Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process
Abstract
1. Introduction
2. Results and Discussion
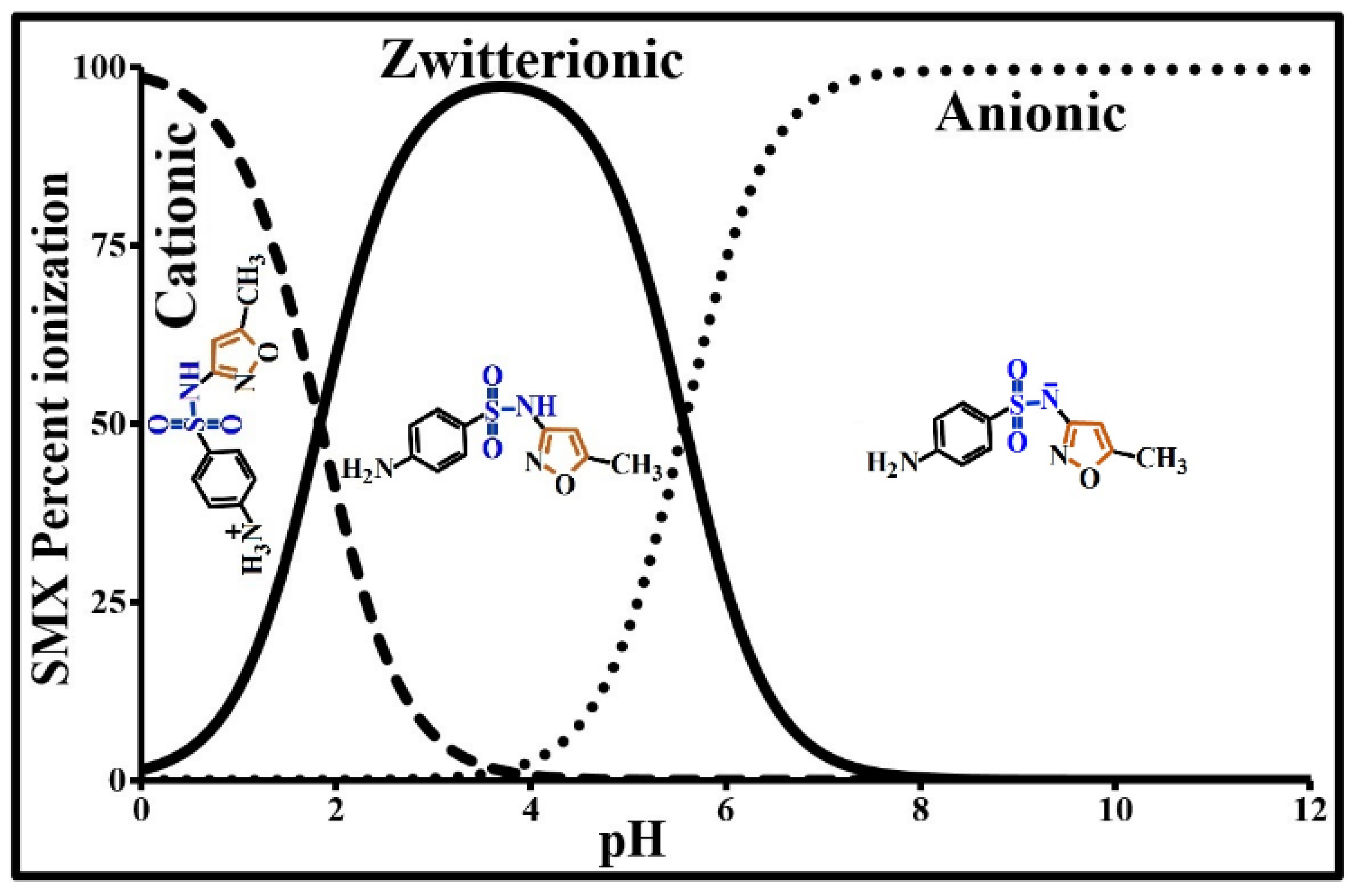
2.1. SMX Characterization
2.2. Activated Carbon Characteristics
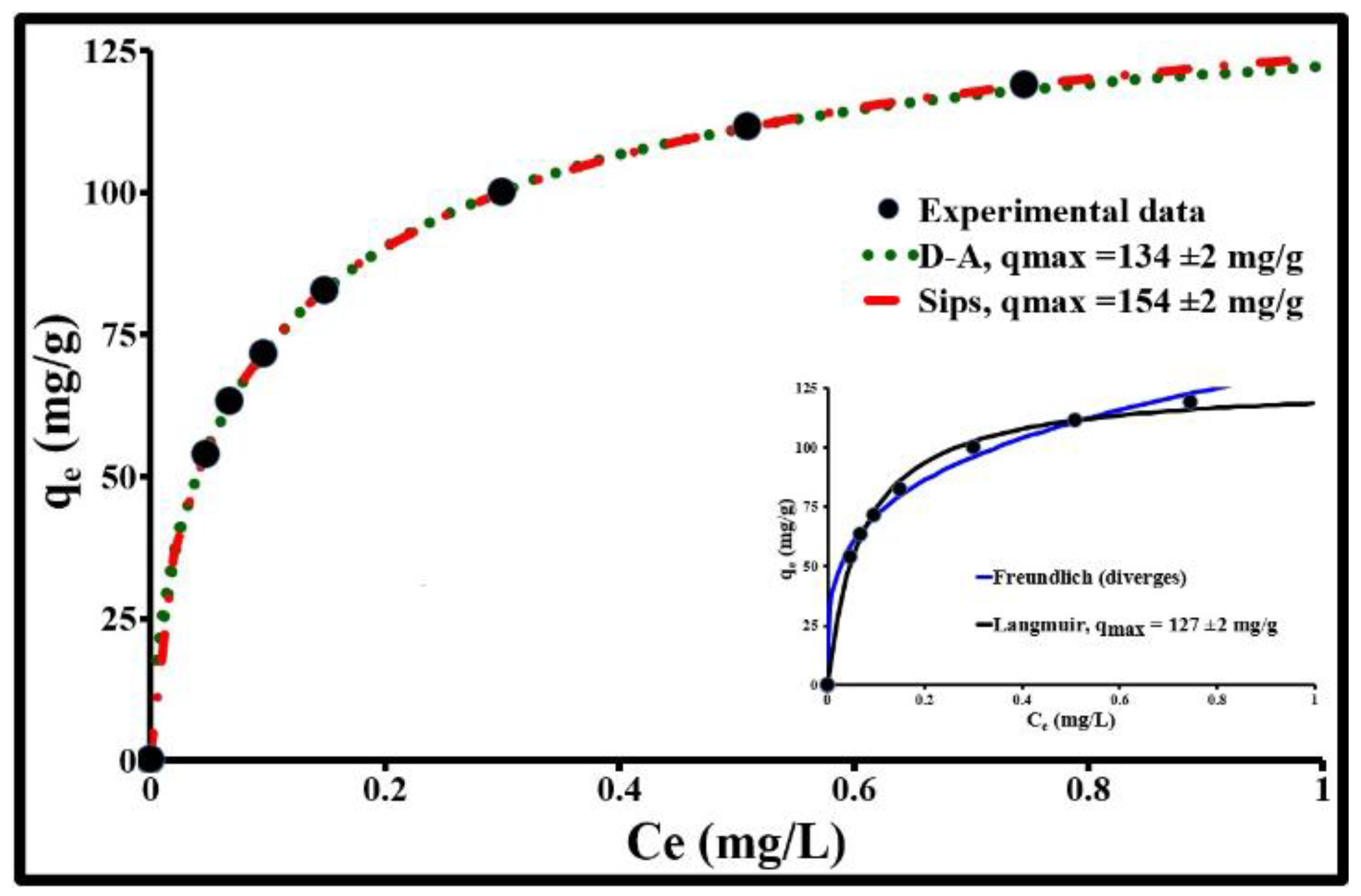
2.3. Equilibrium Isotherm and Adsorption Mechanism
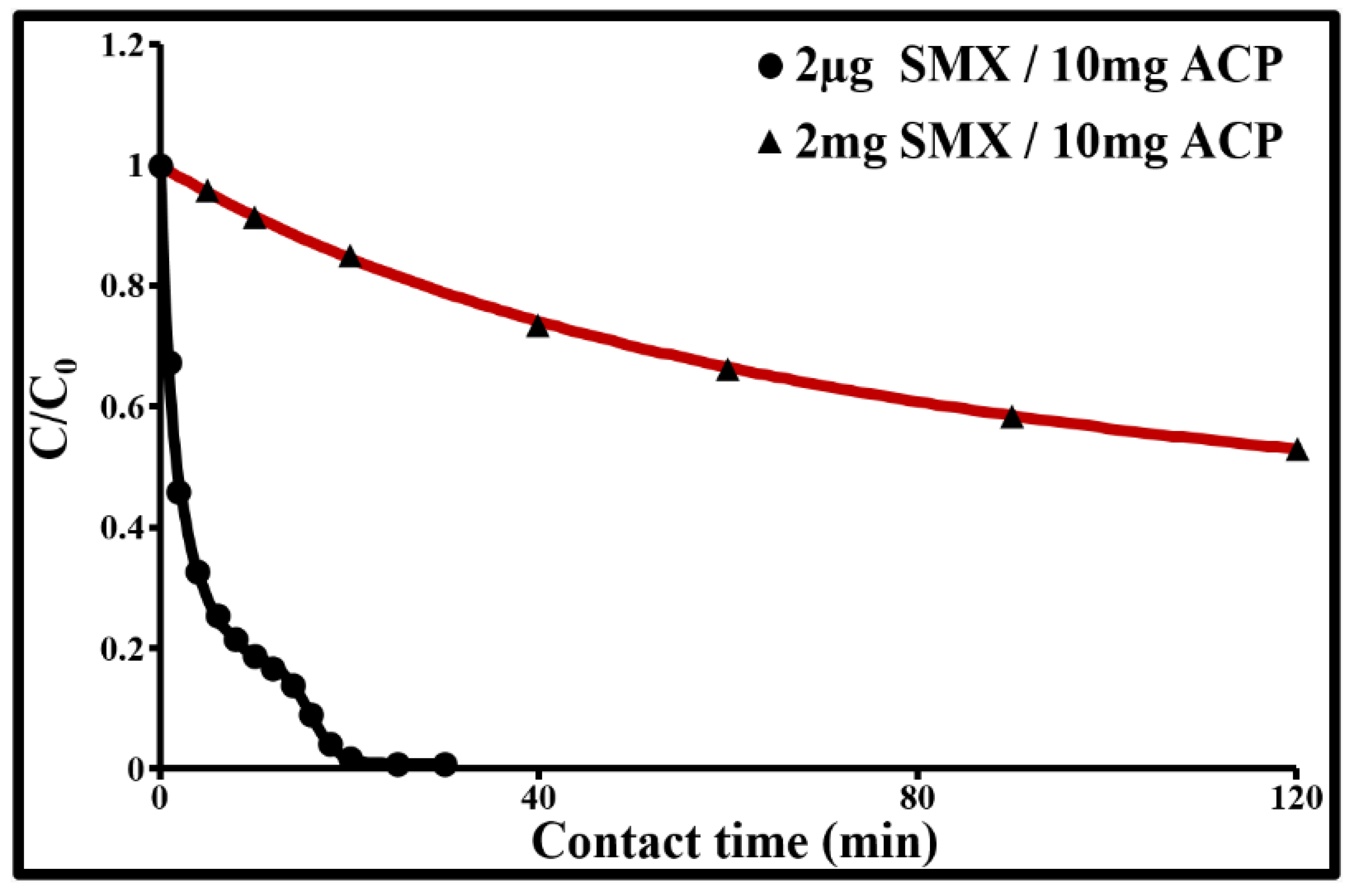
2.4. Sorption Dynamics
2.4.1. Initial Adsorption Rate
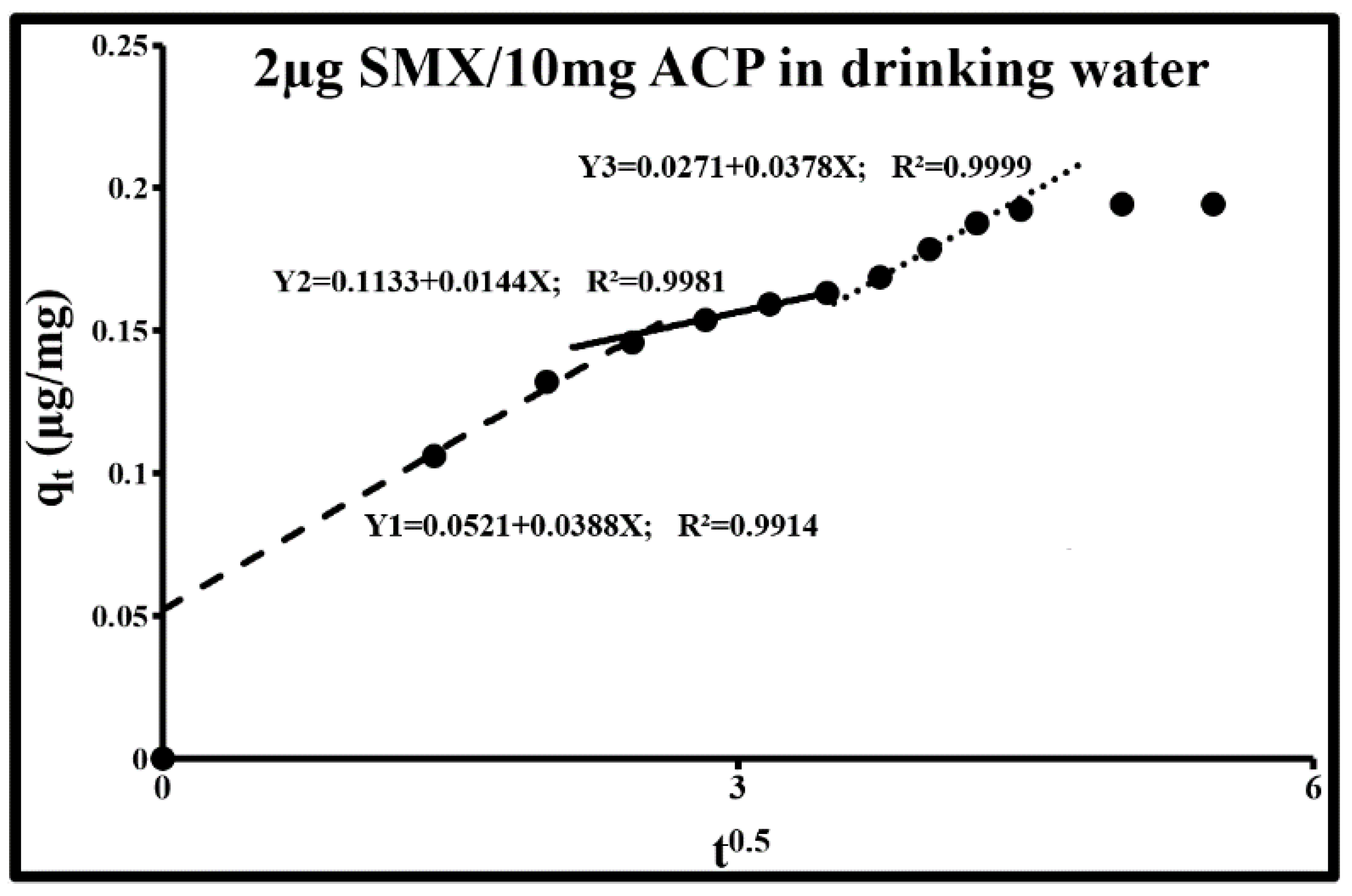
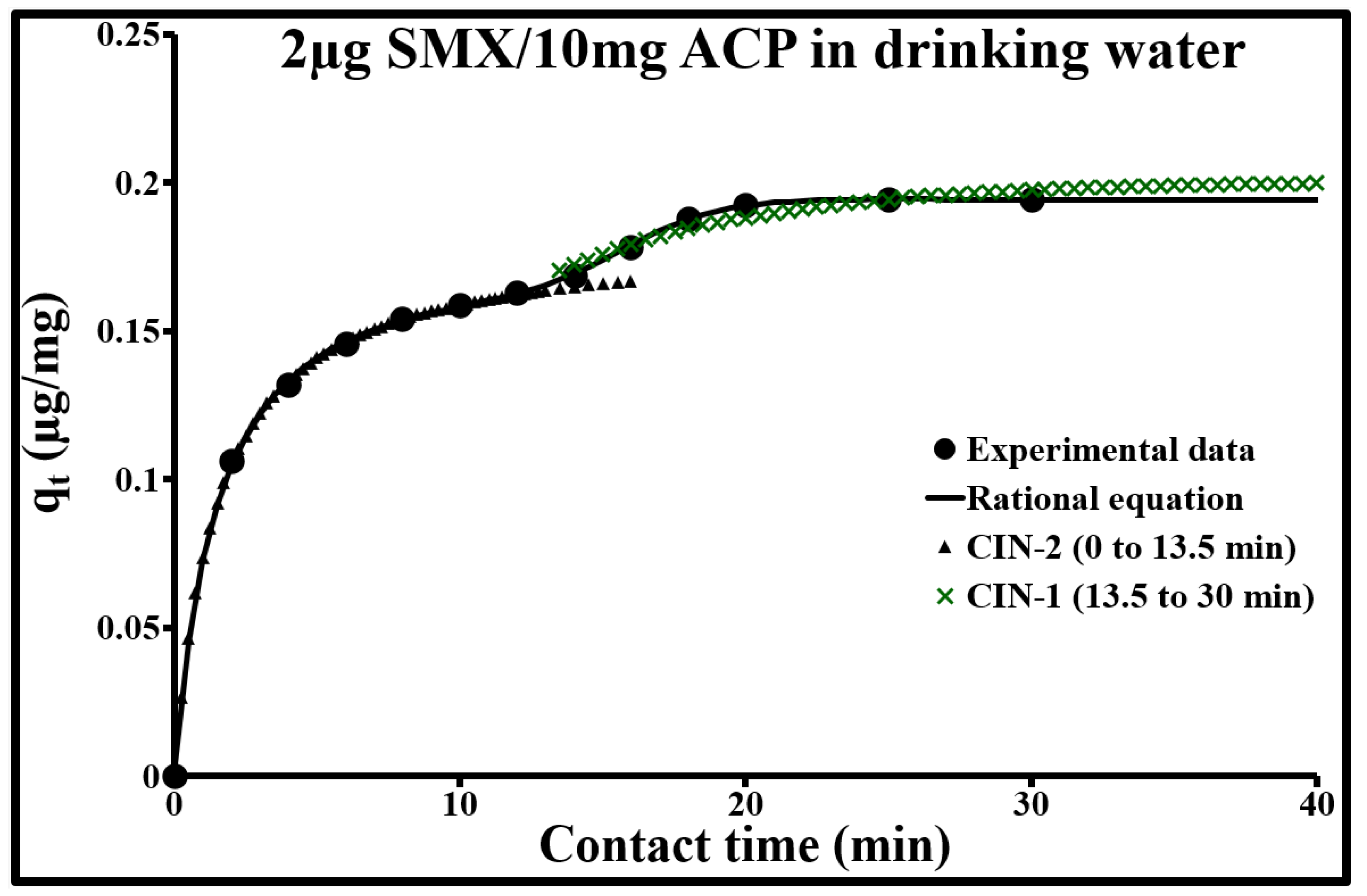
2.4.2. Sorption Diffusion Mechanisms
- -
- Diffusion through the film around the solid particles of adsorbate towards the outer surface, known as external diffusion (external mass transfer);
- -
- Diffusion into the pores of the adsorbate, known as “intraparticle diffusion”;
- -
- The adsorption/desorption reaction, i.e., the “surface reaction”.
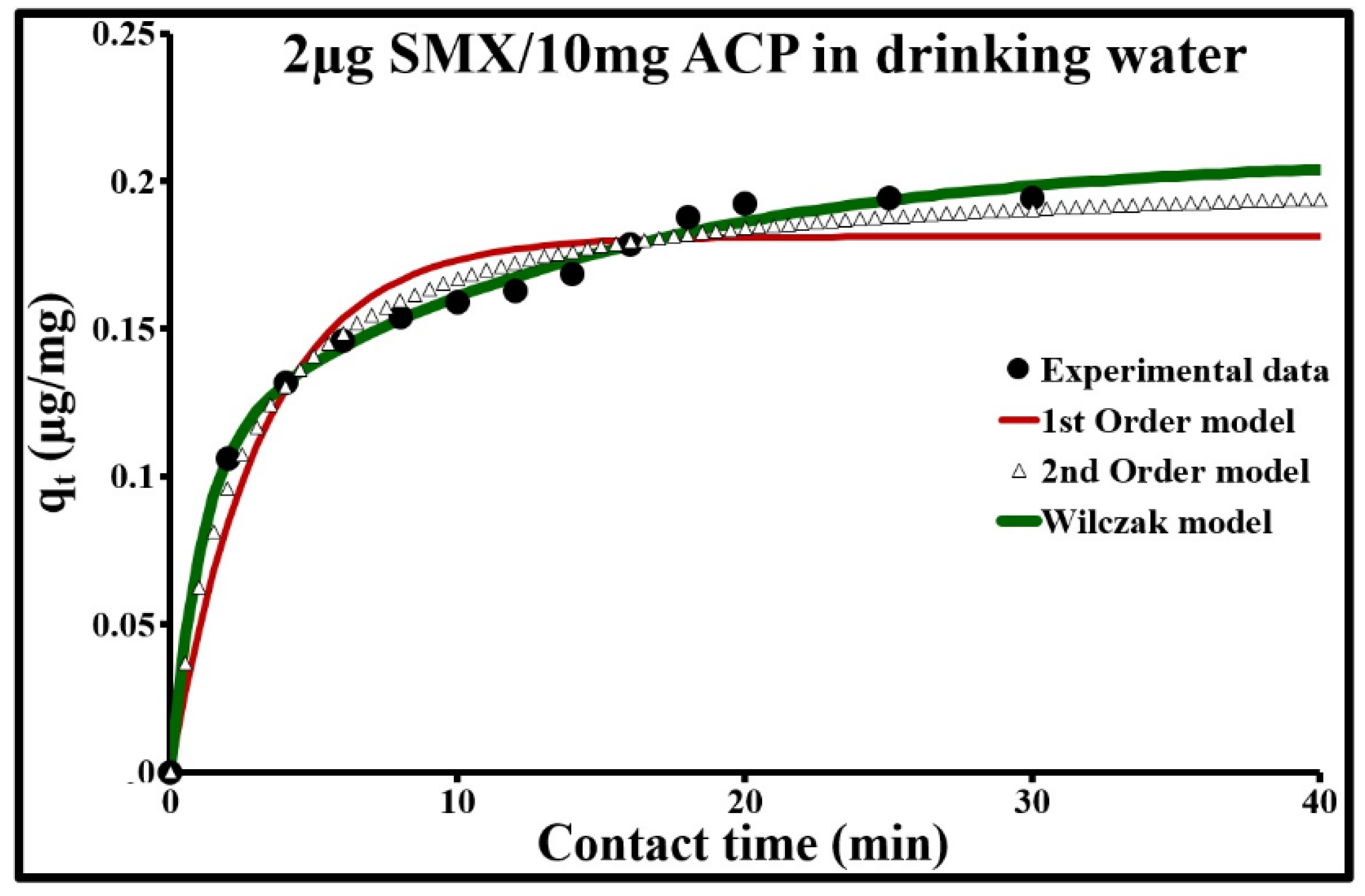
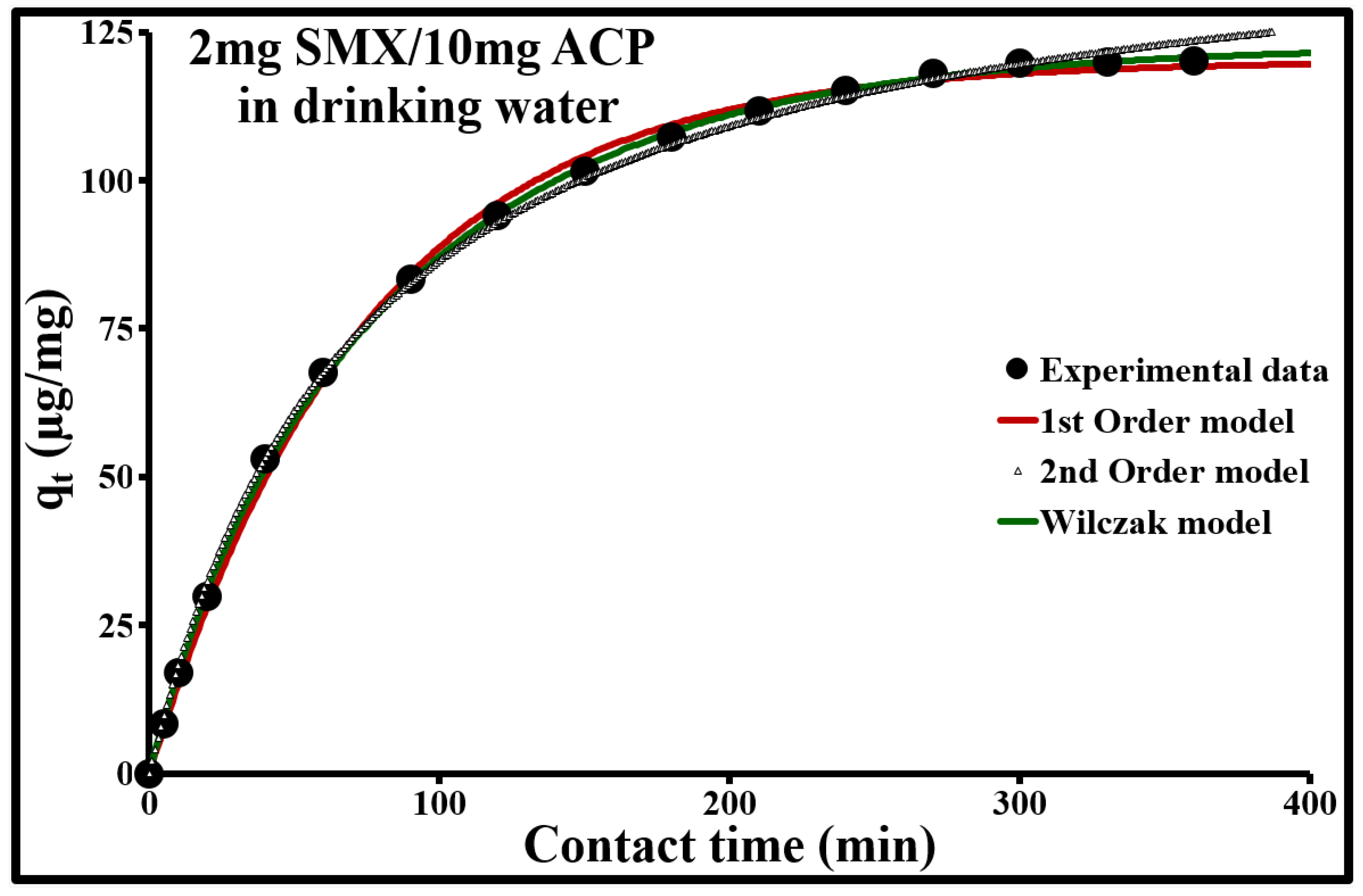
2.4.3. Adsorption Kinetic Modeling
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Characterization Techniques
3.2.2. Adsorption Method and Models
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- aus der Beek, T.; Weber, F.A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the Environment: Global Occurrence and Perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Kibuye, F.A.; Gall, H.E.; Elkin, K.R.; Ayers, B.; Veith, T.L.; Miller, M.; Jacob, S.; Hayden, K.R.; Watson, J.E.; Elliott, H.A. Fate of pharmaceuticals in a spray-irrigation system: From wastewater to groundwater. Sci. Total. Environ. 2019, 654, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Verlicchi, P.; Galletti, A.; Petrovic, M.; Barceló, D. Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J. Hydrol. 2010, 389, 416–428. [Google Scholar] [CrossRef]
