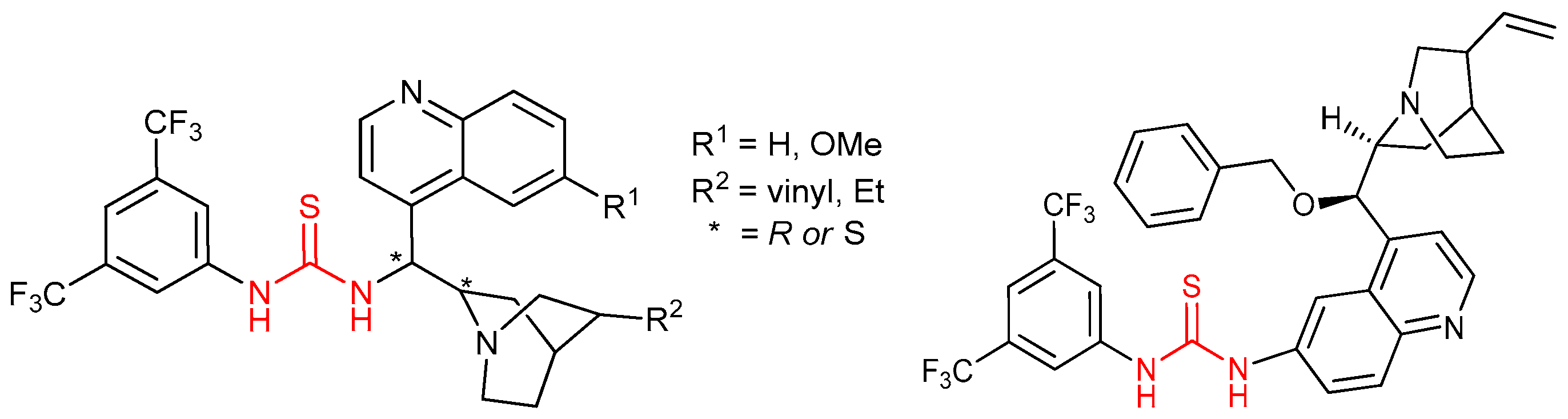Chiral Thioureas—Preparation and Significance in Asymmetric Synthesis and Medicinal Chemistry
Abstract
1. Introduction
2. Synthesis of Chiral Thioureas
2.1. Reaction of Isothiocyanates with Amines
2.2. Reaction of Amines with Dithiocarbamates
2.3. The Use of Carbon Disulfide
2.4. Application of Other Compounds Containing C=S Bond
2.5. Preparation of Chiral Thioureas from Other Thioureas
2.6. C=S Bond Formation
3. Types of Chiral Thioureas and Selected Applications in Asymmetric Synthesis
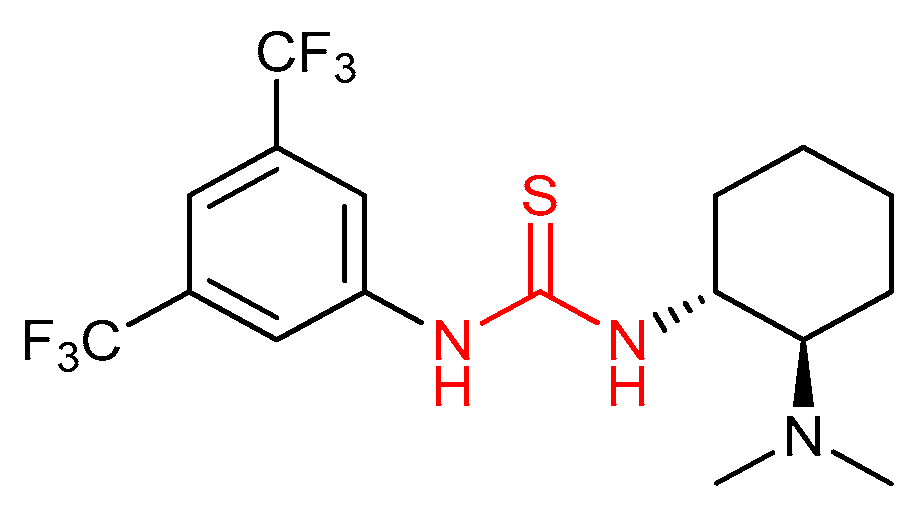
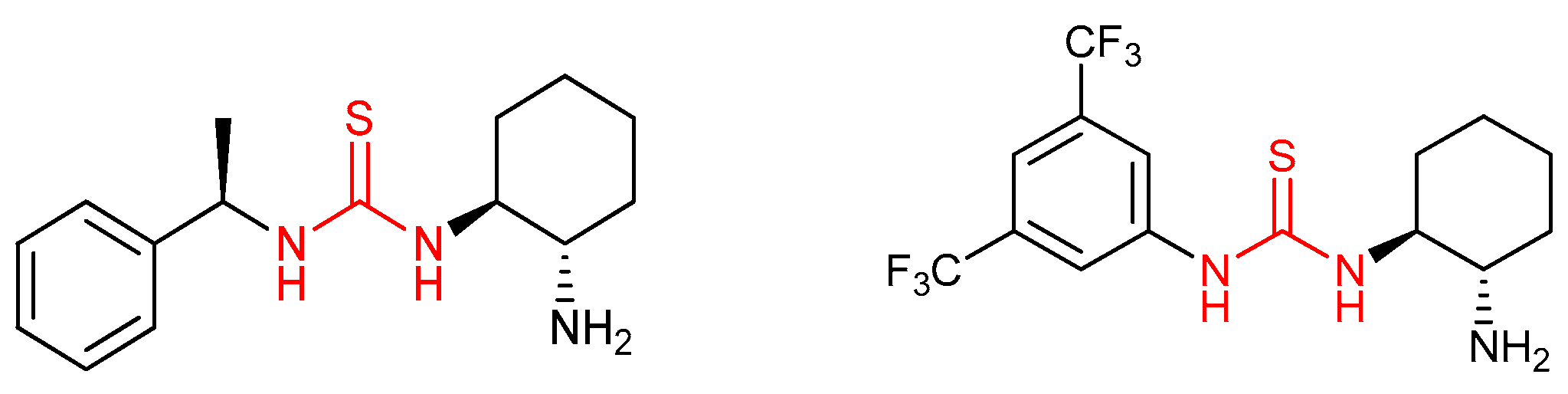
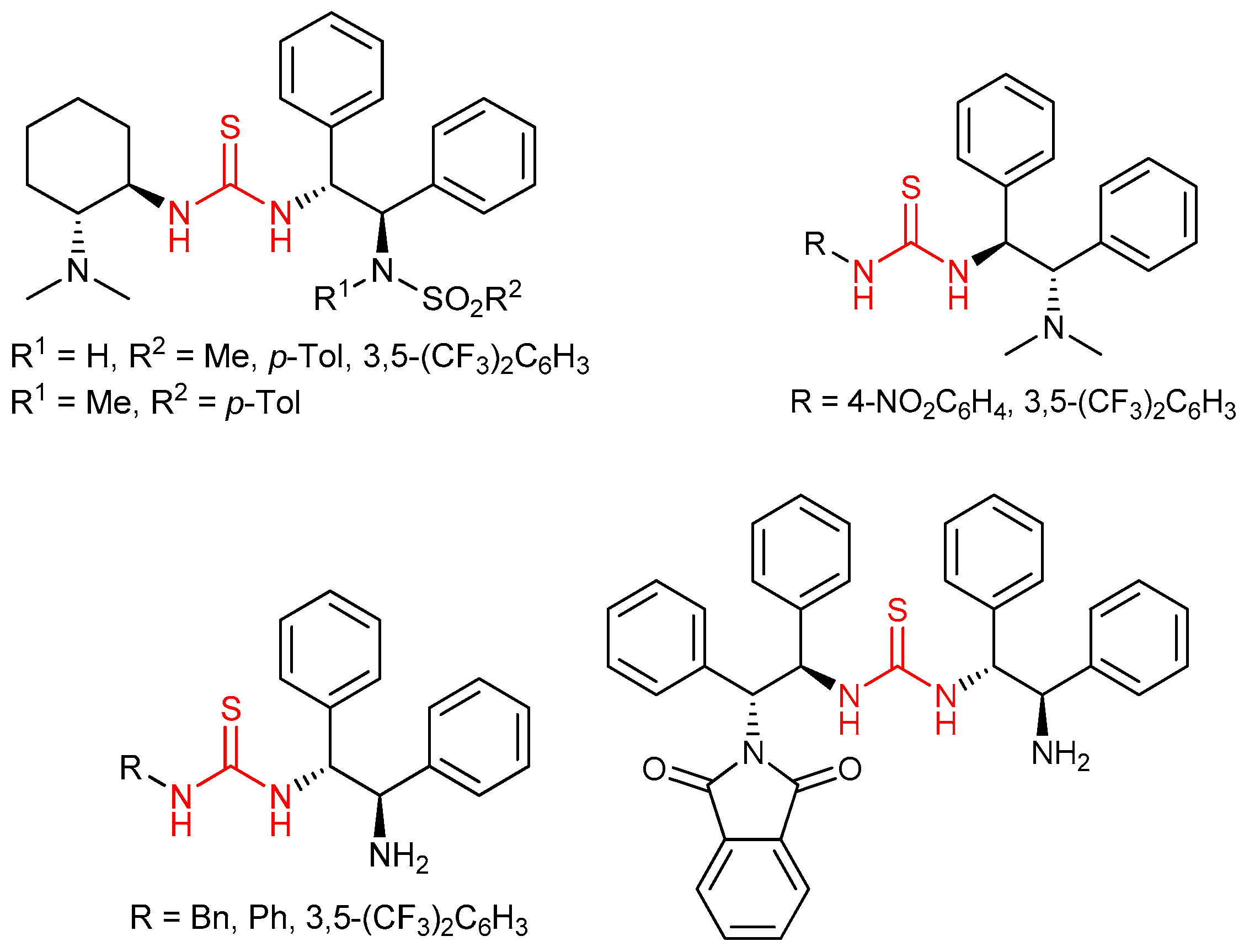
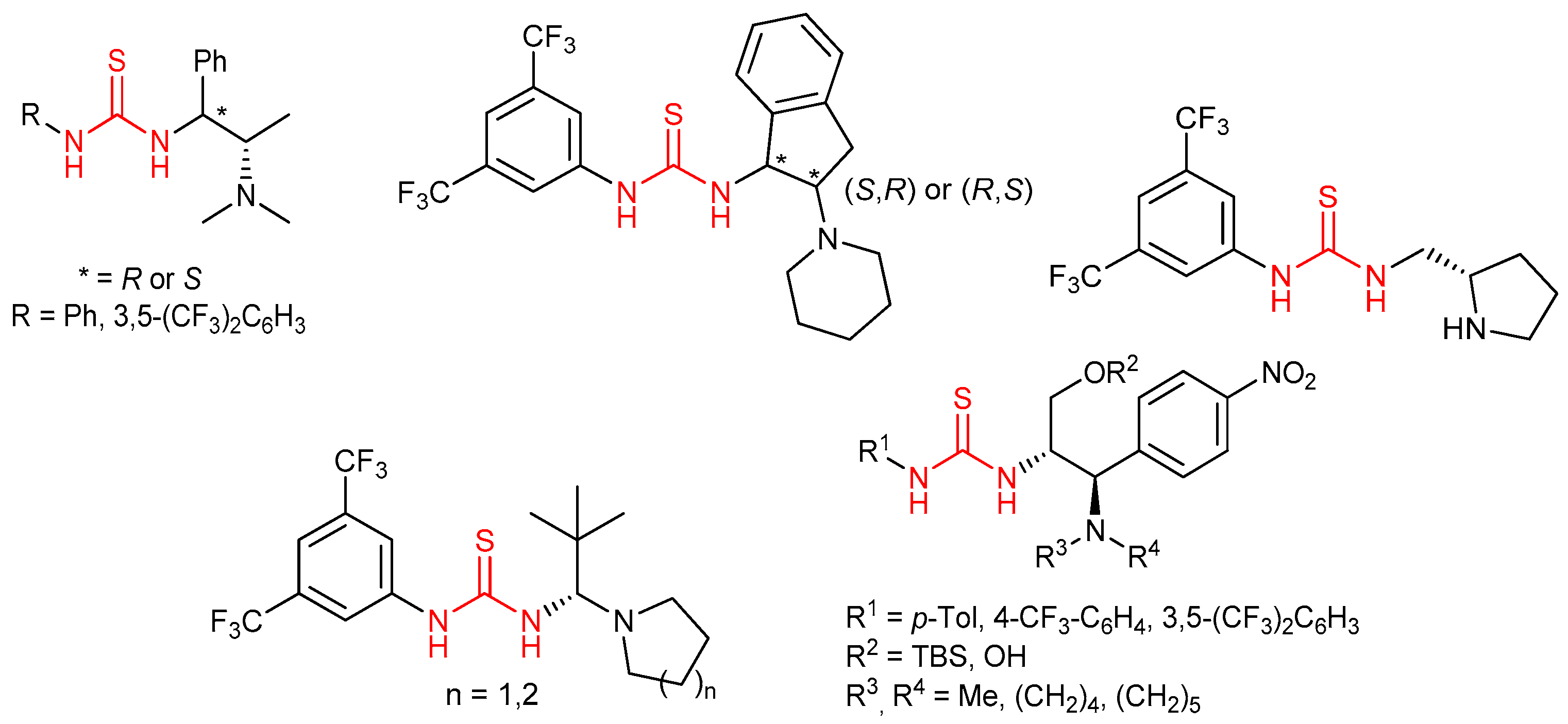
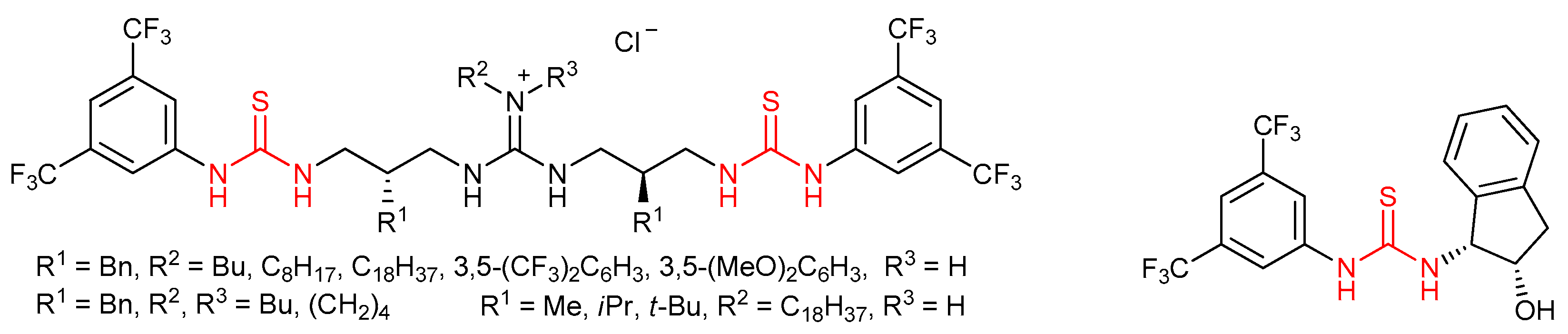
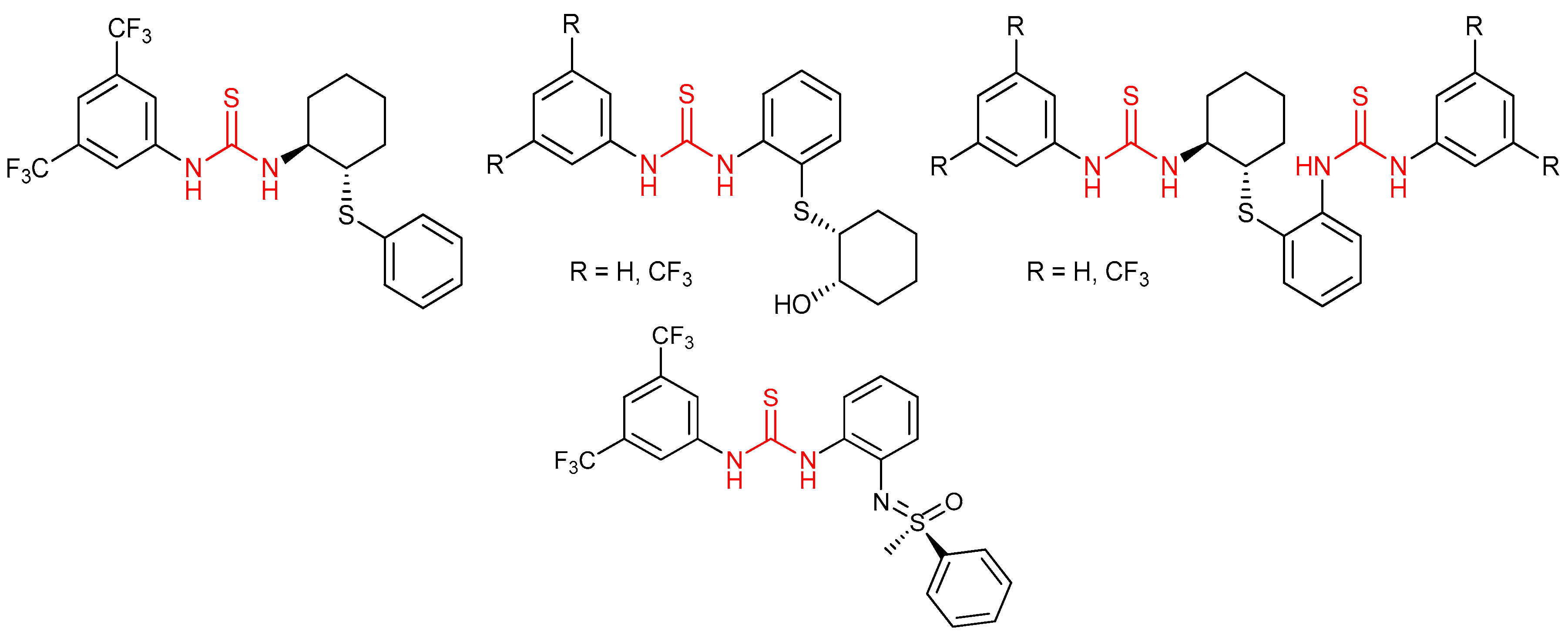
3.1. Thioureas Containing trans-1,2-diaminocyclohexane (DACH) Skeleton and Other Chiral Diamines
3.2. Thioureas Containing Cinchona Alkaloids
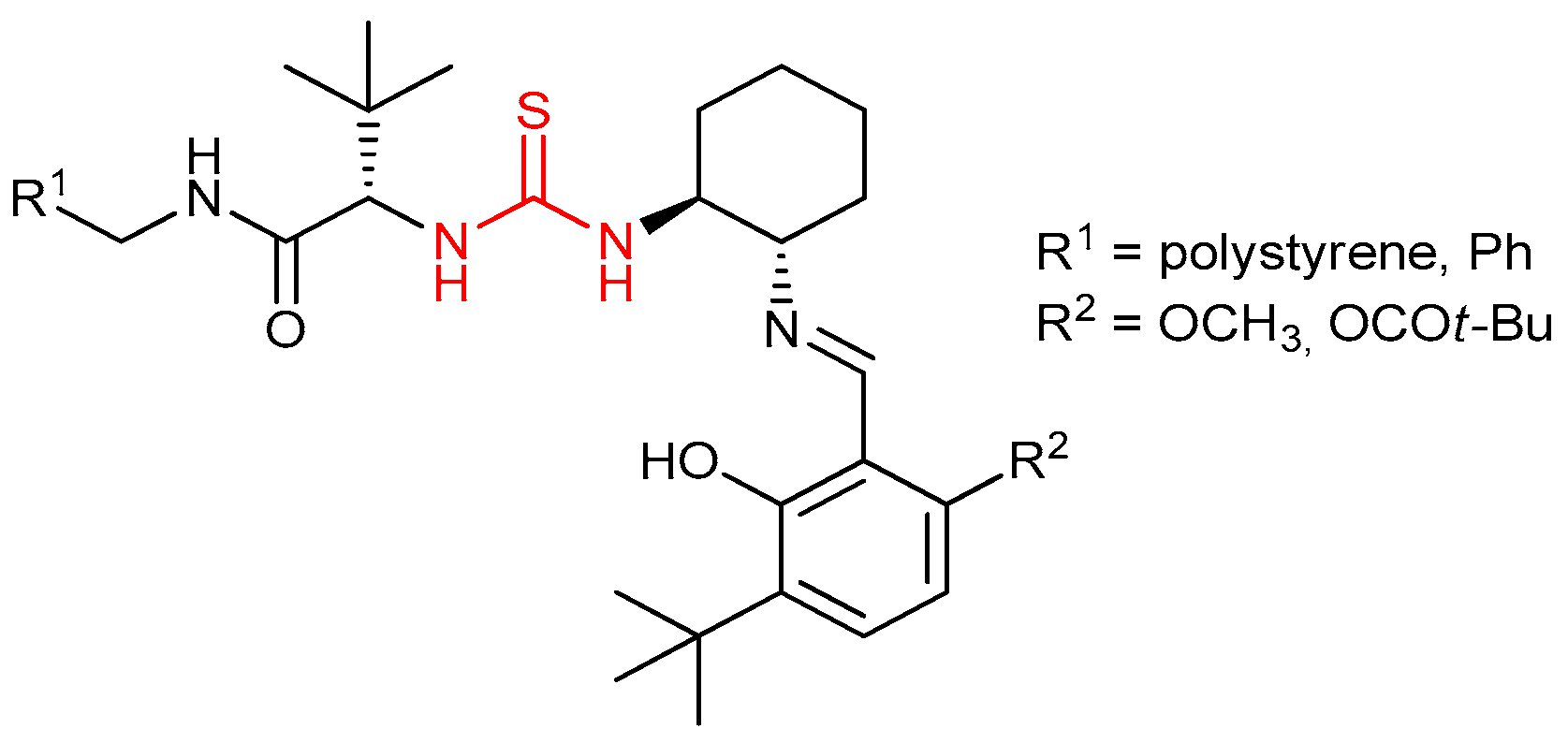
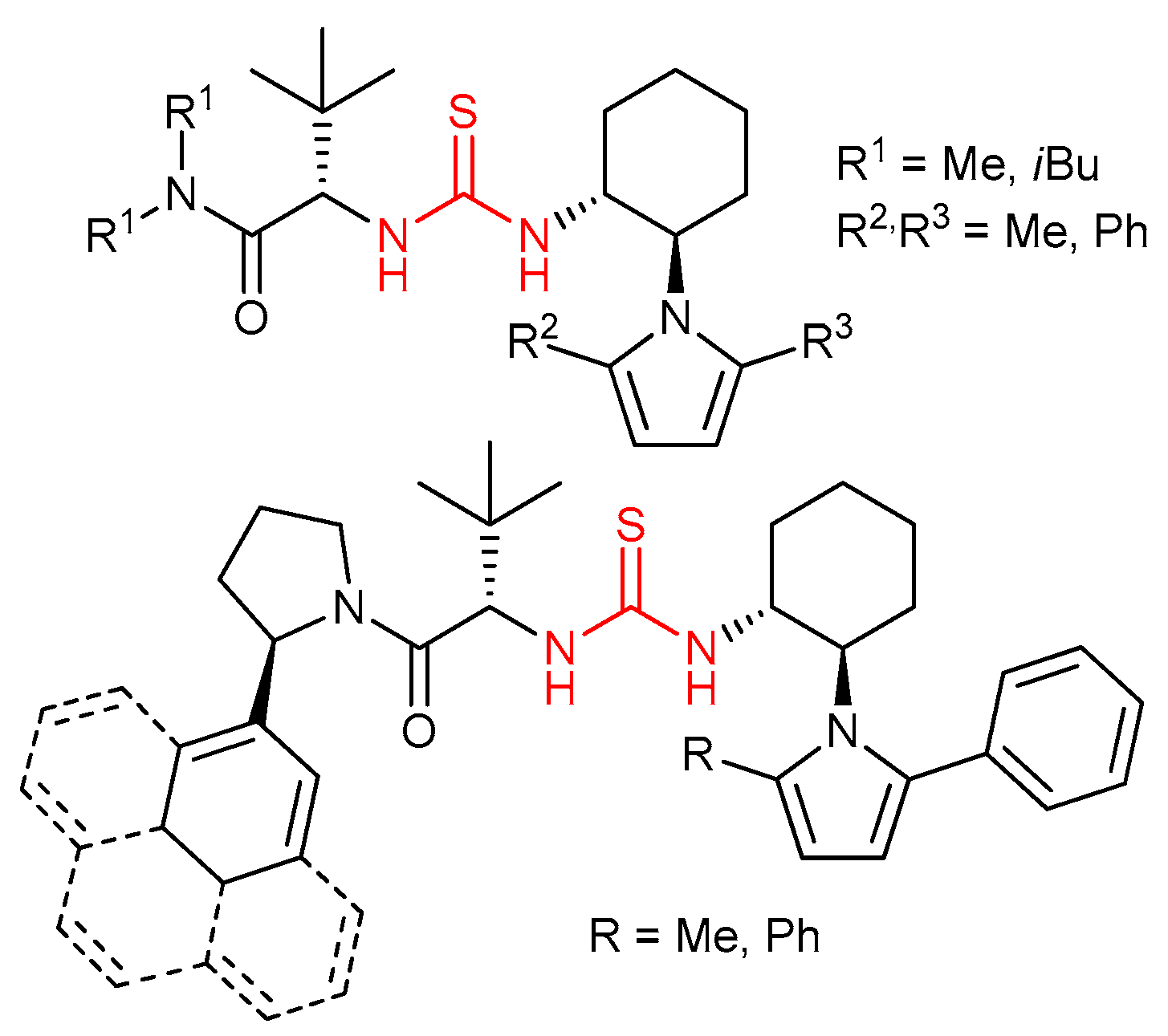
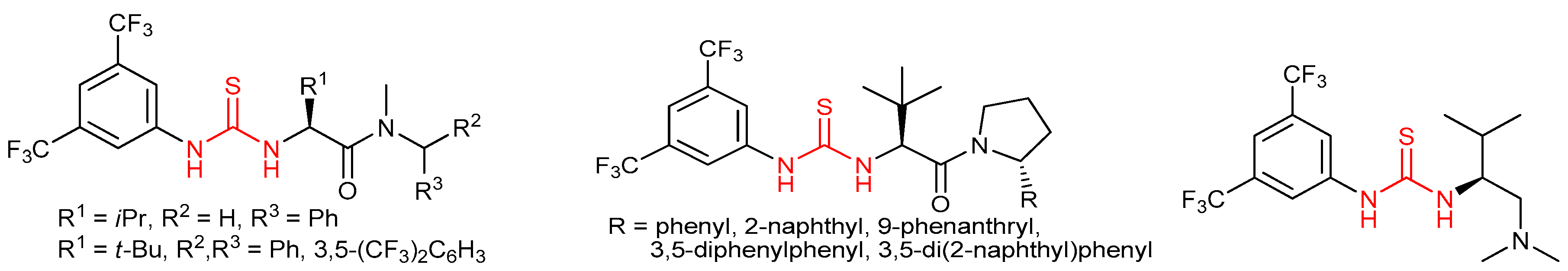
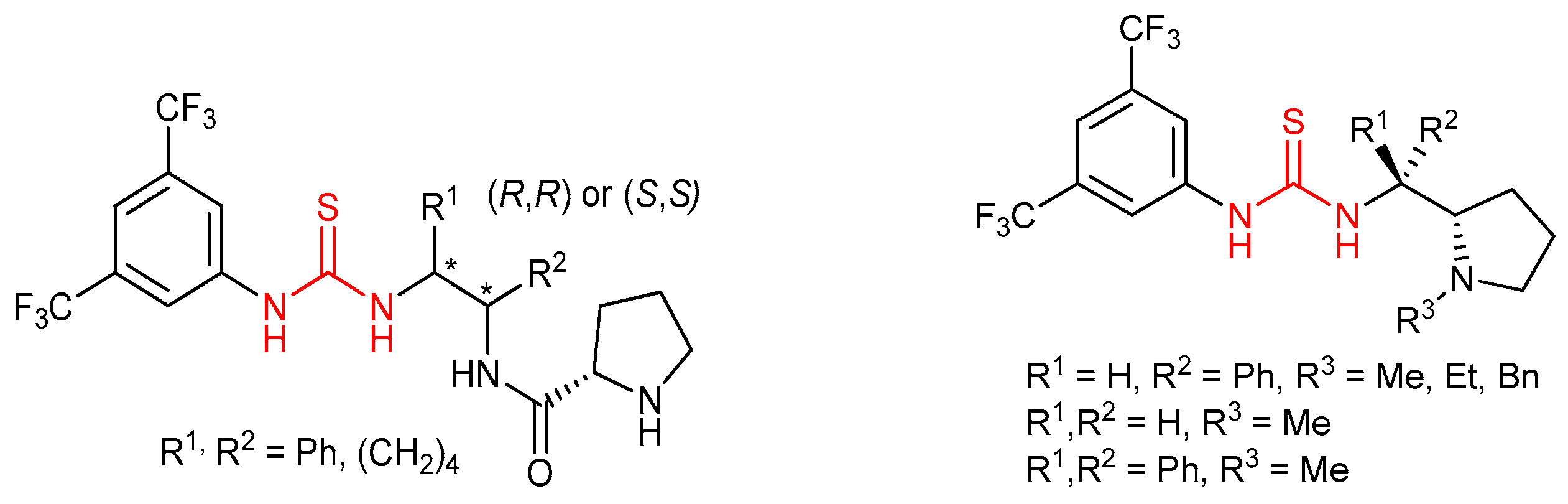
3.3. Thioureas Derived from Amino Acids and Peptides
3.4. Carbohydrate-Based Chiral Thioureas
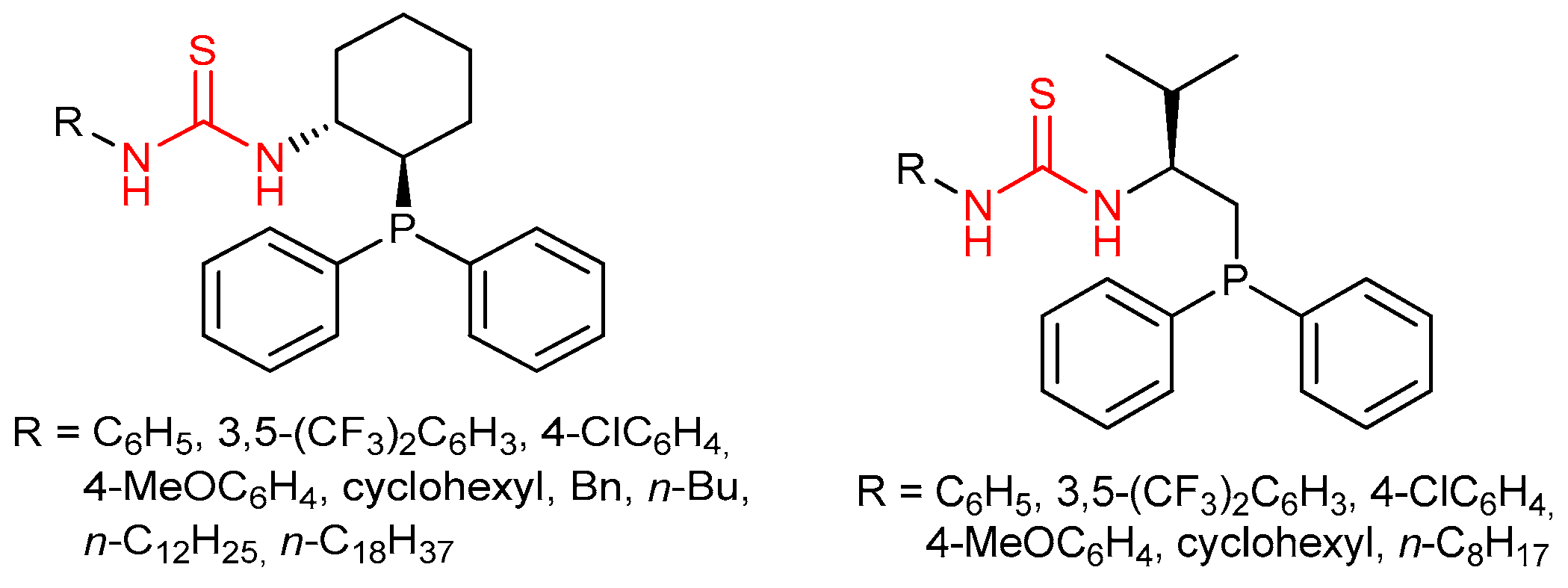
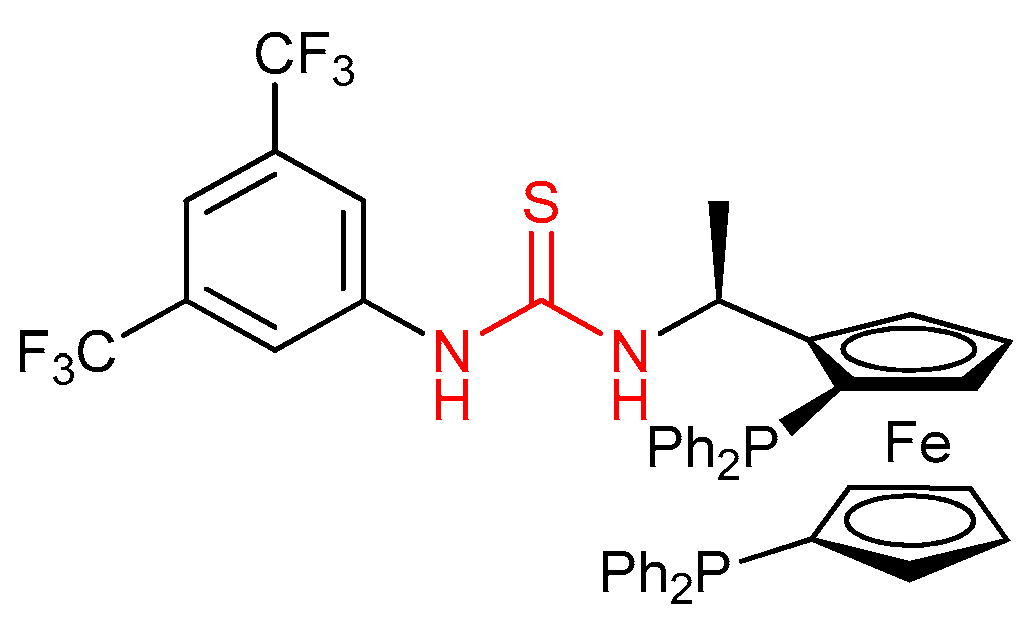
3.5. Chiral Phosphine-Bearing Thioureas
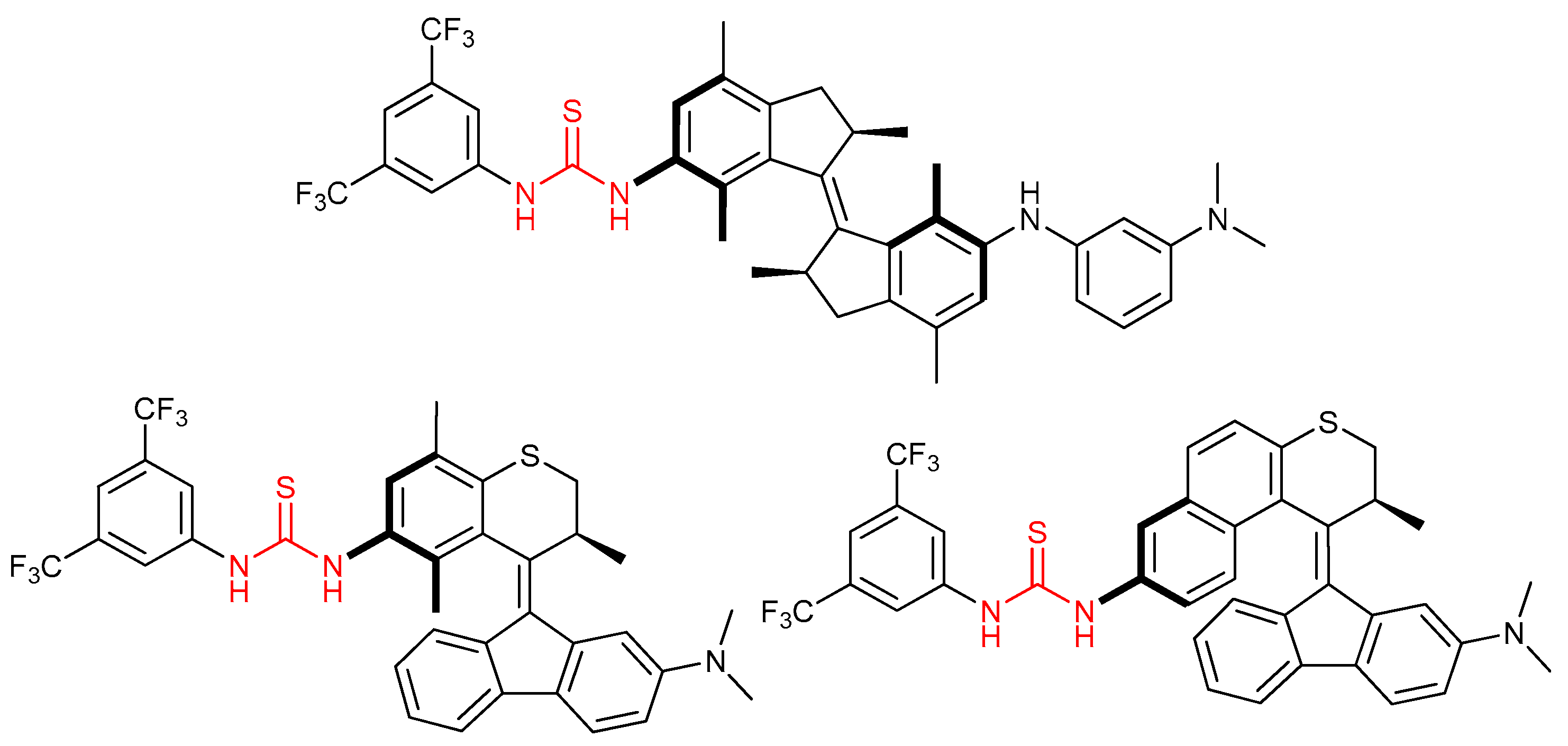
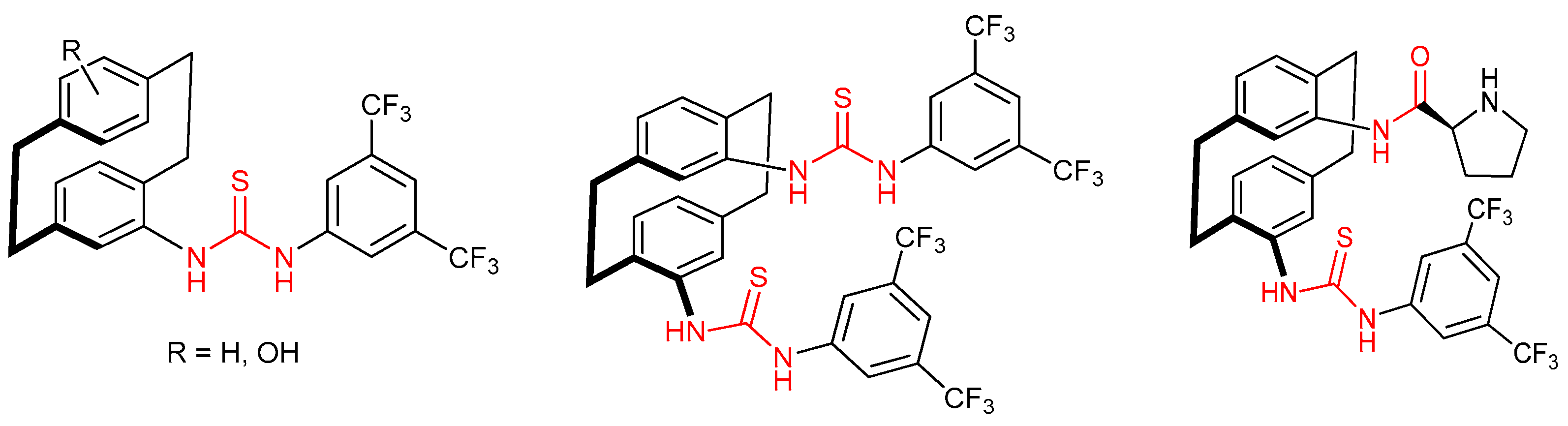
3.6. Thioureas and Bis-Thioureas with Axial, Planar or Helical Chirality
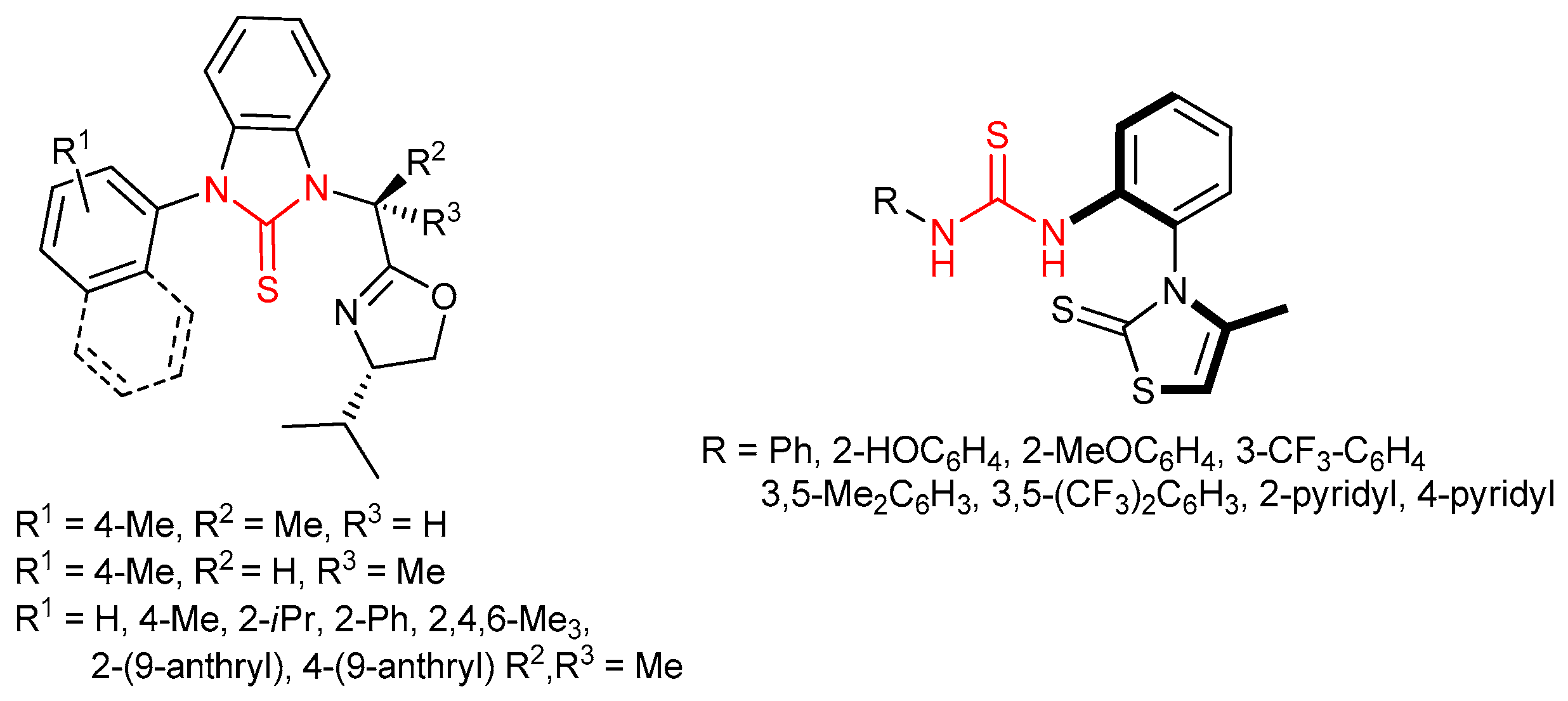
3.7. Thioureas Containing Other Functional Groups
4. Biological Activity of Chiral Thioureas
4.1. Antiviral Thioureas
4.2. Anticancer Thioureas
4.3. Anti-Allergic Thioureas
4.4. Antimicrobial Thioureas
5. Summary
Funding
Conflicts of Interest
References
- Schroeder, D.C. Thioureas. Chem. Rev. 1955, 55, 181–228. [Google Scholar] [CrossRef]
- Gómez, D.E.; Fabbrizzi, L.; Licchelli, M.; Monzani, E. Urea vs. thiourea in anion recognition. Org. Biomol. Chem. 2005, 3, 1495–1500. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, W. Hydrogen-bond-mediated asymmetric catalysis. Chem. Asian J. 2008, 3, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Ruder, F.J.; Guyer, W.; Benson, J.A.; Kayser, H. The thiourea insecticide/acaricide diafenthiuron has a novel mode of action: Inhibition of mitochondrial respiration by its carbodiimide product. Pestic. Biochem. Physiol. 1991, 41, 207–219. [Google Scholar] [CrossRef]
- Xiao, S.; Wei, L.; Hong, Z.; Rao, L.; Ren, Y.; Wan, J.; Feng, L. Design, synthesis and algicides activities of thiourea derivatives as the novel scaffold aldolase inhibitors. Bioorg. Med. Chem. 2019, 27, 805–812. [Google Scholar] [CrossRef]
- Shakeel, A.; Altaf, A.A.; Qureshi, A.M.; Badshah, A. Thiourea derivatives in drug design and medicinal chemistry: A short review. J. Drug Des. Med. Chem. 2016, 2, 10–20. [Google Scholar] [CrossRef]
- Choi, J.; Jee, J.-G. Repositioning of thiourea-containing drugs as tyrosinase inhibitors. Int. J. Mol. Sci. 2015, 16, 28534–28548. [Google Scholar] [CrossRef]
- Subramanyam, N.C.; Sheshadri, B.S.; Mayanna, S.M. Thiourea and substituted thioureas as corrosion inhibitors for aluminium in sodium nitrite solution. Corros. Sci. 1993, 34, 563–571. [Google Scholar] [CrossRef]
- Edrah, S.; Hasan, S. Studies on thiourea derivatives as corrosion inhibitor for aluminum in sodium hydroxide solution. J. Appl. Sci. Res. 2010, 6, 1045–1049. [Google Scholar]
- Loto, R.T.; Loto, C.A.; Popoola, A.P.I. Corrosion inhibition of thiourea and thiadiazole derivatives: A Review. J. Mater. Environ. Sci 2012, 3, 885–894. [Google Scholar]
- Fatima, S.; Sharma, R.; Asghar, F.; Kamal, A.; Badshah, A.; Kraatz, H.-B.B. Study of new amphiphiles based on ferrocene containing thioureas as efficient corrosion inhibitors: Gravimetric, electrochemical, SEM and DFT studies. J. Ind. Eng. Chem. 2019, 76, 374–387. [Google Scholar] [CrossRef]
- Kotke, M.; Schreiner, P.R. Organocatalysts. In Hydrogen Bonding in Organic Synthesis; Pihko, P.M., Ed.; Wiley-VCH: Hoboken, NJ, USA, 2009; pp. 141–251. [Google Scholar]
- Takemoto, Y. Recognition and activation by ureas and thioureas: Stereoselective reactions using ureas and thioureas as hydrogen-bonding donors. Org. Biomol. Chem. 2005, 3, 4299–4306. [Google Scholar] [CrossRef] [PubMed]
- Connon, S.J. Asymmetric catalysis with bifunctional Cinchona alkaloid-based urea and thiourea organocatalysts. Chem. Commun. 2008, 2499–2510. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.-K.; Liu, Y.F.; Lan, Z.; Wen, L.-R.; Li, M. Nickle catalysis enables access to thiazolidines from thioureas via oxidative double isocyanide insertion reactions. Org. Lett. 2018, 20, 7158–7162. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Bhowmik, S.; Batra, S. Synthesis of 4-substituted imino-4H-benzo[d][1,3] thiazin-2-amines via palladium-catalysed isocyanide insertion in 2-bromophenylthioureas. RSC Adv. 2014, 4, 41433–41436. [Google Scholar] [CrossRef]
- Goncalves, I.L.; de Azambuja, G.O.; Kawano, D.F.; Eifler-Lima, V.L. Thioureas as building blocks for the generation of heterocycles and compounds with pharmacological activity: An overview. Mini. Rev. Org. Chem. 2018, 15, 28–35. [Google Scholar] [CrossRef]
- Koch, K.R. New chemistry with old ligands: N-alkyl- and N,N-dialkyl-N′-acyl(aroyl)thioureas in co-ordination, analytical and process chemistry of the platinum group metals. Coord. Chem. Rev. 2001, 216–217, 473–488. [Google Scholar] [CrossRef]
- Mingji, D.; Liang, B.; Wang, C.; You, Z.; Xiang, J.; Dong, G.; Chen, J.; Yang, Z. A novel thiourea ligand applied in the Pd-catalyzed Heck, Suzuki and Suzuki carbonylative reactions. Adv. Synth. Catal. 2004, 346, 1669–1673. [Google Scholar] [CrossRef]
- Li, J.; Shi, L.-L.; Chen, J.; Gong, J.; Yang, Z. Thioureas as ligands in organometallic reactions. Synthesis 2014, 46, 2007–2023. [Google Scholar] [CrossRef]
- Saeed, A.; Flörke, U.; Erben, M.F. A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas. J. Sulfur Chem. 2014, 35, 318–355. [Google Scholar] [CrossRef]
- Metlushka, K.E.; Sadkova, D.N.; Shaimardanova, L.N.; Nikitina, K.A.; Ivshin, K.A.; Islamov, D.R.; Kataeva, O.N.; Alfonsov, A.V.; Kataev, V.E.; Voloshina, A.D. First coordination polymers on the bases of chiral thiophosphorylated thioureas. Inorg. Chem. Commun. 2016, 66, 11–14. [Google Scholar] [CrossRef]
- Blažek Bregović, V.; Basarić, N.; Mlinarić-Majerski, K. Anion binding with urea and thiourea derivatives. Coord. Chem. Rev. 2015, 295, 80–124. [Google Scholar] [CrossRef]
- Nencki, M. Zur Kenntniss des Sulfoharnstoffs. Ber. Dtsch. Chem. Ges. 1873, 6, 598–600. [Google Scholar] [CrossRef]
- Brückner, A. Vorläufige Mittheilung. Ber. Dtsch. Chem. Ges. 1873, 6, 1103–1104. [Google Scholar] [CrossRef]
- Brown, E.L.; Campbell, N. Studies in qualitative organic analysis. identification of alkyl halides, amines, and acids. J. Chem. Soc. 1937, 1699–1701. [Google Scholar] [CrossRef]
- Luskin, L.S.; Gantert, G.E.; Craig, W.E. t-Carbinamines, RR’R″CNH2 IV. The addition of isothiocyanic acid to olefinic compounds. J. Am. Chem. Soc. 1956, 78, 4965–4967. [Google Scholar] [CrossRef]
- Wang, J.; Li, H.; Yu, X.; Zu, L.; Wang, W. Chiral binaphthyl-derived amine-thiourea organocatalyst-promoted asymmetric Morita−Baylis−Hillman reaction. Org. Lett. 2005, 7, 4293–4296. [Google Scholar] [CrossRef]
- Fleming, E.M.; McCabe, T.; Connon, S.J. Novel axially chiral bis-arylthiourea-based organocatalysts for asymmetric Friedel–Crafts type reactions. Tetrahedron Lett. 2006, 47, 7037–7042. [Google Scholar] [CrossRef]
- Liu, X.-G.G.; Jiang, J.-J.J.; Shi, M. Development of axially chiral bis(arylthiourea)-based organocatalysts and their application in the enantioselective Henry reaction. Tetrahedron: Asymmetry 2007, 18, 2773–2781. [Google Scholar] [CrossRef]
- Schneider, J.F.; Falk, F.C.; Fröhlich, R.; Paradies, J. Planar-chiral thioureas as hydrogen-bond catalysts. Eur. J. Org. Chem. 2010, 2265–2269. [Google Scholar] [CrossRef]
- Kitagaki, S.; Ueda, T.; Mukai, C. Planar chiral [2.2]paracyclophane-based bis(thiourea) catalyst: Application to asymmetric Henry reaction. Chem. Commun. 2013, 49, 4030–4032. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Ma, Y.; Yang, S.; Ma, M.; Chu, H.; Song, C. Synthesis of planar chiral [2.2]paracyclophane-based amino thioureas and their application in asymmetric aldol reactions of ketones with isatins. Tetrahedron: Asymmetry 2013, 24, 1082–1088. [Google Scholar] [CrossRef]
- Didier, D.; Sergeyev, S. Thiourea derivatives of Tröger’s base: Synthesis, enantioseparation and evaluation in organocatalysis of Michael addition to nitroolefins. Arkivoc 2009, 124–134. [Google Scholar] [CrossRef]
- Caille, S.; Boni, J.; Cox, G.B.; Faul, M.M.; Franco, P.; Khattabi, S.; Klingensmith, L.M.; Larrow, J.F.; Lee, J.K.; Martinelli, M.J.; et al. Comparison of large-scale routes to manufacture ciral exo -2-norbornyl thiourea. Org. Process Res. Dev. 2010, 14, 133–141. [Google Scholar] [CrossRef]
- Ozturk, T.; Ertas, E.; Mert, O. Use of Lawesson’s Reagent in Organic Syntheses. Chem. Rev. 2007, 107, 5210–5278. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, T.; Ertas, E.; Mert, O. A Berzelius Reagent, Phosphorus Decasulfide (P4S10), in Organic Syntheses. Chem. Rev. 2010, 110, 3419–3478. [Google Scholar] [CrossRef] [PubMed]
- Hodgekins, J.E.; Ettlinger, M.G.; Hodgkins, J.E.; Ettlinger, M.G. The synthesis of isothiocyanates from amines. J. Org. Chem. 1956, 21, 404–405. [Google Scholar] [CrossRef]
- Sureshbabu, V.V.; Naik, S.A.; Hemantha, H.P.; Narendra, N.; Das, U.; Guru Row, T.N. N-urethane-protected amino alkyl isothiocyanates: Synthesis, isolation, characterization, and application to the synthesis of thioureidopeptides. J. Org. Chem. 2009, 74, 5260–5266. [Google Scholar] [CrossRef]
- Liu, M.; Ji, N.; Wang, L.; Liu, P.; He, W. d-Mannitol-derived novel chiral thioureas: Synthesis and application in asymmetric Henry reactions. Tetrahedron Lett. 2018, 59, 999–1004. [Google Scholar] [CrossRef]
- Frings, M.; Thomé, I.; Bolm, C. Synthesis of chiral sulfoximine-based thioureas and their application in asymmetric organocatalysis. Beilstein J. Org. Chem. 2012, 8, 1443–1451. [Google Scholar] [CrossRef]
- Cruz-Hernández, C.; Martínez-Martínez, E.; Hernández-González, P.E.; Juaristi, E. Synthesis of a new N-diaminophosphoryl-N’-[(2S)-2-pyrrolidinylmethyl]thiourea as a chiral organocatalyst for the stereoselective Michael addition of cyclohexanone to nitrostyrenes and chalcones—Application in cascade processes for the synthesis of Polyc. Eur. J. Org. Chem. 2018, 6890–6900. [Google Scholar] [CrossRef]
- Kaupp, G.; Schmeyers, J.; Boy, J. Quantitative solid-state reactions of amines with carbonyl compounds and isothiocyanates. Tetrahedron 2000, 56, 6899–6911. [Google Scholar] [CrossRef]
- Li, J.-P.P.; Wang, Y.-L.L.; Wang, H.; Luo, Q.-F.F.; Wang, X.-Y.Y. A new and efficient solid state synthesis of diaryl thioureas. Synth. Commun. 2001, 31, 781–785. [Google Scholar] [CrossRef]
- Štrukil, V.; Igrc, M.D.; Fábián, L.; Eckert-Maksić, M.; Childs, S.L.; Reid, D.G.; Duer, M.J.; Halasz, I.; Mottillo, C.; Friščić, T. A model for a solvent-free synthetic organic research laboratory: Click-mechanosynthesis and structural characterization of thioureas without bulk solvents. Green Chem. 2012, 14, 2462–2473. [Google Scholar] [CrossRef]
- Štrukil, V. Mechanochemical synthesis of thioureas, ureas and guanidines. Beilstein J. Org. Chem. 2017, 13, 1828–1849. [Google Scholar] [CrossRef]
- Štrukil, V.; Igrc, M.D.; Eckert-Maksić, M.; Friščić, T. Click mechanochemistry: Quantitative synthesis of “ready to use” chiral organocatalysts by efficient two-fold thiourea coupling to vicinal diamines. Chem. Eur. J. 2012, 18, 8464–8473. [Google Scholar] [CrossRef]
- Bhattacharjee, J.; Das, S.; Kottalanka, R.K.; Panda, T.K. Hydroamination of carbodiimides, isocyanates, and isothiocyanates by a bis(phosphinoselenoic amide) supported titanium(IV) complex. Dalton Trans. 2016, 45, 17824–17832. [Google Scholar] [CrossRef]
