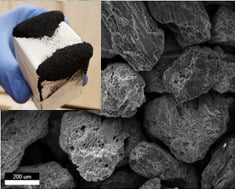
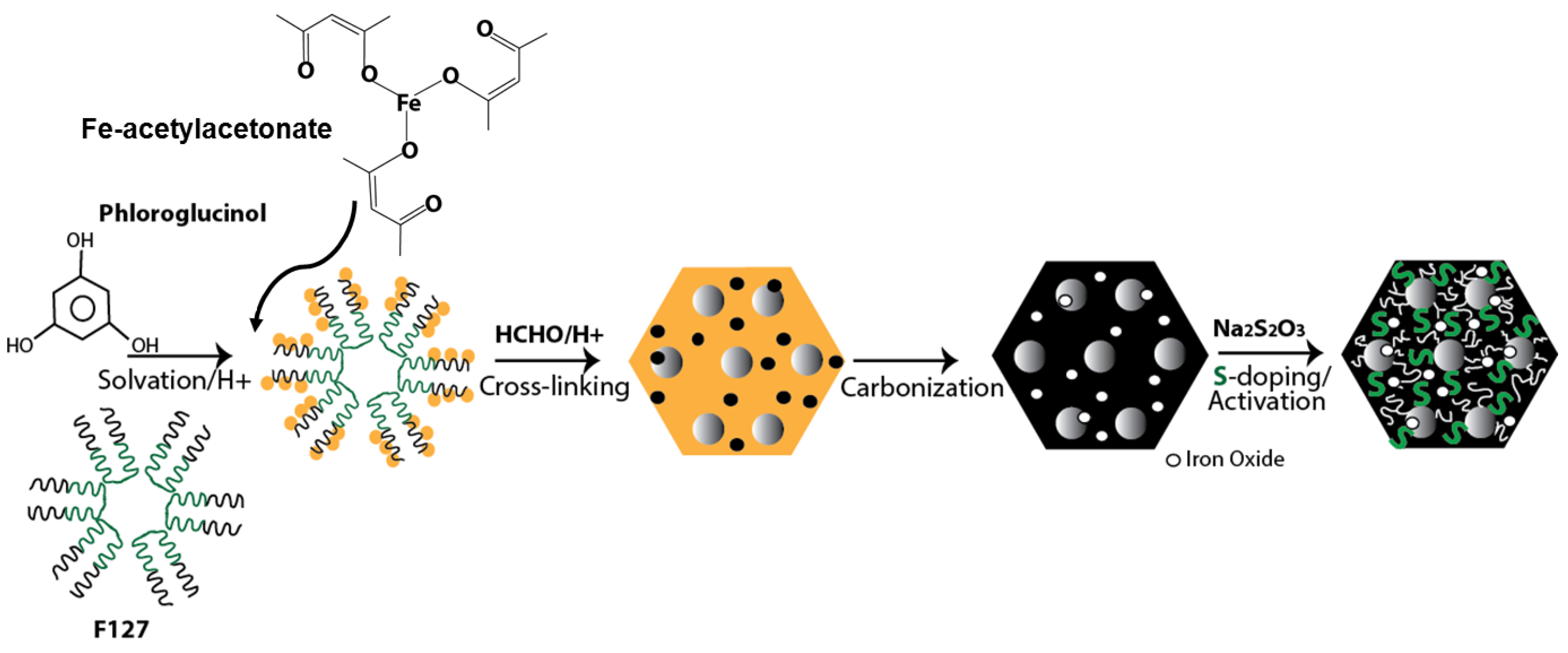
Soft-Templating of Sulfur and Iron Dual-Doped Mesoporous Carbons: Lead Adsorption in Mixtures
Abstract
1. Introduction
2. Experimental
2.1. Synthesis
2.2. Materials Characterization
2.3. Adsorption Studies
3. Results and Discussion
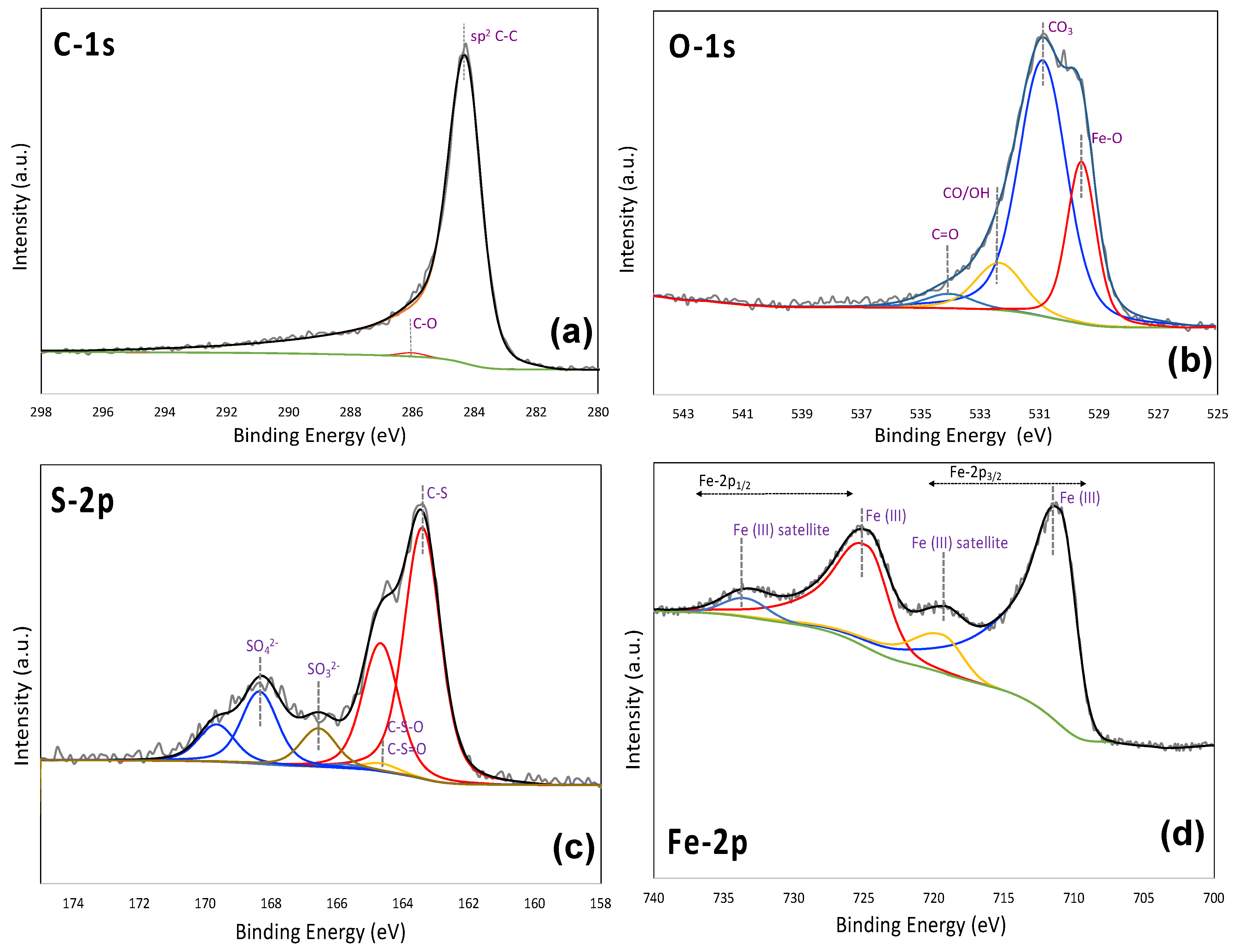
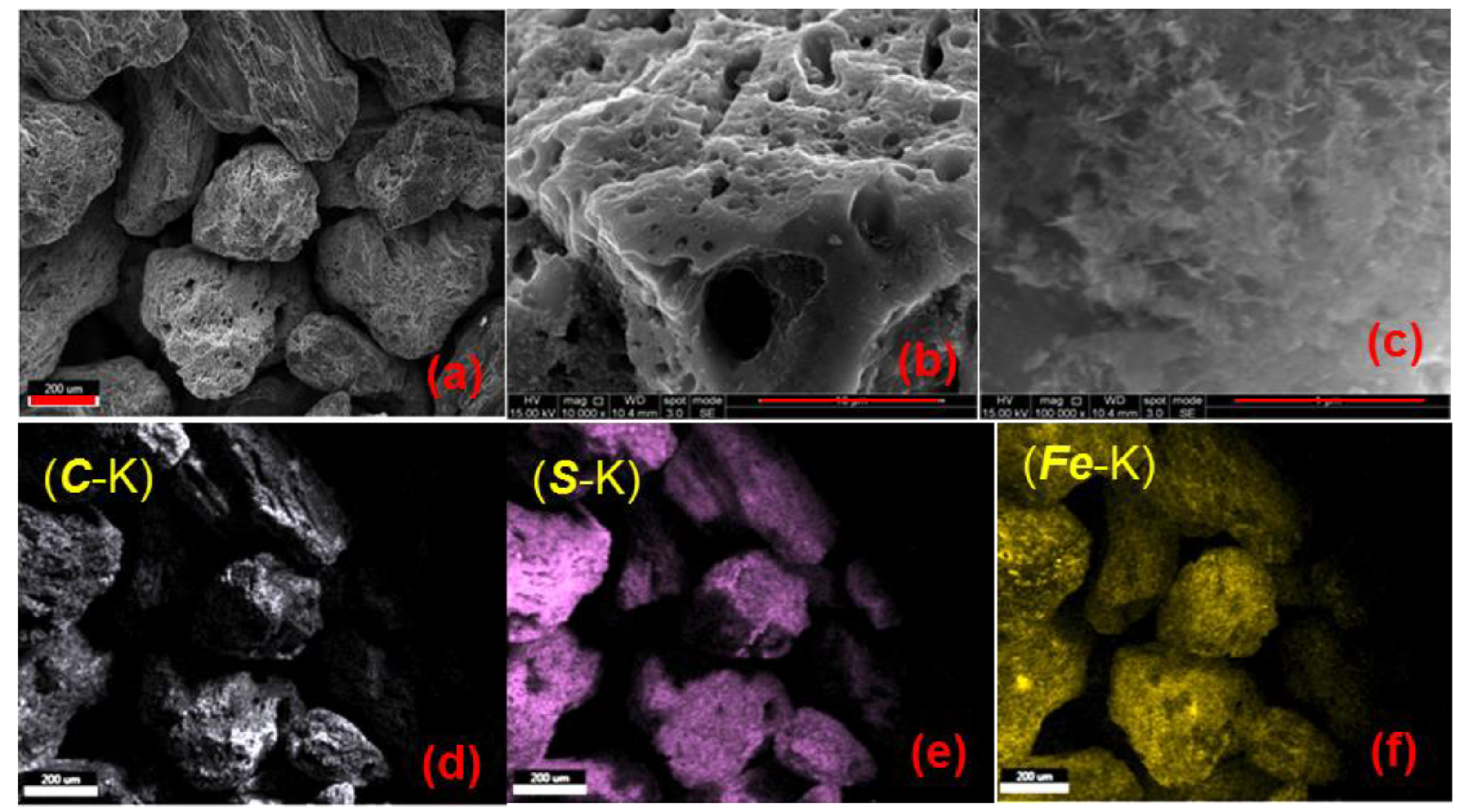
3.1. Materials Characteristics
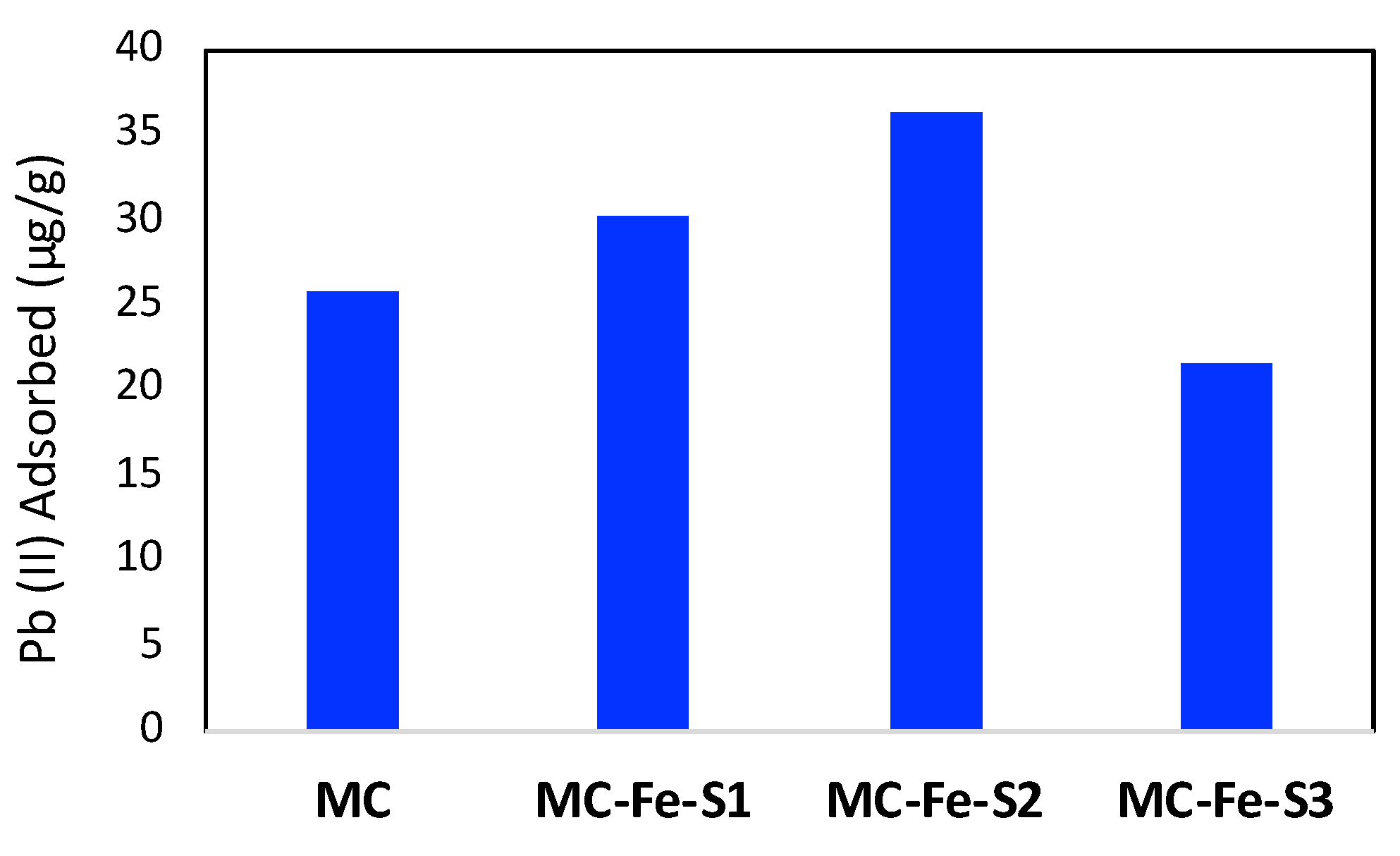
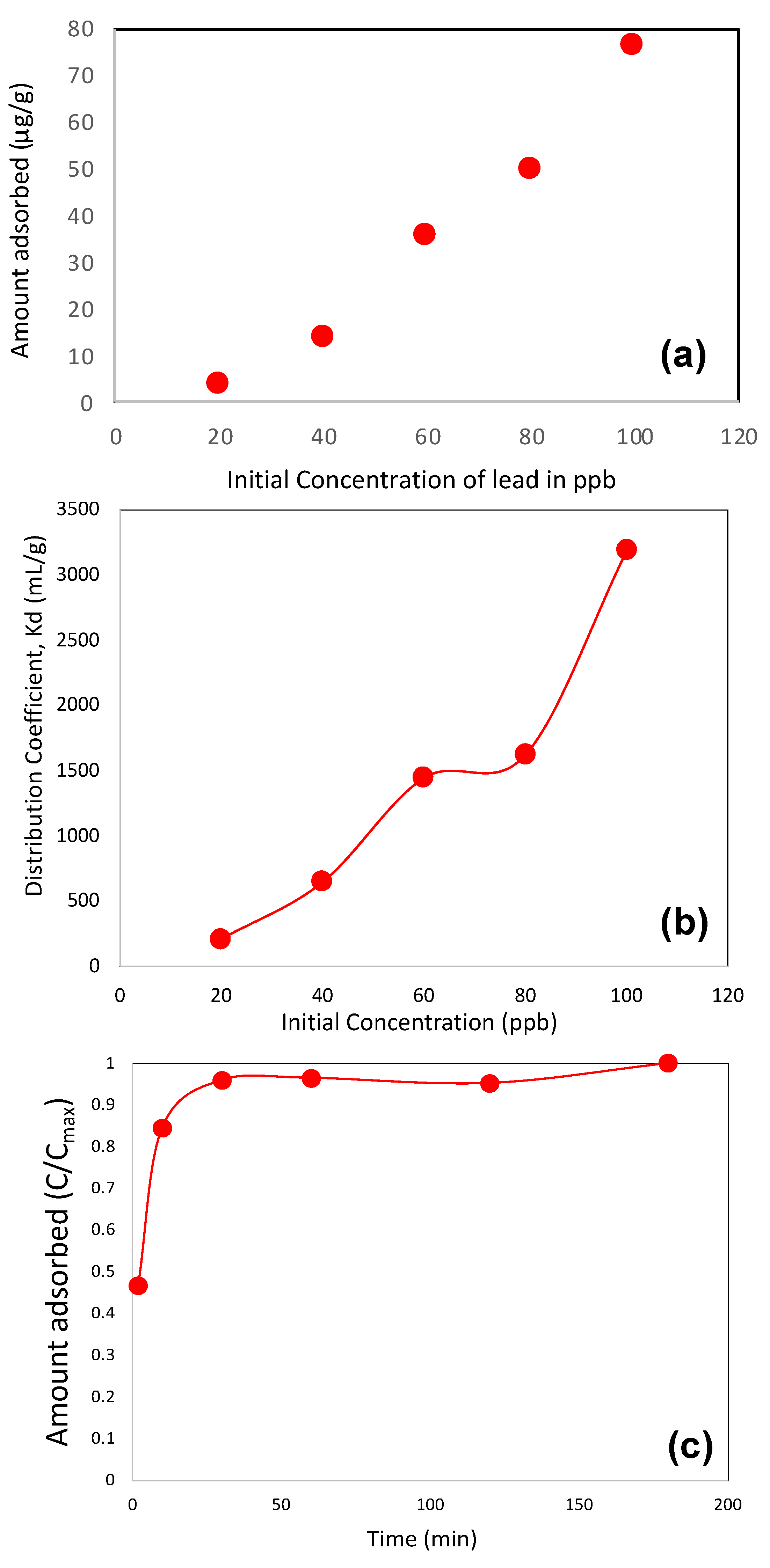
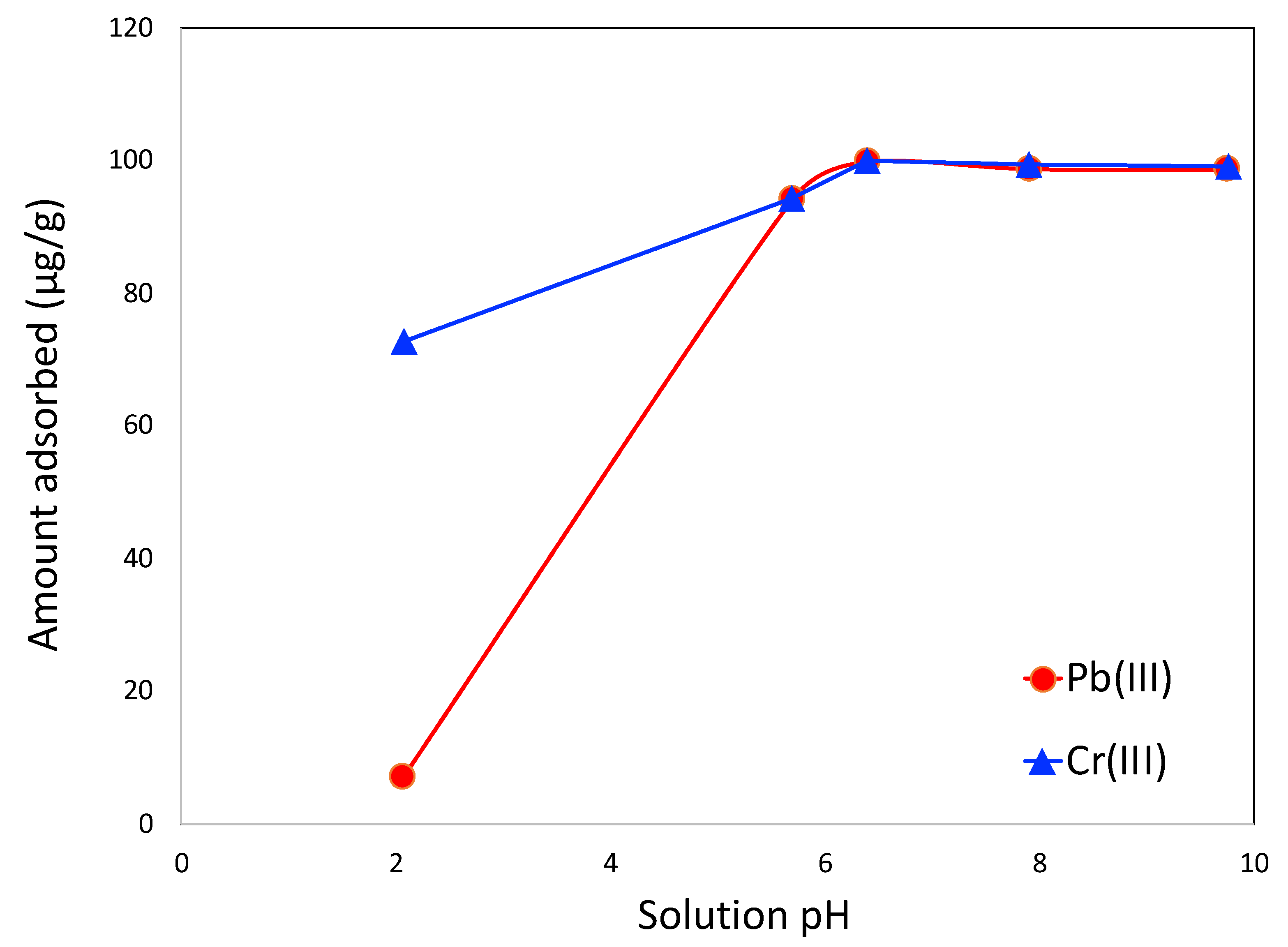
3.2. Adsorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sun, E.-B.; Poo, K.M.; Chang, J.S.; Chae, K.J. Heavy metal removal from aqueous solutions using engineered magnetic biochars derived from waste marine macro-algal biomass. Sci. Total Environ. 2019, 615, 161–168. [Google Scholar] [CrossRef]
- Gupta, V.K.; Gupta, M.; Sharma, S. Process development for the removal of lead and lead from aqueous solutions using redmud-an aluminium industrywaste. Water Res. 2001, 35, 1125–1134. [Google Scholar] [CrossRef]