- Deblonde, T.; Cossu-Leguille, C.; Hartemann, P. Emerging pollutants in wastewater: A review of the literature. Int. J. Hyg. Environ. Health 2011, 214, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Furlong, E.T.; Batt, A.L.; Glassmeyer, S.T.; Noriega, M.C.; Kolpin, D.; Mash, H.; Schenck, K.M. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States: Pharmaceuticals. Sci. Total. Environ. 2017, 579, 1629–1642. [Google Scholar] [CrossRef]
- Glassmeyer, S.T.; Furlong, E.T.; Kolpin, D.W.; Batt, A.L.; Bensond, R.; Boone, J.S.; Conerly, O.; Donohue, M.J.; King, D.N.; Kostich, M.S.; et al. Nationwide reconnaissance of contaminants of emerging concern in source and treated drinking waters of the United States. Sci. Total Environ. 2017, 909–922. [Google Scholar] [CrossRef]
- Paíga, P.; Correia, M.; Fernandes, M.J.; Silva, A.; Carvalho, M.; Vieira, J.S.; Jorge, S.; Silva, J.G.; Freire, C.; Delerue-Matos, C. Assessment of 83 pharmaceuticals in WWTP influent and euent samples by UHPLC-MS/MS: Hourly variationSci. Sci. Total Environ. 2019, 648, 582–600. [Google Scholar] [CrossRef]
- Kibuye, F.A.; Gall, H.E.; Elkin, K.R.; Swistock, B.; Veith, T.L.; Watson, J.E.; Elliott, H.A. Occurrence, Concentrations, and Risks of Pharmaceutical Compounds in Private Wells in Central Pennsylvania. J. Environ. Qual. 2019, 48, 1057–1066. [Google Scholar] [CrossRef]
- Barnes, K.K.; Kolpin, D.W.; Furlong, E.T.; Zaugg, S.D.; Meyer, M.T.; Barber, L.B. A national reconnaissance of pharmaceuticals and other organic wastewater contaminants in the United States—I) Groundwater. Sci. Total. Environ. 2008, 402, 192–200. [Google Scholar] [CrossRef]
- Benotti, M.J.; Brownawell, B.J. Distributions of Pharmaceuticals in an Urban Estuary during both Dry- and Wet-Weather Conditions. Environ. Sci. Technol. 2007, 41, 5795–5802. [Google Scholar] [CrossRef]
- Chang, X.; Meyer, M.T.; Liu, X.; Zhao, Q.; Chen, H.; Chen, J.-A.; Qiu, Z.; Yang, L.; Cao, J.; Shu, W. Determination of antibiotics in sewage from hospitals, nursery and slaughter house, wastewater treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ. Pollut. 2010, 158, 1444–1450. [Google Scholar] [CrossRef] [PubMed]