- Arafa, W.A.A.; Ibrahim, H.M. A sustainable strategy for the synthesis of bis-2-iminothiazolidin-4-ones utilizing novel series of asymmetrically substituted bis-thioureas as viable precursors. RSC Adv. 2018, 8, 10516–10521. [Google Scholar] [CrossRef]
- Herr, J.R.; Kuhler, J.L.; Meckler, H.; Opalka, C.J. A convenient method for the preparation of primary and symmetrical N,N’-disubstituted thioureas. Synthesis 2000, 1569–1574. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Y.; Zhang, G.; Fu, D.; Zhang, F.; Kai, M.; Wang, R. Enantioselective synthesis of cyclic thioureas via mannich reaction and concise synthesis of highly optically active methylthioimidazolines: Discovery of a more potent antipyretic agent. Adv. Synth. Catal. 2011, 353, 1787–1796. [Google Scholar] [CrossRef]
- Azizi, N.; Aryanasab, F.; Saidi, M.R. Straightforward and highly efficient catalyst-free one-pot synthesis of dithiocarbamates under solvent-free conditions. Org. Lett. 2006, 8, 5275–5277. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Aryanasab, F.; Torkiyan, L.; Ziyaei, A.; Saidi, M.R. One-pot synthesis of dithiocarbamates accelerated in water. J. Org. Chem. 2006, 71, 3634–3635. [Google Scholar] [CrossRef] [PubMed]
- Ziyaei-Halimjani, A.; Saidi, M.R. Synthesis of β-hydroxy dithiocarbamate derivatives via regioselective addition of dithiocarbamate anion to epoxide in water. Can. J. Chem. 2006, 84, 1515–1519. [Google Scholar] [CrossRef]
- Ziyaei Halimehjani, A.; Martens, J.; Schlüter, T. A one-pot three-component synthesis of dithiocarbamates starting from vinyl pyridines and vinyl pyrazine under solvent- and catalyst-free conditions. Tetrahedron 2016, 72, 3958–3965. [Google Scholar] [CrossRef]
- Ziyaei Halimehjani, A.; Klepetářová, B.; Beier, P. Synthesis of novel dithiocarbamates and xanthates using dialkyl azodicarboxylates: S–N bond formation. Tetrahedron 2018, 74, 1850–1858. [Google Scholar] [CrossRef]
- Li, T.-T.T.; Song, X.-H.H.; Wang, M.-S.S.; Ma, N. Cerium ammonium nitrate-catalyzed aerobic oxidative coupling of dithiocarbamates: Facile synthesis of thioureas and bis(aminothiocarbonyl)disulfides. RSC Adv. 2014, 4, 40054–40060. [Google Scholar] [CrossRef]
- Cao, Q.; Liu, F.; Wang, M.; Xu, W.; Zeng, M.-T.; Liu, M.; Li, Y.-S.; Dong, Z.-B. Facile synthesis of substituted arylthioureas in the presence of sodium hydride. J. Chem. Res. 2017, 41, 301–303. [Google Scholar] [CrossRef]
- Gan, S.-F.; Wan, J.-P.; Pan, Y.-J.; Sun, C.-R. Highly efficient and catalyst-free synthesis of substituted thioureas in water. Mol. Divers. 2011, 15, 809–815. [Google Scholar] [CrossRef]
- Ziyaei Halimehjani, A.; Pourshojaei, Y.; Saidi, M.R. Highly efficient and catalyst-free synthesis of unsymmetrical thioureas under solvent-free conditions. Tetrahedron Lett. 2009, 50, 32–34. [Google Scholar] [CrossRef]
- Dirksen, A.; Nieuwenhuizen, P.J.; Hoogenraad, M.; Haasnoot, J.G.; Reedijk, J. New mechanism for the reaction of amines with zinc dithiocarbamates. J. Appl. Polym. Sci. 2001, 79, 1074–1083. [Google Scholar] [CrossRef]
- Maddani, M.; Prabhu, K.R. A convenient method for the synthesis of substituted thioureas. Tetrahedron Lett. 2007, 48, 7151–7154. [Google Scholar] [CrossRef]
- Üngören, Ş.H.; Sırça, F. Novel self -condensation of ammonium dithiocarbamates leading to symmetrical substituted thioureas. Phosphorus. Sulfur. Silicon Relat. Elem. 2017, 192, 28–33. [Google Scholar] [CrossRef]
- Liang, F.; Tan, J.; Piao, C.; Liu, Q. Carbon tetrabromide promoted reaction of amines with carbon disulfide: Facile and efficient synthesis of thioureas and thiuram disulfides. Synthesis 2008, 3579–3584. [Google Scholar] [CrossRef]
- Fardpour, M.; Shafie, A.; Bahadorikhalili, S.; Larijani, B.; Mahdavi, M. Utilizing amines and carbon disulfide to obtain nitrogen- and sulfur-containing compounds under green conditions: A review. Curr. Org. Chem. 2018, 22, 2315–2380. [Google Scholar] [CrossRef]
- Maddani, M.R.; Prabhu, K.R. A concise synthesis of substituted thiourea derivatives in aqueous medium. J. Org. Chem. 2010, 75, 2327–2332. [Google Scholar] [CrossRef]
- Azizi, N.; Khajeh-Amiri, A.; Ghafuri, H.; Bolourtchian, M. Toward a practical and waste-free synthesis of thioureas in water. Mol. Divers. 2011, 15, 157–161. [Google Scholar] [CrossRef]
- Azizi, N.; Rahimzadeh-Oskooee, A.; Yadollahy, Z.; Ourimi, A.G. Ultrasound-assisted rapid sustainable synthesis of substituted thiourea. Monatsh. Chem. 2014, 145, 1675–1680. [Google Scholar] [CrossRef]
- Wan, G.-X.; Xu, L.; Ma, X.-S.; Ma, N. Silica gel promoted synthesis of N-sulfonylcyclothioureas in water. Tetrahedron Lett. 2011, 52, 6250–6254. [Google Scholar] [CrossRef]
- Jangale, A.D.; Kumavat, P.P.; Wagh, Y.B.; Tayade, Y.A.; Mahulikar, P.P.; Dalal, D.S. Green process development for the synthesis of aliphatic symmetrical N,N′-disubstituted thiourea derivatives in aqueous medium. Synth. Commun. 2015, 45, 236–244. [Google Scholar] [CrossRef]
- Milosavljević, M.M.; Vukićević, I.M.; Drmanić, S.Ž.; Nikolić, J.B.; Marinković, A.D.; Krstić, S.S.; Petrović, S.D. Simple one-pot synthesis of thioureas from amines, carbon disulfide and oxidants in water. J. Serb. Chem. Soc 2016, 81, 219–231. [Google Scholar] [CrossRef]
- Kumavat, P.P.; Jangale, A.D.; Patil, D.R.; Dalal, K.S.; Meshram, J.S.; Dalal, D.S. Green synthesis of symmetrical N,N’-disubstituted thiourea derivatives in water using solar energy. Environ. Chem. Lett. 2013, 11, 177–182. [Google Scholar] [CrossRef]
- Ranu, B.C.; Dey, S.S.; Bag, S. A simple and green procedure for the synthesis of symmetrical N,N’-disubstituted thioureas on the surface of alumina under microwave irradiation. Arkivoc 2003, 14–20. [Google Scholar] [CrossRef]
- Chau, C.-M.M.; Chuan, T.-J.J.; Liu, K.-M.M. A highly efficient one-pot method for the synthesis of thioureas and 2-imino-4-thiazolidinones under microwave conditions. RSC Adv. 2014, 4, 1276–1282. [Google Scholar] [CrossRef]
- Ziyaei Halimehjani, A.; Farahbakhsh, F. Synthesis of thioureas in ionic liquid medium. J. Sulfur Chem. 2013, 34, 284–288. [Google Scholar] [CrossRef]
- Azizi, N.; Farhadi, E. Rapid and highly efficient synthesis of thioureas in biocompatible basic choline hydroxide. J. Sulfur Chem. 2017, 38, 548–554. [Google Scholar] [CrossRef]
- Vázquez, J.; Bernès, S.; Reyes, Y.; Moya, M.; Sharma, P.; Alvarez, C.; Gutiérrez, R. Solvent-free synthesis of chiral N,N′-disubstituted thioureas by ‘just mixing’ the reagents. Synthesis 2004, 1955–1958. [Google Scholar] [CrossRef]
- Dutta, S.; Mondal, M.; Ghosh, T.; Saha, A. Unprecedented thiocarbamidation of nitroarenes a facile one-pot route to unsymmetrical thioureas. Org. Chem. Front. 2019, 6, 70–74. [Google Scholar] [CrossRef]
- Kumar, L.R.; Panduranga, V.; Vishwanatha, T.M.; Shekharappa; Sureshbabu, V.V. Synthesis of thioureido peptidomimetics employing alkyl azides and dithiocarbamates. Org. Biomol. Chem. 2018, 16, 2258–2263. [Google Scholar] [CrossRef]
- Sharma, S. Thiophosgene in organic synthesis. Synthesis 1978, 803–820. [Google Scholar] [CrossRef]
- Dai, M.; Liang, B.; Wang, C.; Chen, J.; Yang, Z. Synthesis of a novel C2-symmetric thiourea and its application in the Pd-catalyzed cross-coupling reactions with arenediazonium salts under aerobic conditions. Org. Lett. 2004, 6, 221–224. [Google Scholar] [CrossRef]
- Gao, Y.; Chang, L.; Shi, H.; Liang, B.; Wongkhan, K.; Chaiyaveij, D.; Batsanov, A.S.; Marder, T.B.; Li, C.; Yang, Z.; et al. A thiourea-oxazoline library with axial chirality: Ligand synthesis and studies of the palladium-catalyzed enantioselective bis(methoxycarbonylation) of terminal olefins. Adv. Synth. Catal. 2010, 352, 1955–1966. [Google Scholar] [CrossRef]
- Mohanta, P.K.; Dhar, S.; Samal, S.K.; Ila, H.; Junjappa, H. 1-(Methyldithiocarbonyl)imidazole: A useful thiocarbonyl transfer reagent for synthesis of substituted thioureas. Tetrahedron 2000, 56, 629–637. [Google Scholar] [CrossRef]
- Ramadas, K.; Srinivasan, N. A convenient route to substituted thiocarbamides. Synth. Commun. 1995, 25, 3381–3387. [Google Scholar] [CrossRef]
- Li, Z.; Liu, D.; Chen, Y.; Yin, Y.; Wang, Z.; Sun, X. Practical synthesis of symmetrical thioureas and heterocyclic thiones in water. J. Chem. Res. 2016, 40, 515–518. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Yin, Y.; Wang, Z.; Sun, X. Convenient synthesis of unsymmetrical N,N′-disubstituted thioureas in water. J. Chem. Res. 2016, 40, 670–673. [Google Scholar] [CrossRef]
- da Silva, T.L.; Miolo, L.M.F.; Sousa, F.S.S.; Brod, L.M.P.; Savegnago, L.; Schneider, P.H. New thioureas based on thiazolidines with antioxidant potential. Tetrahedron Lett. 2015, 56, 6674–6680. [Google Scholar] [CrossRef]
- Liang, B.; Liu, J.; Gao, Y.X.; Wongkhan, K.; Shu, D.X.; Lan, Y.; Li, A.; Batsanov, A.S.; Howard, J.A.H.; Marder, T.B.; et al. Synthesis of thiourea-oxazolines, a new class of chiral S,N-heterobidentate ligands: Application in Pd-catalyzed asymmetric bis(methoxycarbonylation) of terminal olefins. Organometallics 2007, 26, 4756–4762. [Google Scholar] [CrossRef]
- Yin, B.-L.; Liu, Z.-G.; Zhang, J.-C.; Li, Z.-R. N,N-Di-Boc-substituted thiourea as a novel and mild thioacylating agent applicable for the synthesis of thiocarbonyl compounds. Synthesis 2010, 991–999. [Google Scholar] [CrossRef]
- Cohrt, A.E.; Nielsen, T.E. Solid-phase synthesis of peptide thioureas and thiazole-containing macrocycles through Ru-catalyzed ring-closing metathesis. ACS Comb. Sci. 2014, 16, 71–77. [Google Scholar] [CrossRef][Green Version]
- Katritzky, A.R.; Ledoux, S.; Witek, R.M.; Nair, S.K. 1-(Alkyl/arylthiocarbamoyl)benzotriazoles as stable isothiocyanate equivalents: Synthesis of di- and trisubstituted thioureas. J. Org. Chem. 2004, 69, 2976–2982. [Google Scholar] [CrossRef]
- Kang, I.-J.; Wang, L.-W.; Yeh, T.-K.; Lee, C.-C.; Lee, Y.-C.; Hsu, S.-J.; Wu, Y.-S.; Wang, J.-C.; Chao, Y.-S.; Yueh, A.; et al. Synthesis, activity, and pharmacokinetic properties of a series of conformationally-restricted thiourea analogs as novel hepatitis C virus inhibitors. Bioorg. Med. Chem. 2010, 18, 6414–6421. [Google Scholar] [CrossRef] [PubMed]
- Katritzky, A.; Kirichenko, N.; Rogovoy, B.; Kister, J.; Tao, H. Synthesis of mono- and N,N-disubstituted thioureas and N-acylthioureas. Synthesis 2004, 1799–1805. [Google Scholar] [CrossRef]
- Koketsu, M.; Fukuta, Y.; Ishihara, H. Preparation of N,N-unsubstituted selenoureas and thioureas from cyanamides. Tetrahedron Lett. 2001, 42, 6333–6335. [Google Scholar] [CrossRef]
- Tan, W.; Wei, J.; Jiang, X. Thiocarbonyl surrogate via combination of sulfur and chloroform for thiocarbamide and oxazolidinethione construction. Org. Lett. 2017, 19, 2166–2169. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.-C.C.; Gao, Y.-C.C.; Zhu, Z.; Xu, L.; Li, Z.-D.D.; Tang, R.-Y.Y. Sulfite-promoted synthesis of N-difluoromethylthioureas via the reaction of azoles with bromodifluoroacetate and elemental sulfur. Org. Lett. 2019, 21, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.-H.H.; Xu, X.-P.P.; Cao, J.-J.J.; Wei, T.-Q.Q.; Wang, S.-Y.Y.; Ji, S.-J.J. Cobalt(II)-catalyzed isocyanide insertion reaction with amines under ultrasonic conditions: A divergent synthesis of ureas, thioureas and azaheterocycles. Adv. Synth. Catal. 2014, 356, 509–518. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ermolenko, L.; Al-Mourabit, A. Three-component reaction between isocyanides, aliphatic amines and elemental sulfur: Preparation of thioureas under mild conditions with complete atom economy. Synthesis 2014, 46, 3172–3179. [Google Scholar] [CrossRef]
- Angyal, A.; Demjén, A.; Wölfling, J.; Puskás, L.G.; Kanizsai, I. A green, isocyanide-based three-component reaction approach for the synthesis of multisubstituted ureas and thioureas. Tetrahedron Lett. 2018, 59, 54–57. [Google Scholar] [CrossRef]