- Andrew, S.C.; Taylor, M.P.; Lundregan, S.; Lien, S.; Jensen, H.; Griffith, S.C. Signs of adaptation to trace metal contamination in a common urban bird. Sci. Total Environ. 2019, 650, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Momčilović, M.; Purenović, M.; Bojić, A.; Zarubica, A.; Ranđelović, M. Removal of lead (II) ions from aqueous solutions by adsorption onto pine cone activated carbon. Desalination 2011, 276, 53–59. [Google Scholar] [CrossRef]
- Gercel, O.; Gercel, H.F. Adsorption of lead (II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem. Eng. J. 2007, 132, 289–297. [Google Scholar] [CrossRef]
- Acharya, J.; Sahu, J.N.; Mohanty, C.R.; Meikap, B.C. Removal of lead(II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chem. Eng. J. 2009, 149, 249–262. [Google Scholar] [CrossRef]
- Sulaymon, A.H.; Abid, B.A.; Al-Najar, J.A. Removal of lead, copper, chromium and cobalt onto granular activated carbon in batch and fixed-bed adsorbers. Chem. Eng. J. 2009, 155, 647–653. [Google Scholar] [CrossRef]
- Husein, M.M.; Vera, J.H.; Weber, M.E. Removal of lead from aqueous solutions with sodium caprate. Sep. Sci. Technol. 1998, 33, 1889–1904. [Google Scholar] [CrossRef]
- Lin, S.W.; Navarro, R.M.F. An innovative method for removing Hg2+ and Pb2+ in ppm concentrations from aqueous media. Chemosphere 1999, 39, 1809–1817. [Google Scholar] [CrossRef]