- Kümmerer, K. Resistance in the environment. J. Antimicrob. Chemother. 2004, 54, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, S.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-H.; Cerniglia, C.E. Influence of erythromycin A on the microbial populations in aquaculture sediment microcosms. Aquat. Toxicol. 2005, 73, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Ying, G.G.; Su, H.C.; Stauber, J.L.; Adams, M.S.; Binet, M.T. Growth-inhibiting effects of 12 antibacterial agents and their mixtures on the freshwater microalga Pseudokirchneriella subcapitata. Environ. Toxicol. Chem. 2008, 27, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ernst, F.; Conkle, J.L.; Gan, J. Comparative uptake and translocation of pharmaceutical and personal care products (PPCPs) by common vegetables. Environ. Int. 2013, 60, 15–22. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Hawkes, S. Effects of membrane fouling on the nanofiltration of pharmaceutically active compounds (PhACs): Mechanisms and role of membrane pore size. Sep. Purif. Technol. 2007, 57, 176–184. [Google Scholar] [CrossRef]
- Trovó, A.G.; Nogueira, R.F.; Agüera, A.; Fernández-Alba, A.R.; Sirtori, C.; Malato, S. Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res. 2009, 43, 3922–3931. [Google Scholar] [CrossRef]
- Garoma, T.; Umamaheshwar, S.K.; Mumper, A. Removal of sulfadiazine, sulfamethizole, sulfamethoxazole, and sulfathiazole from aqueous solution by ozonation. Chemosphere 2010, 79, 814–820. [Google Scholar] [CrossRef]
