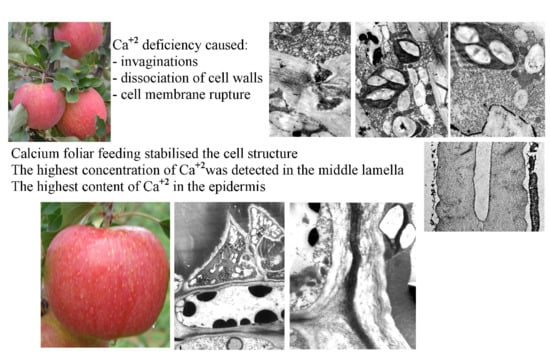
Ultrastructure of Cells and Microanalysis in Malus domestica Borkh. ‘Szampion’ Fruit in Relation to Varied Calcium Foliar Feeding
Abstract
1. Introduction
1.1. Role of Calcium in Plants
1.2. Foliar Feeding
1.3. Calcium Content
2. Results
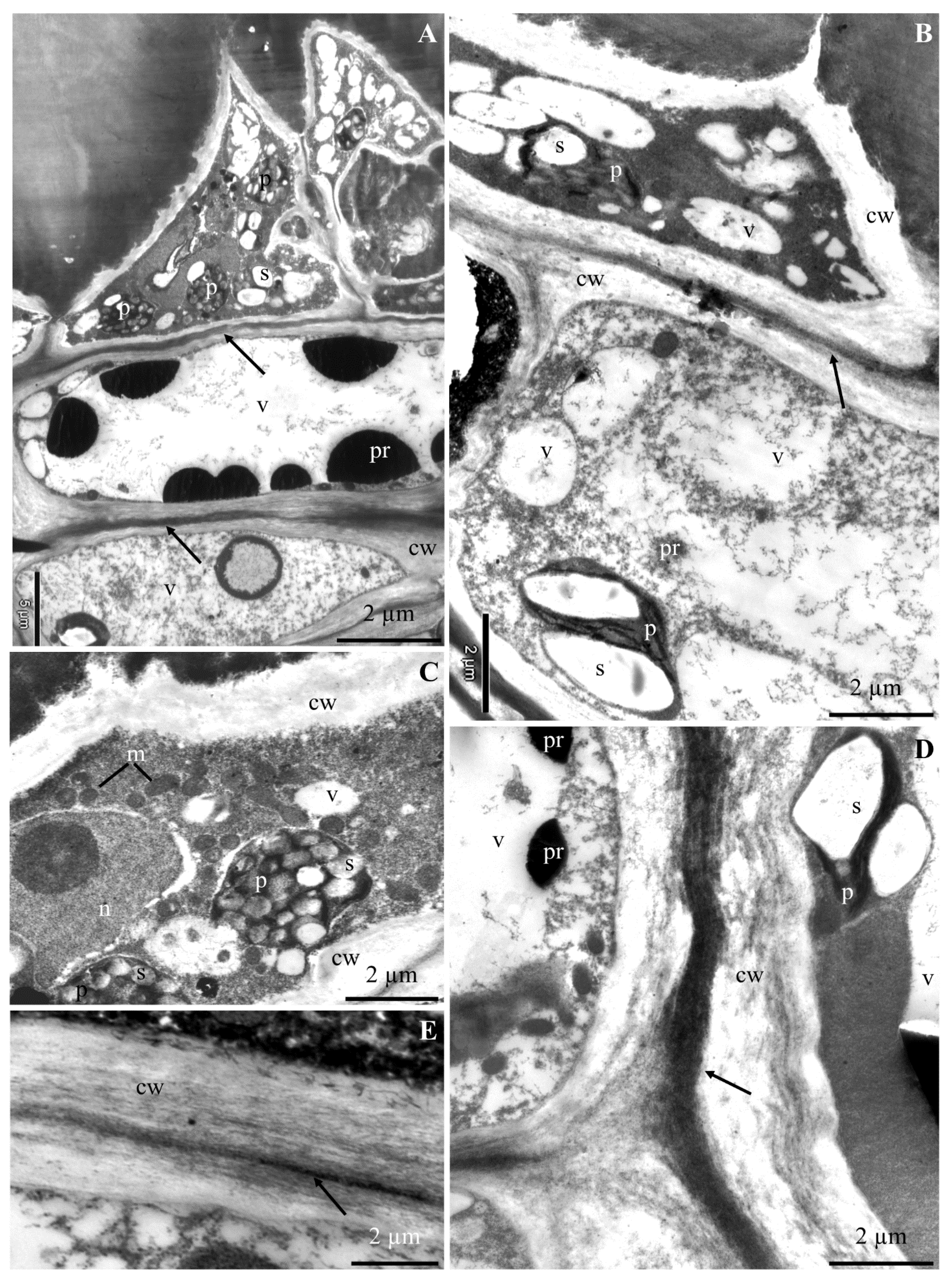
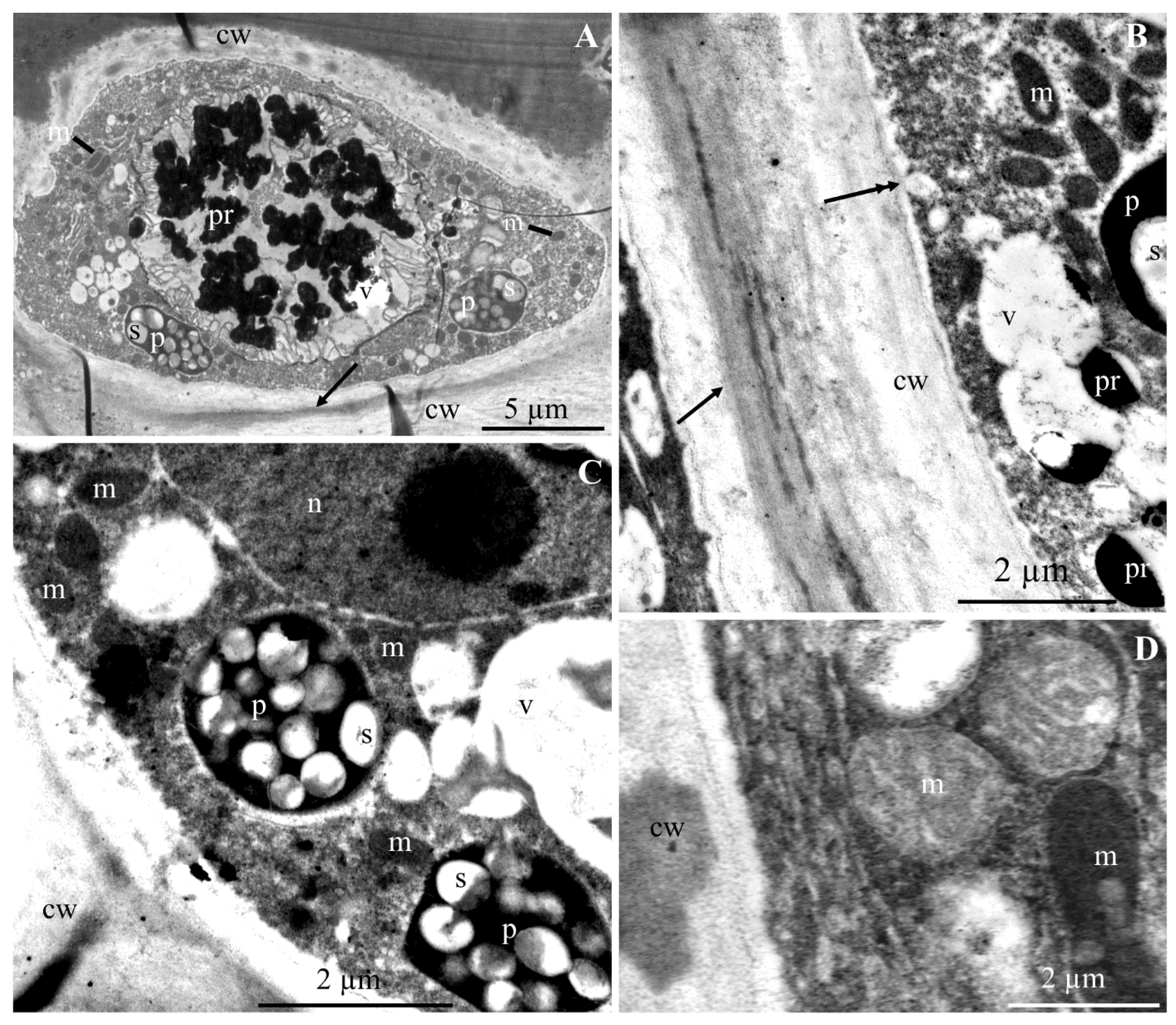
2.1. Ultrastructure of Epidermis and Hypodermis Cells in Malus Domestica L. ‘Szampion’ Apples
2.1.1. Epidermis
2.1.2. Hypodermis
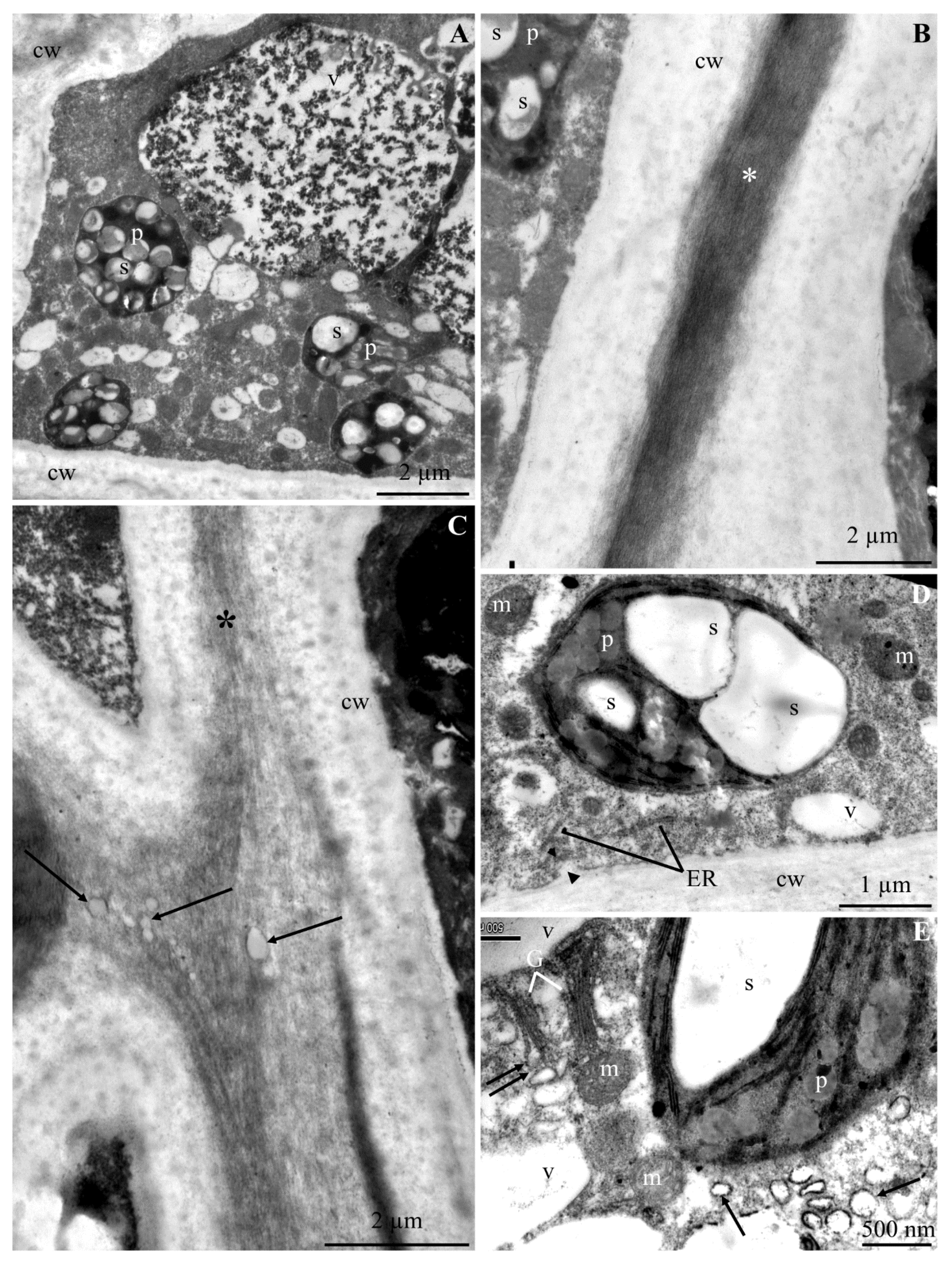
2.2. Calcium Content at the Epidermis Ultrastructure Level
2.2.1. Calcium Content in Selected Cell Elements
2.2.2. Calcium Content in Selected Fragments of Epidermis Cells
2.3. Ca2+ Mapping at the Level of Epidermis Cell Ultrastructure
3. Discussion
3.1. Impact of Ca2+ Cations on the Cell Ultrastructure
3.2. Content of Ca2+ Cations at the Cellular Level
4. Material and Research Methods
4.1. Study Area and Experimental Treatments
4.2. Plant Material
4.3. Fixation of Plant Samples
4.4. Microscopic Study Methods
4.4.1. Bright-Field Microscopy
4.4.2. Transmission Electron Microscopy
4.5. Microanalysis of Ca2+ Cations at the Cell Ultrastructure Level
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, T.; Zhan, X.; Xu, Y.; Zhou, L.; Zong, L. Roles of calcium in stress-tolerance of plants and its ecological significance. J. Appl. Ecol. 2005, 16, 971–976. [Google Scholar]
- Song, W.Y.; Zhang, Z.B.; Shao, H.B.; Guo, X.L.; Cao, H.X.; Zhao, H.B.; Hu, X.J. Relationship between calcium decoding elements and plant abiotic-stress resistance. Int. J. Biol. Sci. 2008, 4, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.S.N.; Ali, G.S.; Celesnik, H.; Day, I.S. Coping with stresses: Roles of calcium- and calcium/calmodulin-regulated gene expression. Plant Cell 2011, 23, 2010–2032. [Google Scholar] [CrossRef] [PubMed]
- Delian, E.; Chira, A.; Dulescu, L.B.; Chira, L. Calcium alleviates stress in plants: Insight into regulatory mechanisms. AgroLife Sci. J. 2014, 3, 19–28. [Google Scholar]
- Ferguson, I.B.; Watkins, C.B. Cation distribution and balance in apple fruit in relation to calcium treatments for bitter pit. Sci. Hort. 1983, 19, 301–310. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Calcium in plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Gilliham, M.; Dayod, M.; Hocking, B.J.; Xu, B.; Conn, S.J.; Kaiser, B.N.; Leigh, R.A.; Tyerman, S.D. Calcium delivery and storage in plant leaves: Exploring the link with water flow. J. Exp. Bot. 2011, 62, 2233–2250. [Google Scholar] [CrossRef]
- Tuteja, N.; Mahajan, S. Calcium signalling network in plants: An overview. Plant Signal. Behav. 2007, 2, 79–85. [Google Scholar] [CrossRef]
