, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef]
- Connor, B.; Dragunow, M. The role of neuronal growth factors in neurodegenerative disorders of the human brain. Brain Res. Rev. 1998, 27, 1–39. [Google Scholar] [CrossRef]
- Cheng, L.; Ye, Y.; Xiang, L.; Osada, H.; Qi, J. Lindersin B from Lindernia crustacea induces neuritogenesis by activation of tyrosine kinase A/phosphatidylinositol 3 kinase/extracellular signal-regulated kinase signaling pathway. Phytomedicine 2017, 24, 31–38. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Maggi, F.; Papa, F.; Kaya, E.; Dikmen, M.; Öztürk, Y. Isofuranodiene: A neuritogenic compound isolated from wild celery (Smyrnium olusatrum L., Apiaceae). Food Chem. 2016, 192, 782–787. [Google Scholar] [CrossRef]
- Foot, M.; Cruz, T.F.; Clandinin, M.T. Influence of dietary fat on the lipid composition of rat brain synaptosomal and microsomal membranes. Biochem. J. 1982, 208, 631–640. [Google Scholar] [CrossRef]
- Wurtman, R.J. Synapse formation and cognitive brain development: Effect of docosahexaenoic acid and other dietary constituents. Metabolism 2008, 57, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.Y.; Moriyama, T.; Uezu, E.; Uezu, K.; Hirata, R.; Yohena, N.; Masuda, Y.; Kokubu, T.; Yamamoto, S. Administration of phosphatidylcholine increases brain acetylcholine concentration and improves memory in mice with dementia. J. Nutr. 1995, 125, 1484–1489. [Google Scholar] [PubMed]
- Carter, J.M.; Waite, K.A.; Campenot, R.B.; Vance, J.E.; Vance, D.E. Enhanced expression and activation of CTP: Phosphocholine cytidylyltransferase β2 during neurite outgrowth. J. Biol. Chem. 2003, 278, 44988–44994. [Google Scholar] [CrossRef]
- Araki, W.; Wurtman, R.J. Control of membrane phosphatidylcholine biosynthesis by diacylglycerol levels in neuronal cells undergoing neurite outgrowth. Proc. Natl. Acad. Sci. USA 1997, 94, 11946–11950. [Google Scholar] [CrossRef]
- Yen, C.L.; Mar, M.H.; Meeker, R.B.; Fernandes, A.; Zeisel, S.H. Choline deficiency induces apoptosis in primary cultures of fetal neurons. FASEB J. 2001, 15, 1704–1710. [Google Scholar] [CrossRef]
- de Chaves, E.D.; Vance, D.E.; Campenot, R.B.; Vance, J.E. Axonal synthesis of phosphatidylcholine is required for normal axonal growth in rat sympathetic neurons. J. Cell Biol. 1995, 128, 913–918. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, L.; Elena, C.; Domizi, P.; Banchio, C. Role of phosphatidylcholine during neuronal differentiation. IUBMB Life 2011, 63, 714–720. [Google Scholar] [CrossRef]
- Duan, L.; Hope, J.M.; Guo, S.; Ong, Q.; François, A.; Kaplan, L.; Scherrer, G.; Cui, B. Optical activation of trkA signaling. ACS Synth. Biol. 2018, 7, 1685–1693. [Google Scholar] [CrossRef]
- Vaudry, D.; Stork, P.J.; Lazarovici, P.; Eiden, L.E. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002, 296, 1648–1649. [Google Scholar] [CrossRef]
- Schulze, I.; Perez-Polo, J.R. Nerve growth factor and cyclic AMP: Opposite effects on neuroblastoma-substrate adhesion. J. Neurosci. Res. 1982, 8, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Gunning, P.W.; Landreth, G.E.; Bothwell, M.A.; Shooter, E.M. Differential and synergistic actions of nerve growth factor and cyclic AMP in PC12 cells. J. Cell Biol. 1981, 89, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S.E.; Shokoohinia, Y.; Mehramiri, P. Isolation and characterization of steroids, phthalide and essential oil of the fruits of Kelussia odoratissima Mozaff., an endemic mountain celery. Res. Pharm. Sci. 2013, 8, 35–41. [Google Scholar]
- Chaturvedula, V.S.; Prakash, I. Isolation of stigmasterol and β-sitosterol from the dichloromethane extract of Rubus suavissimus. Int. Curr. Pharm. J. 2012, 1, 239–242. [Google Scholar] [CrossRef]
- Luhata, L.P.; Munkombwe, N.M. Isolation and characterization of stigmasterol and β-sitosterol from Odontonema Strictum (Acanthaceae). J. Innov. Pharm. Biol. Sci. 2015, 2, 88–95. [Google Scholar]
