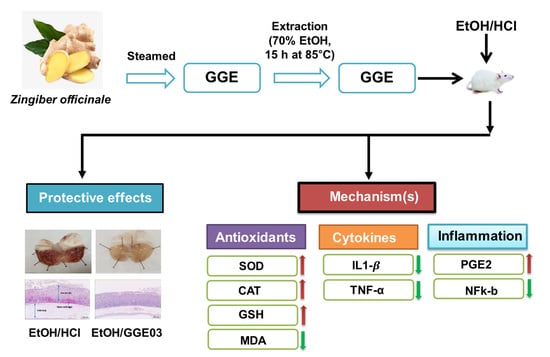
Antiulcer Activity of Steamed Ginger Extract against Ethanol/HCl-Induced Gastric Mucosal Injury in Rats
Abstract
:1. Introduction
2. Results
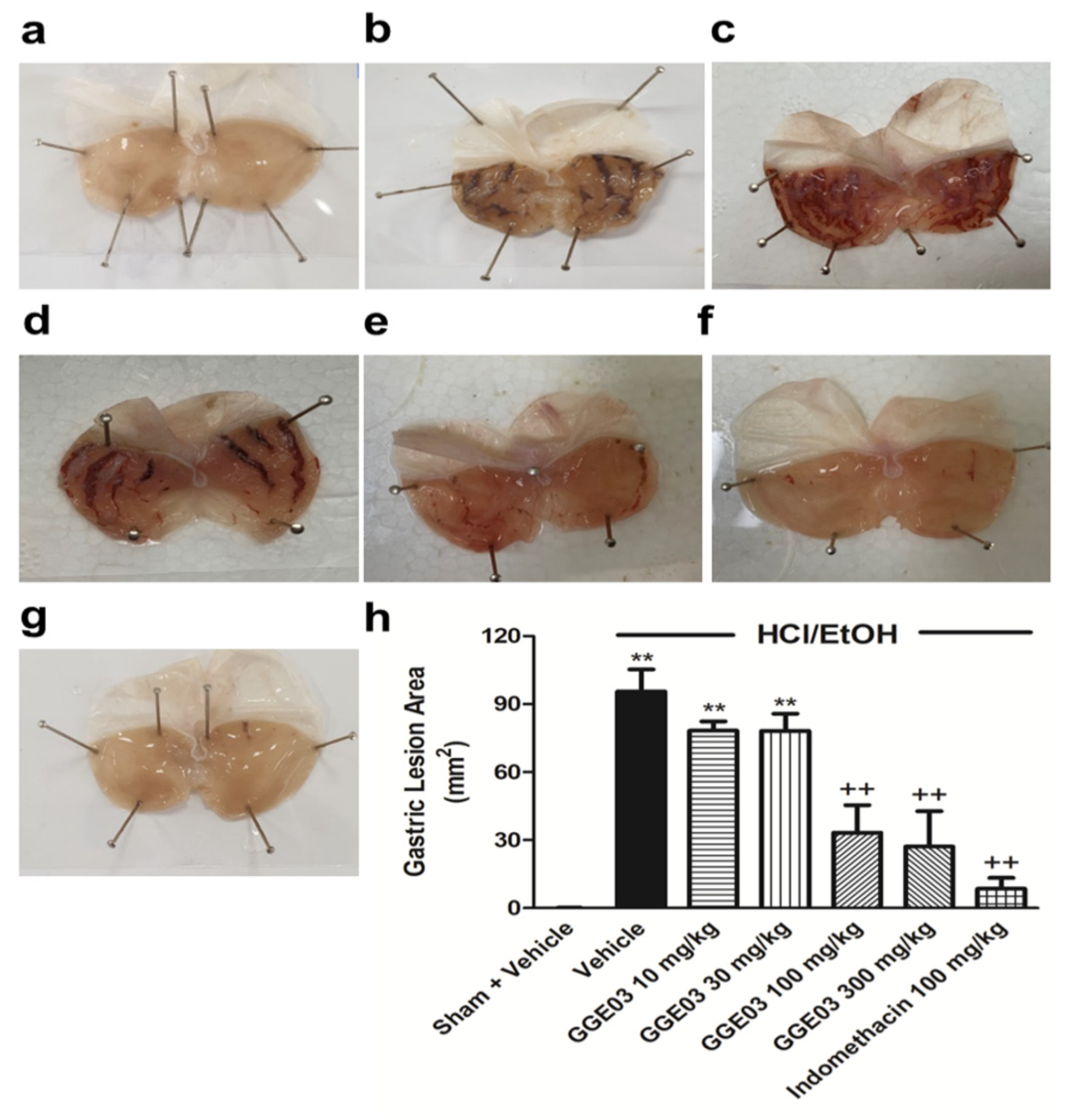
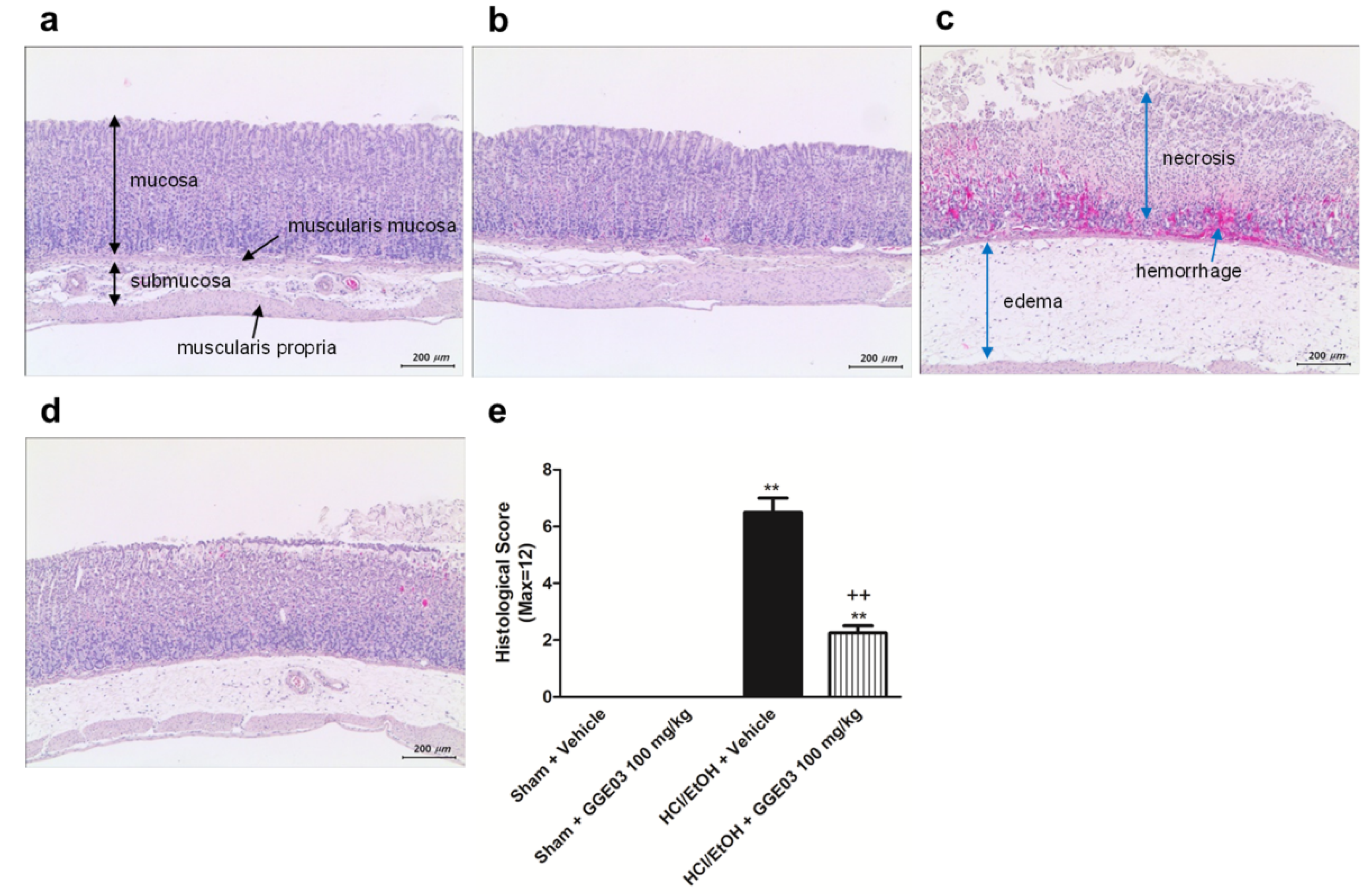
2.1. Macroscopic and Histological Effect of GGE03 on EtOH/HCl-Induced Gastric Mucosal Injury
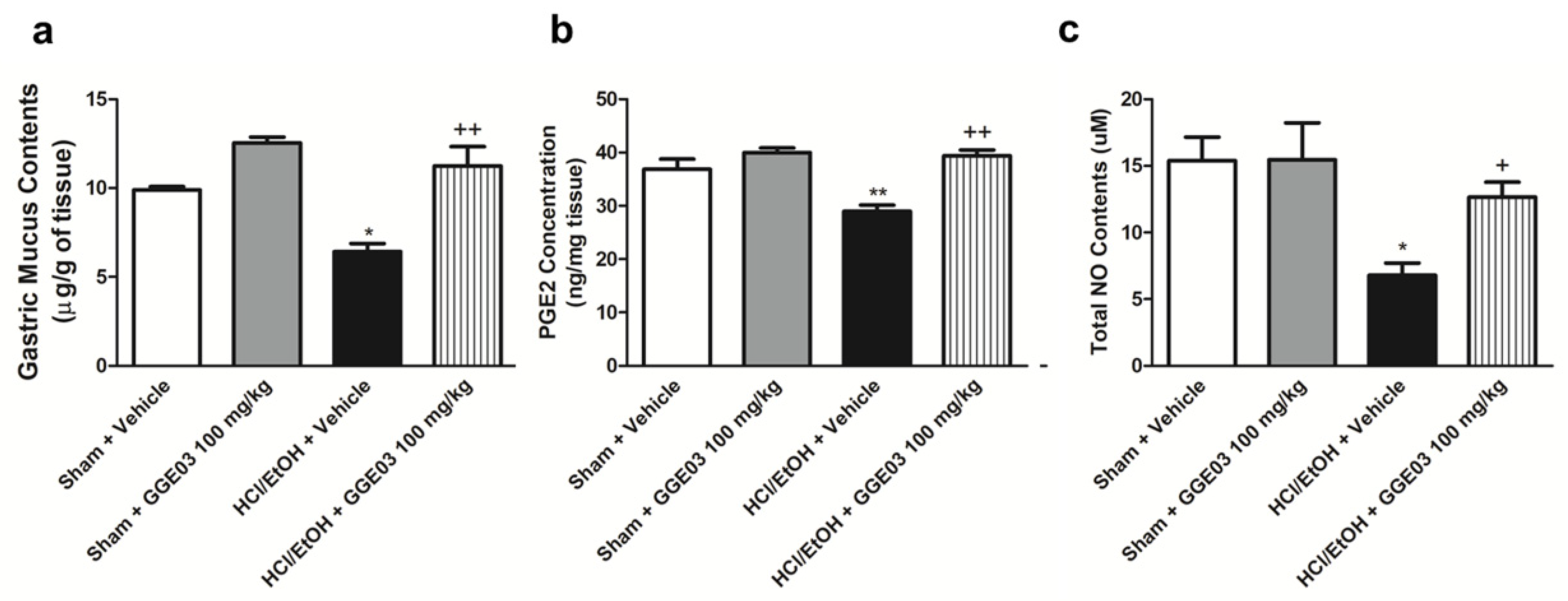
2.2. Effect of GGE03 on Mucosal Defensive Factors in EtOH/HCl-Treated Rats
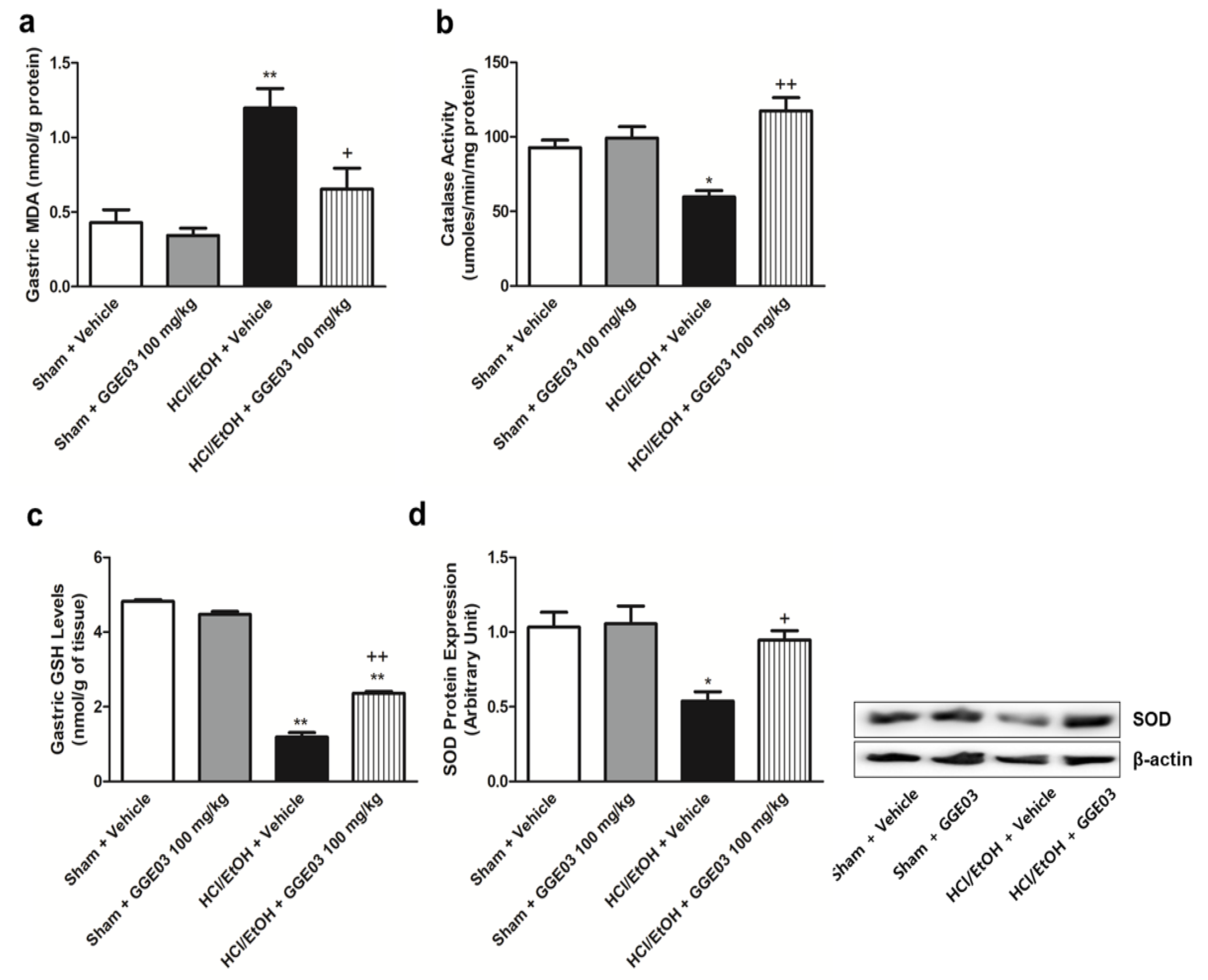
2.3. Effect of GGE03 on Antioxidants Activities in EtOH/HCl-Treated Rats
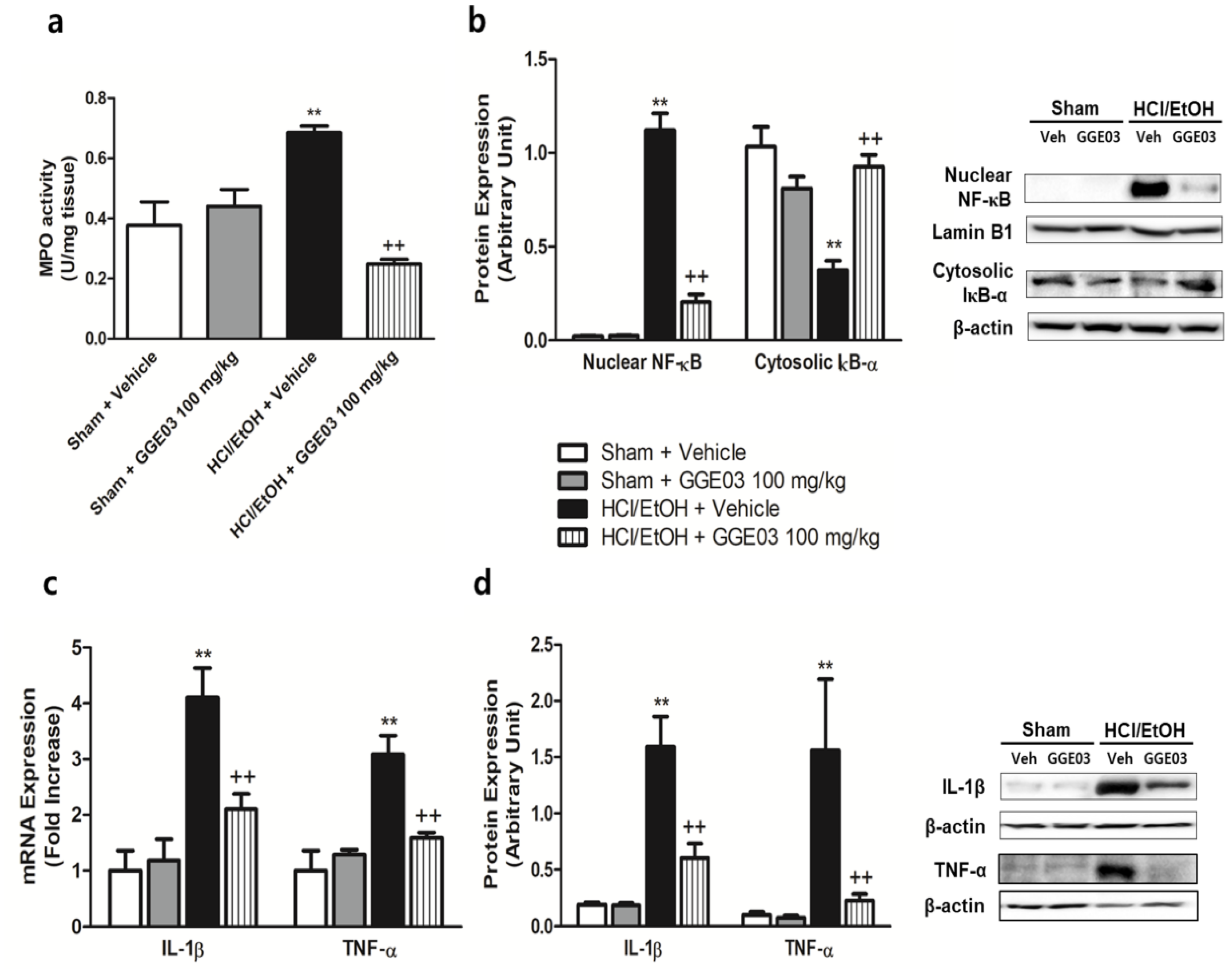
2.4. Effect of GGE03 on Anti-Inflammatory Properties in EtOH/HCl-Treated Rats
3. Discussion
4. Materials and Methods
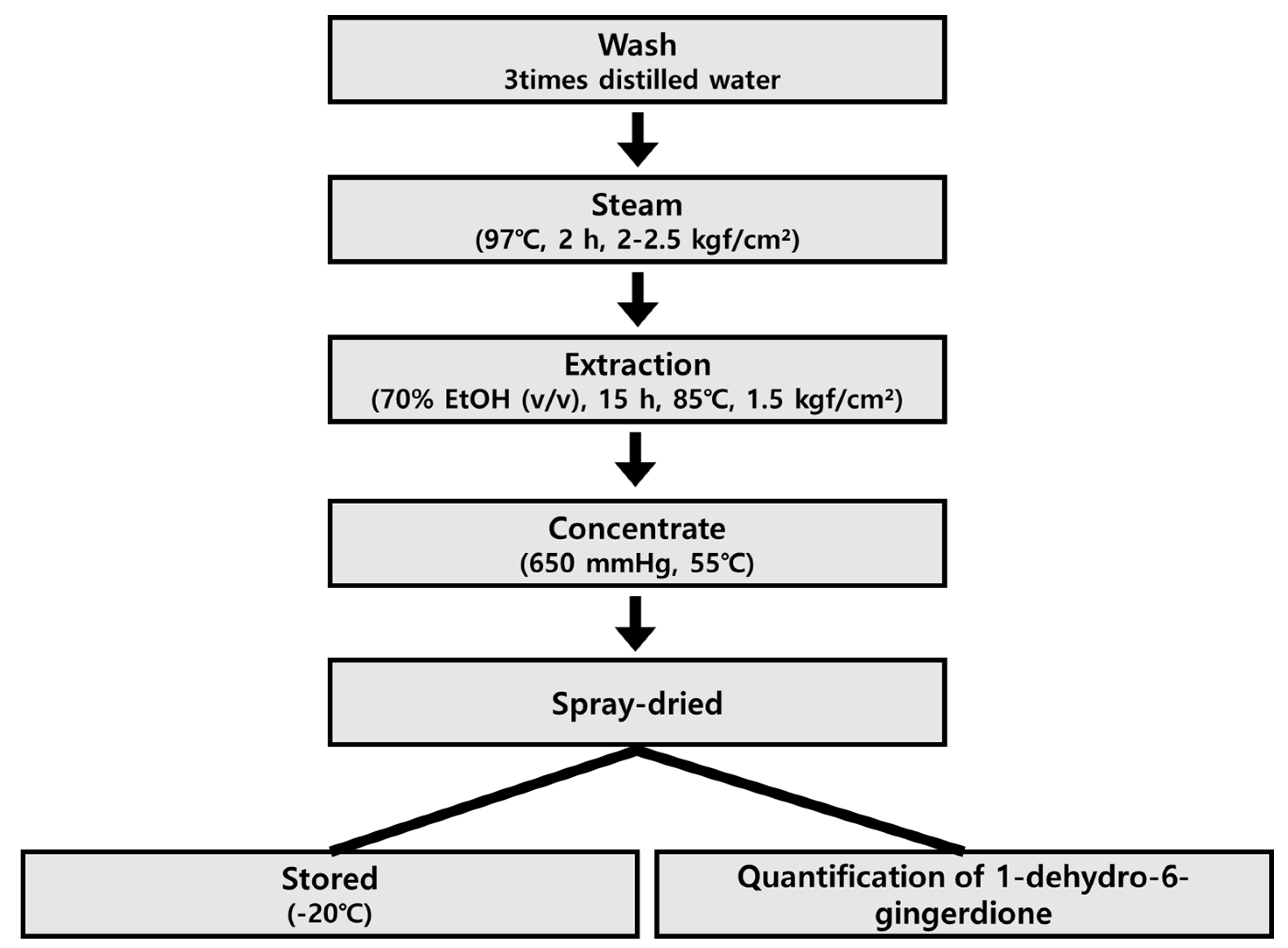
4.1. Preparation of Steamed Ginger Extracts (Golden Ginger, GG)
4.2. Quantitative Analysis of the Major Component of GGE
4.3. Animal Treatment
4.4. Macroscopic Assessment of Gastric Ulcers
4.5. Histological Analysis
4.6. Gastric Wall Mucus Contents
4.7. Measurement of Gastric Mucosal NO and Enzyme-Linked Immunosorbent Assay for PGE2
4.8. Catalase Activity
4.9. Lipid Peroxidation and Glutathione Contents
4.10. Measurement of Gastric MPO Activity
4.11. Whole, Cytoplasmic, and Nuclear Protein Extraction of Stomach Tissue
4.12. Protein Extraction and Immunoblots
4.13. Determination of mRNA Extraction in Gastric Tissue
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| ROS | Reactive oxygen species |
| SOD | Superoxide dismutase |
| GPx | Glutathione peroxidase |
| CAT | Catalase |
| TNF-α | Tumor necrosis factor-α |
| IL-1β | Interleukin-1β |
| PGs | Prostaglandins |
| NO | Nitric oxide |
| NF-κB | nuclear factor κ-activated B |
| EtOH | Ethanol |
| GGE | Golden ginger |
| MDA | malondialdehyde |
| TBA | thiobarbituric acid |
| MPO | Myeloperoxidase |
| TBS/T | Tris-buffered saline |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| COX | cyclooxygenase |
| NOS | nitric oxide synthase |
| GSH | glutathione |
References
- Kenneth, T.; Søreide, J.A.; Kvaløy, J.T.; Glomsaker, T.; Søreide, K. Epidemiology of perforated peptic ulcer: Age- and gender-adjusted analysis of incidence and mortality. World J. Gastroenterol. 2013, 19, 347–354. [Google Scholar]