- Yu, W.; Zhang, P.; Yang, J.; Li, M.; Hu, Y.; Liang, S.; Wang, J.; Li, S.; Xiao, K.; Hou, H.; et al. A low-emission strategy to recover lead compound products directly from spent lead-acid battery paste: Key issue of impurities removal. J. Clean. Prod. 2019, 210, 1534–1544. [Google Scholar] [CrossRef]
- Saeed, A.; Iqbal, M.; Akhtar, M.W. Removal and recovery of lead (II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J. Hazard. Mater. 2005, 117, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Doyurum, S.; Celik, A. Pb(II) and Cd(II) removal fromaqueous solutions by olive cake. J. Hazard. Mater. 2006, 138, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Boonamnuayvitaya, V.; Chaiya, C.; Tanthapanichakoon, W.; Jarudilokkul, S. Removal of heavy metals by adsorbent prepared from pyrolyzed coffee residues and clay. Sep. Purif. Technol. 2004, 35, 11–22. [Google Scholar] [CrossRef]
- Teles de Vasconcelos, L.A.; Gonzalez Beca, C.G. Adsorption equilibria between pine bark and several ions in aqueous solution, 1. Pb(II). Eur. Water Poll. Control 1994, 4, 41–51. [Google Scholar]
- Ahmedna, M.; Marshall, W.E.; Husseiny, A.A.; Rao, R.M.; Goktepe, I. The use of nutshell carbons in drinking water filters for removal of trace metals. Water Res. 2004, 38, 1062–1068. [Google Scholar] [CrossRef]
- Saha, D.; Barakat, S.; Van Bramer, S.; Nelson, K.A.; Hensley, D.K.; Chen, J. Non-competitive and competitive adsorption of heavy metals in sulfur-functionalized ordered mesoporous carbon. ACS Appl. Mater. Interfaces 2016, 8, 34132–34142. [Google Scholar] [CrossRef]
- Hadi, P.; To, M.-H.; Hui, C.-W.; Lin, C.S.Z.; McKay, G. Aqueous mercury adsorption by activated carbons. Water Res. 2015, 73, 37–55. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Wu, W.; Wei, S.; Xu, Y.; Kuang, Y. Studies of heavy metal ion adsorption on Chitosan/Sulfydryl functionalized graphene oxide composites. J. Colloid Interface Sci. 2015, 448, 389–397. [Google Scholar] [CrossRef]
- Chapman, V.L. The relationship of lead and sulfur in a chemical reaction. J. Chem. Educ. 1977, 54, 436. [Google Scholar] [CrossRef]
- Gonick, H.C. Lead-binding proteins: A review. J. Toxicol. 2011, 2011. [Google Scholar] [CrossRef]