- Singh, K.; Sharma, S. An isocyanide based multi-component reaction under catalyst- and solvent-free conditions for the synthesis of unsymmetrical thioureas. Tetrahedron Lett. 2017, 58, 197–201. [Google Scholar] [CrossRef]
- Cui, H.-L.; Chouthaiwale, P.V.; Yin, F.; Tanaka, F. Reaction-based mechanistic investigations of asymmetric hetero-Diels-Alder reactions of enones with isatins catalyzed by amine-based three-component catalyst systems. Asian J. Org. Chem. 2016, 5, 153–161. [Google Scholar] [CrossRef]
- Serdyuk, O.V.; Heckel, C.M.; Tsogoeva, S.B. Bifunctional primary amine-thioureas in asymmetric organocatalysis. Org. Biomol. Chem. 2013, 11, 7051–7071. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Asymmetric organocatalysis. Tetrahedron 2007, 63, 9267–9331. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Giacalone, F. (Eds.) Catalytic Methods in Asymmetric Synthesis: Advanced Materials, Techniques, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 9781118087992. [Google Scholar]
- Alemán, J.; Cabrera, S. Applications of asymmetric organocatalysis in medicinal chemistry. Chem. Soc. Rev. 2013, 42, 774–793. [Google Scholar] [CrossRef] [PubMed]
- Volla, C.M.R.R.; Atodiresei, I.; Rueping, M. Catalytic C–C bond-forming multi-component cascade or domino reactions: Pushing the boundaries of complexity in asymmetric organocatalysis. Chem. Rev. 2014, 114, 2390–2431. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Sun, W.; Yang, D.; Li, G.; Wang, R. Additive effects on asymmetric catalysis. Chem. Rev. 2016, 116, 4006–4123. [Google Scholar] [CrossRef]
- Tsogoeva, S.B. Recent advances in asymmetric organocatalytic 1,4-conjugate additions. Eur. J. Org. Chem. 2007, 1701–1716. [Google Scholar] [CrossRef]
- Doyle, A.G.; Jacobsen, E.N. Small-molecule H-bond donors in asymmetric catalysis. Chem. Rev. 2007, 107, 5713–5743. [Google Scholar] [CrossRef]
- Takemoto, Y.; Miyabe, H. The amino thiourea-catalyzed asymmetric nucleophilic reactions. Chimia 2007, 61, 269–275. [Google Scholar] [CrossRef]
- Miyabe, H.; Takemoto, Y. Discovery and application of asymmetric reaction by multi-functional thioureas. Bull. Chem. Soc. Jpn. 2008, 81, 785–795. [Google Scholar] [CrossRef]
- Takemoto, Y. Development of Chiral Thiourea Catalysts and Its Application to Asymmetric Catalytic Reactions. Chem. Pharm. Bull. 2010, 58, 593–601. [Google Scholar] [CrossRef]
- Sun, Y.L.; Wei, Y.; Shi, M. Applications of chiral thiourea-amine/phosphine organocatalysts in catalytic asymmetric reactions. Chem. Cat. Chem. 2017, 9, 718–727. [Google Scholar] [CrossRef]
- Ricci, P.; Khotavivattana, T.; Pfeifer, L.; Médebielle, M.; Morphy, J.R.; Gouverneur, V. The dual role of thiourea in the thiotrifluoromethylation of alkenes. Chem. Sci. 2017, 8, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Ellis, G.P. (Ed.) Chromenes, Chromanones, and Chromones. In Chemistry of Heterocyclic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 1977. [Google Scholar] [CrossRef]
- Koufaki, M.; Kiziridi, C.; Alexi, X.; Alexis, M.N. Design and synthesis of novel neuroprotective 1,2-dithiolane/chroman hybrids. Bioorg. Med. Chem. 2009, 17, 6432–6441. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.C. Asymmetric synthesis of chiral chromans. Tetrahedron 2009, 65, 3931–3952. [Google Scholar] [CrossRef]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; del Rio, D.; Clifford, M.N. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, H.; Xu, P.-F. Asymmetric catalytic cascade reactions for constructing diverse scaffolds and complex molecules. Acounts Chem. Res. 2015, 48, 1832–1844. [Google Scholar] [CrossRef]
- Sonsona, I.; Marqués-López, E.; Herrera, R.P. Enantioselective organocatalyzed synthesis of 2-amino-3-cyano-4H-chromene derivatives. Symmetry 2015, 7, 1519–1535. [Google Scholar] [CrossRef]
- Núnez, M.G.; García, P.; Moro, R.F.; Díez, D. Asymmetric organocatalytic synthesis of six-membered oxygenated heterocycles. Tetrahedron 2010, 66, 2089–2109. [Google Scholar] [CrossRef]
- Sigman, M.S.; Jacobsen, E.N. Schiff base catalysts for the asymmetric Strecker reaction identified and optimized from parallel synthetic libraries. J. Am. Chem. Soc. 1998, 120, 4901–4902. [Google Scholar] [CrossRef]
- Sigman, M.S.; Vachal, P.; Jacobsen, E.N. A general catalyst for the asymmetric Strecker reaction. Angew. Chem. Int. Ed. 2000, 39, 1279–1281. [Google Scholar] [CrossRef]
- Vachal, P.; Jacobsen, E.N. Structure-based analysis and optimization of a highly enantioselective catalyst for the Strecker reaction. J. Am. Chem. Soc. 2002, 124, 10012–10014. [Google Scholar] [CrossRef] [PubMed]
- Joly, G.D.; Jacobsen, E.N. Thiourea-catalyzed enantioselective hydrophosphonylation of imines: Practical access to enantiomerically enriched α-amino phosphonic acids. J. Am. Chem. Soc. 2004, 126, 4102–4103. [Google Scholar] [CrossRef] [PubMed]
- Peterson, E.A.; Jacobsen, E.N. Enantioselective, thiourea-catalyzed intermolecular addition of indoles to cyclic N-acyl iminium ions. Angew. Chem. Int. Ed. 2009, 48, 6328–6331. [Google Scholar] [CrossRef]
- Zuend, S.J.; Coughlin, M.P.; Lalonde, M.P.; Jacobsen, E.N. Scaleable catalytic asymmetric Strecker syntheses of unnatural α-amino acids. Nature 2009, 461, 968–970. [Google Scholar] [CrossRef]
- Zuend, S.J.; Jacobsen, E.N. Mechanism of amido-thiourea catalyzed enantioselective imine hydrocyanation: Transition state stabilization via multiple non-covalent interactions. J. Am. Chem. Soc. 2009, 131, 15358–15374. [Google Scholar] [CrossRef]
- Pan, S.C.; List, B. The catalytic acylcyanation of imines. Chem. Asian J. 2008, 3, 430–437. [Google Scholar] [CrossRef]
- Tsogoeva, S.B.; Yalalov, D.A.; Hateley, M.J.; Weckbecker, C.; Huthmacher, K. Asymmetric organocatalysis with novel chiral thiourea derivatives: Bifunctional catalysts for the Strecker and nitro-Michael reactions. Eur. J. Org. Chem. 2005, 19, 4995–5000. [Google Scholar] [CrossRef]
- Lalonde, M.P.; Chen, Y.; Jacobsen, E.N. A chiral primary amine thiourea catalyst for the highly enantioselective direct conjugate addition of α,α-disubstituted aldehydes to nitroalkenes. Angew. Chem. Int. Ed. 2006, 45, 6366–6370. [Google Scholar] [CrossRef]
- Taylor, M.S.; Jacobsen, E.N. Highly enantioselective catalytic acyl-Pictet-Spengler reactions. J. Am. Chem. Soc. 2004, 126, 10558–10559. [Google Scholar] [CrossRef]
- Brown, A.R.; Uyeda, C.; Brotherton, C.A.; Jacobsen, E.N. Enantioselective thiourea-catalyzed intramolecular Cope-type hydroamination. J. Am. Chem. Soc. 2013, 135, 6747–6749. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.; Syed, S.; Barbas III, C.F. Thiourea-catalyzed highly enantio- and diastereoselective additions of oxindoles to nitroolefins: Application to the formal synthesis of (+)-physostigmine. J. Am. Chem. Soc. 2009, 131, 8758–8759. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Hoashi, Y.; Takemoto, Y. Enantioselective Michael reaction of malonates to nitroolefins catalyzed by bifunctional organocatalysts. J. Am. Chem. Soc. 2003, 125, 12672–12673. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Hoashi, Y.; Furukawa, T.; Xu, X.; Takemoto, Y. Enantio- and diastereoselective Michael reaction of 1,3-dicarbonyl compounds to nitroolefins catalyzed by a bifunctional thiourea. J. Am. Chem. Soc. 2005, 127, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hoashi, Y.; Okino, T.; Takemoto, Y. Enantioselective Michael addition to α,β-unsaturated imides catalyzed by a bifunctional organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 4032–4035. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, T.; Hoashi, Y.; Takemoto, Y. Thiourea-catalyzed asymmetric Michael addition of activated methylene compounds to α,β-unsaturated imides: Dual activation of imide by intra- and intermolecular hydrogen bonding. J. Am. Chem. Soc. 2006, 128, 9413–9419. [Google Scholar] [CrossRef] [PubMed]
- Okino, T.; Nakamura, S.; Furukawa, T.; Takemoto, Y. Enantioselective aza-Henry reaction catalyzed by a bifunctional organocatalyst. Org. Lett. 2004, 6, 625–627. [Google Scholar] [CrossRef]
- Sakamoto, S.; Inokuma, T.; Takemoto, Y. Organocatalytic asymmetric Neber reaction for the synthesis of 2H-azirine carboxylic esters. Org. Lett. 2011, 13, 6374–6377. [Google Scholar] [CrossRef]
- Sakamoto, S.; Kazumi, N.; Kobayashi, Y.; Tsukano, C.; Takemoto, Y. Asymmetric synthesis of trisubstituted oxazolidinones by the thiourea-catalyzed aldol reaction of 2-isocyanatomalonate diester. Org. Lett. 2014, 16, 4758–4761. [Google Scholar] [CrossRef]
- Wang, J.-J.; Lao, J.-H.; Hu, Z.-P.; Lu, R.-J.; Nie, S.-Z.; Du, Q.-S.; Yan, M. Organocatalytic asymmetric conjugate addition of cyclic 1,3-dicarbonyl compounds to β,γ-unsaturated α-ketoesters. Arkivoc 2010, 229–243. [Google Scholar] [CrossRef]
- Zea, A.; Valero, G.; Alba, A.N.R.; Moyano, A.; Rios, R. Bifunctional thiourea-catalyzed asymmetric addition of anthrones to maleimides. Adv. Synth. Catal. 2010, 352, 1102–1106. [Google Scholar] [CrossRef]
- Enders, D.; Göddertz, D.P.; Beceño, C.; Raabe, G. Asymmetric synthesis of polyfunctionalized pyrrolidines via a thiourea catalyzed domino Mannich/aza-Michael reaction. Adv. Synth. Catal. 2010, 352, 2863–2868. [Google Scholar] [CrossRef]
- Liao, Y.H.; Liu, X.L.; Wu, Z.J.; Du, X.L.; Zhang, X.M.; Yuan, W.C. Thiourea-catalyzed highly diastereo- and enantioselective conjugate additions of α-substituted cyanoacetates to maleimides: Efficient construction of vicinal quaternary-tertiary stereocenters. Adv. Synth. Catal. 2011, 353, 1720–1728. [Google Scholar] [CrossRef]
- Ma, C.H.; Kang, T.R.; He, L.; Liu, Q.Z. Highly enantioselective Michael addition of malonates to β-CF3-β-(3-indolyl)nitroalkenes: Construction of trifluoromethylated all-carbon quaternary stereogenic centres. Eur. J. Org. Chem. 2014, 3981–3985. [Google Scholar] [CrossRef]
- Monari, M.; Montroni, E.; Nitti, A.; Lombardo, M.; Trombini, C.; Quintavalla, A. Highly Stereoselective [4+2] and [3+2] Spiroannulations of 2-(2-Oxoindolin-3-ylidene)acetic Esters Catalyzed by Bifunctional Thioureas. Chem. Eur. J. 2015, 21, 11038–11049. [Google Scholar] [CrossRef]
- Lutete, L.M.; Miyamoto, T.; Ikemoto, T. Tertiary amino thiourea-catalyzed asymmetric cross aldol reaction of aryl methyl ketones with aryl trifluoromethyl ketones. Tetrahedron Lett. 2016, 57, 1220–1223. [Google Scholar] [CrossRef]
- Yang, J.; Sun, W.; He, Z.; Yu, C.; Bao, G.; Li, Y.; Liu, Y.; Hong, L.; Wang, R. Access to α, γ-diamino diacid derivatives via organocatalytic asymmetric 1,4-addition of azlactones and dehydroalanines. Org. Lett. 2018, 20, 7080–7084. [Google Scholar] [CrossRef]
- Orhan, B.; Tschan, M.J.-L.; Wirotius, A.-L.; Dove, A.P.; Coulembier, O.; Taton, D. Isoselective ring-opening polymerization of rac-lactide from chiral Takemoto’s organocatalysts: Elucidation of stereocontrol. ACS Macro Lett. 2018, 7, 1413–1419. [Google Scholar] [CrossRef]
- Miyabe, H.; Tuchida, S.; Yamauchi, M.; Takemoto, Y. Reaction of nitroorganic compounds using thiourea catalysts anchored to polymer support. Synthesis 2006, 3295–3300. [Google Scholar] [CrossRef]
- Yamaoka, Y.; Miyabe, H.; Takemoto, Y. Catalytic enantioselective Petasis-type reaction of quinolines catalyzed by a newly designed thiourea catalyst. J. Am. Chem. Soc. 2007, 129, 6686–6687. [Google Scholar] [CrossRef]
- Azuma, T.; Murata, A.; Kobayashi, Y.; Inokuma, T.; Takemoto, Y. A dual arylboronic acid-aminothiourea catalytic system for the asymmetric intramolecular hetero-Michael reaction of α,β-unsaturated carboxylic acids. Org. Lett. 2014, 16, 4256–4259. [Google Scholar] [CrossRef] [PubMed]
- Hayama, N.; Kuramoto, R.; Földes, T.; Nishibayashi, K.; Kobayashi, Y.; Pápai, I.; Takemoto, Y. Mechanistic insight into asymmetric hetero-Michael addition of α,β-unsaturated carboxylic acids catalyzed by multifunctional thioureas. J. Am. Chem. Soc. 2018, 140, 12216–12225. [Google Scholar] [CrossRef] [PubMed]