- Chauhan, A.K.; Kang, S.C. Therapeutic potential and mechanism of thymol action against ethanol-induced gastric mucosal injury in rat model. Alcohol 2015, 49, 739–745. [Google Scholar] [CrossRef] [PubMed]
- Kurata, J.H.; Nogawa, A.N. Meta-analysis of risk factors for peptic ulcer. Nonsteroidal antiinflammatory drugs, Helicobacter pylori, and smoking. J. Clin. Gastroenterol. 1997, 24, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Yeomans, N.D.; Hawkey, C.J.; Brailsford, W.; Naesdal, J. Gastroduodenal toxicity of low-dose acetylsalicylic acid: A comparison with non-steroidal anti-inflammatory drugs. Curr. Med. Res. Opin. 2009, 25, 2785–2793. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J. Gastroenterol. 2012, 18, 2147–2160. [Google Scholar] [CrossRef]
- Bode, C.; Bode, J.C. Alcohol’s role in gastrointestinal tract disorders. Alcohol Health Res. World 1997, 21, 76–83. [Google Scholar]
- Kountouras, J.; Chatzopoulos, D.; Zavos, C. Reactive oxygen metabolites and upper gastrointestinal diseases. Hepatogastroenterology 2001, 48, 743–751. [Google Scholar]
- Mandrekar, P.; Szabo, G. Signalling pathways in alcohol-induced liver inflammation. J. Hepatol. 2009, 50, 1258–1266. [Google Scholar] [CrossRef] [Green Version]
- Al Batran, R.; Al-Bayaty, F.; Al-Obaidi, M.M.J.; Abdualkader, A.M.; Hadi, H.A.; Ali, H.M.; Abdulla, M.A. In vivo antioxidant and anti-ulcer activity of Parkia speciosa ethanolic leaf extract against ethanol-induced gastric ulcer in rats. PLoS ONE 2013, 8, e64751. [Google Scholar] [CrossRef]
- Wasman, S.Q.; Mahmood, A.A.; Salehhuddin, H.; Zahra, A.A.; Salmah, I. Cytoprotective activities of Polygonum minus aqueous leaf extract on ethanol-induced gastric ulcer in rats. J. Med. Plants Res. 2010, 4, 2658–2665. [Google Scholar]
- Hall, W.H. Letter: Breast changes in males on cimetidine. N. Engl. J. Med. 1976, 295, 841. [Google Scholar] [PubMed]