- Cangelosi, V.; Ruckthong, L.; Pecoraro, V.L. Lead (II) binding in natural and artificial proteins. In Lead—Its Effects on Environment and Health; Sigel, A., Sigel, H., Sigel, R.K.O., Eds.; Walter de Gruyter GmbH & Co KG: Berlin, Germany; Boston, MA, USA, 2017; pp. 271–318. [Google Scholar] [CrossRef]
- Saha, D.; Orkoulas, G.; Chen, J.; Hensley, D.K. Adsorptive separation of CO2 in sulfur-doped nanoporous carbons: Selectivity and breakthrough simulation. Microporous Mesoporous Mater. 2017, 241, 226–237. [Google Scholar] [CrossRef]
- Saha, D.; Spurri, A.; Chen, J.; Helsely, D.K. Controlled release of alendronate from nitrogen-doped mesoporous carbons. Microporous Mesoporous Mater. 2016, 229, 8–13. [Google Scholar] [CrossRef]
- Saha, D.; Moken, T.; Chen, J.; Henseley, D.; Delaney, K.; Hunt, M.A.; Nelson, K.A.; Spurri, A.; Benham, L.; Brice, R.; et al. Micro-/mesoporous carbons for controlled release of antipyrine and indomethacin. RSC Adv. 2015, 5, 23699–23707. [Google Scholar] [CrossRef]
- Saha, D.; Zacharia, R.; Naskar, A.K. Soft-templated mesoporous carbons: Chemistry and Characteristics, Polymer derived carbon, 2014. ACS Symp. Ser. 2014, 1173. [Google Scholar] [CrossRef]
- Remy, H. Treatise, on Inorganic Chemistry; Elsevier: Amsterdam, The Netherlands, 1956. [Google Scholar]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases, HSAB, part II: Underlying theories. J. Chem. Educ. 1968, 45, 643. [Google Scholar] [CrossRef]