- Mezghich, S.; Ayari, F.; Sarakha, M. Photochemical Degradation of the Antimicrobial Sulfamethoxazole upon Solar Light Excitation: Kinetics and Elucidation of Byproducts Using LC/ESI+/MS2 Technique. Mass Spectrom. Purif. Tech. 2017, 3, 1000118. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, X.; Jiang, J.; Ma, J.; Liu, G.; Cao, Y.; Liu, W.; Li, J.; Pang, S.; Kong, X.; et al. Degradation of sulfamethoxazole by UV, UV/H2O2 and UV/persulfate (PDS): Formation of oxidation products and effect of bicarbonate. Water Res. 2017, 118, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Sui, Q.; Mei, X.; Cheng, X. Efficient elimination of sulfonamides by an anaerobic/anoxic/oxic-membrane bioreactor process: Performance and influence of redox condition. Sci. Total Environ. 2018, 633, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Sun, Q.; Wang, C.; Yang, Z.; Xue, Q. Removal of Sulfamethoxazole, Sulfathiazole and Sulfamethazine in their Mixed Solution by UV/H2O2 Process. Int. J. Environ. Res. Public Health 2019, 16, 1797. [Google Scholar] [CrossRef] [PubMed]
- Hu, A.; Zhang, X.; Luong, D.; Oakes, K.D.; Servos, M.R.; Liang, R.; Kurdi, S.; Peng, P.; Zhou, Y. Adsorption and Photocatalytic Degradation Kinetics of Pharmaceuticals by TiO2 Nanowires During Water Treatm. Waste Biomass Valorization 2012, 3, 443–449. [Google Scholar] [CrossRef]
- Li, H.; He, J.; Chen, K.; Shi, Z.; Li, M.; Guo, P.; Wu, L. Dynamic Adsorption of Sulfamethoxazole from Aqueous Solution by Lignite Activated Coke. Materials 2020, 13, 1785. [Google Scholar] [CrossRef]
- Prasannamedha, G.; Kumar, P.S. A review on contamination and removal of sulfamethoxazole from aqueous solution using cleaner techniques: Present and future perspective. J. Clean. Prod. 2020, 250, 119553. [Google Scholar] [CrossRef]
- Baan, R.A. Carcinogenic Hazards from Inhaled Carbon Black, Titanium Dioxide, and Talc not Containing Asbestos or Asbestiform Fibers: Recent Evaluations by anIARC MonographsWorking Group. Inhal. Toxicol. 2007, 19, 213–228. [Google Scholar] [CrossRef]