- He, L.; Li, B.; Lu, X.; Yuan, L.; Yang, Y.; Yuan, Y.; Du, J.; Guo, S. The effect of exogenous calcium on mitochondria, respiratory metabolism enzymes and ion transport in cucumber roots under hypoxia. Sci. Rep. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta Bioenerg. 2015, 1847, 993–1003. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, S.; Wan, S.; Li, X. The significance of calcium in photosynthesis. Int. J. Mol. Sci. 2019, 20, 1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Bi, A.; Amombo, E.; Li, H.; Zhang, L.; Cheng, C.; Hu, T.; Fu, J. Exogenous calcium enhances the photosystem II photochemistry response in salt stressed tall fescue. Front. Plant Sci. 2017, 8, 2032. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; wei Meng, Q.; Brestic, M.; Olsovska, K.; Yang, X. Photosynthesis is improved by exogenous calcium in heat-stressed tobacco plants. J. Plant Physiol. 2011, 168, 2063–2071. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, P.; Zhang’, W.; Yang, Z.; Liu, H.; Ahammed, G.J.; Cui, J. Calcium is involved in exogenous NO-induced enhancement of photosynthesis in cucumber (Cucumis sativus L.) seedlings under low temperature. Sci. Hortic. 2020, 261, 108953. [Google Scholar] [CrossRef]
- Hajihashemi, S.; Skalicky, M.; Brestic, M.; Pavla, V. Cross-talk between nitric oxide, hydrogen peroxide and calcium in salt-stressed Chenopodium quinoa Willd. At seed germination stage. Plant Physiol. Biochem. 2020, 154, 657–664. [Google Scholar] [CrossRef]
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hort. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Ge, L.L.; Xie, C.T.; Tian, H.Q.; Russell, S.D. Distribution of calcium in the stigma and style of tobacco during pollen germination and tube elongation. Sex. Plant Reprod. 2009, 22, 87–96. [Google Scholar] [CrossRef]
- El Habbasha, S.F.; Faten, M.I. Calcium: Physiological function, deficiency and absorption. Inter. J. Chem. Tech. Res. 2015, 8, 196–202. [Google Scholar]
- Schlegel, T.K.; Schönherr, J.; Schreiber, L. Rates of foliar penetration of chelated Fe (III): Role of light, stomata, species, and leaf age. J. Agric. Food Chem. 2006, 54, 6809–6813. [Google Scholar] [CrossRef]
- Fageira, N.K.; Gheyi, H.R.; Moreira, A. Nutrient bioavailability in salt affected soils. J. Plant Nutr. 2011, 34, 945–962. [Google Scholar] [CrossRef]
- Harker, F.R.; Ferguson, I.B. Effects of surfactants on calcium penetration of cuticles isolated from apple fruit. Sci. Hortic. 1991, 46, 225–233. [Google Scholar] [CrossRef]
- Papadakis, I.E.; Protopapadakis, E.; Therios, I.N.; Tsirakoglou, V. Foliar treatment of Mn deficient ‘Washington navel’ orange trees with two Mn sources. Sci. Hortic. 2005, 106, 70–75. [Google Scholar] [CrossRef]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Avestan, S.; Naseri, L.; Najafzadeh, R. Improvement of in vitro proliferation of apple (Malus domestica Borkh.) by enriched nano chelated iron fertilizer. Int. J. Hortic. Sci. Technol. 2018, 5, 43–51. [Google Scholar]