- Gomez-Serrano, V.; Macias-Garcia, A.; Espinosa-Mansilla, A.; Valenzuela-Calahorro, C. Adsorption of mercury, cadmium and lead from aqueous solution on heat-treated and sulphurized activated carbon. Water Res. 1998, 32, 1–4. [Google Scholar] [CrossRef]
- Saha, D.; Akkoyunlu, S.D.; Thorpe, R.; Hensley, D.K.; Chen, J. Adsorptive recovery of neodymium and dysprosium in phosphorous functionalized nanoporous carbon. J. Environ. Chem. Eng. 2017, 5, 4684–4692. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles and Adsorption and Adsorption Processes; Willey Interscience: New York, NY, USA, 1984. [Google Scholar]
- Pan, S.; Shen, H.; Xu, Q.; Luo, J.; Hu, M. Surface mercapto engineered magnetic Fe3O4 nanoadsorbent for the removal of mercury from aqueous solutions. J. Colloid Interface Sci. 2012, 365, 204–212. [Google Scholar] [CrossRef]
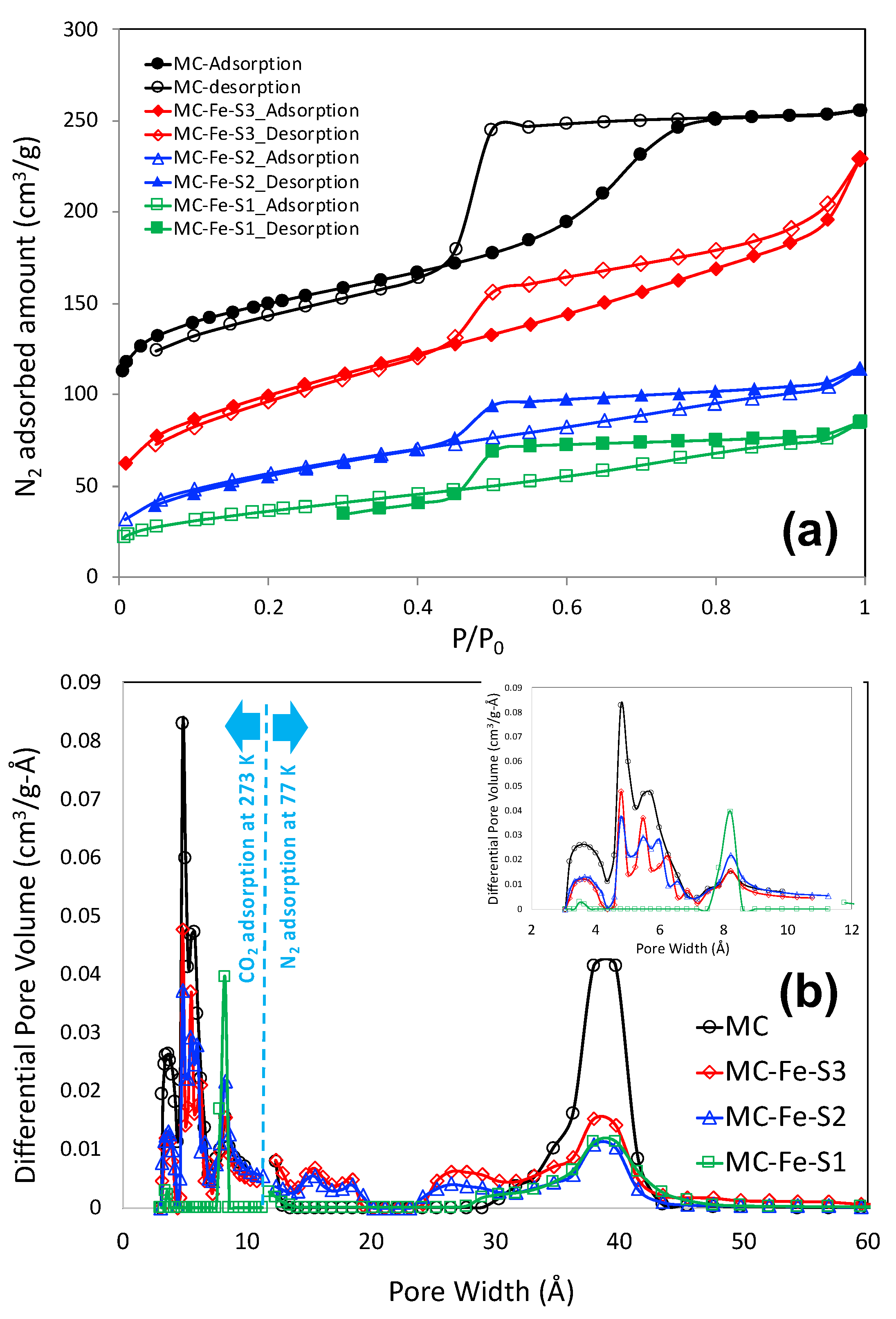


| Adsorbents | BET SSA (m2/g) | Micropore Volume (cm3/g) | Mesopore Volume (cm3/g) | Total Pore Volume (cm3/g) |
|---|---|---|---|---|
| MC | 509 | 0.147 | 0.216 | 0.363 |
| MC-Fe-S1 | 130 | 0.0019 | 0.115 | 0.117 |