- Ettrup, K.; Kounina, A.; Hansen, S.F.; Meesters, J.A.J.; Vea, E.B.; Laurent, A. Development of comparative toxicity potentials of TiO2 nanoparticles for use in life cycle assessment. Environ. Sci. Technol. 2017, 51, 4027–4037. [Google Scholar] [CrossRef]
- Bizi, M. Activated Carbon and the Principal Mineral Constituents of a Natural Soil in the Presence of Carbamazepine. Water 2019, 11, 2290. [Google Scholar] [CrossRef]
- Bizi, M.; El Bachra, F. Evaluation of the ciprofloxacin adsorption capacity of common industrial minerals and application to tap water treatment. Powder Technol. 2020, 362, 323–333. [Google Scholar] [CrossRef]
- Boehm, H.P.; Diehl, E.; Heck, W.; Sappok, R. Surface oxides de carbon. Angew. Chem. Int. Ed. Engl. 1964, 3, 667–677. [Google Scholar] [CrossRef]
- Boehm, H.P. Chemical Identification of Surface Groups, Advances in Catalysis; Eley, D.D., Pines, H., Weisz, P.B., Eds.; Academic Press: New York, NY, USA, 1966; Volume 16, pp. 179–274. [Google Scholar]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, F.A.; Wang, Y. Solubilities of Sulfadiazine, Sulfamethazine, Sulfadimethoxine, Sulfamethoxydiazine, Sulfamonomethoxine, Sulfamethoxazole, and Sulfachloropyrazine in Water from (298.15 to 333.15 K). J. Chem. Eng. Data 2007, 52, 1563–1566. [Google Scholar] [CrossRef]
- Finqueneisel, G.; Zimny, T.; Albiniak, A.; Siemieniewska, T.; Vogt, D.; Weber, J.V. Cheap adsorbent. Part 1: Active cokes from lignites and improvement of their adsorptive properties by mild oxidation. Fuel 1998, 77, 549–556. [Google Scholar] [CrossRef]
- Giles, C.H.; Smith, D.; Huitson, A. A general treatment and classification of thesolute adsorption isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Everett, D.H.; Powl, J.C. Adsorption in slit-like and cylindrical micropores in the henry’s law region. A model for the microporosity of carbons. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1976, 72, 619–636. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon 2004, 42, 83–94. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Swiatkowski, A.; Biniak, S.; Walczyk, M. Effect of properties of chemically modified activated carbon and aromatic adsorbate molecule on adsorption from liquid phase. Colloids Surf. A Phys. Eng. Asp. 2008, 327, 1–8. [Google Scholar] [CrossRef]
- Coughlin, R.W.; Ezra, F.S. Role of Surface Acidity in the Adsorption of Organic Pollutants on the Surface of Carbon. Environ. Sci. Technol. 1968, 2, 291–293. [Google Scholar] [CrossRef]
- Mattson, S.; Mark, H.B.; Malbin, M.D.; Weber, W.J.; Crittenden, J.C. Surface Chemistry of Active Carbon: Specific Adsorption of Phenols. J. Colloid Interface Sci. 1969, 31, 116–130. [Google Scholar] [CrossRef]
- Hunter, C.A.; Singh, J.; Thornton, J.M. π-π interactions: The geometry and energetics of phenylalanine-phenylalanine interactions in proteins. J. Mol. Biol. 1991, 218, 837–846. [Google Scholar] [CrossRef]
- Haydar, S.; Ferro-García, M.A.; Rivera-Utrilla, J.; Joly, J.P. A dsorption of p-nitrophenol on an activated carbon with different oxidations. Carbon 2003, 41, 387–395. [Google Scholar] [CrossRef]
- Friedman, H.L.; Krishnan, C.V. Thermodynamics of ionichydration. In Water: A Comprehensive Treatise; Franks, F., Ed.; PlenumPress: New York, NY, USA, 1973. [Google Scholar]
- Ikeda, T.; Boero, M.; Terakura, K. Hydration properties of magnesium and calcium ions from constrained first principles molecular dynamics. J. Chem. Phys. 2007, 127, 074503. [Google Scholar] [CrossRef]
- de Courcy, B.; Pedersen, L.G.; Parisel, O.; Gresh, N.; Silvi, B.; Pilmé, J.; Piquemal, J.P. Understanding selectivity of hard and soft metal cations within biological systems using the subvalence concept. 1. Application to blood coagulation: Direct cation− protein electronic effects versus indirect interactions through water networks. J. Chem. Theory Comput. 2010, 6, 1048–1063. [Google Scholar] [CrossRef]