- Li, D.R.; He, A.; Falck, J.R. Enantioselective, organocatalytic reduction of ketones using bifunctional thiourea-amine catalysts. Org. Lett. 2010, 12, 1756–1759. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Lin, A.; Shi, Y.; Mao, H.; Li, W.; Cheng, Y.; Zhu, C. Chiral tertiary amine thiourea-catalyzed asymmetric inverse-electron-demand Diels-Alder reaction of chromone heterodienes using 3-vinylindoles as dienophiles. J. Org. Chem. 2013, 78, 10233–10239. [Google Scholar] [CrossRef] [PubMed]
- Ravi Kumar, G.; Ramesh, B.; Yarlagadda, S.; Sridhar, B.; Reddy, B.V.S. Organocatalytic enantioselective Mannich reaction: Direct access to chiral β-amino esters. ACS Omega 2019, 4, 2168–2177. [Google Scholar] [CrossRef]
- Lu, R.J.; Wei, W.T.; Wang, J.J.; Nie, S.Z.; Zhang, X.J.; Yan, M. Organocatalytic conjugate addition of α-nitroacetates to β,γ-unsaturated α-keto esters and subsequent decarboxylation: Synthesis of optically active δ-nitro-α-keto esters. Tetrahedron 2012, 68, 9397–9404. [Google Scholar] [CrossRef]
- Zhou, M.-Q.; Zuo, J.; Cui, B.-D.; Zhao, J.-Q.; You, Y.; Bai, M.; Chen, Y.-Z.; Zhang, X.-M.; Yuan, W.-C. Organocatalytic asymmetric double Michael reaction of Nazarov reagents with alkylidene azlactones for the construction of spiro-fused cyclohexanone/5-oxazolone system. Tetrahedron 2014, 70, 5787–5793. [Google Scholar] [CrossRef]
- Zhang, Z.; Lippert, K.M.; Hausmann, H.; Kotke, M.; Schreiner, P.R. Cooperative thiourea-Brønsted acid organocatalysis: Enantioselective cyanosilylation of aldehydes with TMSCN. J. Org. Chem. 2011, 76, 9764–9776. [Google Scholar] [CrossRef]
- Peng, F.-Z.; Shao, Z.-H.; Fan, B.-M.; Song, H.; Li, G.-P.; Zhang, H.-B. Addition of 2,4-pentandione to nitroalkenes promoted by bifunctional thioureas with central and axial chiral elements. J. Org. Chem. 2008, 73, 5202–5205. [Google Scholar] [CrossRef]
- Guo, H.-M.; Li, J.-G.; Qu, G.-R.; Zhang, X.-M.; Yuan, W.-C. Organocatalytic enantioselective Michael addition of malononitrile to nitroolefins catalyzed by bifunctional thiourea. Chirality 2011, 23, 514–518. [Google Scholar] [CrossRef]
- Mei, K.; Jin, M.; Zhang, S.; Li, P.; Liu, W.; Chen, X.; Xue, F.; Duan, W.; Wang, W. Simple cyclohexanediamine-derived primary amine thiourea catalyzed highly enantioselective conjugate addition of nitroalkanes to enones. Org. Lett. 2009, 11, 2864–2867. [Google Scholar] [CrossRef] [PubMed]
- Dudziński, K.; Pakulska, A.M.; Kwiatkowski, P. An efficient organocatalytic method for highly enantioselective Michael addition of malonates to enones catalyzed by readily accessible primary amine-thiourea. Org. Lett. 2012, 14, 4222–4225. [Google Scholar] [CrossRef] [PubMed]
- Moritaka, M.; Miyamae, N.; Nakano, K.; Ichikawa, Y.; Kotsuki, H. Highly efficient asymmetric Michael addition reaction of malonates to α,β-unsaturated ketones promoted by a chiral thiourea/PPY dual-catalyst system. Synlett 2012, 23, 2554–2558. [Google Scholar] [CrossRef]
- Miyamae, N.; Watanabe, N.; Moritaka, M.; Nakano, K.; Ichikawa, Y.; Kotsuki, H. Asymmetric organocatalytic desymmetrization of 4,4-disubstituted cyclohexadienones at high pressure: A new powerful strategy for the synthesis of highly congested chiral cyclohexenones. Org. Biomol. Chem 2014, 12, 5847–5855. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, S.; Lao, J.; Du, G.; Yan, M.; Chan, A.S.C.C. Asymmetric conjugate addition of carbonyl compounds to nitroalkenes catalyzed by chiral bifunctional thioureas. Tetrahedron: Asymmetry 2009, 20, 1451–1458. [Google Scholar] [CrossRef]
- Li, B.-L.L.; Wang, Y.-F.F.; Luo, S.-P.P.; Zhong, A.-G.G.; Li, Z.-B.B.; Du, X.-H.H.; Xu, D.-Q.Q. Enantioselective Michael addition of aromatic ketones to nitroolefins catalyzed by bifunctional thioureas and mechanistic insight. Eur. J. Org. Chem. 2010, 656–662. [Google Scholar] [CrossRef]
- Retini, M.; Bergonzini, G.; Melchiorre, P. Dioxindole in asymmetric catalytic synthesis: Direct access to 3-substituted 3-hydroxy-2-oxindoles via 1,4-additions to nitroalkenes. Chem. Commun. 2012, 48, 3336–3338. [Google Scholar] [CrossRef]
- Guo, X.-T.; Sha, F.; Wu, X.-Y. Highly enantioselective Michael addition of aromatic ketones to nitrodienes and the application to the synthesis of chiral γ-aminobutyric acid. Synthesis 2017, 49, 647–656. [Google Scholar] [CrossRef][Green Version]
- Jiménez, E.I.; Vallejo Narváez, W.E.; Román-Chavarría, C.A.; Vazquez-Chavez, J.; Rocha-Rinza, T.; Hernández-Rodríguez, M. Bifunctional thioureas with α-trifluoromethyl or methyl groups: Comparison of catalytic performance in Michael additions. J. Org. Chem. 2016, 81, 7419–7431. [Google Scholar] [CrossRef]
- Sohtome, Y.; Tanatani, A.; Hashimoto, Y.; Nagasawa, K. Development of bis-thiourea-type organocatalyst for asymmetric Baylis-Hillman reaction. Tetrahedron Lett. 2004, 45, 5589–5592. [Google Scholar] [CrossRef]
- Sohtome, Y.; Takemura, N.; Takagi, R.; Hashimoto, Y.; Nagasawa, K. Thiourea-catalyzed Morita-Baylis-Hillman reaction. Tetrahedron 2008, 64, 9423–9429. [Google Scholar] [CrossRef]
- Mayr, F.; Brimioulle, R.; Bach, T. A chiral thiourea as a template for enantioselective intramolecular [2 + 2] photocycloaddition reactions. J. Org. Chem. 2016, 81, 6965–6971. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Zhang, Z.-H.; Dong, X.-Q.; Wu, X.-J. Chiral amine-thioureas bearing multiple hydrogen bonding donors: Highly efficient organocatalysts for asymmetric Michael addition of acetylacetone to nitroolefins. Chem. Commun. 2008, 1431–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-J.; Dong, X.-Q.; Zhang, Z.-H.; Xue, Z.-Y.; Teng, H.-L. Highly anti-selective asymmetric nitro-mannich reactions catalyzed by bifunctional amine-thiourea-bearing multiple hydrogen-bonding donors. J. Am. Chem. Soc. 2008, 130, 8606–8607. [Google Scholar] [CrossRef]
- Liao, Y.-H.; Liu, X.-L.; Wu, Z.-J.; Cun, L.-F.; Zhang, X.-M.; Yuan, W.-C. Highly diastereo- and enantioselective Michael additions of 3-substituted oxindoles to maleimides catalyzed by chiral bifunctional thiourea-tertiary amine. Org. Lett. 2010, 12, 2896–2899. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Long, J.; Li, B.-J.; Jiang, L.; Li, R.; Wu, Y.; Ding, L.-S.; Chen, Y.-C. Enantioselective construction of quaternary carbon centre catalysed by bifunctional organocatalyst. Org. Biomol. Chem. 2006, 4, 2097–2099. [Google Scholar] [CrossRef]
- Dong, L.-T.; Du, Q.-S.; Lou, C.-L.; Zhang, J.-M.; Lu, R.-J.; Yan, M. Asymmetric synthesis of nitrocyclopropanes catalyzed by chiral primary amines. Synlett 2010, 266–270. [Google Scholar] [CrossRef]
- Mei, R.-Q.; Xu, X.-Y.; Li, Y.-C.; Fu, J.-Y.; Huang, Q.-C.; Wang, L.-X. Highly effective and enantioselective Michael addition of 4-hydroxycoumarin to α,β-unsaturated ketones promoted by simple chiral primary amine thiourea bifunctional catalysts. Tetrahedron Lett. 2011, 52, 1566–1568. [Google Scholar] [CrossRef]
- Kang, J.Y.; Johnston, R.C.; Snyder, K.M.; Cheong, P.H.-Y.; Carter, R.G. Construction of stereogenic α,α-disubstituted cycloalkanones via 1° amine thiourea dual catalysis: Experimental scope and computational analyses. J. Org. Chem. 2016, 81, 3629–3637. [Google Scholar] [CrossRef]
- Mei, R.-Q.Q.; Xu, X.-Y.Y.; Peng, L.; Wang, F.; Tian, F.; Wang, L.-X.X. Asymmetric Michael/cyclization tandem reaction of 4-hydroxycoumarin with β-nitroalkenes catalyzed by chiral bifunctional thioureas. Org. Biomol. Chem. 2013, 11, 1286–1289. [Google Scholar] [CrossRef]
- Basak, A.K.; Shimada, N.; Bow, W.F.; Vicic, D.A.; Tius, M.A. An asymmetric organocatalytic Nazarov cyclization. J. Am. Chem. Soc. 2010, 132, 8266–8267. [Google Scholar] [CrossRef] [PubMed]
- Asari, A.H.; Lam, Y.-H.; Tius, M.A.; Houk, K.N. Origins of the stereoselectivity in a thiourea-primary amine-catalyzed Nazarov cyclization. J. Am. Chem. Soc. 2015, 137, 13191–13199. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Roland, K.; Neudörfl, J.M. Asymmetric Morita-Baylis-Hillman reaction catalyzed by isophoronediamine-derived bis(thio)urea organocatalysts. Org. Lett. 2006, 8, 4195–4198. [Google Scholar] [CrossRef] [PubMed]
- Flock, A.M.; Krebs, A.; Bolm, C. Ephedrine- and pseudoephedrine-derived thioureas in asymmetric Michael additions of keto esters and diketones to nitroalkenes. Synlett 2010, 1219–1222. [Google Scholar] [CrossRef]
- Ren, Q.; Gao, Y.; Wang, J. Enantioselective synthesis of densely functionalized pyranochromenes via an unpredictable cascade Michael-oxa-Michael-tautomerization sequence. Chem. Eur. J. 2010, 16, 13594–13598. [Google Scholar] [CrossRef]
- Cao, C.-L.; Ye, M.-C.; Sun, X.-L.; Tang, Y. Pyrrolidine—Thiourea as a bifunctional organocatalyst: Highly enantioselective Michael addition of cyclohexanone to nitroolefins. Org. Lett. 2006, 8, 2901–2904. [Google Scholar] [CrossRef]
- Zhi, Y.; Zhao, K.; von Essen, C.; Rissanen, K.; Enders, D. Thiourea-catalyzed domino Michael-Mannich [3+2] cycloadditions: A strategy for the asymmetric synthesis of 3,3′-pyrrolidinyl-dispirooxindoles. Synlett 2017, 28, 2876–2880. [Google Scholar] [CrossRef]
- Yan, L.; Wang, H.; Xiong, F.; Tao, Y.; Wu, Y.; Chen, F. Chloramphenicol base chemistry. Part 11: Chloramphenicol base-derived thiourea-catalyzed enantioselective Michael addition of malononitrile to α,β-unsaturated ketones. Tetrahedron: Asymmetry 2017, 28, 921–929. [Google Scholar] [CrossRef]
- Wang, S.-X.; Chen, F.-E. A novel cost-effective thiourea bifunctional organocatalyst for highly enantioselective alcoholysis of meso-cyclic anhydrides: Enhanced enantioselectivity by configuration inversion. Adv. Synth. Catal. 2009, 351, 547–552. [Google Scholar] [CrossRef]
- Yan, L.-J.; Wang, H.-F.; Chen, W.-X.; Tao, Y.; Jin, K.-J.; Chen, F.-E. Development of bifunctional thiourea organocatalysts derived from a chloramphenicol base scaffold and their use in the enantioselective alcoholysis of meso cyclic anhydrides. Chem. Cat. Chem. 2016, 8, 2249–2253. [Google Scholar] [CrossRef]
- Li, X.-J.; Liu, K.; Ma, H.; Nie, J.; Ma, J.-A. Highly enantioselective Michael addition of malonates to nitroolefins catalyzed by chiral bifunctional tertiary amine-thioureas based on saccharides. Synlett 2008, 3242–3246. [Google Scholar] [CrossRef]
- Chen, F.-X.; Shao, C.; Liu, Q.; Gong, P.; Liu, C.-L.; Wang, R. Asymmetric Michael addition of trisubstituted carbanion to nitroalkenes catalyzed by sodium demethylquinine salt in water. Chirality 2009, 21, 600–603. [Google Scholar] [CrossRef] [PubMed]
- McGarraugh, P.G.; Brenner, S.E. Novel bifunctional sulfonamides catalyze an enantioselective conjugate addition. Tetrahedron 2009, 65, 449–455. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nie, S.-Z.; Hu, Z.-P.; Xuan, Y.-N.; Wang, J.-J.; Li, X.-M.; Yan, M. Organocatalytic asymmetric conjugate addition of malonates to 3-nitro-2H-chromenes. Tetrahedron: Asymmetry 2010, 21, 2055–2059. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R. Recent developments in catalytic asymmetric inverse-electron-demand Diels−Alder reaction. Chem. Rev. 2013, 113, 5515–5546. [Google Scholar] [CrossRef]
- Johansson, H.; Jørgensen, T.B.; Gloriam, D.E.; Bräuner-Osborne, H.; Pedersen, D.S. 3-Substituted 2-phenyl-indoles: Privileged structures for medicinal chemistry. RSC Adv. 2013, 3, 945–960. [Google Scholar] [CrossRef]
- Guo, H.-C.; Ma, J.-A. Catalytic asymmetric tandem transformations triggered by conjugate additions. Angew. Chem. Int. Ed. 2006, 45, 354–366. [Google Scholar] [CrossRef]
- Pellissier, H. Asymmetric domino reactions. Part B: Reactions based on the use of chiral catalysts and biocatalysts. Tetrahedron 2006, 62, 2143–2173. [Google Scholar] [CrossRef]
- Alba, A.-N.; Companyo, X.; Viciano, M.; Rios, R. Organocatalytic domino reactions. Curr. Org. Chem. 2009, 13, 1432–1474. [Google Scholar] [CrossRef]
- Zu, L.; Zhang, S.; Xie, H.; Wang, W. Catalytic asymmetric oxa-Michael-Michael cascade for facile construction of chiral chromans via an aminal intermediate. Org. Lett. 2009, 11, 1627–1630. [Google Scholar] [CrossRef]