- Lam, J.R.; Schneider, J.L.; Zhao, W.; Corley, D.A. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA 2013, 310, 2435–2442. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Ashraf, A. Hypercalcemic crisis induced by calcium carbonate. Clin. Kidney J. 2012, 5, 288–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozza, A.L.; Hiruma-Lima, C.A.; Tanimoto, A.; Pellizzon, C.H. Morphologic and pharmacological investigations in the epicatechin gastroprotective effect. Evid. Based Complement. Alternat. Med. 2012, 2012, 708156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terry, R.; Posadzki, P.; Watson, L.K.; Ernst, E. The use of ginger (Zingiber officinale) for the treatment of pain: A systematic review of clinical trials. Pain Med. 2011, 12, 1808–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathai, K.; Anand, S.; Aravind, A.; Dinatius, P.; Krishnan, A.V.; Mathai, M. Antimicrobial effect of ginger, garlic, honey, and lemon extracts on Streptococcus mutans. J. Contemp. Dent. Pract. 2017, 18, 1004–1008. [Google Scholar] [PubMed]
- Si, W.; Chen, Y.P.; Zhang, J.; Chen, Z.-Y.; Chung, H.Y. Antioxidant activities of ginger extract and its constituents toward lipids. Food Chem. 2018, 239, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Cakir, U.; Tayman, C.; Serkant, U.; Yakut, H.I.; Cakir, E.; Ates, U.; Koyuncu, I.; Karaogul, E. Ginger (Zingiber officinale Roscoe) for the treatment and prevention of necrotizing enterocolitis. J. Ethnopharmacol. 2018, 225, 297–308. [Google Scholar] [CrossRef]
- Vipin, A.V.; Rao, R.; Kurrey, N.K.; KA, A.A.; Venkateswaran, G. Protective effects of phenolics rich extract of ginger against Aflatoxin B1-induced oxidative stress and hepatotoxicity. Biomed. Pharmacother. 2017, 91, 415–424. [Google Scholar]
- Honarvar, N.M.; Zarezadeh, M.; Khorshidi, M.; Arzati, M.M.; Yekaninejad, M.S.; Abdollahi, M.; Effatpanah, M.; Hashemi, R.; Saedisomeolia, A. The effect of an oral ginger supplementation on NF-κB concentration in peripheral blood mononuclear cells and anthropomorphic data of patients with type 2 diabetes: A randomized double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2019, 42, 7–11. [Google Scholar] [CrossRef]
- Karampour, N.S.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaghlool, S.S.; Shehata, B.A.; Abo-Seif, A.A.; El-Latif, H.A.A. Protective effects of ginger and marshmallow extracts on indomethacin-induced peptic ulcer in rats. J. Nat. Sci. Biol. Med. 2015, 6, 421–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalil, M.S. The postulated mechanism of the protective effect of ginger on the aspirin induced gastric ulcer: Histological and immunohistochemical studies. Histol. Histopathol. 2015, 30, 855–864. [Google Scholar]