- Harker, F.R.; Ferguson, I.B. Transport of calcium across cuticles isolated from apple fruit. Sci. Hortic. 1988, 36, 205–217. [Google Scholar] [CrossRef]
- Santier, S.; Chamel, A. Reassessment of the role of cuticular waxes in the transfer of organic molecules through plant cuticles. Plant Physiol. Biochem. 1998, 36, 225–231. [Google Scholar] [CrossRef]
- Riederer, M.; Müller, C. Biology of the Plant Cuticle; Blackwell Publishing Ltd.: Oxford, UK, 2008. [Google Scholar]
- Khanal, B.P.; Grimm, E.; Knoche, M. Russeting in apple and pear: A plastic periderm replaces a stiff cuticle. AoB Plants 2013, 5, 1–12. [Google Scholar] [CrossRef]
- Khanal, B.P.; Shrestha, R.; Hückstädt, L.; Knoche, M. Russeting in apple seems unrelated to the mechanical properties of the cuticle at maturity. HortScience 2013, 48, 1135–1138. [Google Scholar] [CrossRef]
- Lara, I.; Belge, B.; Goulao, L.F. The fruit cuticle as a modulator of postharvest quality. Postharvest Biol. Technol. 2014, 87, 103–112. [Google Scholar] [CrossRef]
- Martin, L.B.; Rose, J.K. There’s more than one way to skin a fruit: Formation and functions of fruit cuticles. J. Exp. Bot. 2014, 65, 4639–4651. [Google Scholar] [CrossRef]
- Fogelman, E.; Stern, R.A.; Ginzberg, I. Benzyladenine and gibberellin treatment of developing “Pink Lady” apples results in mature fruits with a thicker cuticle comprising clusters of epidermal cells. Protoplasma 2015, 252, 1009–1017. [Google Scholar] [CrossRef]
- Ginzberg, I.; Stern, R.A. Strengthening fruit-skin resistance to growth strain by application of plant growth regulators. Sci. Hortic. 2016, 198, 150–153. [Google Scholar] [CrossRef]
- Joshi, M.; Baghel, R.S.; Fogelman, E.; Stern, R.A.; Ginzberg, I. Identification of candidate genes mediating apple fruit-cracking resistance following the application of gibberellic acids 4 + 7 and the cytokinin 6-benzyladenine. Plant Physiol. Biochem. 2018, 127, 436–445. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Dai, M.S.; Cai, D.Y.; Zhang, S.; Shi, Z.B. A review for the molecular research of russet/semi-russet of sand pear exocarp and their genetic characters. Sci. Hortic. 2016, 210, 138–142. [Google Scholar] [CrossRef]
- Ferguson, I. Calcium in apple fruit. In Proceedings of the Washington Tree Fruit; Postharvest Conference, Wenatchee, DC, USA, 13–14 March; 2001. [Google Scholar]
- Eren, İ.; Çalhan, Ö.; Onursal, E. Postharvest practices of apples and cherries in Turkey. In Workshop book New Approaches on Apple and Cherry Growing and Breeding Techniques; 22–24 May 2014; Küden, A.B., Ed.; Çukurova Üniversitesi Türkiye: Adana, Turkey, 2014; Volume 76, pp. 124–137. [Google Scholar]
- Andziak, J.; Tomala, K.; Sadowski, A.; Dziuban, R. Nutritional status and quality of Sampion apples depending rootstock. Acta Sci. Pol. Hortorum Cultus 2004, 3, 111–122. [Google Scholar]