- Moyano, A.; Rios, R. Asymmetric organocatalytic cyclization and cycloaddition reactions. Chem. Rev. 2011, 111, 4703–4832. [Google Scholar] [CrossRef] [PubMed]
- Chanda, T.; Zhao, J.C.-G. Recent progress in organocatalytic asymmetric domino transformations. Adv. Synth. Catal. 2018, 360, 2–79. [Google Scholar] [CrossRef]
- Bella, M.; Gasperi, T. Organocatalytic formation of quaternary stereocenters. Synthesis 2009, 1583–1614. [Google Scholar] [CrossRef]
- Kumar, K.; Waldmann, H. Synthesis of natural product inspired compound collections. Angew. Chem. Int. Ed. 2009, 48, 3224–3242. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Chin, Y.-W.; Chai, H.; Keller, W.J.; Kinghorn, A.D. Anthraquinones with quinone reductase-inducing activity and benzophenones from morinda citrifolia (noni) roots. J. Nat. Prod. 2007, 70, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S.; Desta, Z.Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef] [PubMed]
- Dalpozzo, R.; Bartoli, G.; Bencivenni, G. Recent advances in organocatalytic methods for the synthesis of disubstituted 2- and 3-indolinones. Chem. Soc. Rev. 2012, 41, 7247–7290. [Google Scholar] [CrossRef]
- Mao, H.; Lin, A.; Tang, Y.; Shi, Y.; Hu, H.; Cheng, Y.; Zhu, C. Organocatalytic oxa/aza-Michael–Michael cascade strategy for the construction of spiro [chroman/tetrahydroquinoline-3,3′-oxindole] scaffolds. Org. Lett. 2013, 15, 4062–4065. [Google Scholar] [CrossRef]
- Hong, B.-C.; Kotame, P.; Lee, G.-H. Asymmetric synthesis of 3,4-dihydrocoumarin motif with an all-carbon quaternary stereocenter via a Michael–acetalization sequence with bifunctional amine-thiourea organocatalysts. Org. Lett. 2011, 13, 5758–5761. [Google Scholar] [CrossRef]
- Wang, H.; Luo, J.; Han, X.; Lu, Y. Enantioselective synthesis of chromanones via a tryptophan-derived bifunctional thiourea-catalyzed oxa-Michael-Michael cascade reaction. Adv. Synth. Catal. 2011, 353, 2971–2975. [Google Scholar] [CrossRef]
- Yoon, T.P.; Jacobsen, E.N. Privileged chiral catalysts. Science 2003, 299, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, K.; Gawroński, J. Cinchona alkaloids and their derivatives: Versatile catalysts and ligands in asymmetric synthesis. Synthesis 2001, 961–998. [Google Scholar] [CrossRef]
- McCooey, S.H.; Connon, S.J. Urea- and thiourea-substituted Cinchona alkaloid derivatives as highly efficient bifunctional organocatalysts for the asymmetric addition of malonate to nitroalkenes: Inversion of configuration at C9 dramatically improves catalyst performance. Angew. Chem. Int. Ed. 2005, 44, 6367–6370. [Google Scholar] [CrossRef] [PubMed]
- Vakulya, B.; Varga, S.; Csámpai, A.; Soós, T. Highly enantioselective conjugate addition of nitromethane to chalcones using bifunctional Cinchona organocatalysts. Org. Lett. 2005, 7, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
- Vakulya, B.; Varga, S.; Soós, T. Epi -Cinchona based thiourea organocatalyst family as an efficient assymmetric Michael addition promoter: Enantioselective conjugate addition of nitroalkanes to chalcones and α,β-unsaturated N-acylpyrroles. J. Org. Chem. 2008, 73, 3475–3480. [Google Scholar] [CrossRef]
- Varga, S.; Jakab, G.; Drahos, L.; Holczbauer, T.; Czugler, M.; Soós, T. Double diastereocontrol in bifunctional thiourea organocatalysis: Iterative Michael-Michael-Henry sequence regulated by the configuration of chiral catalysts. Org. Lett. 2011, 13, 5416–5419. [Google Scholar] [CrossRef]
- Li, P.; Wen, S.; Yu, F.; Liu, Q.; Li, W.; Wang, Y.; Liang, X.; Ye, J. Enantioselective organocatalytic Michael addition of malonates to α, β-unsaturated ketones. Org. Lett. 2009, 11, 753–756. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Yu, C.; Song, X.; Wang, W. Organocatalytic asymmetric synthesis of chiral fluorinated quaternary carbon containing β-ketoesters. Chem. Commun. 2009, 2136–2138. [Google Scholar] [CrossRef]
- Han, X.; Luo, J.; Liu, C.; Lu, Y. Asymmetric generation of fluorine-containing quaternary carbons adjacent to tertiary stereocenters: Uses of fluorinated methines as nucleophiles. Chem. Commun. 2009, 2044–2046. [Google Scholar] [CrossRef]
- Fan, L.-P.; Li, P.; Li, X.-S.; Xu, D.-C.; Ge, M.-M.; Zhu, W.-D.; Xie, J.-W.W. Facile domino access to chiral mono-, bi-, and tricyclic 2,3-dihydrofurans. J. Org. Chem. 2010, 75, 8716–8719. [Google Scholar] [CrossRef]
- Pham, T.S.; Balázs, L.; Petneházy, I.; Jászay, Z. Enantioselective Michael addition of diethyl cyanomethylphosphonate to chalcones using bifunctional Cinchona-derived organocatalysts: Synthesis of chiral precursors of α-substituted β-aminophosphonates. Tetrahedron: Asymmetry 2010, 21, 346–351. [Google Scholar] [CrossRef]
- Mancheño, O.G.; Tangen, P.; Rohlmann, R.; Fröhlich, R.; Alemán, J. Synthesis of chiral cyclic nitrones by asymmetric addition of β-ketosulfones to nitroalkenes followed by reductive cyclization. Chem. Eur. J. 2011, 17, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-Z.Z.; Cheng, R.-L.L.; Xu, P.-F.F. Asymmetric michael addition of 1-acetylindolin-3-ones to β-nitrostyrenes catalyzed by bifunctional thioureas: A simple access to 2-functionalized indoles. J. Org. Chem. 2011, 76, 2884–2887. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-J.; Hu, Z.-P.; Lou, C.-L.; Liu, J.-L.; Li, X.-M.; Yan, M. Asymmetric synthesis of trifluoromethyl substituted dihydropyrans via organocatalytic cascade Michael-hemiketalization reaction. Tetrahedron 2011, 67, 4578–4583. [Google Scholar] [CrossRef]
- Chiarucci, M.; Lombardo, M.; Trombini, C.; Quintavalla, A. Enantioselective conjugate addition of nitroalkanes to alkylidenemalonates promoted by thiourea-based bifunctional organocatalysts. Adv. Synth. Catal. 2012, 354, 364–370. [Google Scholar] [CrossRef]
- Curti, C.; Rassu, G.; Zambrano, V.; Pinna, L.; Pelosi, G.; Sartori, A.; Battistini, L.; Zanardi, F.; Casiraghi, G. Bifunctional Cinchona alkaloid/thiourea catalyzes direct and enantioselective vinylogous Michael addition of 3-alkylidene oxindoles to nitroolefins. Angew. Chem. Int. Ed. 2012, 51, 6200–6204. [Google Scholar] [CrossRef]
- Guo, Z.-W.; Li, X.-S.; Zhu, W.-D.; Xie, J.-W. Construction of chiral multi-functionalized polyheterocyclic benzopyran derivatives by using an asymmetric organocatalytic domino reaction. Eur. J. Org. Chem. 2012, 6924–6932. [Google Scholar] [CrossRef]
- Molleti, N.; Allu, S.; Ray, S.K.; Singh, V.K. Bifunctional chiral urea catalyzed highly enantioselective Michael addition of cyclic 1,3-dicarbonyl compounds to 2-enoylpyridines. Tetrahedron Lett. 2013, 54, 3241–3244. [Google Scholar] [CrossRef]
- Kwiatkowski, J.; Lu, Y. Highly enantioselective preparation of fluorinated phosphonates by Michael addition of α-fluoro-β-ketophosphonates to nitroalkenes. Asian J. Org. Chem. 2014, 3, 458–461. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Cholewiak, A.; Kasztelan, A. Efficient and highly enantioselective construction of trifluoromethylated quaternary stereogenic centers via high-pressure mediated organocatalytic conjugate addition of nitromethane to β,β-disubstituted enones. Org. Lett. 2014, 16, 5930–5933. [Google Scholar] [CrossRef]
- Konda, S.; Zhao, J.C.-G. High enantioselective Michael addition of malonates to β,γ-unsaturated α-ketoesters catalyzed by bifunctional thioureas. Tetrahedron Lett. 2014, 55, 5216–5218. [Google Scholar] [CrossRef]
- Chang, H.-H.; Chu, K.-T.; Chiang, M.-H.; Han, J.-L. Organocatalytic enantioselective Michael reaction of 1,3-dicarbonyls with α-substituted β-nitroacrylates. Tetrahedron 2017, 73, 727–734. [Google Scholar] [CrossRef]
- Bhagat, U.K.; Peddinti, R.K. Asymmetric organocatalytic approach to 2,4-disubstituted 1,2,3-triazoles by N2-selective aza-Michael addition. J. Org. Chem. 2018, 83, 793–804. [Google Scholar] [CrossRef]
- Reddy, S.N.; Reddy, V.R.; Dinda, S.; Nanubolu, J.B.; Chandra, R. Asymmetric reaction of p-quinone diimide: Organocatalyzed Michael addition of α-cyanoacetates. Org. Lett. 2018, 20, 2572–2575. [Google Scholar] [CrossRef]
- Yuan, J.-N.; Liu, H.-X.; Tian, Q.-Q.; Ji, N.; Shen, K.; He, W. Highly enantioselective Michael addition of dithiomalonates to nitroolefins catalyzed by new bifunctional chiral thioureas. Synthesis 2018, 50, 2577–2586. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Wu, S.; Karmaker, P.G.; Sohail, M.; Wang, Q.; Chen, F.-X. Enantioselective synthesis of trifluoromethylated tertiary thioethers through organocatalytic sulfa-Michael addition of thiols to β-trifluoromethyl β,β-disubstituted enones. Synthesis 2015, 47, 1147–1153. [Google Scholar] [CrossRef]
- Bacsó, A.; Szigeti, M.; Varga, S.; Soós, T. Bifunctional thiourea-catalyzed stereoablative retro-sulfa-Michael reaction: Concise and diastereoselective access to chiral 2,4-diarylthietanes. Synthesis 2017, 49, 429–439. [Google Scholar] [CrossRef][Green Version]
- Konda, S.; Guo, Q.-S.; Abe, M.; Huang, H.; Arman, H.; Zhao, J.C.-G. Organocatalyzed asymmetric aldol reactions of ketones and β,γ-unsaturated α-ketoesters and phenylglyoxal hydrates. J. Org. Chem. 2015, 80, 806–815. [Google Scholar] [CrossRef]
- Ji, S.; Alkhalil, A.E.; Su, Y.; Xia, X.; Chong, S.; Wang, K.-H.; Huang, D.; Fu, Y.; Hu, Y. Bifunctional thiourea catalyzed asymmetric Mannich reaction using trifluoromethyl aldimine as trifluoromethyl building blocks. Synlett 2015, 26, 1725–1731. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Shi, T.-D.; Zhou, F.; Zhao, X.-L.; Wang, X.; Zhou, J. Organocatalytic asymmetric Strecker reaction of di- and trifluoromethyl ketoimines. Remarkable fluorine effect. Org. Lett. 2011, 13, 3826–3829. [Google Scholar] [CrossRef]
- Xie, H.; Song, A.; Song, X.; Zhang, X.; Wang, W. Organocatalytic enantioselective Strecker reaction of cyclic trifluoromethyl-ketoimines. Tetrahedron Lett. 2013, 54, 1409–1411. [Google Scholar] [CrossRef]
- Xie, H.; Song, A.; Zhang, X.; Chen, X.; Li, H.; Sheng, C.; Wang, W. Quinine-thiourea catalyzed enantioselective hydrophosphonylation of trifluoromethyl 2(1H)-quinazolinones. Chem. Commun. 2013, 49, 928–930. [Google Scholar] [CrossRef] [PubMed]
- Du, F.; Zhou, J.; Peng, Y. Asymmetric reaction of α-diazomethylphosphonates with α-ketoesters to access optically active α-diazo-β-hydroxyphosphonate derivatives. Org. Lett. 2017, 19, 1310–1313. [Google Scholar] [CrossRef] [PubMed]
- Amere, M.; Lasne, M.-C.; Rouden, J. Highly enantioselective decarboxylative protonation of α-aminomalonates mediated by thiourea Cinchona alkaloid derivatives: Access to both enantiomers of cyclic and acyclic α-aminoacids. Tetrahedron: Asymmetry 2007, 9, 2621–2624. [Google Scholar] [CrossRef]
- Xu, J.; Hu, Y.; Huang, D.; Wang, K.-H.; Xu, C.; Niu, T. Thiourea-catalyzed enantioselective fluorination of β-keto esters. Adv. Synth. Catal. 2012, 354, 515–526. [Google Scholar] [CrossRef]
- Hu, X.-Y.; Hu, F.-Z.; Chen, H.; Xu, X.-Y.; Yuan, W.-C.; Zhang, X.-M. Enantioselective α-arylation of cyclic β-ketoamides with a quinone monoimine. ChemistrySelect 2018, 3, 3975–3977. [Google Scholar] [CrossRef]
- Marcelli, T.; van Der Haas, R.N.S.; van Maarseveen, J.H.; Hiemstra, H. Asymmetric organocatalytic Henry reaction. Angew. Chem. Int. Ed. 2006, 45, 929–931. [Google Scholar] [CrossRef]
- Oh, J.-S.; Lee, J.-W.; Ryu, T.H.; Lee, J.H.; Song, C.E. Self-association free bifunctional thiourea organocatalysts: Synthesis of chiral α-amino acids via dynamic kinetic resolution of racemic azlactones. Org. Biomol. Chem. 2012, 10, 1052–1055. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Asymmetric organocatalysis: From infancy to adolescence. Angew. Chem. Int. Ed. 2008, 47, 4638–4660. [Google Scholar] [CrossRef]
- Tárkányi, G.; Király, P.; Varga, S.; Vakulya, B.; Soós, T. Edge-to-face CH/π aromatic interaction and molecular self-recognition in epi-Cinchona-based bifunctional thiourea organocatalysis. Chem. Eur. J. 2008, 14, 6078–6086. [Google Scholar] [CrossRef]