- Chan, E.C.Y.; Yap, S.-L.; Lau, A.-J.; Leow, P.-C.; Toh, D.-F.; Koh, H.-L. Ultra-performance liquid chromatography/time-of-flight mass spectrometry based metabolomics of raw and steamed Panax notoginseng. Rapid Commun. Mass Spectrom. 2007, 21, 519–528. [Google Scholar] [CrossRef]
- Qi, L.-W.; Wang, C.-Z.; Yuan, C.-S. American ginseng: Potential structure-function relationship in cancer chemoprevention. Biochem. Pharmacol. 2010, 80, 947–954. [Google Scholar] [CrossRef]
- Wang, C.-Z.; Zhang, B.; Song, W.-X.; Wang, A.; Ni, M.; Luo, X.; Aung, H.H.; Xie, J.-T.; Tong, R.; He, T.-C.; et al. Steamed American ginseng berry: Ginsenoside analyses and anticancer activities. J. Agric. Food Chem. 2006, 54, 9936–9942. [Google Scholar] [CrossRef]
- Nam, Y.H.; Hong, B.N.; Rodriguez, I.; Park, M.S.; Jeong, S.Y.; Lee, Y.-G.; Shim, J.H.; Yasmin, T.; Kim, N.W.; Koo, Y.T.; et al. Steamed ginger may enhance insulin secretion through KATP channel closure in pancreatic β-cells potentially by increasing 1-dehydro-6-gingerdione content. Nutrients 2020, 12, 324. [Google Scholar] [CrossRef] [Green Version]
- Whittle, B.J.; Lopez-Belmonte, J. Actions and interactions of endothelins, prostacyclin and nitric oxide in the gastric mucosa. J. Physiol. Pharmacol. 1993, 44, 91–107. [Google Scholar]
- Ghosh, S.; Acharyya, M.; Majumder, T.; Bagchi, A. Metabolic signatures of oxidative stress and their relationship with erythrocyte membrane surface roughness among workers of manual materials handling (MMH). N. Am. J. Med. Sci. 2015, 7, 558–566. [Google Scholar] [CrossRef] [Green Version]
- Njålsson, R.; Norgren, S. Physiological and pathological aspects of GSH metabolism. Acta Paediatr. 2005, 94, 132–137. [Google Scholar] [CrossRef]
- Suo, H.; Zhao, X.; Qian, Y.; Sun, P.; Zhu, K.; Li, J.; Sun, B. Lactobacillus fermentum Suo attenuates HCl/ethanol induced gastric injury in mice through its antioxidant effects. Nutrients 2016, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Xu, H.-D.; Zhao, B.-T.; Chang, H.-I.; Rhee, H.-I. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol 2008, 42, 683–687. [Google Scholar] [CrossRef] [PubMed]