- Lin, C.E.; Chang, C.C.; Lin, W.C. Migration behavior and separation of sulfonamides in capillary zone electrophoresis III. Citrate buffer as a background electrolyte. J. Chromatogr. A 1997, 768, 105–112. [Google Scholar]
- Dubinin, M.M.; Stoeckli, H.F. Homogeneous and heterogeneous micropore structures in carbonaceous adsorbents. J. Colloid Interface Sci. 1980, 75, 34–42. [Google Scholar] [CrossRef]
- Mular, A.L.; Roberts, R.B. A Simplified method to determine isoelectric points of oxides. Trans. Can. Inst. Min. Metall. 1966, 69, 438–439. [Google Scholar]
- Sips, R. On the structure of a catalyst surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Micropore structure of activated carbons prepared from a Spanish subbituminous coalstudied by CO2, benzene, and cyclohexane adsorption. Langmuir 1995, 11, 247–252. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solutions. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Moussout, H.; Ahlafi, H.; Aazza, M.; Maghat, H. Critical of linear and nonlinear equations of pseudo-first order and pseudo-second order kinetic models. Karbala Int. J. Mod. Sci. 2018, 4, 244–254. [Google Scholar] [CrossRef]
Sample Availability: Activated carbon is available from the author. |


| da µm | PZC | SSBET m2/g | SSµP-t m2/g | SSmP-t m2/g | SSMP-t m2/g | Lµ-t nm | VµP-t cm3/g | VmP-t cm3/g | VMP-t cm3/g | SSµP-DA * m2/g | VµP-DA * cm3/g |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 | 6.8 | 957 | 695 | 203 | 60 | 1.75 | 0.30 | 0.17 | 0.10 | 711 | 0.31 |
| Functional Group | Density µmol/m2 | Number of Sites/nm2 |
|---|---|---|
| Carboxylic | 0.28 | 0.17 |
| Carbonyl | 0.46 | 0.28 |
| Anhydride | 0.17 | 0.10 |
| Lactone | 0.06 | 0.03 |
| Phenol | 0.53 | 0.32 |
| Total electron donor | 0.96 | |
| Total electron acceptor | 0.80 |
| External Diffusion | Intraparticle Diffusion | |||||||
|---|---|---|---|---|---|---|---|---|