- Neilsen, G.H.; Neilsen, D.; Dong, S.; Toivonen, P.; Peryea, F. Application of CaCl2 sprays earlier in the season may reduce bitter pit incidence in ‘Braeburn’ apple. Hort. Sci. 2005, 40, 1850–1853. [Google Scholar] [CrossRef]
- Lanauskas, J.; Kviklienė, N. Effect of calcium fertilizer sprays on storage quality of ‘Shampion’ apples. Sodinink. Daržinink. 2005, 24, 20–28. [Google Scholar]
- Dilmaghani, M.R.; Malakouti, M.J.; Neilsen, G.H.; Fallahi, E. Interactive effects of potassium and calcium on K/Ca ratio and its consequences on apple fruit quality in calcareous soils of Iran. J. Plant Nutr. 2005, 25, 1149–1162. [Google Scholar] [CrossRef]
- Domagała-Świątkiewicz, I. Effect of foliar calcium nitrate spray on the mineral nutrient content in leaves and fruit of ‘Elise’ apples. Acta Agrophys. 2006, 7, 867–877. [Google Scholar]
- Fallahi, E.; Conway, W.S.; Hickey, K.D.; Sams, C.E. The role of calcium and nitrogen in postharvest quality and disease resistance of apples. Hort Sci. 1997, 32, 831–835. [Google Scholar] [CrossRef]
- Haynes, R.J.; Goh, K.M. Variation in the nutrient content of leaves and fruit with season and crown position for two apple varieties. Aust. J. Agric. Res. 1980, 31, 739–748. [Google Scholar] [CrossRef]
- Val, J.; Monge, E.; Risco, D.; Blanco, A. Effect of pre-harvest calcium sprays on calcium concentrations in the skin and flesh of apples. J. Plant Nutr. 2008, 31, 1889–1905. [Google Scholar] [CrossRef]
- Kadir, S.A. Fruit quality at harvest of ‘Jonathan’ apple treated with foliarly-applied calcium chloride. J. Plant Nutr. 2005, 27, 1991–2006. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Konstankiewicz, K. Calcium effect on mechanical properties of model cell walls and apple tissue. J. Food Eng. 2011, 102, 217–223. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. Calcium at the cell wall cytoplast interface. J. Int. Plant Biol. 2010, 52, 147–160. [Google Scholar] [CrossRef] [PubMed]
- Glenn, G.M.; Reddy, A.S.N.; Poovaiah, B.W. Effect of calcium on cell wall structure, protein phosphorylation and protein profile in senescing apples. Plant Cell Physiol. 1988, 29, 565–572. [Google Scholar]
- Glenn, G.M.; Poovaiah, B.W. Calcium-mediated postharvest changes in texture and cell wall structure and composition in ‘Golden Delicious’ apples. J. Am. Soc. 1990, 115, 962–968. [Google Scholar] [CrossRef]
- Willats, W.G.; McCartney, L.; Mackie, W.; Knox, J.P. Pectin: Cell biology and prospects for functional analysis. Plant Mol. Biol. 2001, 47, 9–27. [Google Scholar] [CrossRef]
- Hocking, B.; Tyerman, S.D.; Burton, R.A.; Gilliham, M. Fruit calcium: Transport and physiology. Front. Plant Sci. 2016, 7, 569. [Google Scholar] [CrossRef]
- Edel, K.H.; Marchadier, E.; Brownlee, C.; Kudla, J.; Hetherington, A.M. The evolution of calcium-based signalling in plants. Curr. Biol. 2017, 27, R667–R679. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef]
- Singh, R. Calcium in plant biology: Nutrient and second messenger. Int. J. Biol. Innov. 2020, 2, 31–35. [Google Scholar] [CrossRef]