- AlRashdi, A.S.; Salama, S.M.; Alkiyumi, S.S.; Abdulla, M.A.; Hadi, A.H.A.; Abdelwahab, S.I.; Taha, M.M.; Hussiani, J.; Asykin, N. Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/ethanol-induced gastric mucosal injury in rats. Evid. Based Complement. Alternat. Med. 2012, 2012, 786426. [Google Scholar] [CrossRef] [Green Version]
- Júnior, F.E.B.; de Oliveira, D.R.; Boligon, A.A.; Athayde, M.L.; Kamdem, J.P.; Macedo, G.E.; da Silva, G.F.; de Menezes, I.R.A.; Costa, J.G.M.; Coutinho, H.D.M.; et al. Protective effects of Croton campestris A. St-Hill in different ulcer models in rodents: Evidence for the involvement of nitric oxide and prostaglandins. J. Ethnopharmacol. 2014, 153, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordin, N.; Salama, S.M.; Golbabapour, S.; Hajrezaie, M.; Hassandarvish, P.; Kamalidehghan, B.; Majid, N.A.; Hashim, N.M.; Omar, H.; Fadaienasab, M.; et al. Anti-ulcerogenic effect of methanolic extracts from Enicosanthellum pulchrum (King) Heusden against ethanol-induced acute gastric lesion in animal models. PLoS ONE 2014, 9, e111925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Maraghy, S.A.; Rizk, S.M.; Shahin, N.N. Gastroprotective effect of crocin in ethanol-induced gastric injury in rats. Chem. Biol. Interact. 2015, 229, 26–35. [Google Scholar] [CrossRef]
- Wallace, J.L. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself? Physiol. Rev. 2008, 88, 1547–1565. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, A.; Roy, S.; Bera, B.; Bandyopadhyay, S.K. L-Theanine healed NSAID-induced gastric ulcer by modulating pro/antioxidant balance in gastric ulcer margin. J. Nat. Med. 2014, 68, 699–708. [Google Scholar] [CrossRef]
- Li, H.-L.; Sun, B.-Z.; Ma, F.-C. Expression of COX-2, iNOS, p53 and Ki-67 in gastric mucosa-associated lymphoid tissue lymphoma. World J. Gastroenterol. 2004, 10, 1862–1866. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, D.-M.; Kim, J.Y. Ginger extract suppresses inflammatory response and maintains barrier function in human colonic epithelial Caco-2 cells exposed to inflammatory mediators. J. Food Sci. 2017, 82, 1264–1270. [Google Scholar] [CrossRef]