- Oh, S.H.; Rho, H.S.; Lee, J.W.; Lee, J.E.; Youk, S.H.; Chin, J.; Song, C.E. A highly reactive and enantioselective bifunctional organocatalyst for the methanolytic desymmetrization of cyclic anhydrides: Prevention of catalyst aggregation. Angew. Chem. Int. Ed. 2008, 47, 7872–7875. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.-X.; Luo, Y.-C.; Cheng, X.-N.; Xu, P.-F.; Gu, Y.-C. Organocatalyzed Michael–Michael cascade reaction: Asymmetric synthesis of polysubstituted chromans. J. Org. Chem. 2013, 78, 6488–6494. [Google Scholar] [CrossRef] [PubMed]
- Saha, P.; Biswas, A.; Molleti, N.; Singh, V.K. Enantioselective synthesis of highly substituted chromans via the oxa-Michael–Michael cascade reaction with a bifunctional organocatalyst. J. Org. Chem. 2015, 80, 11115–11122. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.M.; Manzano, R.; Pedrosa, R. Novel bifunctional chiral urea and thiourea derivatives as organocatalysts: Enantioselective nitro-Michael reaction of malonates and diketones. Chem. Eur. J. 2008, 14, 5116–5119. [Google Scholar] [CrossRef]
- Chen, X.-K.; Zheng, C.-W.; Zhao, S.-L.; Chai, Z.; Yang, Y.-Q.; Zhao, G.; Cao, W.-G. Highly enantioselective Michael addition of cyclic 1,3-dicarbonyl compounds to β,γ-unsaturated α-keto esters. Adv. Synth. Catal. 2010, 352, 1648–1652. [Google Scholar] [CrossRef]
- Cui, H.-F.; Li, P.; Wang, X.-W.; Zhu, S.-Z.; Zhao, G. Asymmetric Michael addition of α-fluoro-α-phenylsulfonyl ketones to nitroolefins catalyzed by phenylalanine-based bifunctional thioureas. J. Fluor. Chem. 2012, 133, 120–126. [Google Scholar] [CrossRef]
- Massolo, E.; Benaglia, M.; Orlandi, M.; Rossi, S.; Celentano, G. Enantioselective organocatalytic reduction of β-trifluoromethyl nitroalkenes: An efficient strategy for the synthesis of chiral β-trifluoromethyl amines. Chem. Eur. J. 2015, 21, 3589–3595. [Google Scholar] [CrossRef]
- Klausen, R.S.; Jacobsen, E.N. Weak Brønsted acid-thiourea co-catalysis: Enantioselective, catalytic protio-Pictet-Spengler reactions. Org. Lett. 2009, 11, 887–890. [Google Scholar] [CrossRef]
- Klausen, R.S.; Kennedy, C.R.; Hyde, A.M.; Jacobsen, E.N. Chiral thioureas promote enantioselective Pictet-Spengler cyclization by stabilizing every intermediate and transition state in the carboxylic acid-catalyzed reaction. J. Am. Chem. Soc. 2017, 139, 12299–12309. [Google Scholar] [CrossRef]
- Lee, Y.; Klausen, R.S.; Jacobsen, E.N. Thiourea-catalyzed enantioselective iso-pictet-spengler reactions. Org. Lett. 2011, 13, 5564–5567. [Google Scholar] [CrossRef]
- Yeung, C.S.; Ziegler, R.E.; Porco, J.A.; Jacobsen, E.N. Thiourea-catalyzed enantioselective addition of indoles to pyrones: Alkaloid cores with quaternary carbons. J. Am. Chem. Soc. 2014, 136, 13614–13617. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, A.R.; Mukherjee, S. Enantioselective dearomatization of isoquinolines by anion-binding catalysis en route to cyclic a-aminophosphonates. Chem. Sci. 2016, 7, 6940–6945. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-J.; Du, W.; Chen, Y.-C. Construction of furan derivatives with a trifluoromethyl stereogenic center: Enantioselective Friedel−Crafts alkylations via formal trienamine catalysis. J. Org. Chem. 2016, 81, 10056–10061. [Google Scholar] [CrossRef] [PubMed]
- Manzano, R.; Andrés, J.M.; Muruzábal, M.D.; Pedrosa, R. Stereocontrolled construction of quaternary stereocenters by inter- and intramolecular nitro-michael additions catalyzed by bifunctional thioureas. Adv. Synth. Catal. 2010, 352, 3364–3372. [Google Scholar] [CrossRef]
- Manzano, R.; Andrés, J.M.; Álvarez, R.; Muruzábal, M.D.; de Lera, Á.R.; Pedrosa, R. Enantioselective conjugate addition of nitro compounds to α,β-unsaturated ketones: An experimental and computational study. Chem. Eur. J. 2011, 17, 5931–5938. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.M.; Ceballos, M.; Maestro, A.; Sanz, I.; Pedrosa, R. Supported bifunctional thioureas as recoverable and reusable catalysts for enantioselective nitro-Michael reactions. Beilstein J. Org. Chem. 2016, 12, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Andrés, J.M.; González, M.; Maestro, A.; Naharro, D.; Pedrosa, R. Recyclable chiral bifunctional thioureas derived from [60]fullerene and their use as highly efficient organocatalysts for the asymmetric Nitro-Michael reaction. Eur. J. Org. Chem. 2017, 2683–2691. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Q.; Xu, X.-Y.; Zhou, Y.; Bai, J.; Wang, F.; Wang, L. A highly asymmetric direct aldol reaction catalyzed by chiral proline amide – thiourea bifunctional catalysts. Can. J. Chem. 2011, 89, 1312–1318. [Google Scholar] [CrossRef]
- Vargas-Caporali, J.; Cruz-Hernández, C.; Juaristi, E. Synthesis of versatile bifunctional derivatives of chiral diamines obtained through anchimerically assisted nucleophilic substitution reactions on diastereomeric phenylprolinols. Heterocylces 2012, 86, 1275–1300. [Google Scholar] [CrossRef]
- Vinayagam, P.; Vishwanath, M.; Kesavan, V. New class of bifunctional thioureas from l-proline: Highly enantioselective Michael addition of 1,3-dicarbonyls to nitroolefins. Tetrahedron: Asymmetry 2014, 25, 568–577. [Google Scholar] [CrossRef]
- Hielkema, J.U.; Ticheler, J.; Jörres, M.; Schiffers, I.; Atodiresei, I.; Bolm, C. Asymmetric Michael additions of α-nitrocyclohexanone to aryl nitroalkenes catalyzed by natural amino acid-derived bifunctional thioureas. Org. Lett. 2012, 14, 4518–4521. [Google Scholar] [CrossRef]
- Lattanzi, A. Asymmetric Morita-Baylis-Hillman reaction catalyzed by simple amino alcohol derived thioureas. Synlett 2007, 2106–2110. [Google Scholar] [CrossRef]
- Wanka, L.; Cabrele, C.; Vanejews, M.; Schreiner, P.R. γ-aminoadamantanecarboxylic acids through direct C-H bond amidations. Eur. J. Org. Chem. 2007, 1474–1490. [Google Scholar] [CrossRef]
- Huang, Y.; Zheng, C.; Chai, Z.; Zhao, G. Synthesis of spiro[chroman/tetrahydrothiophene-3,3′-oxindole] scaffolds via heteroatom-Michael-Michael reactions: Easily controlled enantioselectivity via bifunctional catalysts. Adv. Synth. Catal. 2014, 356, 579–583. [Google Scholar] [CrossRef]
- Zhao, K.; Zhi, Y.; Shu, T.; Valkonen, A.; Rissanen, K.; Enders, D. Organocatalytic domino oxa-Michael/1,6-addition reactions: Asymmetric synthesis of chromans bearing oxindole scaffolds. Angew. Chem. Int. Ed. 2016, 55, 12104–12108. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Y.-X.; Zhang, J.-M.; Qian, C.; Chen, X.-Z. Improved synthesis of 4-benzylidene-2,6-di-tert-butylcyclohexa-2,5-dienone and its derivatives. Monatsh. Chem. 2014, 145, 1941–1945. [Google Scholar] [CrossRef]
- Hughes, B.; Howat, D.; Lisle, H.; Holbrook, M.; James, T.; Gozzard, N.; Blease, K.; Hughes, P.; Kingaby, R.; Warrellow, G.; et al. The inhibition of antigen-induced eosinophilia and bronchoconstriction by CDP840, a novel stereo-selective inhibitor of phosphodiesterase type 4. Br. J. Pharmacol. 1996, 118, 1183–1191. [Google Scholar] [CrossRef][Green Version]
- Rovner, E.S.; Wein, A.J. Once-daily, extended-release formulations of antimuscarinic agents in the treatment of overactive bladder: A review. Eur. Urol. 2002, 41, 6–14. [Google Scholar] [CrossRef]
- Davidson, S.J.; Barker, D. Synthesis of various lignans via the rearrangements of 1,4-diarylbutane-1,4-diols. Tetrahedron Lett. 2015, 56, 4549–4553. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, S.; Wang, J.; Mei, G.; Shi, F. Catalytic Asymmetric [4+2] Cyclization of para-quinone methide derivatives with 3-alkyl-2-vinylindoles. Adv. Synth. Catal. 2018, 360, 4225–4235. [Google Scholar] [CrossRef]
- Li, W.; Yuan, H.; Liu, Z.; Zhang, Z.; Cheng, Y.; Li, P. NHC-Catalyzed enantioselective [4 + 3] cycloaddition of ortho-hydroxyphenyl substituted para -quinone methides with isatin-derived enals. Adv. Synth. Catal. 2018, 360, 2460–2464. [Google Scholar] [CrossRef]
- Sun, M.; Ma, C.; Zhou, S.-J.; Lou, S.-F.; Xiao, J.; Jiao, Y.; Shi, F. Catalytic asymmetric (4 + 3) cyclizations of in situ generated ortho-quinone methides with 2-indolylmethanols. Angew. Chem. Int. Ed. 2019, 58, 8703–8708. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-H.; Zhang, X.-Y.; You, Y.; Zhao, J.-Q.; Zhou, M.-Q.; Zhang, X.-M.; Xu, X.-Y.; Yuan, W.-C. Efficient construction of polycyclic chromans through 4-methylbenzenesulfonic acid mediated domino 1,6-addition/oxa-Mannich reaction of ortho-hydroxyphenyl substituted para-quinone methides and cyclic enamides. Tetrahedron 2019, 75, 3456–3462. [Google Scholar] [CrossRef]
- Cheng, Y.-C.; Wang, C.-S.; Li, T.-Z.; Gao, F.; Jiao, Y.; Shi, F. Organocatalytic [4 + 2] cyclizations of para -quinone methide derivatives with isocyanates. Org. Biomol. Chem. 2019, 17, 6662–6670. [Google Scholar] [CrossRef]
- Liu, K.; Cui, H.-F.; Nie, J.; Dong, K.-Y.; Li, X.-J.; Ma, J.-A. Highly enantioselective Michael addition of aromatic ketones to nitroolefins promoted by chiral bifunctional primary amine-thiourea catalysts based on saccharides. Org. Lett. 2007, 9, 923–925. [Google Scholar] [CrossRef]
- Ma, H.; Liu, K.; Zhang, F.-G.; Zhu, C.-L.; Nie, J.; Ma, J.-A. Chiral bifunctional thiourea-catalyzed enantioselective Michael addition of ketones to nitrodienes. J. Org. Chem. 2010, 75, 1402–1409. [Google Scholar] [CrossRef]
- Qiao, B.; Huang, Y.-J.; Nie, J.; Ma, J.-A. Highly regio-, diastereo-, and enantioselective Mannich reaction of allylic ketones and cyclic ketimines: Access to chiral benzosultam. Org. Lett. 2015, 17, 4608–4611. [Google Scholar] [CrossRef]
- Liu, Y.-J.; Li, J.-S.; Nie, J.; Ma, J.-A. Organocatalytic asymmetric decarboxylative Mannich reaction of β-keto acids with cyclic α-ketiminophosphonates: Access to quaternary α-aminophosphonates. Org. Lett. 2018, 20, 3643–3646. [Google Scholar] [CrossRef]
- Li, F.; Sun, L.; Teng, Y.; Yu, P.; Zhao, J.C.-G.; Ma, J.-A. Highly diastereo- and enantioselective organocatalytic one-pot sequential 1,4-addition/dearomative-fluorination transformation. Chem. Eur. J. 2012, 18, 14255–14260. [Google Scholar] [CrossRef]
- Meng, W.-T.; Zheng, Y.; Nie, J.; Xiong, H.-Y.; Ma, J.-A. Organocatalytic asymmetric one-pot sequential conjugate addition/dearomative fluorination: Synthesis of chiral fluorinated isoxazol-5(4H)-ones. J. Org. Chem. 2013, 78, 559–567. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, Z.; Tang, C. Novel bifunctional chiral thiourea catalyzed higly enantioselective aza-Henry reaction. Org. Lett. 2008, 10, 1707–1710. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Zhang, J.; Liu, S.; Yu, C.; Miao, Z. Asymmetric synthesis of spiro[chroman-3,3′-pyrazol] scaffolds with an all-carbon quaternary stereocenter via a oxa-Michael–Michael cascade strategy with bifunctional amine-thiourea organocatalysts. RSC Adv. 2015, 5, 91108–91113. [Google Scholar] [CrossRef]
- V. Kouznetsov, V.; R. Merchan Arenas, D.; Arvelo, F.; S. Bello Forero, J.; Sojo, F.; Munoz, A. 4-Hydroxy-3-methoxyphenyl substituted 3-methyl-tetrahydroquinoline derivatives obtained through imino Diels-Alder reactions as potential antitumoral agents. Lett. Drug Des. Discov. 2010, 7, 632–639. [Google Scholar] [CrossRef]
- Breschi, M.C.; Calderone, V.; Martelli, A.; Minutolo, F.; Rapposelli, S.; Testai, L.; Tonelli, F.; Balsamo, A. New benzopyran-based openers of the mitochondrial ATP-sensitive potassium channel with potent anti-ischemic properties. J. Med. Chem. 2006, 49, 7600–7602. [Google Scholar] [CrossRef] [PubMed]
- Rapposelli, S.; Da Settimo, F.; Digiacomo, M.; La Motta, C.; Lapucci, A.; Sartini, S.; Vanni, M. Synthesis and biological evaluation of 2′-oxo-2,3-dihydro-3′H- spiro[chromene-4,5′-[1,3]oxazolidin]-3′yl]acetic acid derivatives as aldose reductase inhibitors. Arch. Pharm. 2011, 344, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Madhavachary, R.; Prasad, M.S. Observation of neighboring ortho-hydroxyl group participation in organocatalytic asymmetric sequential Michael-lactonization reactions: Synthesis of highly substituted chiral spirodihydrocoumarins. Org. Biomol. Chem. 2012, 10, 5825–5829. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.-L.; Shi, M. Chiral thiourea-phosphine organocatalysts in the asymmetric aza-Morita-Baylis-Hillman reaction. Adv. Synth. Catal. 2007, 349, 2129–2135. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Pei, C.-K.; Shi, M. Multifunctional chiral phosphines-catalyzed highly diastereoselective and enantioselective substitution of Morita-Baylis-Hillman adducts with oxazolones. Org. Biomol. Chem. 2011, 9, 3349–3358. [Google Scholar] [CrossRef]