- Jemrić, T.; Fruk, I.; Fruk, M.; Radman, S.; Sinkovič, L.; Fruk, G. Bitter pit in apples: Pre-and postharvest factors: A review. Span. J. Agric. Res. 2016, 14, 15. [Google Scholar]
- Yang, T.; Poovaiah, B.W. Calcium/calmodulin-mediated signal network in plants. Trends Plant Sci. 2003, 1, 505–512. [Google Scholar] [CrossRef]
- Kim, M.C.; Chung, W.S.; Yun, D.J.; Cho, M.J. Calcium and calmodulin-mediated regulation of gene expression in plants. Mol. Plant 2009, 2, 13–21. [Google Scholar] [CrossRef]
- Türkan, I.; Demiral, T. Recent developments in understanding salinity tolerance. Environ. Exp. Bot. 2009, 67, 2–9. [Google Scholar] [CrossRef]
- Quiles, A.; Hernando, I.; Pérez-Munuera, I.; Llorca, E.; Larrea, V.; Ángeles Lluch, M. The effect of calcium and cellular permeabilization on the structure of the parenchyma of osmotic dehydrated ‘Granny Smith’ apple. J. Sci. Food Agric. 2004, 84, 1765–1770. [Google Scholar] [CrossRef]
- Wang, L.L.; Chen, A.P.; Zhong, N.Q.; Liu, N.; Wu, X.M.; Wang, F.; Yang, C.L.; Romero, M.F.; Xia, G.X. The Thellungiella salsuginea tonoplast aquaporin TsTIP1; 2 functions in protection against multiple abiotic stresses. Plant Cell Physiol. 2014, 55, 148–161. [Google Scholar] [CrossRef]
- Fukumoto, M.; Venis, M.A. ATP-dependent Ca2+ transport in tonoplast vesicles from apple fruit. Plant Cell Physiol. 1986, 27, 491–497. [Google Scholar]
- Quiles, A.; Hernando, I.; Pérez-Munuera, I.; Lluch, M.Á. Effect of calcium propionate on the microstructure and pectin methy-lesterase activity in the parenchyma of fresh-cut Fuji apples. J. Sci. Food Agric. 2007, 87, 511–519. [Google Scholar] [CrossRef]
- Mauro, M.A.; Dellarosa, N.; Tylewicz, U.; Tappi, S.; Laghi, L.; Rocculi, P.; Dalla Rosa, M. Calcium and ascorbic acid affect cellular structure and water mobility in apple tissue during osmotic dehydration in sucrose solutions. Food Chem. 2016, 195, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, V.R.; Nakata, P.A. Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Nakata, P.A. Plant calcium oxalate crystal formation, function, and its impact on human health. Front. Biol. 2012, 7, 254–266. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef]
- Cuéllar-Cruz, M.; Pérez, K.S.; Mendoza, M.E.; Moreno, A. Biocrystals in plants: A short review on biomineralization processes and the role of phototropins into the uptake of calcium. Crystals 2020, 10, 591. [Google Scholar] [CrossRef]
- Bush, D.S.; Biswas, A.K.; Jones, R.L. Gibberellic acid-stimulated Ca2+ accumulation in endoplasmic reticulum of barley aleurone - Ca2+ transport and steady state levels. Planta 1989, 178, 411–420. [Google Scholar] [CrossRef]
- Yuk, J.B.; Choi, S.H.; Lee, T.H.; Jang, M.U.; Park, J.; Yi, A.R.; Svensson, B.; Kim, T.J. Effects of calcium ion concentration on starch hydrolysis of barley α-amylase isozymes. J. Microbiol. Biotechnol. 2008, 18, 730–734. [Google Scholar]