- Jabeur, H.; Zribi, A.; Makni, J.; Rebai, A.; Abdelhedi, R.; Bouaziz, M. Detection of chemlali extra-virgin olive oil adulteration mixed with soybean oil, corn oil, and sunflower oil by using GC and HPLC. J. Agric. Food Chem. 2014, 62, 4893–4904. [Google Scholar] [CrossRef]
- Valitova, J.N.; Sulkarnayeva, A.G.; Minibayeva, F.V. Plant sterols: Diversity, biosynthesis, and physiological functions. Biochemistry (Moscow) 2016, 81, 819–834. [Google Scholar] [CrossRef]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Bioactive compounds in banana and their associated health benefits—A review. Food Chem. 2016, 206, 1–11. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A. Edible seed from Cucurbitaceae family as potential functional foods: Immense promises, few concerns. Biomed. Pharmacother. 2017, 91, 330–337. [Google Scholar] [CrossRef]
- Lee, Y.M.; Haastert, B.; Scherbaum, W.; Hauner, H. A phytosterol-enriched spread improves the lipid profile of subjects with type 2 diabetes mellitus. A randomized controlled trial under free-living conditions. Eur. J. Nutr. 2003, 42, 111–117. [Google Scholar] [CrossRef]
- Tatematsu, K.; Fuma, S.Y.; Nagase, T.; Ichikawa, Y.; Fujii, Y.; Okuyama, H. Factors other than phytosterols in some vegetable oils affect the survival of SHRSP rats. Food Chem. Toxicol. 2004, 42, 1443–1451. [Google Scholar] [CrossRef] [PubMed]
- Al-Okbi, S.Y.; Mohamed, D.A.; Hamed, T.E.; Esmail, R.S.; Donya, S.M. Prevention of renal dysfunction by nutraceuticals prepared from oil rich plant foods. Asian Pac. J. Trop. Biomed. 2014, 4, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.A.S.; Baggio, S.R.; Mariutti, L.R.B.; Bragagnolo, N. One-step rapid extraction of phytosterols from vegetable oils. Food Res. Int. 2020, 130, 108891. [Google Scholar] [CrossRef] [PubMed]
- Bin Sayeed, M.S.; Ameen, S.S. Beta-sitosterol: A promising but orphan nutraceutical to fight against cancer. Nutr. Cancer 2015, 67, 1214–1220. [Google Scholar] [CrossRef] [PubMed]
- Muti, P.; Awad, A.B.; Schünemann, H.; Fink, C.S.; Hovey, K.; Freudenheim, J.L.; Wu, Y.W.; Bellati, C.; Pala, V.; Berrino, F. A plant food-based diet modifies the serum β-sitosterol concentration in hyperandrogenic postmenopausal women. J. Nutr. 2003, 133, 4252–4255. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Awad, A.B.; Roy, R.; Fink, C.S. β-Sitosterol, a plant sterol, induces apoptosis and activates key caspases in MDA-MB-231 human breast cancer cells. Oncol. Rep. 2003, 10, 497–500. [Google Scholar] [CrossRef]
- Ayaz, M.; Junaid, M.; Ullah, F.; Subhan, F.; Sadiq, A.; Ali, G.; Ovais, M.; Shahid, M.; Ahmad, A.; Wadood, A.; et al. Anti-Alzheimer’s studies on β-sitosterol isolated from Polygonum hydropiper L. Front. Pharmacol. 2017, 8, 697. [Google Scholar] [CrossRef]
- Shi, C.; Liu, J.; Wu, F.; Zhu, X.; Yew, D.T.; Xu, J. β-Sitosterol inhibits high cholesterol-induced platelet β-amyloid release. J. Bioenerg. Biomembr. 2011, 43, 691–697. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Weingärtner, O.; van der Pol, S.; Husche, C.; Kerksiek, A.; Friedrichs, S.; Sijbrands, E.; Steinbusch, H.; Grimm, M.; Hartmann, T.; et al. Dietary intake of plant sterols stably increases plant sterol levels in the murine brain. J. Lipid Res. 2012, 53, 726–735. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koga, T.; Sakamoto, T.; Sakuradani, E.; Tai, A. Neurite Outgrowth-Promoting Activity of Compounds in PC12 Cells from Sunflower Seeds. Molecules 2020, 25, 4748. https://doi.org/10.3390/molecules25204748
Koga T, Sakamoto T, Sakuradani E, Tai A. Neurite Outgrowth-Promoting Activity of Compounds in PC12 Cells from Sunflower Seeds. Molecules. 2020; 25(20):4748. https://doi.org/10.3390/molecules25204748
Chicago/Turabian StyleKoga, Takeru, Takaiku Sakamoto, Eiji Sakuradani, and Akihiro Tai. 2020. "Neurite Outgrowth-Promoting Activity of Compounds in PC12 Cells from Sunflower Seeds" Molecules 25, no. 20: 4748. https://doi.org/10.3390/molecules25204748
APA StyleKoga, T., Sakamoto, T., Sakuradani, E., & Tai, A. (2020). Neurite Outgrowth-Promoting Activity of Compounds in PC12 Cells from Sunflower Seeds. Molecules, 25(20), 4748. https://doi.org/10.3390/molecules25204748