| MC-Fe-S2 | 205 | 0.0046 | 0.152 | 0.157 |
| MC-Fe-S3 | 352 | 0.0083 | 0.298 | 0.306 |
| MC-Fe-S1 | MC-Fe-S2 | MC-Fe-S3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | 63.0% | C-C sp2 | 57.1% | C | 60.5% | C-C sp2 | 59.7% | C | 67.6% | C-C sp2 | 53.0% |
| C-C sp3 | 2.6% | C-C sp3 | <0.1% | C-C sp3 | 10.2% | ||||||
| C-O | 3.2% | C-O | 0.4% | C-O | 1.9% | ||||||
| C=O COOH O-C=O | <0.1% | C=O COOH O-C=O | 0.4% | C=O COOH O-C=O | 2.5% | ||||||
| S | 4.4% | Fe-S | <0.1% | S | 6.1% | Fe-S | <0.1% | S | 5.9% | Fe-S | <0.1% |
| C-S | 2.6% | C-S | 4.1% | C-S | 4.0% | ||||||
| C-S-O C-S=O | 0.4% | C-S-O C-S=O | 0.1% | C-S-O C-S=O | 0.7% | ||||||
| -SOx | 1.4% | -SOx | 1.8% | -SOx | 1.2% | ||||||
| O | 24.1% | Fe-O | 5.2% | O | 24.4% | Fe-O | 5.8% | O | 18.7% | Fe-O | 5.3% |
| Other | 18.9% | Other | 18.6% | Other | 13.4% | ||||||
| Fe | 8.6% | Fe2O3 | ~5.2% | Fe | 9.0% | Fe2O3 | ~5.8% | Fe | 7.8% | Fe2O3 | ~5.3% |
| FeSO4 | ~1.4% | FeSO4 | ~1.8% | FeSO4 | ~1.2% | ||||||
| Metals | Diffusion Time Constant (Dc/rc2) (s−1) | Pseudosecond Order Rate Constant (k2) (g−1mg−1s−1) |
|---|---|---|
| Pb (II), pure | 1.52 × 10-4 | 8.83 × 10−5 |
| Pb (II), mix | 1.01 × 10-4 | 1.18 × 10−4 |