- Raese, J.T.; Drake, S.R. Effects of preharvest calcium sprays on apple and pear quality. J. Plant Nutr. 1993, 16, 1807–1819. [Google Scholar] [CrossRef]
- Rab, A.; Haq, I. Foliar application of calcium chloride and borax influences plant growth, yield, and quality of tomato (Lycopersicon esculentum Mill.) fruit. Turk. J. Agric. For. 2012, 36, 695–701. [Google Scholar]
- Toivonen, P.M.A.; Bowen, P.A. The effect of preharvest foliar sprays of calcium on quality and shelf life of two cultivars of sweet bell peppers (Capsicum annuum L.) grown in plasticulture. Can. J. Plant Sci. 1999, 79, 411–416. [Google Scholar] [CrossRef]
- Tzortzakis, N.G. Influence of NaCl and calcium nitrate on lettuce and endive growth using nutrientfilm technique. Intl. J. Veg. Sci. 2009, 15, 1–13. [Google Scholar]
- Fernández, V.; Eichert, T. Uptake of hydrophilic solutes through plant leaves: Current state of knowledge and perspectives of foliar fertilization. Crit. Rev. Plant Sci. 2009, 28, 36–68. [Google Scholar] [CrossRef]
- Wójcik, P. Effect of addition of amino acids to solution of calcium chloride on phytotoxicity and absorption of calcium by ‘Šampion’ apples. Zesz. Nauk. Inst. Ogrod. 2016, 24, 141–152. [Google Scholar]
- Sekhon, B.S. Chelates for micronutrient nutrition among crops. Resonance 2003, 8, 46–53. [Google Scholar] [CrossRef]
- Inal, A.; Günes, A.; Alpaslan, M.; Demir, K. Nitrate versus chloride nutrition effects in a soil-plant system on the growth, nitrate accumulation, and nitrogen, potassium, sodium, calcium, and chloride content of carrot. J. Plant Nutr. 1998, 21, 2001–2011. [Google Scholar] [CrossRef]
- Schönherr, J. Cuticular penetration of calcium salts: Effects of humidity, anions, and adjuvants. J. Plant Nutr. Soil Sci. 2001, 164, 225–231. [Google Scholar]
- Chondraki, S.C.; Tzerakis, N.; Tzortzakis, N. Influence of sodium chloride and calcium foliar spray on hydroponically grown parsley in nutrient film technique system. J. Plant Nutr. 2012, 35, 1457–1467. [Google Scholar] [CrossRef]
- Mayr, U.; Schröder, M. Influence of calcium sprays with different concentrations; spray timing and combinations with prohexadione-Ca on the mineral content in ‘Boskoop’ and ‘Elstar’ apples. In International Symposium on Foliar Nutrition of Perennial Fruit Plants; 1–15 September 2001; Tagliavini, M., Drahorad, W., Eds.; International Society of Horticultura Science: Meran, Italy, 2001; Volume 594, pp. 553–556. [Google Scholar]
- Qiu, Y.; Nishina, M.S.; Paull, R.E. Papaya fruit growth, calcium uptake, and fruit ripening. J. Am. Soc. Hortic. Sci. 1995, 120, 246–253. [Google Scholar] [CrossRef]
- Madani, B.; Wall, M.; Mirshekari, A.; Bah, A.; Mohamed, M.T.M. Influence of calcium foliar fertilization on plant growth, nutrient concentrations, and fruit quality of papaya. HortTechnology 2015, 25, 496–504. [Google Scholar] [CrossRef]