| Micropore Diffusion | Mesopore Diffusion | |||||||
| CACP mg/L | C0 mg/L | ki1 mg/g min0.5 | C1 mg/L | ki2 mg/g min0.5 | C2 mg/g | ki3 mg/g min0.5 | C3 mg/g | R2 |
| 10 | 0.002 | 0.039 | 0.052 | 0.014 | 0.113 | 0.038 | 0.027 | 0.99 |
| Pseudo-First Order | Pseudo-Second Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C0 mg/L | qe.exp mg/g | qe mg/g | k1 min−1 | R2 | ∆q % | qe mg/g | k2 g/mg min | R2 | ∆q % |
| 0.002 | 0.198 | 0.181 | 0.313 | 0.948 | 8.8 | 0.205 | 2.146 | 0.985 | 4.6 |
| 2 | 120.5 | 120.16 | 0.013 | 0.999 | 4.4 | 147.9 | 9.5 × 10−5 | 0.999 | 1.9 |
| Double-Exponential Model | |||||||||
| C0 mg/L | qe.exp mg/g | q0 mg/g | qe1 mg/g | qe2 mg/g | qe1 + qe2 mg/g | kD1 min−1 | kD2 min−1 | R2 | ∆q % |
| 0.002 | 0.198 | 1.5 × 10−7 | 0.102 | 0.107 | 0.209 | 0.075 | 0.979 | 0.996 | 1.9 |
| 2 | 120.5 | 1.5 × 10−5 | 107.7 | 15.0 | 122.7 | 0.011 | 0.049 | 0.999 | 2.5 |
| 0 to 13.5 min Pseudo-Second Order | 13.5 to 30 min Pseudo-First Order | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| C0 mg/L | qe.exp mg/g | qe mg/g | k2 g/mg min | R2 | ∆q % | qe mg/g | k1 min−1 | R2 | ∆q % |
| 0.002 | 0.198 | 0.181 | 3.730 | 0.999 | 0.6 | 0.200 | 0.140 | 0.998 | 1.6 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
BIZI, M. Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process. Molecules 2020, 25, 4656. https://doi.org/10.3390/molecules25204656
BIZI M. Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process. Molecules. 2020; 25(20):4656. https://doi.org/10.3390/molecules25204656
Chicago/Turabian StyleBIZI, Mohamed. 2020. "Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process" Molecules 25, no. 20: 4656. https://doi.org/10.3390/molecules25204656
APA StyleBIZI, M. (2020). Sulfamethoxazole Removal from Drinking Water by Activated Carbon: Kinetics and Diffusion Process. Molecules, 25(20), 4656. https://doi.org/10.3390/molecules25204656