- Kwiecien, S.S.; Pawlik, M.W.; Brzozowski, T.; Konturek, P.C.; Sliwowski, Z.; Pawlik, W.W.; Konturek, S.J. Nitric oxide (NO)-releasing aspirin and (NO) donors in protection of gastric mucosa against stress. J. Physiol. Pharmacol. 2008, 59, 103–115. [Google Scholar]
- Chatterjee, A.; Chatterjee, S.; Biswas, A.; Bhattacharya, S.; Chattopadhyay, S.; Bandyopadhyay, S.K. Gallic acid enriched fraction of Phyllanthus emblica potentiates indomethacin-induced gastric ulcer healing via e-NOS-dependent pathway. Evid. Based Complement. Alternat. Med. 2012, 2012, 487380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral delivery of nanoparticles loaded with ginger active compound, 6-shogaol, attenuates ulcerative colitis and promotes wound healing in a murine model of ulcerative colitis. J. Crohns Colitis 2018, 12, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Hasegawa, J.; Wang, X.; Matsuda, A.; Tokuda, T.; Miura, N.; Watanabe, T. Protective effects of ginger against aspirin-induced gastric ulcers in rats. Yonago Acta Med. 2011, 54, 11–19. [Google Scholar]
- Yoshikawa, T.; Naito, Y. The role of neutrophils and inflammation in gastric mucosal injury. Free Radic. Res. 2000, 33, 785–794. [Google Scholar] [CrossRef]
- Sampson, A.P. The role of eosinophils and neutrophils in inflammation. Clin. Exp. Allergy 2000, 30, 22–27. [Google Scholar] [CrossRef]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [Green Version]
- Albensi, B.C.; Mattson, M.P. Evidence for the involvement of TNF and NF-κB in hippocampal synaptic plasticity. Synapse 2000, 35, 151–159. [Google Scholar] [CrossRef]
- Gilmore, T.D. Introduction to NF-κB: Players, pathways, perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [Green Version]
- Kunnumakkara, A.B.; Sailo, B.L.; Banik, K.; Harsha, C.; Prasad, S.; Gupta, S.C.; Bharti, A.C.; Aggarwal, B.B. Chronic diseases, inflammation, and spices: How are they linked? J. Transl. Med. 2018, 16, 14. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Park, S.H.; Lee, M.; Kim, H.-J.; Ryu, S.Y.; Kim, N.D.; Hwang, B.Y.; Hong, J.T.; Han, S.