- Yuan, K.; Zhang, L.; Song, H.-L.; Hu, Y.; Wu, X.-Y. Chiral phosphinothiourea organocatalyst in the enantioselective Morita-Baylis-Hillman reactions of aromatic aldehydes with methyl vinyl ketone. Tetrahedron Lett. 2008, 49, 6262–6264. [Google Scholar] [CrossRef]
- Yuan, K.; Song, H.-L.; Hu, Y.; Wu, X.-Y. Chiral phosphinothiourea-catalyzed asymmetric Morita-Baylis-Hillman reactions of acrylates with aromatic aldehydes. Tetrahedron 2009, 65, 8185–8190. [Google Scholar] [CrossRef]
- Gong, J.-J.; Yuan, K.; Wu, X.-Y. Valine-derived phosphinothiourea as organocatalyst in enantioselective Morita-Baylis-Hillman reactions of acrylates with aromatic aldehydes. Tetrahedron: Asymmetry 2009, 20, 2117–2120. [Google Scholar] [CrossRef]
- Mita, T.; Jacobsen, E. Bifunctional asymmetric catalysis with hydrogen chloride: Enantioselective ring opening of aziridines catalyzed by a phosphinothiourea. Synlett 2009, 1680–1684. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Wei, W.; Lin, A.; Yao, H. Organocatalyzed asymmetric 1,6-conjugate addition of para-quinone methides with dicyanoolefins. Org. Lett. 2016, 18, 428–431. [Google Scholar] [CrossRef] [PubMed]
- Su, H.Y.; Taylor, M.S. P-Stereogenic β-aminophosphines: Preparation and applications in enantioselective organocatalysis. J. Org. Chem. 2017, 82, 3173–3182. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, G. Enantioselective Mannich reaction of γ-malonate-substituted α,β-unsaturated esters with N-Boc imines catalyzed by chiral bifunctional thiourea-phosphonium salts. Tetrahedron 2019, 75, 1697–1705. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Huang, K.; Wang, R.; Zhang, X. A novel chiral bisphosphine-thiourea ligand for asymmetric hydrogenation of β,β-disubstituted nitroalkenes. Org. Lett. 2013, 15, 4014–4017. [Google Scholar] [CrossRef]
- Zhao, Q.; Wen, J.; Tan, R.; Huang, K.; Metola, P.; Wang, R.; Anslyn, E.V.; Zhang, X. Rhodium-catalyzed asymmetric hydrogenation of unprotected NH imines assisted by a thiourea. Angew. Chem. Int. Ed. 2014, 53, 8467–8470. [Google Scholar] [CrossRef]
- Han, Z.; Li, P.; Zhang, Z.; Chen, C.; Wang, Q.; Dong, X.Q.; Zhang, X. Highly enantioselective synthesis of chiral succinimides via Rh/bisphosphine-thiourea-catalyzed asymmetric hydrogenation. ACS Catal. 2016, 6, 6214–6218. [Google Scholar] [CrossRef]
- Li, P.; Hu, X.; Dong, X.Q.; Zhang, X. Rhodium/bisphosphine-thiourea-catalyzed enantioselective hydrogenation of α,β-unsaturated N-acylpyrazoles. Chem. Commun. 2016, 52, 11677–11680. [Google Scholar] [CrossRef]
- Li, P.; Huang, Y.; Hu, X.; Dong, X.-Q.; Zhang, X. Access to chiral seven-member cyclic amines via Rh-catalyzed asymmetric hydrogenation. Org. Lett. 2017, 19, 3855–3858. [Google Scholar] [CrossRef]
- Han, Z.; Wang, R.; Gu, G.; Dong, X.-Q.; Zhang, X. Asymmetric hydrogenation of maleic anhydrides catalyzed by Rh/bisphosphine-thiourea: Efficient construction of chiral succinic anhydrides. Chem. Commun. 2017, 53, 4226–4229. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, P.; Dong, X.-Q.; Zhang, X. Synthesis of chiral seven-membered β-substituted lactams via Rh-catalyzed asymmetric hydrogenation. Org. Biomol. Chem. 2018, 16, 8819–8823. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Han, Z.; Dong, X.-Q.; Zhang, X. Rh-catalyzed asymmetric hydrogenation of β-substituted-β-thio-α,β-unsaturated esters: Expeditious access to chiral organic sulfides. Org. Lett. 2018, 20, 5636–5639. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, Y.; Yi, Z.; Liu, G.; Dong, X.-Q.; Zhang, X. Enantioselective access to chiral cyclic sulfamidates through iridium-catalyzed asymmetric hydrogenation. Adv. Synth. Catal. 2019, 361, 1582–1586. [Google Scholar] [CrossRef]
- Han, Z.; Liu, G.; Wang, R.; Dong, X.-Q.; Zhang, X. Highly efficient Ir-catalyzed asymmetric hydrogenation of benzoxazinones and derivatives with a Brønsted acid cocatalyst. Chem. Sci. 2019, 10, 4328–4333. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.-Q.; Zhao, Q.; Li, P.; Chen, C.; Zhang, X. Metalorganocatalysis: Cooperating transition metal catalysis and organocatalysis through a covalent bond. Org. Chem. Front. 2015, 2, 1425–1431. [Google Scholar] [CrossRef]
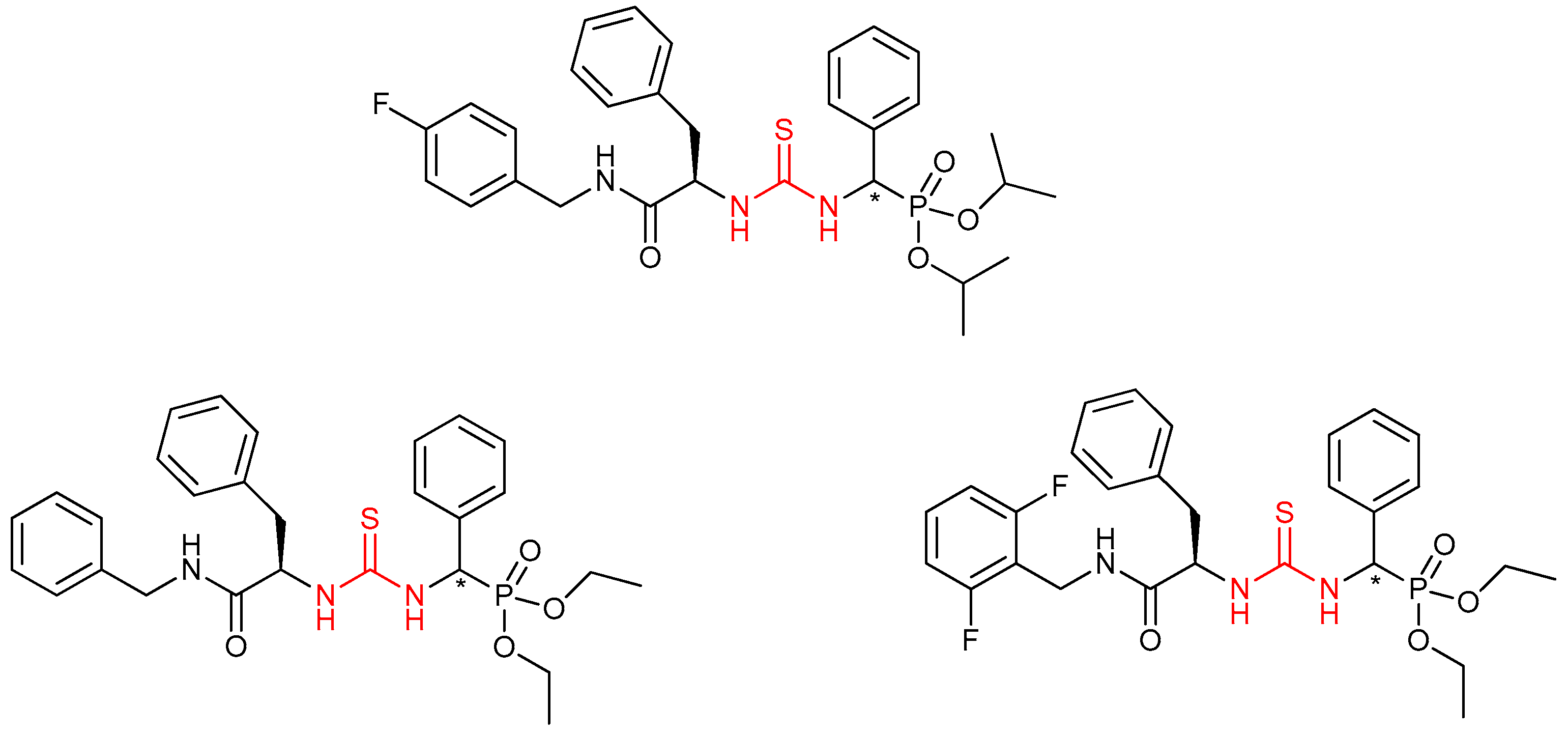
- Chen, M.-H.; Chen, Z.; Song, B.-A.; Bhadury, P.S.; Yang, S.; Cai, X.-J.; Hu, D.-Y.; Xue, W.; Zeng, S. Synthesis and antiviral activities of chiral thiourea derivatives containing an α-aminophosphonate moiety. J. Agric. Food Chem. 2009, 57, 1383–1388. [Google Scholar] [CrossRef]
- Shi, M.; Liu, X.-G. Asymmetric Morita-Baylis-Hillman reaction of arylaldehydes with 2-cyclohexen-1-one catalyzed by chiral bis(thio)urea and DABCO. Org. Lett. 2008, 10, 1043–1046. [Google Scholar] [CrossRef]
- Tan, B.; Candeias, N.R.; Barbas, C.F. Construction of bispirooxindoles containing three quaternary stereocentres in a cascade using a single multifunctional organocatalyst. Nat. Chem. 2011, 3, 473–477. [Google Scholar] [CrossRef]
- Rampalakos, C.; Wulff, W.D.D. A novel bis-thiourea organocatalyst for the asymmetric aza-Henry reaction. Adv. Synth. Catal. 2008, 350, 1785–1790. [Google Scholar] [CrossRef]
- Kang, Y.K.; Yoon, S.J.; Kim, D.Y. Asymmetric Mannich-type reactions of fluorinated ketoesters with binaphthyl-modified thiourea catalysts. Bull. Korean Chem. Soc. 2011, 32, 1195–1200. [Google Scholar] [CrossRef]
- Nakayama, Y.; Hidaka, Y.; Ito, K. Asymmetric Henry reactions of aldehydes using chiral biaryl-based bis(thiourea) organocatalysts. Synlett 2013, 24, 883–885. [Google Scholar] [CrossRef]
- Otevrel, J.; Bobal, P. Biphenyl-based bis(thiourea) organocatalyst for asymmetric and syn-selective Henry reaction. Synthesis 2017, 49, 593–603. [Google Scholar] [CrossRef][Green Version]
- Otevrel, J.; Bobal, P. Diamine-tethered bis(thiourea) organocatalyst for asymmetric Henry reaction. J. Org. Chem. 2017, 82, 8342–8358. [Google Scholar] [CrossRef]
- Otevrel, J.; Svestka, D.; Bobal, P. Bianthryl-based organocatalysts for the asymmetric Henry reaction of fluoroketones. Org. Biomol. Chem. 2019, 17, 5244–5248. [Google Scholar] [CrossRef]
- Roussel, C.; Roman, M.; Andreoli, F.; del Rio, A.; Faure, R.; Vanthuyne, N. Non-racemic atropisomeric (thio)ureas as neutral enantioselective anion receptors for amino-acid derivatives: Origin of smaller Kass with thiourea than urea derivatives. Chirality 2006, 18, 762–771. [Google Scholar] [CrossRef]
- Vlatković, M.; Bernardi, L.; Otten, E.; Feringa, B.L. Dual stereocontrol over the Henry reaction using a light- and heat-triggered organocatalyst. Chem. Commun. 2014, 50, 7773–7775. [Google Scholar] [CrossRef]
- Pizzolato, S.F.; Collins, B.S.L.; van Leeuwen, T.; Feringa, B.L. Bifunctional molecular photoswitches based on overcrowded alkenes for dynamic control of catalytic activity in Michael addition reactions. Chem. Eur. J. 2017, 23, 6174–6184. [Google Scholar] [CrossRef]
- Song, C.; Li, L.; Wang, F.; Deng, J.; Yang, W. Novel optically active helical poly(N-propargylthiourea)s: Synthesis, characterization and complexing ability toward Fe(III) ions. Polym. Chem. 2011, 2, 2825–2829. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, W.; Deng, J. Optically active helical polymers with pendent thiourea groups: Chiral organocatalyst for asymmetric Michael addition reaction. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 1816–1823. [Google Scholar] [CrossRef]
- Mayr, F.; Mohr, L.-M.; Rodriguez, E.; Bach, T. Synthesis of chiral thiourea-thioxanthone hybrids. Synthesis 2017, 49, 5238–5250. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, Y.; Chan, A.S.C.; Wang, R. Highly enantioselective synthesis of γ-nitro heteroaromatic ketones in a doubly stereocontrolled manner catalyzed by bifunctional thiourea catalysts based on dehydroabietic amine: A doubly stereocontrolled approach to pyrrolidine carboxylic acids. Org. Lett. 2009, 11, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, Y.; Liu, X.; Zhang, G.; Lai, L.; Wu, L.; Zhang, J.; Wang, R. Enantio- and diastereoselective asymmetric addition of 1,3-dicarbonyl compounds to nitroalkenes in a doubly stereocontrolled manner catalyzed by bifunctional rosin-derived amine thiourea catalysts. J. Org. Chem. 2009, 74, 5562–5567. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, Y.; Wu, L.; Zhang, G.; Liu, X.; Zhang, H.; Fu, D.; Wang, R. Doubly stereocontrolled asymmetric aza-Henry reaction with in situ generation of N-Boc-imines catalyzed by novel rosin-derived amine thiourea catalysts. Adv. Synth. Catal. 2009, 351, 2096–2100. [Google Scholar] [CrossRef]
- Jiang, X.; Fu, D.; Zhang, G.; Cao, Y.; Liu, L.; Song, J.; Wang, R. Highly diastereo- and enantioselective Mannich reaction of lactones with N-Boc-aldimines catalyzed by bifunctional rosin-derived amine thiourea catalysts. Chem. Commun. 2010, 46, 4294–4296. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, G.; Fu, D.; Cao, Y.; Shen, F.; Wang, R. Direct organocatalytic asymmetric aldol reaction of α-isothiocyanato imides to α-ketoesters under low ligand loading: A doubly stereocontrolled approach to cyclic thiocarbamates bearing chiral quaternary stereocenters. Org. Lett. 2010, 12, 1544–1547. [Google Scholar] [CrossRef]
- Liu, L.; Zhong, Y.; Zhang, P.; Jiang, X.; Wang, R. Core scaffold-inspired stereoselective synthesis of spiropyrazolones via an organocatalytic Michael/cyclization sequence. J. Org. Chem. 2012, 77, 10228–10234. [Google Scholar] [CrossRef]
- Shi, X.M.; Dong, W.P.; Zhu, L.P.; Jiang, X.X.; Wang, R. Asymmetric vinylogous Michael addition/cyclization cascade reaction for the construction of diversely structured spiro-oxindole skeletons. Adv. Synth. Catal. 2013, 355, 3119–3123. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Jiang, X.; Yan, W.; Wang, R. Highly enantioslective synthesis of multisubstituted polyfunctional dihydropyrrole via an organocatalytic tandem Michael/cyclization sequence. Org. Lett. 2011, 13, 3806–3809. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.; Yan, J.; Chen, R.; Wang, S.; Ma, Y.; Wang, R. One-pot enantioselective synthesis of functionalized pyranocoumarins and 2-amino-4H-chromenes: Discovery of a type of potent antibacterial agent. J. Org. Chem. 2012, 77, 878–888. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, L.; Kai, M.; Zhu, L.; Yao, X.; Wang, R. Asymmetric inverse-electron-demand hetero-Diels-Alder reaction for the construction of bicyclic skeletons with multiple stereocenters by using a bifunctional organocatalytic strategy: An efficient approach to chiral macrolides. Chem. Eur. J. 2012, 18, 11465–11473. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.V.S.; Swain, M.; Reddy, S.M.; Yadav, J.S.; Sridhar, B. Asymmetric Michael/hemiketalization of 5-hydroxy-2-methyl-4H-pyran-4-one to β,γ-unsaturated α-ketoesters catalyzed by a bifunctional rosin-indane amine thiourea catalyst. RSC Adv. 2014, 4, 42299–42307. [Google Scholar] [CrossRef]
- Sohtome, Y.; Hashimoto, Y.; Nagasawa, K. Guanidine-thiourea bifunctional organocatalyst for the asymmetric Henry (nitroaldol) reaction. Adv. Synth. Catal. 2005, 347, 1643–1648. [Google Scholar] [CrossRef]
- Herrera, R.P.; Sgarzani, V.; Bernardi, L.; Ricci, A. Catalytic enantioselective Friedel-Crafts alkylation of indoles with nitroalkenes by using a simple thiourea organocatalyst. Angew. Chem. Int. Ed. 2005, 44, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Nowak, A.E.; Wojaczyńska, E.; Skarewski, J. Enantiopure trans-1-amino-2-(arylsulfanyl)cyclohexanes: Novel chiral motifs for ligands and organocatalysts. Tetrahedron: Asymmetry 2011, 22, 1687–1691. [Google Scholar] [CrossRef]