- Michalczuk, L.; Kubik, M. Influence of several factors on penetration of surface applied calcium into Jonathan apple fruits at different stages of fruit development. Fruit Sci. Rep. 1984, 11, 87–97. [Google Scholar]
- Schlegel, T.K.; Schonherr, J. Stage of development affects penetration of calcium chloride into apple fruits. J. Plant Nutr. Soil Sci. 2002, 165, 738–745. [Google Scholar] [CrossRef]
- Shiri, M.A.; Ghasemnezhad, M.; Fatahi, M.J.; Ebrahimi, R. Efficiency of CaCl2 spray at different fruit development stages on the fruit mineral nutrient accumulation in cv. Hayward kiwifruit. J. Elem. 2016, 21, 195–209. [Google Scholar] [CrossRef]
- Ferguson, I.B. Calcium in plant senescence and fruit ripening. Plant Cell Environ. 1984, 7, 477–489. [Google Scholar] [CrossRef]
- Tuna, A.L.; Kaya, C.; Ashraf, M.; Altunlu, H.; Yokas, I.; Yagmur, B. The effects of calcium sulphate on growth, membrane stability and nutrient uptake of tomato plants grown under salt stress. Environ. Exp. Bot. 2007, 59, 173–178. [Google Scholar] [CrossRef]
- Poovaiah, B.W.; McFadden, J.J.; Reddy, A.S.N. The role of calcium ions in gravity singal perception and transduction. Physiol. Plant. 1987, 71, 401–407. [Google Scholar] [CrossRef]
- Bush, D.S. Calcium regulation in plant cells and its role in signaling. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 95–122. [Google Scholar] [CrossRef]
- Lecourieux, D.; Ranjeva, R.; Pugin, A. Calcium in plant defence signalling pathways. New Phytol. 2006, 171, 249–269. [Google Scholar] [CrossRef]
- Sharma, D.; Jamra, G.; Singh, U.M.; Sood, S.; Kumar, A. Calcium biofortification: Three pronged molecular approaches for dissecting complex trait of calcium nutrition in finger millet (Eleusine coracana) for devising strategies of enrichment of food crops. Front. Plant Sci. 2017, 7, 1–18. [Google Scholar] [CrossRef]
- Palmgren, M.G. Regulation of plant plasma membrane H+-ATPase activity. Physiol. Plant. 1991, 83, 314–323. [Google Scholar] [CrossRef]
- Bush, D.S. Regulation of cytosolic calcium in plants. Plant Physiol. 1993, 103, 7–13. [Google Scholar] [CrossRef]
- Sze, H.; Li, X.; Palmgren, M.G. Energization of plant cell membranes by H+-pumping ATPases: Regulation and biosynthesis. Plant Cell 1999, 11, 677–689. [Google Scholar] [PubMed]
- Smaili, S.S.; Hsu, Y.T.; Youle, R.J.; Russell, J.T. Mitochondria in Ca2+ signaling and apoptosis. J. Bioenerg. Biomembr. 2000, 32, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Luan, S.; Kudla, J.; Rodriguez-Concepcion, M.; Yalovsky, S.; Gruissem, W. Calmodulins and calcineurin B–like proteins: Calcium sensors for specific signal response coupling in plants. Plant Cell 2002, 14 (Suppl. 1), S389–S400. [Google Scholar] [CrossRef] [PubMed]
- Camello-Almaraz, C.; Gomez-Pinilla, P.J.; Pozo, M.J.; Camello, P.J. Mitochondrial reactive oxygen species and Ca2+ signaling. Am. J Physiol. Cell Physiol. 2006, 291, C1082–C1088. [Google Scholar] [CrossRef]