-B.; Kim, Y. 1-Dehydro-[10]-gingerdione from ginger inhibits IKKβ activity for NF-κB activation and suppresses NF-κB-regulated expression of inflammatory genes. Br. J. Pharmacol. 2012, 167, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, K.; Ando, T.; Maeda, O.; Ohmiya, N.; Niwa, Y.; Kadomatsu, K.; Goto, H. Ginger ingredients reduce viability of gastric cancer cells via distinct mechanisms. Biochem. Biophys. Res. Commun. 2007, 362, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Taléns-Visconti, R.; Rius-Pérez, S.; Finamor, I.; Sastre, J. Redox signaling in the gastrointestinal tract. Free Radic. Biol. Med. 2017, 104, 75–103. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-S.; Kim, E.-H.; Hong, H.; Ock, C.Y.; Lee, J.S.; Kim, J.-H.; Hahm, K.-B. Attenuation of cysteamine-induced duodenal ulcer with Cochinchina momordica seed extract through inhibiting cytoplasmic phospholipase A2/5-lipoxygenase and activating γ-glutamylcysteine synthetase. J. Gastroenterol. Hepatol. 2012, 27, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Tandon, R.; Khanna, H.D.; Dorababu, M.; Goel, R.K. Oxidative stress and antioxidants status in peptic ulcer and gastric carcinoma. Indian J. Physiol. Pharmacol. 2004, 48, 115–118. [Google Scholar] [PubMed]
- Kang, J.-W.; Yun, N.; Han, H.-J.; Kim, J.-Y.; Kim, J.-Y.; Lee, S.-M. Protective effect of Flos Lonicerae against experimental gastric ulcers in rats: Mechanisms of antioxidant and anti-inflammatory action. Evid. Based Complement. Alternat. Med. 2014, 2014, 596920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzmán-Gómez, O.; García-Rodríguez, R.V.; Quevedo-Corona, L.; Pérez-Pastén-Borja, R.; Rivero-Ramírez, N.L.; Ríos-Castro, E.; Pérez-Gutiérrez, S.; Pérez-Ramos, J.; Chamorro-Cevallos, G.A. Amelioration of Ethanol-Induced Gastric Ulcers in Rats Pretreated with Phycobiliproteins of Arthrospira (Spirulina) Maxima. Nutrients 2018, 10, 763. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Wang, F.; Luo, H.; Liu, A.; Li, K.; Li, C.; Jiang, Y. Protective effect of butyrate against ethanol-induced gastric ulcers in mice by promoting the anti-inflammatory, antioxidant and mucosal defense mechanisms. Int. Immunopharmacol. 2016, 30, 179–187. [Google Scholar] [CrossRef]