| Cr (III), mix | 1.54 × 10-4 | 4.70 × 10−4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saha, D.; Richards, C.P.; Haines, R.G.; D’Alessandro, N.D.; Kienbaum, M.J.; Griffaton, C.A. Soft-Templating of Sulfur and Iron Dual-Doped Mesoporous Carbons: Lead Adsorption in Mixtures. Molecules 2020, 25, 403. https://doi.org/10.3390/molecules25020403
Saha D, Richards CP, Haines RG, D’Alessandro ND, Kienbaum MJ, Griffaton CA. Soft-Templating of Sulfur and Iron Dual-Doped Mesoporous Carbons: Lead Adsorption in Mixtures. Molecules. 2020; 25(2):403. https://doi.org/10.3390/molecules25020403
Chicago/Turabian StyleSaha, Dipendu, Connelly P. Richards, Robert G. Haines, Nicholas D. D’Alessandro, Madeleine J. Kienbaum, and Christian A. Griffaton. 2020. "Soft-Templating of Sulfur and Iron Dual-Doped Mesoporous Carbons: Lead Adsorption in Mixtures" Molecules 25, no. 2: 403. https://doi.org/10.3390/molecules25020403
APA StyleSaha, D., Richards, C. P., Haines, R. G., D’Alessandro, N. D., Kienbaum, M. J., & Griffaton, C. A. (2020). Soft-Templating of Sulfur and Iron Dual-Doped Mesoporous Carbons: Lead Adsorption in Mixtures. Molecules, 25(2), 403. https://doi.org/10.3390/molecules25020403