- Rodríguez-Serrano, M.; Romero-Puertas, M.C.; Zabalza, A.N.A.; Corpas, F.J.; Gomez, M.; Del Rio, L.A.; Sandalio, L.M. Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots. Imaging of reactive 701 oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ. 2006, 29, 1532–1544. [Google Scholar]
- Harmon, A.C. Calcium-regulated protein kinases of plants. Gravit. Space Res. 2007, 16, 83–90. [Google Scholar]
- Kou, X.H.; Wang, S.; Zhang, Y.; Guo, R.Z.; Wu, M.S.; Chen, Q.; Xue, Z.H. Effects of chitosan and calcium chloride treatments on malic acid-metabolizing enzymes and the related gene expression in post-harvest pear cv. ‘Huang guan’. Sci. Hortic. 2014, 165, 252–259. [Google Scholar] [CrossRef]
- Duque, P.; Arrabaça, J.D. Respiratory metabolism during cold storage of apple fruit. II. Alternative oxidase is induced at the climacteric. Physiol. Plant. 1999, 107, 24–31. [Google Scholar] [CrossRef]
- Lindberg, S.; Kader, M.A.; Yemelyanov, V. Calcium signalling in plant cells under environmental stress. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M.N.V., Eds.; Springer: New York, NY, USA, 2012; pp. 325–360. [Google Scholar]
- Liese, A.; Romeis, T. Biochemical regulation of in vivo function of plant calcium-dependent protein kinases (CDPK). BBA Mol. Cell Res. 2013, 1833, 1582–1589. [Google Scholar] [CrossRef] [PubMed]
- Malakouti, M.J.; Tabatabaei, S.J.; Shahabil, A.; Fallahi, E. Effects of calcium chloride on apple fruit quality of trees grown in calcareous soil. J. Plant Nutr. 1999, 22, 1451–1456. [Google Scholar] [CrossRef]
- Reynolds, R.S. The use of lead citrate at high pH as electron - opaque stain in electron microscopy. J. Cell Biol. 1963, 17, 208–213. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalik, P.; Lipa, T.; Michałojć, Z.; Chwil, M. Ultrastructure of Cells and Microanalysis in Malus domestica Borkh. ‘Szampion’ Fruit in Relation to Varied Calcium Foliar Feeding. Molecules 2020, 25, 4622. https://doi.org/10.3390/molecules25204622
Kowalik P, Lipa T, Michałojć Z, Chwil M. Ultrastructure of Cells and Microanalysis in Malus domestica Borkh. ‘Szampion’ Fruit in Relation to Varied Calcium Foliar Feeding. Molecules. 2020; 25(20):4622. https://doi.org/10.3390/molecules25204622
Chicago/Turabian StyleKowalik, Piotr, Tomasz Lipa, Zenia Michałojć, and Mirosława Chwil. 2020. "Ultrastructure of Cells and Microanalysis in Malus domestica Borkh. ‘Szampion’ Fruit in Relation to Varied Calcium Foliar Feeding" Molecules 25, no. 20: 4622. https://doi.org/10.3390/molecules25204622
APA StyleKowalik, P., Lipa, T., Michałojć, Z., & Chwil, M. (2020). Ultrastructure of Cells and Microanalysis in Malus domestica Borkh. ‘Szampion’ Fruit in Relation to Varied Calcium Foliar Feeding. Molecules, 25(20), 4622. https://doi.org/10.3390/molecules25204622