- Kitagawa, H.; Takeda, F.; Kohei, H. A simple method for estimation of gastric mucus and effects of antiulcerogenic agents on the decrease in mucus during water-immersion stress in rats. Arzneimittelforschung 1986, 36, 1240–1244. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar]
- Anderson, M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985, 113, 548–555. [Google Scholar] [PubMed]
Sample Availability: Samples used in this study are available from the authors. |
| Pathological State | Score | Definition |
|---|---|---|
| Gastric Mucosa Injury | 0 | Intact |
| 1 | Desquamation of epithelial lamina | |
| 2 | Desquamation of superficial lamina propria or 1/3 reduction of gastric glands | |
| 3 | Desquamation of middle lamina propria or 2/3 reduction of gastric glands | |
| 4 | Desquamation of lower lamina propria or >2/3 reduction of gastric glands, even exposure of submucosa | |
| Leucocytes Infiltration | 0 | Absent |
| 1 | 2–10/HPF | |
| 2 | 11–20/HPF | |
| 3 | 21–30/HPF | |
| 4 | >31/HPF | |
| Gastric Hemorrhage | 0 | Absent |
| 1 | <10% of total area/LPF | |
| 2 | 11%–20% of total area/LPF | |
| 3 | 21%–30% of total area/LPF | |
| 4 | >30% of total area/LPF |
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| Tnf-α | GAAAGTCAACTCCATCTGCC | CATAGCACACTACGTTTGCC |
| Il-1β | GCTACCTATGTCTTGCCCGT | GACCATTGCTGTTTCCTAGG |
| Gapdh | CATCTTCCAGGAGCGAGACC | TCCACCACCCTGTTGCTGTA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, J.-K.; Park, J.H.; Kim, K.S.; Kang, T.H.; Kim, H.S. Antiulcer Activity of Steamed Ginger Extract against Ethanol/HCl-Induced Gastric Mucosal Injury in Rats. Molecules 2020, 25, 4663. https://doi.org/10.3390/molecules25204663
Shin J-K, Park JH, Kim KS, Kang TH, Kim HS. Antiulcer Activity of Steamed Ginger Extract against Ethanol/HCl-Induced Gastric Mucosal Injury in Rats. Molecules. 2020; 25(20):4663. https://doi.org/10.3390/molecules25204663
Chicago/Turabian StyleShin, Jun-Kyu, Jae Hyeon Park, Kyeong Seok Kim, Tong Ho Kang, and Hyung Sik Kim. 2020. "Antiulcer Activity of Steamed Ginger Extract against Ethanol/HCl-Induced Gastric Mucosal Injury in Rats" Molecules 25, no. 20: 4663. https://doi.org/10.3390/molecules25204663