- Krasnovskaya, O.O.; Malinnikov, V.M.; Dashkova, N.S.; Gerasimov, V.M.; Grishina, I.V.; Kireev, I.I.; Lavrushkina, S.V.; Panchenko, P.A.; Zakharko, M.A.; Ignatov, P.A. Thiourea modified doxorubicin: A perspective pH-sensitive prodrug. Bioconjug. Chem. 2019, 30, 741–750. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-W.; Liu, Y.-X.; Zhang, W.; Tao, Y.; Zhu, Y.; Tao, J.-C.; Tang, M.-S. Highly enantioselective Michael additions of isobutyraldehyde to nitroalkenes promoted by amphiphilic bifunctional primary amine-thioureas in organic or aqueous medium. Eur. J. Org. Chem. 2011, 6747–6754. [Google Scholar] [CrossRef]
- Ma, Z.-W.; Liu, Y.-X.; Huo, L.-J.; Gao, X.; Tao, J.-C. Doubly stereocontrolled asymmetric Michael addition of acetylacetone to nitroolefins promoted by an isosteviol-derived bifunctional thiourea. Tetrahedron: Asymmetry 2012, 23, 443–448. [Google Scholar] [CrossRef]
- Song, Z.-T.; Zhang, T.; Du, H.-L.; Ma, Z.-W.; Zhang, C.-H.; Tao, J.-C. Highly enantioselective Michael addition promoted by a new diterpene-derived bifunctional thiourea catalyst: A doubly stereocontrolled approach to chiral succinimide derivatives. Chirality 2014, 26, 121–127. [Google Scholar] [CrossRef]
- Grošelj, U.; Golobič, A.; Stare, K.; Svete, J.; Stanovnik, B. Synthesis and structural elucidation of novel camphor-derived thioureas. Chirality 2012, 24, 307–317. [Google Scholar] [CrossRef]
- Khan, S.A.; Singh, N.; Saleem, K. Synthesis, characterization and in vitro antibacterial activity of thiourea and urea derivatives of steroids. Eur. J. Med. Chem. 2008, 43, 2272–2277. [Google Scholar] [CrossRef] [PubMed]
- Ramadas, K.; Suresh, G.; Janarthanan, N.; Masilamani, S. Antifungal activity of 1,3-disubstituted symmetrical and unsymmetrical thioureas. Pestic. Sci. 1998, 52, 145–151. [Google Scholar] [CrossRef]
- Cunha, S.; Macedo, F.C.; Costa, G.A.N.; Rodrigues, M.T.; Verde, R.B.V.; de Souza Neta, L.C.; Vencato, I.; Lariucci, C.; Sá, F.P. Antimicrobial activity and structural study of disubstituted thiourea derivatives. Monatsh. Chem. 2007, 138, 511–516. [Google Scholar] [CrossRef]
- Omar, A.-M.M.E.; Farghaly, A.M.; Hazzai, A.A.B.; Eshba, N.H.; Sharabi, F.M.; Daabees, T.T. Thiourea and thiosemicarbazide derivatives structurally related to hexestrol: Synthesis and anticancer and other pharmacological properties. J. Pharm. Sci. 1981, 70, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Kafarski, P.; Lejczak, B. Biological activity of aminophosphonic acids. Phosphorus. Sulfur. Silicon Relat. Elem. 1991, 63, 193–215. [Google Scholar] [CrossRef]
- Yang, X.; Song, B.; Jin, L.; Wei, X.; Bhadury, S.P.; Li, X.; Yang, S.; Hu, D. Synthesis and antiviral bioactivities of novel chiral bis-thiourea-type derivatives containing α-aminophosphonate moiety. Sci. China Chem. 2011, 54, 103–109. [Google Scholar] [CrossRef]
- Liu, J.; Yang, S.; Li, X.; Fan, H.; Bhadury, P.; Xu, W.; Wu, J.; Wang, Z. Synthesis and antiviral bioactivity of chiral thioureas containing leucine and phosphonate moieties. Molecules 2010, 15, 5112–5123. [Google Scholar] [CrossRef]
- Yan, Z.; Cai, X.; Yang, X.; Song, B.; Chen, Z.; Bhadury, S.P.; Hu, D.; Jin, L.; Xue, W.; Lu, P. Synthesis and antiviral activities of chiral thiourea derivatives. Chin. J. Chem. 2009, 27, 593–601. [Google Scholar] [CrossRef]
- Lewi, P.; Heeres, J.; Ariën, K.; Venkatraj, M.; Joossens, J.; Van der Veken, P.; Augustyns, K.; Vanham, G. Reverse transcriptase inhibitors as microbicides. Curr. HIV Res. 2012, 10, 27–35. [Google Scholar] [CrossRef]
- Jones, T.R.; Lee, S.-W.; Johann, S.V.; Razinkov, V.; Visalli, R.J.; Feld, B.; Bloom, J.D.; O’Connell, J. Specific inhibition of human cytomegalovirus glycoprotein B-mediated fusion by a novel thiourea small molecule. J. Virol. 2004, 78, 1289–1300. [Google Scholar] [CrossRef][Green Version]
- Struga, M.; Kossakowski, J.; Koziol, A.E.; Kedzierska, E.; Fidecka, S.; La Colla, P.; Ibba, C.; Collu, G.; Sanna, G.; Secci, B.; et al. Synthesis, pharmacological and antiviral activity of 1,3-thiazepine derivatives. Eur. J. Med. Chem. 2009, 44, 4960–4969. [Google Scholar] [CrossRef] [PubMed]
- De Clercq, E. Perspectives of non-nucleoside reverse transcriptase inhibitors (NNRTIs) in the therapy of HIV-1 infection. Farmaco 1999, 54, 26–45. [Google Scholar] [CrossRef]
- Robl, J.A.; Sun, C.-Q.; Stevenson, J.; Ryono, D.E.; Simpkins, L.M.; Cimarusti, M.P.; Dejneka, T.; Slusarchyk, W.A.; Chao, S.; Stratton, L.; et al. Dual metalloprotease inhibitors: Mercaptoacetyl-based fused heterocyclic dipeptide mimetics as inhibitors of angiotensin-converting enzyme and neutral endopeptidase. J. Med. Chem. 1997, 40, 1570–1577. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Jain, G.; Sharma, A. Benzothiazepines: Chemistry of a privileged scaffold. RSC Adv. 2015, 5, 70619–70639. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Sudbeck, E.A.; Mao, C.; Uckun, F.M. Stereochemistry of halopyridyl and thiazolyl thiourea compounds is a major determinant of their potency as nonnucleoside inhibitors of HIV-1 reverse transcriptase. Bioorg. Med. Chem. Lett. 2000, 10, 2071–2074. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Mao, C.; Uckun, F.M. Stereochemistry as a major determinant of the anti-HIV activity of chiral naphthyl thiourea compounds. Antivir. Chem. Chemother. 2001, 12, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Bell, F.W.; Cantrell, A.S.; Hoegberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordan, C.L.; Kinnick, M.D.; Lind, P.; Morin, J.M. Phenethylthiazolethiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem. 1995, 38, 4929–4936. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.S.; Engelhardt, P.; Högberg, M.; Jaskunas, S.R.; Johansson, N.G.; Jordan, C.L.; Kangasmetsä, J.; Kinnick, M.D.; Lind, P.; Morin, J.M.; et al. Phenethylthiazolylthiourea (PETT) compounds as a new class of HIV-1 reverse transcriptase inhibitors. 2. Synthesis and further structure−activity relationship studies of PETT analogs. J. Med. Chem. 1996, 39, 4261–4274. [Google Scholar] [CrossRef] [PubMed]
- Venkatachalam, T.K.; Mao, C.; Uckun, F.M. Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 2004, 12, 4275–4284. [Google Scholar] [CrossRef] [PubMed]
- Pingaew, R.; Sinthupoom, N.; Mandi, P.; Prachayasittikul, V.V.; Cherdtrakulkiat, R.; Prachayasittikul, S.; Ruchirawat, S.; Prachayasittikul, V.V. Synthesis, biological evaluation and in silico study of bis-thiourea derivatives as anticancer, antimalarial and antimicrobial agents. Med. Chem. Res. 2017, 26, 3136–3148. [Google Scholar] [CrossRef]
- Rohde, J.M.; Brimacombe, K.R.; Liu, L.; Pacold, M.E.; Yasgar, A.; Cheff, D.M.; Lee, T.D.; Rai, G.; Baljinnyam, B.; Li, Z.; et al. Discovery and optimization of piperazine-1-thiourea-based human phosphoglycerate dehydrogenase inhibitors. Bioorg. Med. Chem. 2018, 26, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Manjula, S.N.; Noolvi, N.M.; Vipan Parihar, K.; Reddy, S.A.M.; Ramani, V.; Gadad, A.K.; Singh, G.; Kutty, N.G.; Rao, C.M. Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem. 2009, 44, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Dinakaran, V.S.; Jacob, D.; Mathew, J.E.; Seekarajapuram, V.; Divya, D.; Jessy, J.; Mathew, E. Synthesis and biological evaluation of novel pyrimidine-2(1H)-ones/thiones as potent anti-inflammatory and anticancer agents. Med. Chem. Res. 2012, 21, 3598–3606. [Google Scholar] [CrossRef]
- Liu, J.-Z.; Song, B.-A.; Fan, H.-T.; Bhadury, P.S.; Wan, W.-T.; Yang, S.; Xu, W.; Wu, J.; Jin, L.-H.; Wei, X.; et al. Synthesis and in vitro study of pseudo-peptide thioureas containing α-aminophosphonate moiety as potential antitumor agents. Eur. J. Med. Chem. 2010, 45, 5108–5112. [Google Scholar] [CrossRef]
- Liu, J.; Liao, P.; Hu, J.; Zhu, H.; Wang, Y.; Li, Y.; Li, Y.; He, B. Synthesis and Antitumor Activities of Chiral Dipeptide Thioureas Containing an Alpha-Aminophosphonate Moiety. Molecules 2017, 22, 238–248. [Google Scholar] [CrossRef]
- Huang, X.-C.; Wang, M.; Pan, Y.-M.; Yao, G.-Y.; Wang, H.-S.; Tian, X.-Y.; Qin, J.-K.; Zhang, Y. Synthesis and antitumor activities of novel thiourea α-aminophosphonates from dehydroabietic acid. Eur. J. Med. Chem. 2013, 69, 508–520. [Google Scholar] [CrossRef]
- Rao, X.; Song, Z.; He, L.; Jia, W. Synthesis, structure analysis and cytotoxicity studies of novel unsymmetrically N,N′-substituted ureas from dehydroabietic acid. Chem. Pharm. Bull. 2008, 56, 1575–1578. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Vassilev, A.O.; Benyunov, A.; Grigoriants, O.O.; Tibbles, H.E.; Uckun, F.M. Stereochemistry as a determinant of the anti-leukemic potency of halopyridyl and thiazolyl thiourea compounds. Lett. Drug Des. Discov. 2007, 4, 318–326. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Qazi, S.; Samuel, P.; Uckun, F.M. Substituted heterocyclic thiourea compounds as a new class of anti-allergic agents inhibiting IgE/FcεRI receptor mediated mast cell leukotriene release. Bioorg. Med. Chem. 2003, 11, 1095–1105. [Google Scholar] [CrossRef]
- Wasserman, S.I. Mast cell biology. J. Allergy Clin. Immunol. 1990, 86, 590–593. [Google Scholar] [CrossRef]
- Bildirici, I.; Cetin, A.; Menges, N.; Alan, Y. Synthesis and SAR studies of pyrazole-3-carboxamides and -3-carbonyl thioureides including chiral moiety: Novel candidates as antibacterial agents. J. Serb. Chem. Soc. 2018, 83, 795–807. [Google Scholar] [CrossRef]
- O’Dwyer, P.J.; King, S.A.; Plowman, J.; Grieshaber, C.K.; Hoth, D.F.; Leyland-Jones, B. Pyrazole: Preclinical reassessment. Invest. New Drugs 1988, 6, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Rashid, N.; Jones, P.G.; Ali, M.; Hussain, R. Synthesis, characterization and biological evaluation of some thiourea derivatives bearing benzothiazole moiety as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2010, 45, 1323–1331. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Tiwari, O.P. Brijog Synthesis and antimicrobial activity of some methyl 4-(1H-benzo[d] imidazol-2-yl) phenyl carbamodithioate amine derivatives. Int. J. Pharm. Sci. Res. 2018, 9, 1194–1200. [Google Scholar] [CrossRef]
- Madabhushi, S.; Mallu, K.K.R.; Vangipuram, V.S.; Kurva, S.; Poornachandra, Y.; Kumar, C.G. Synthesis of novel benzimidazole functionalized chiral thioureas and evaluation of their antibacterial and anticancer activities. Bioorg. Med. Chem. Lett. 2014, 24, 4822–4825. [Google Scholar] [CrossRef] [PubMed]
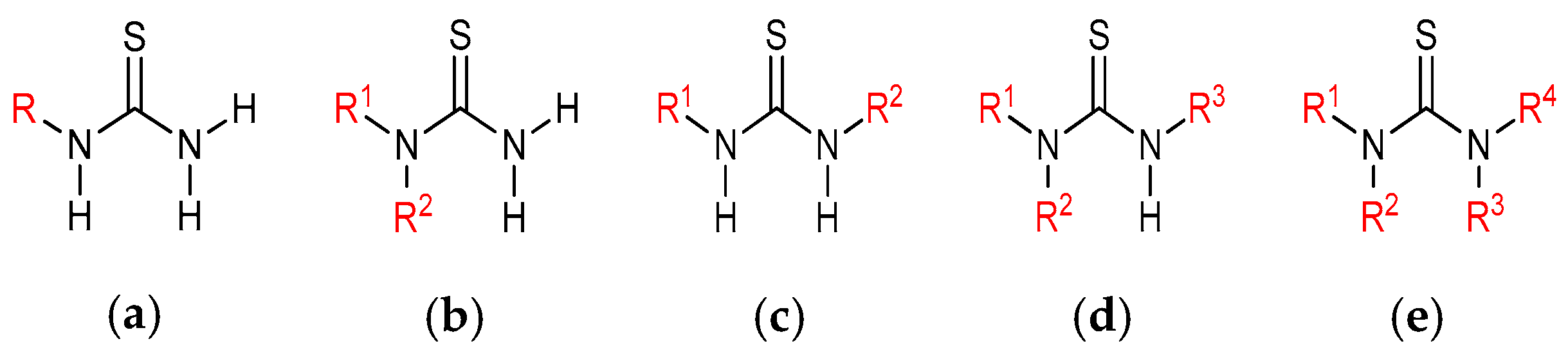

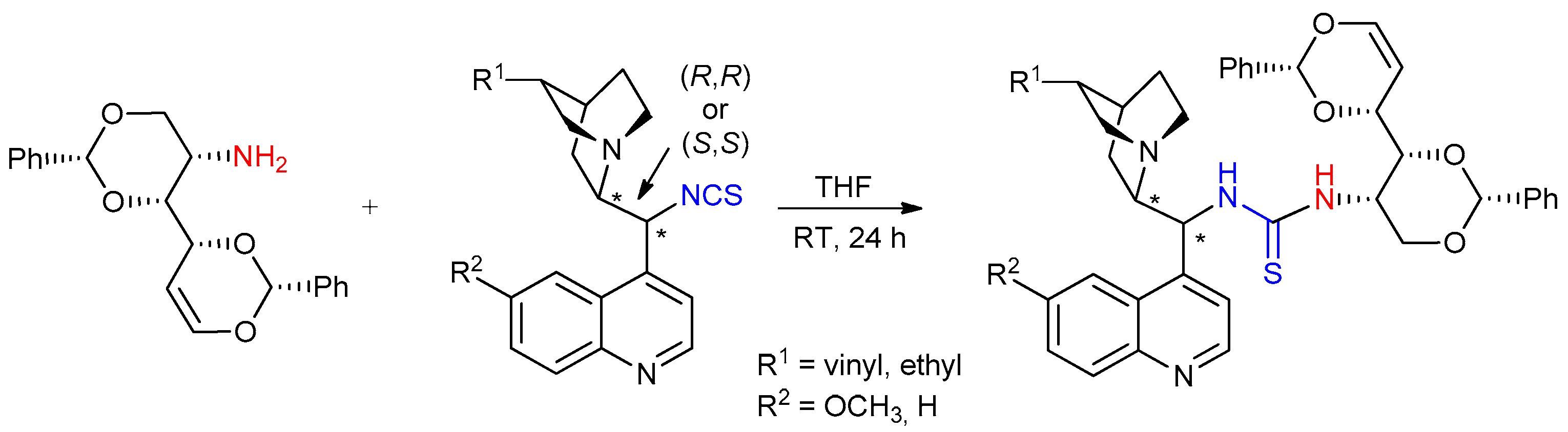
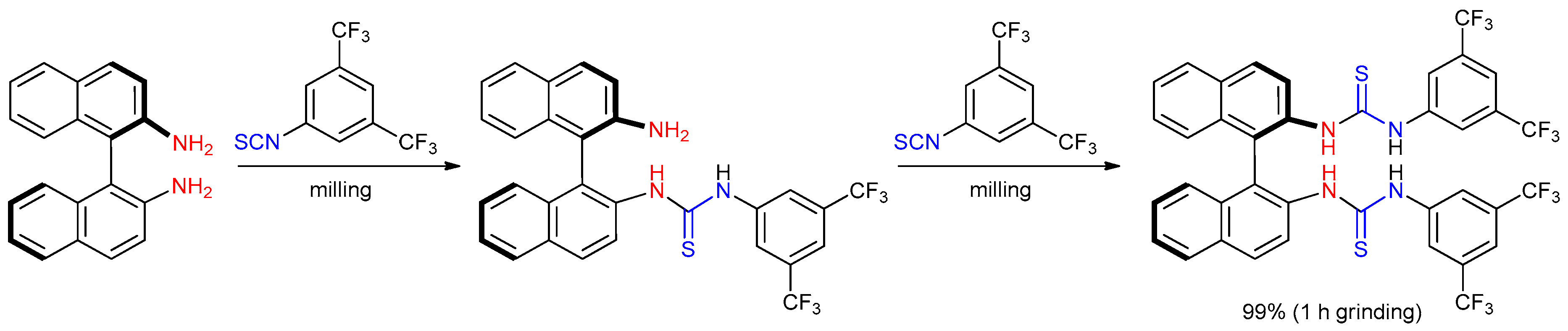
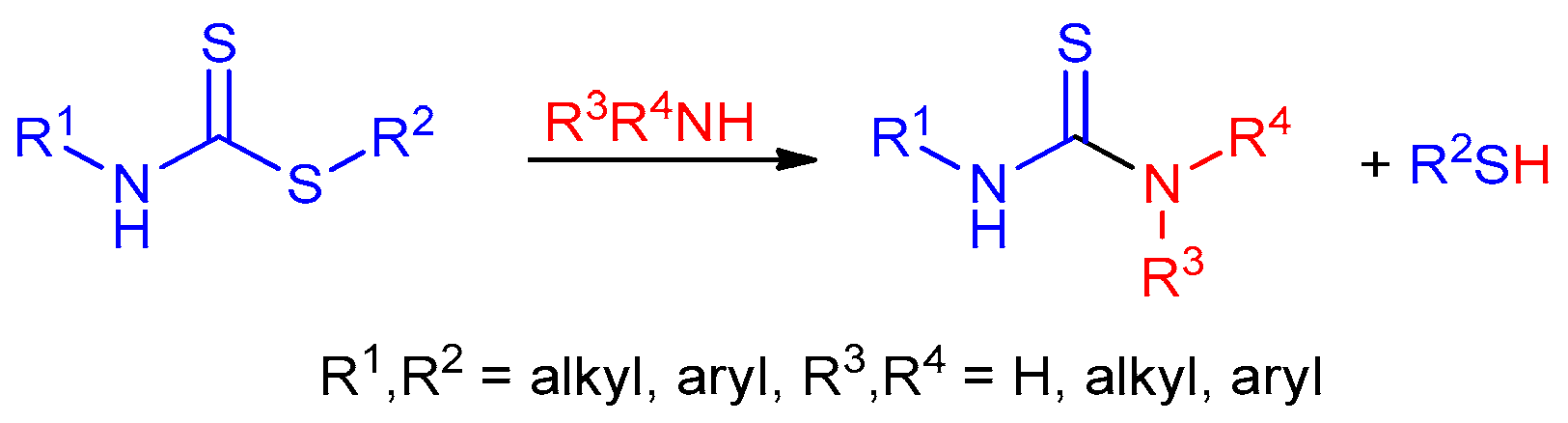
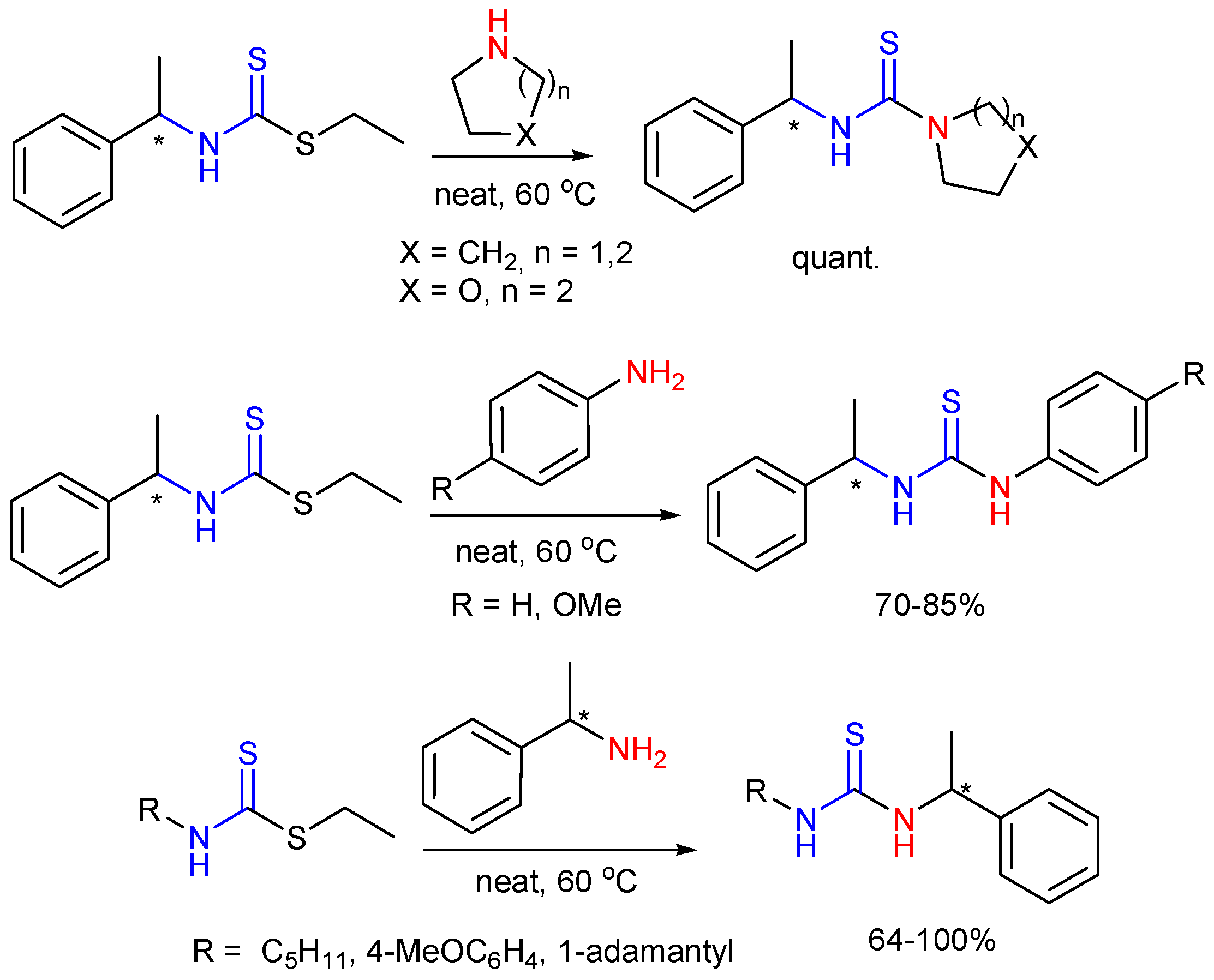
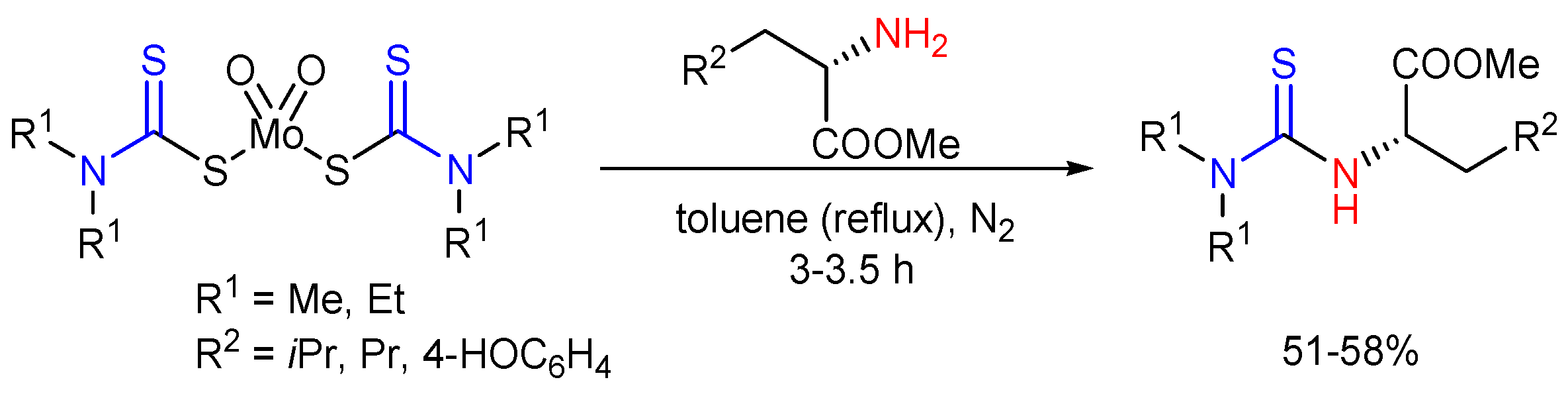
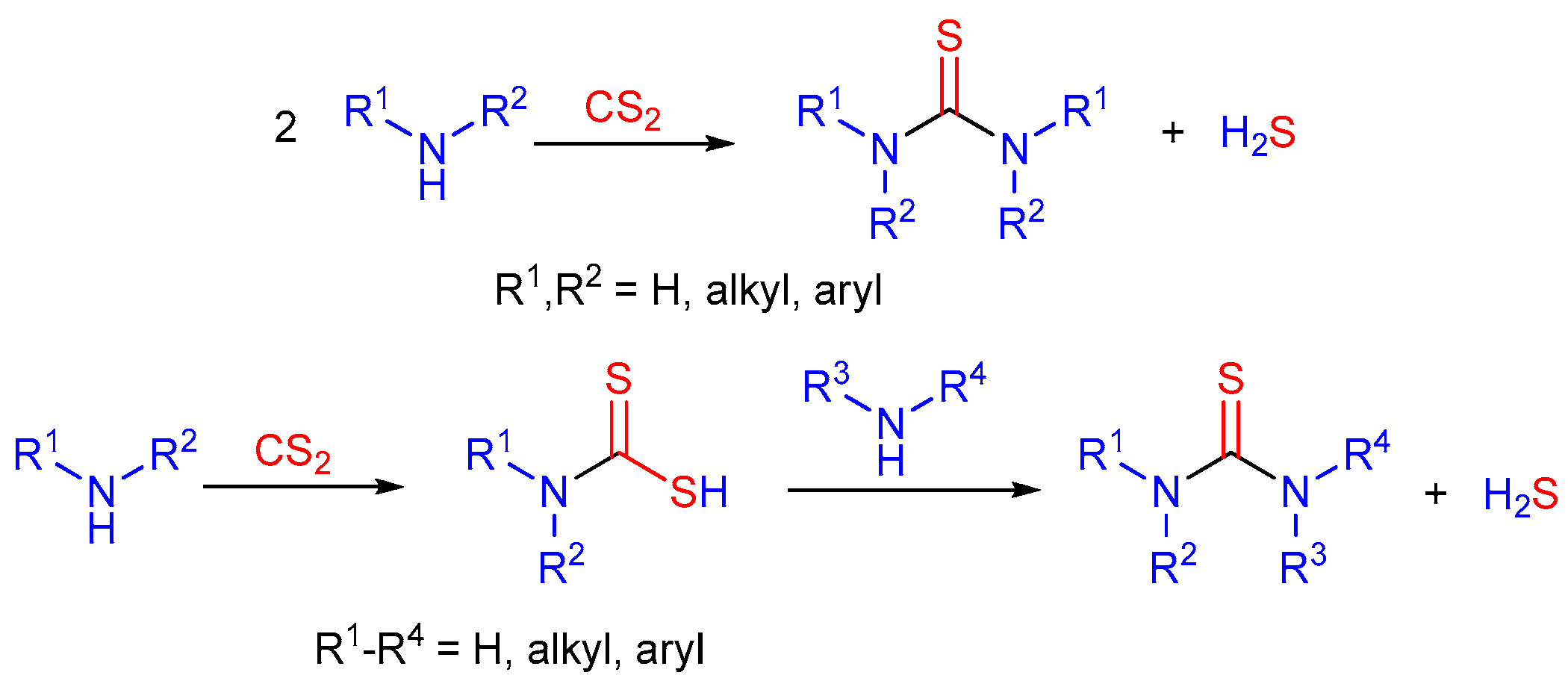
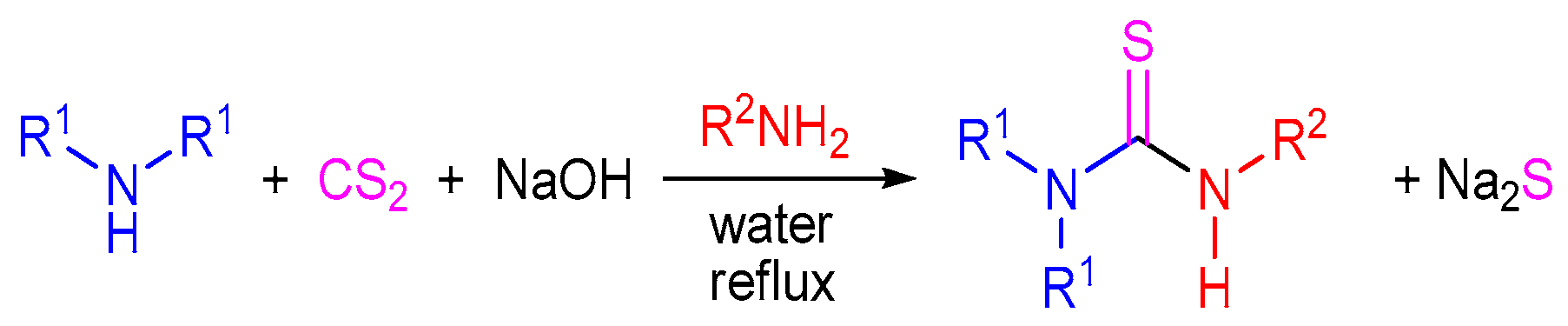
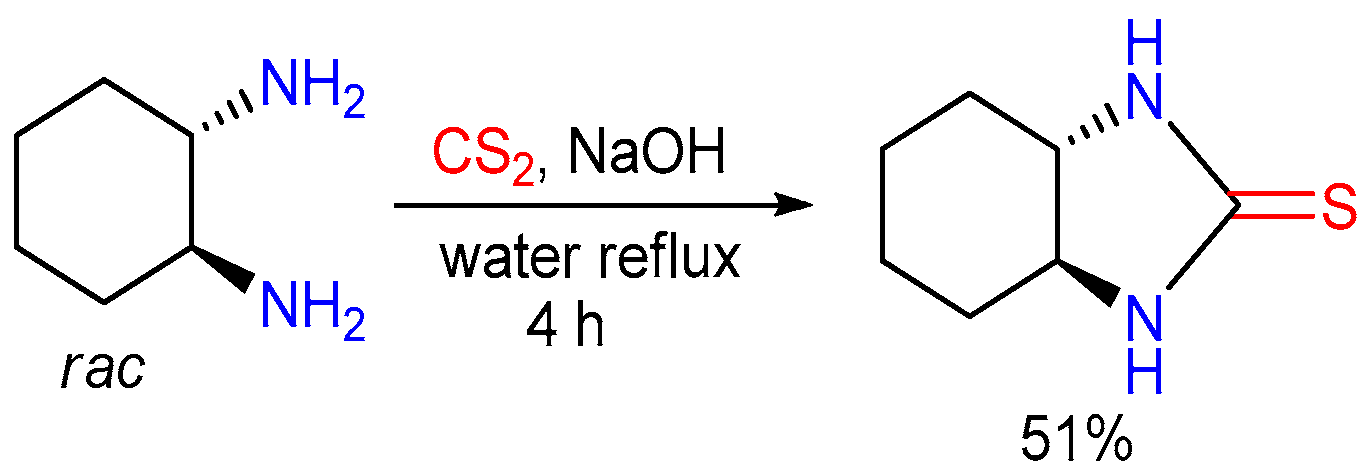
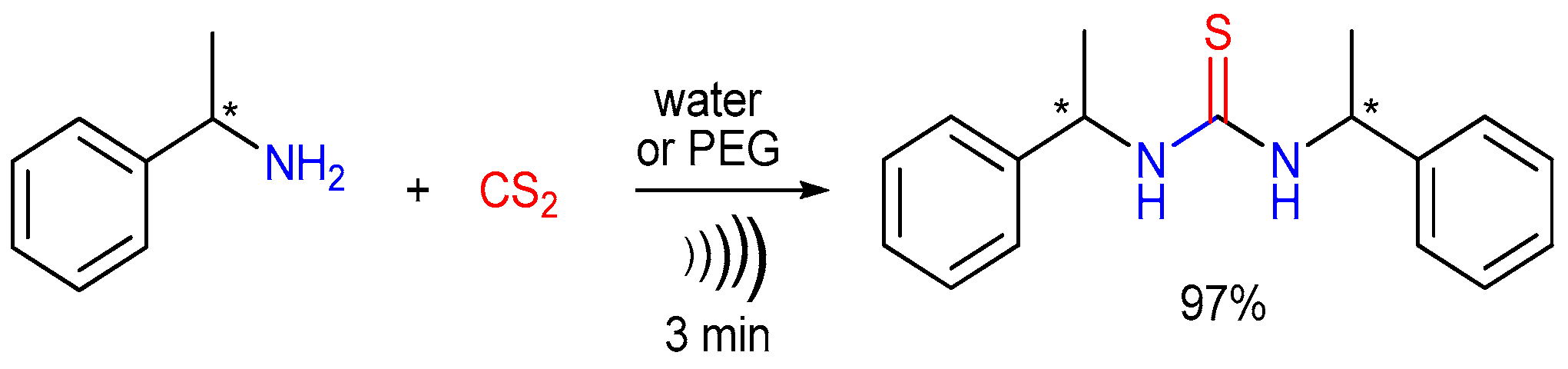


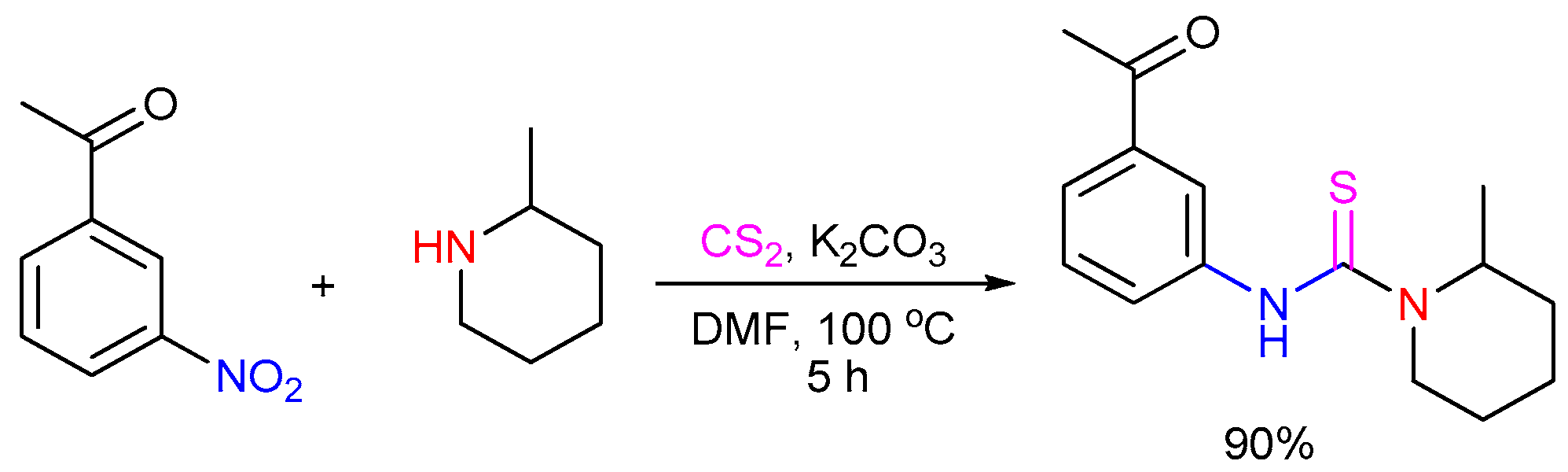
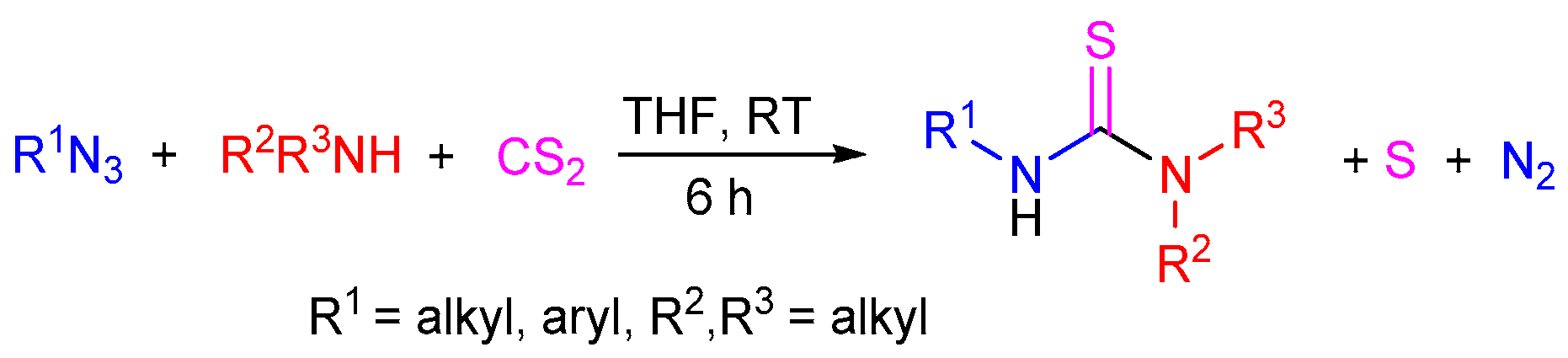
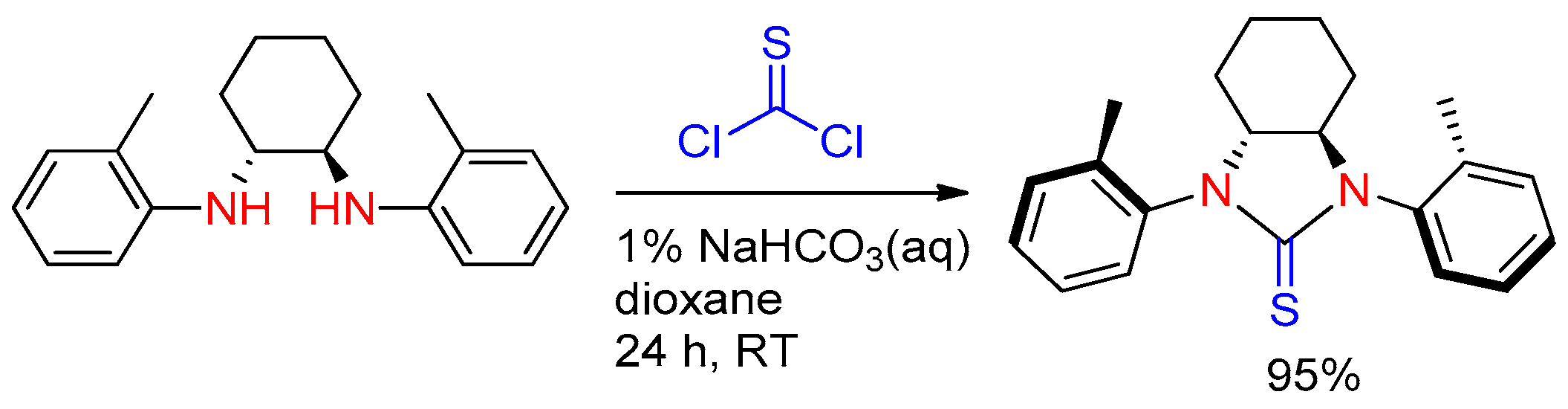
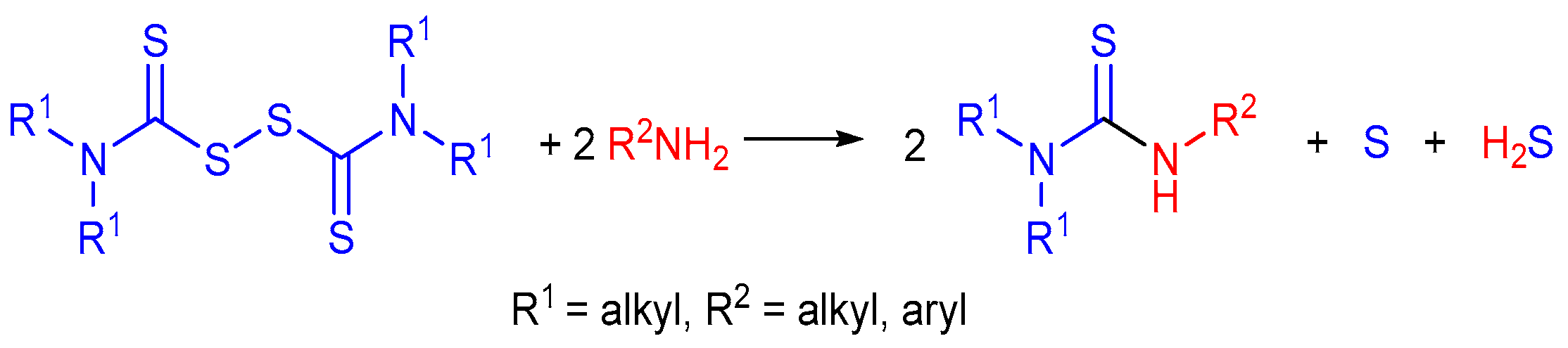
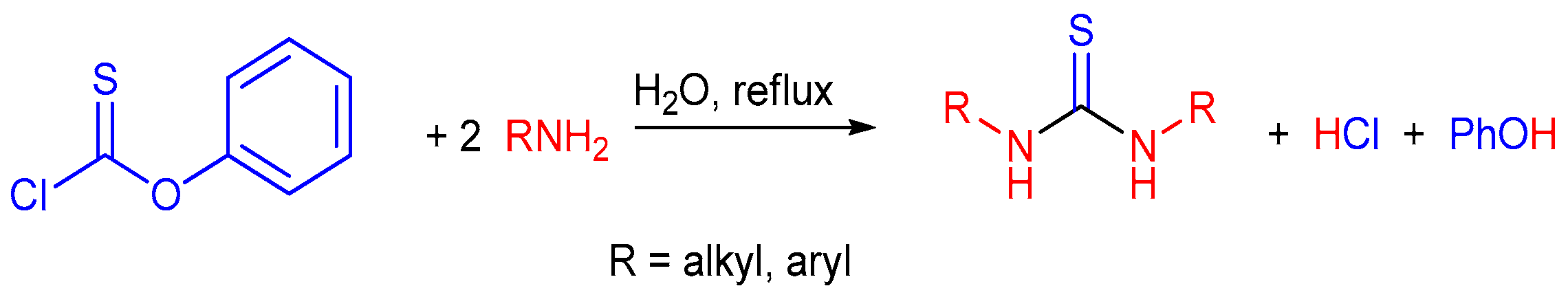
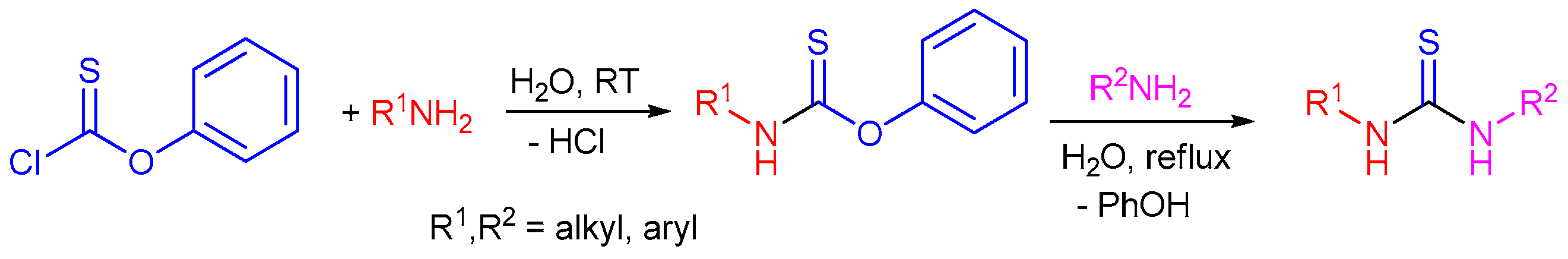
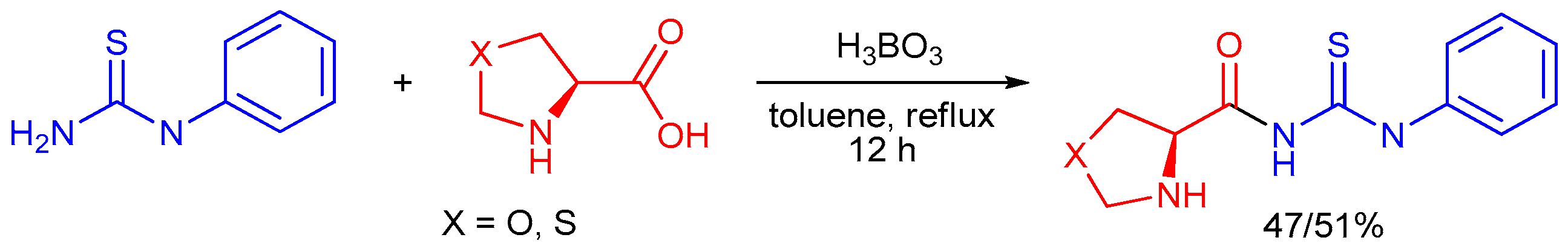
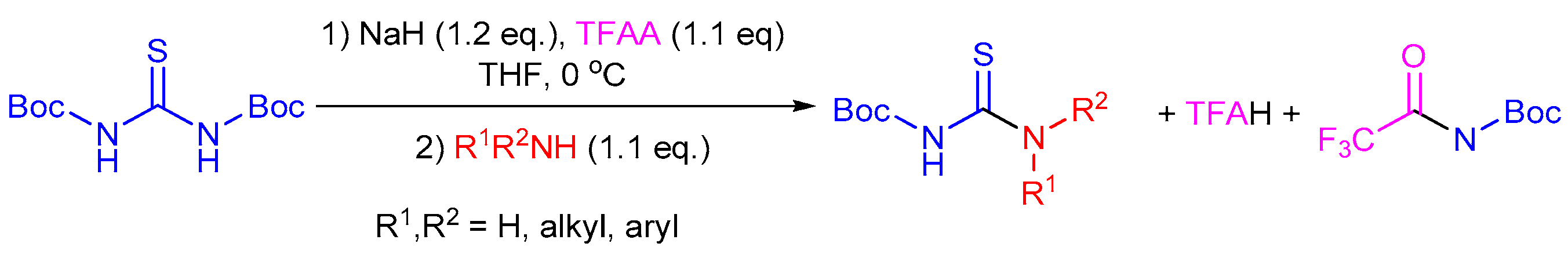
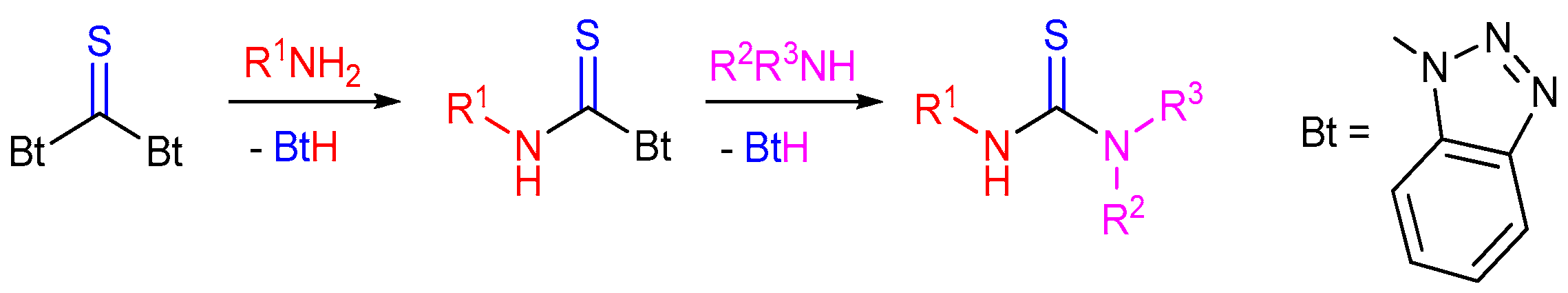
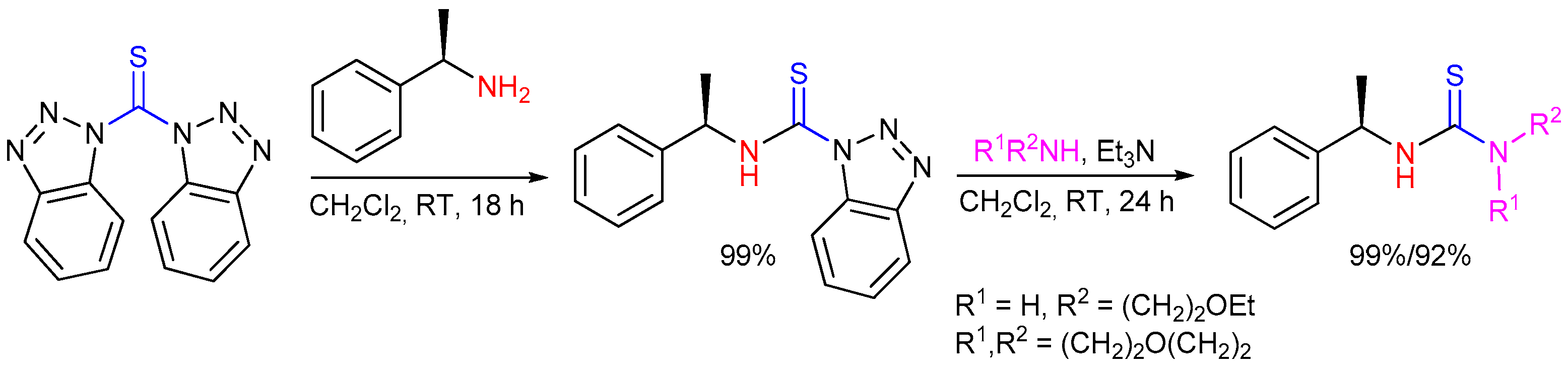

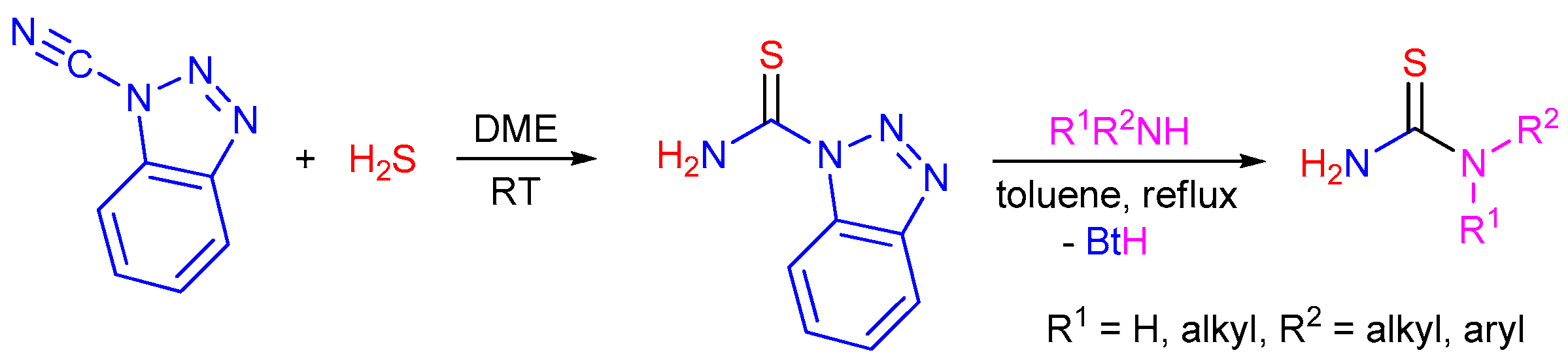
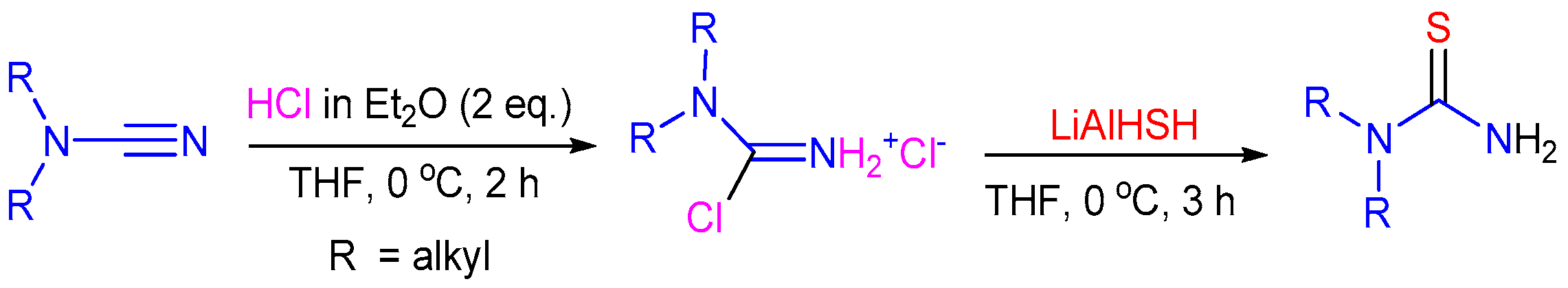

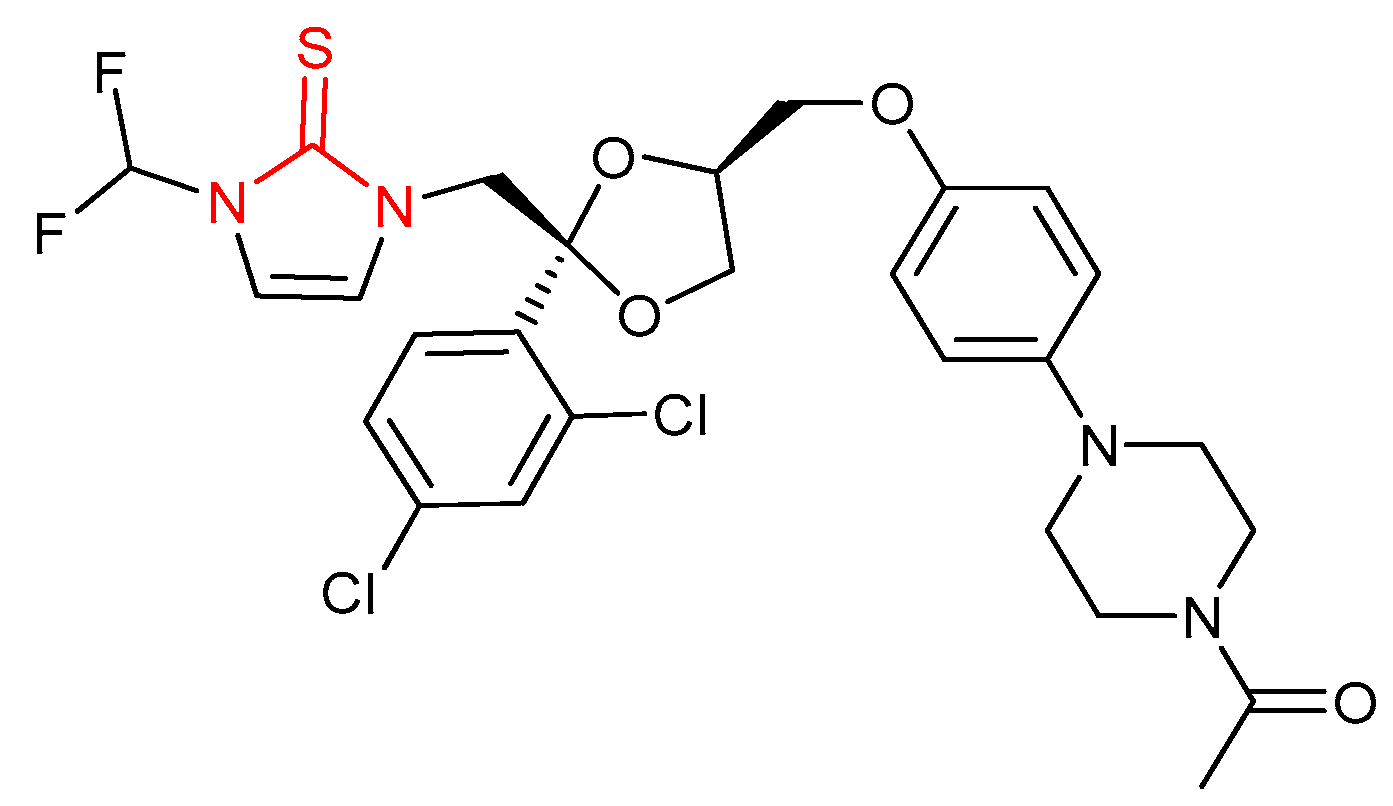
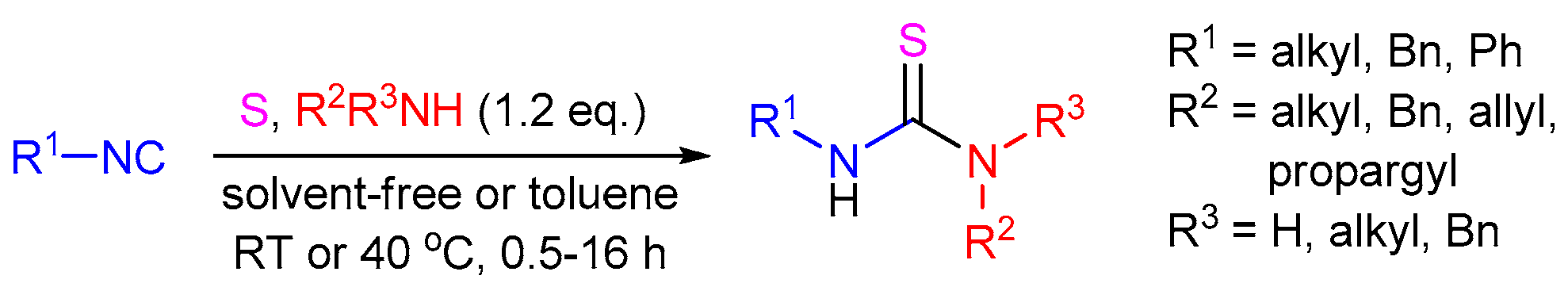

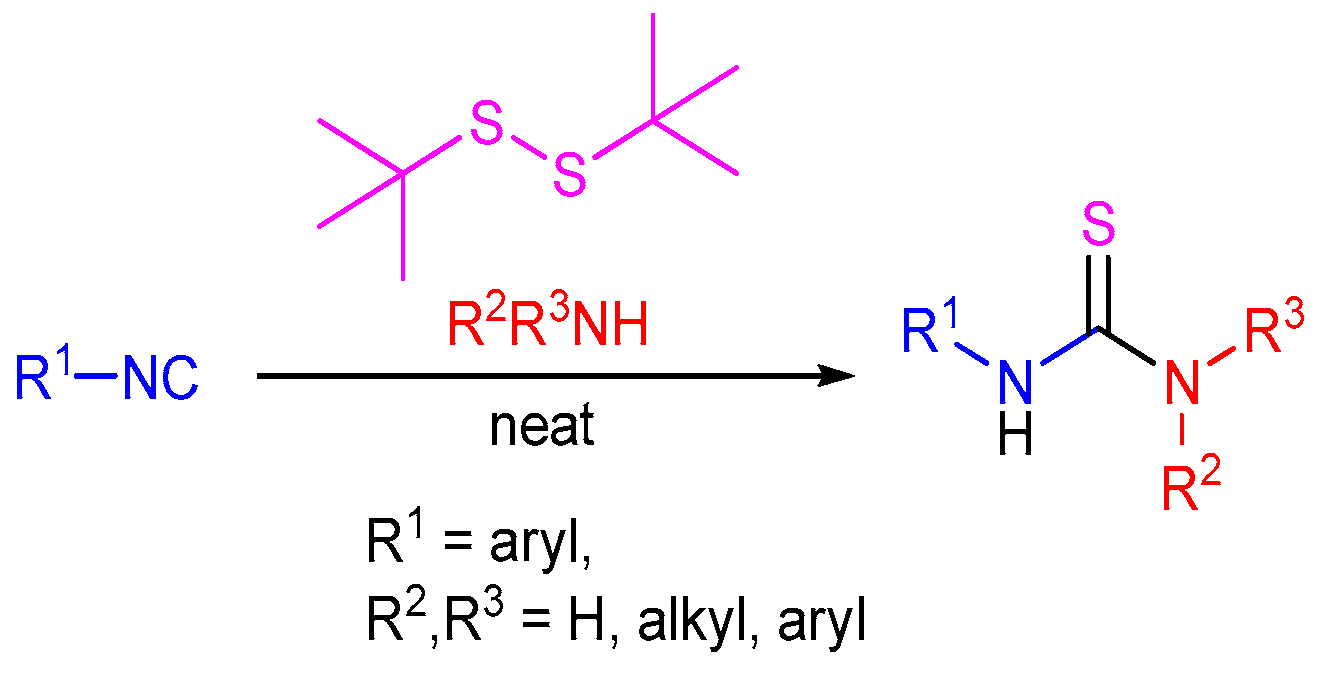
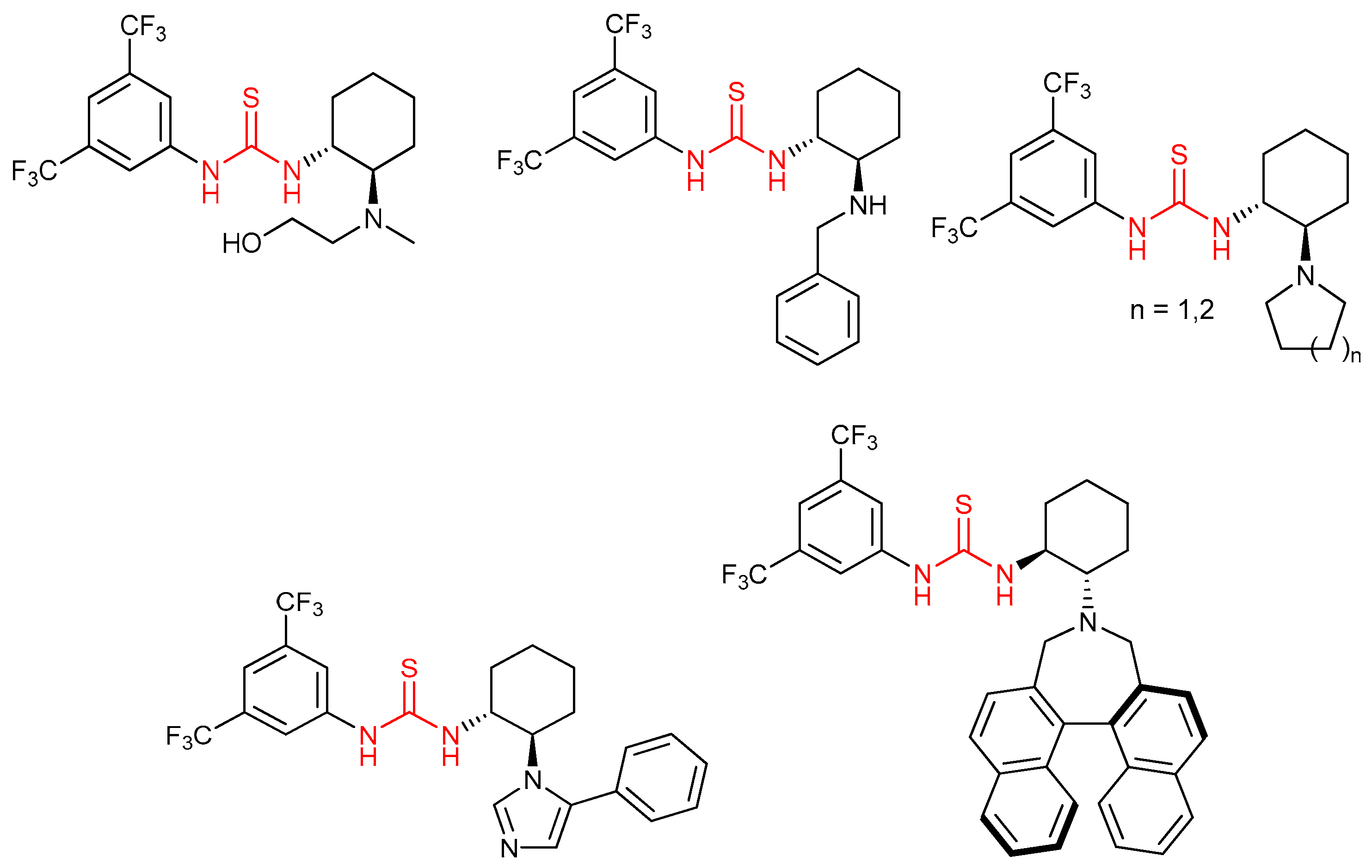
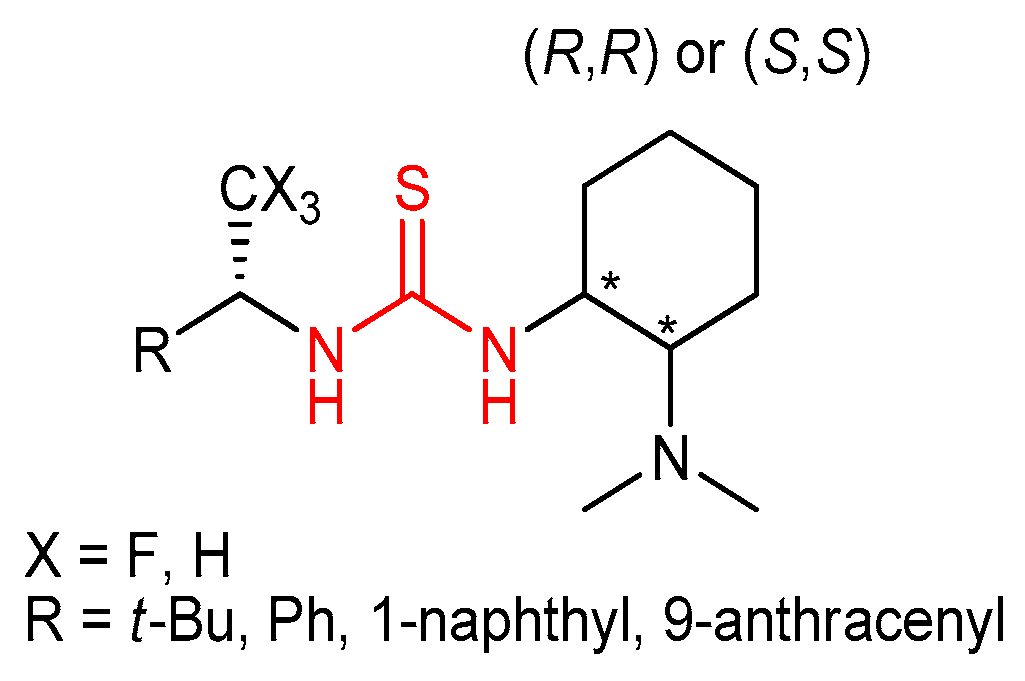
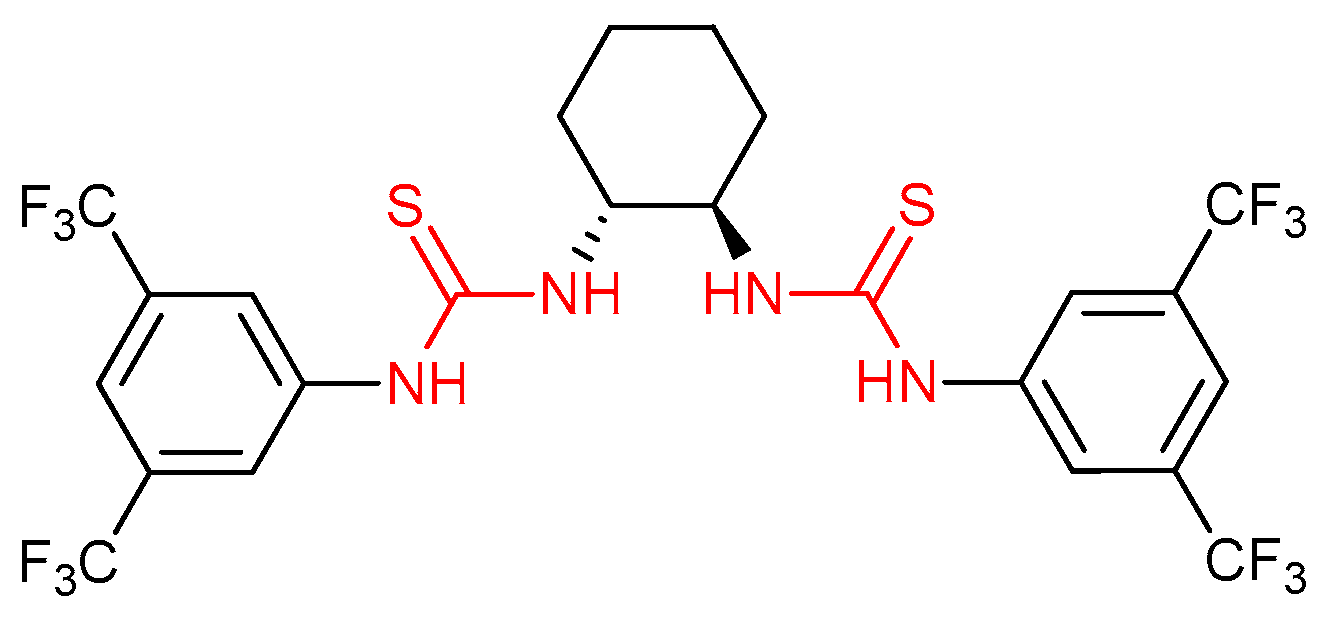


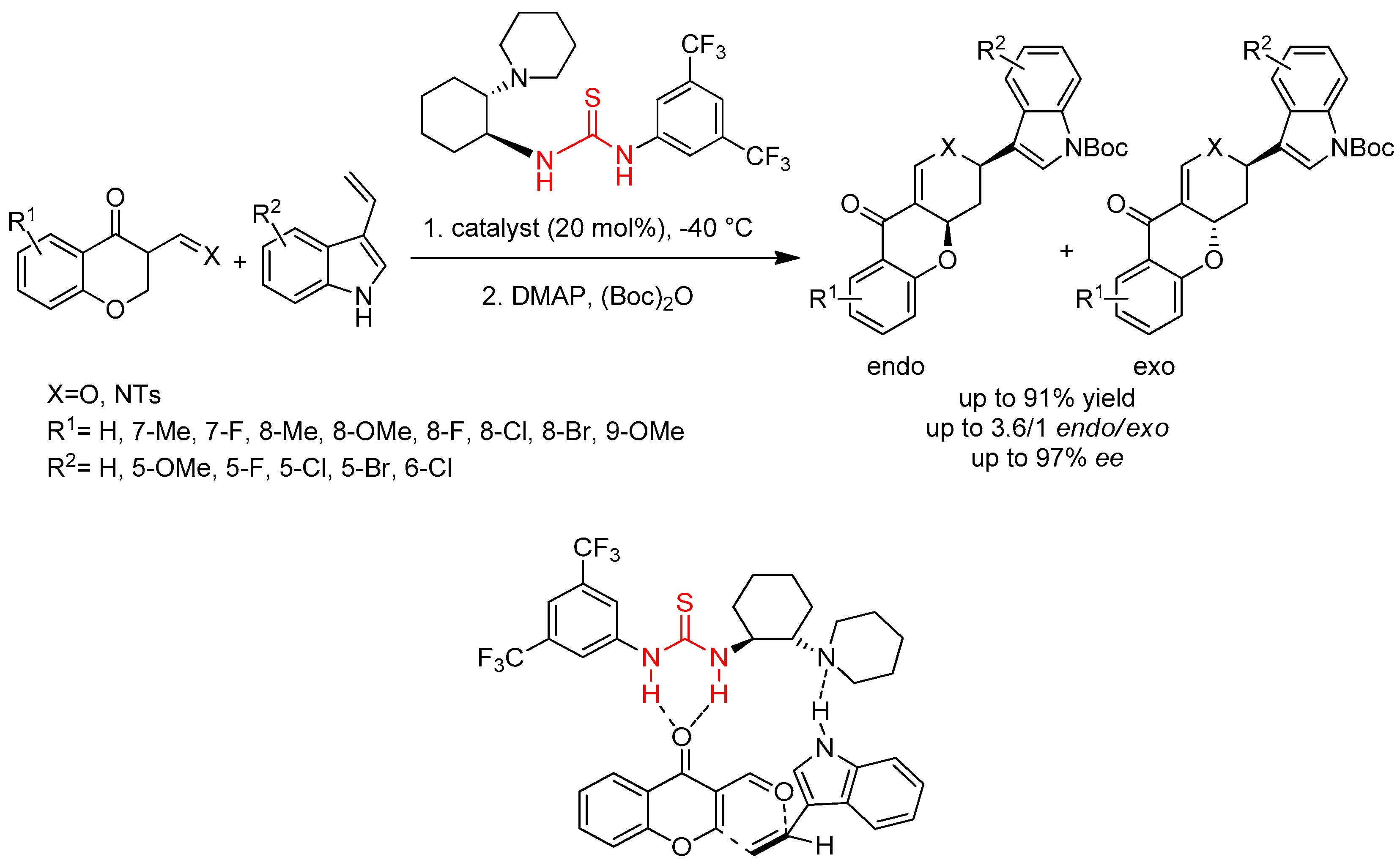
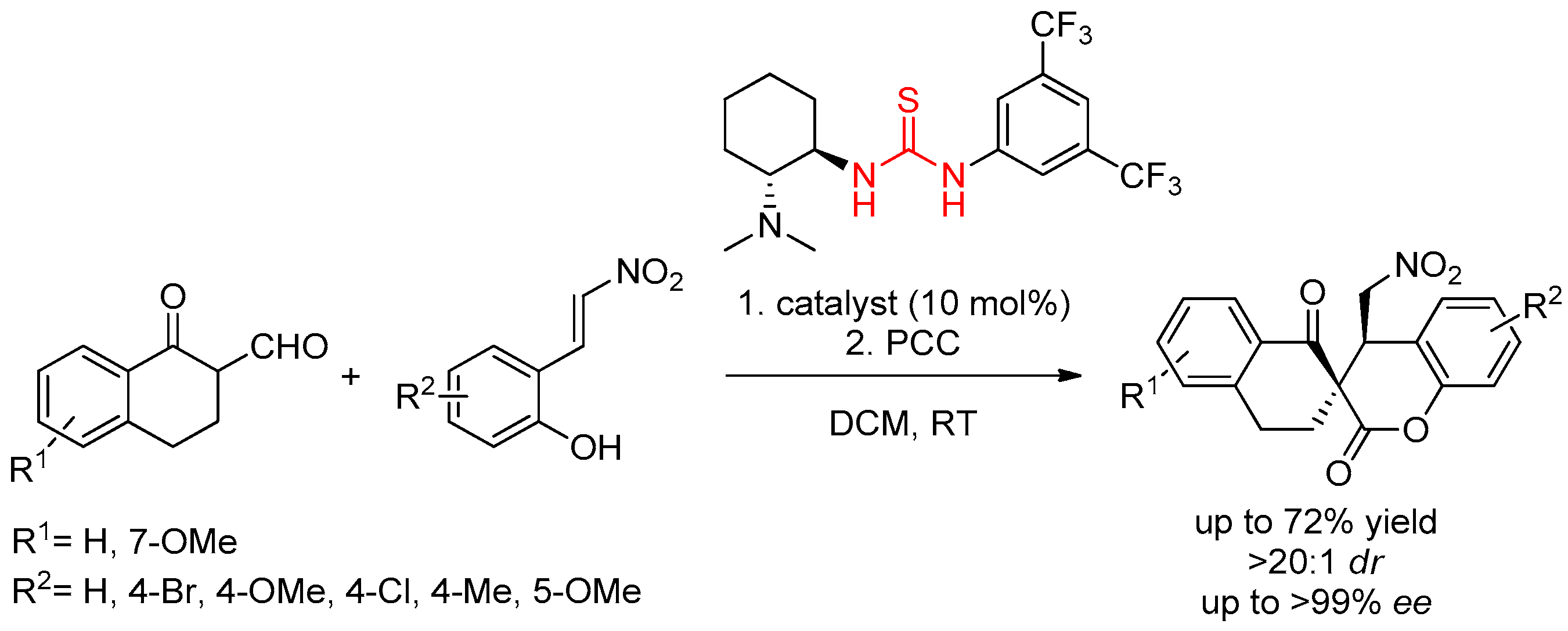
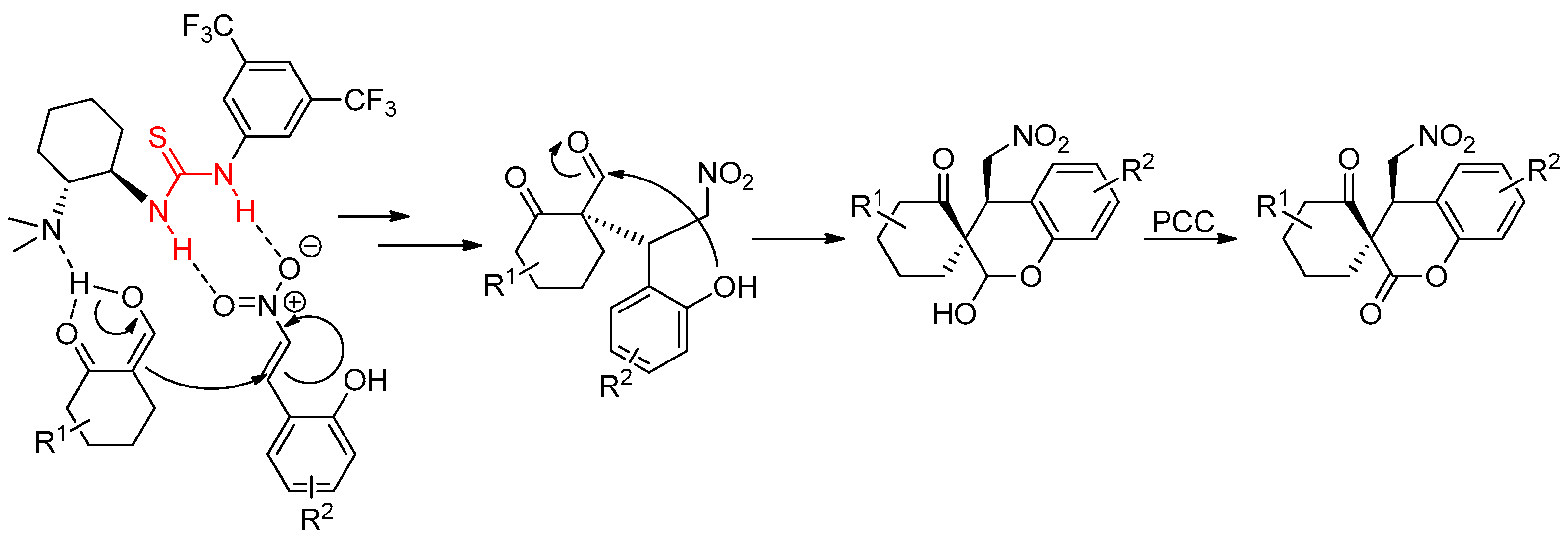
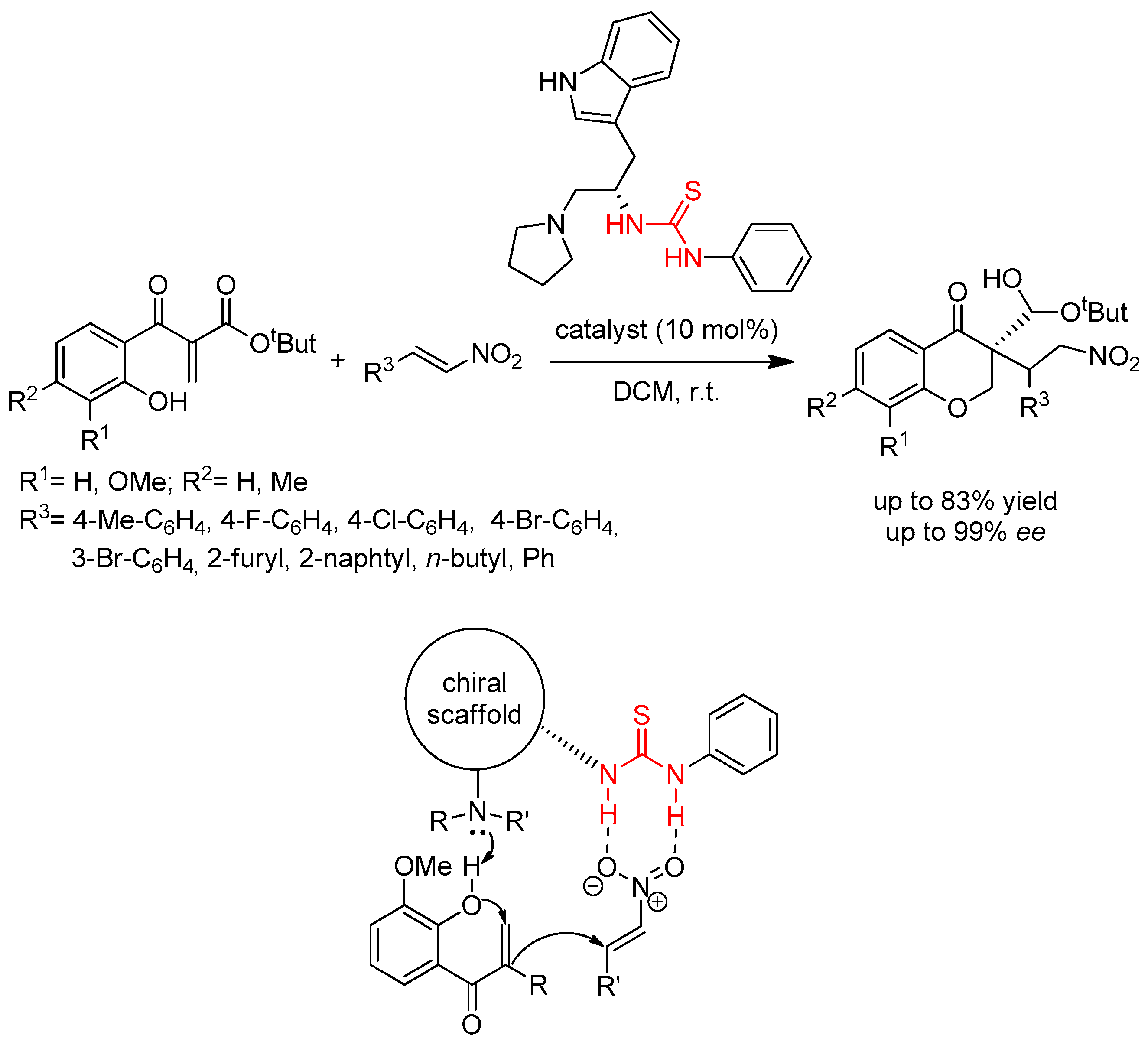
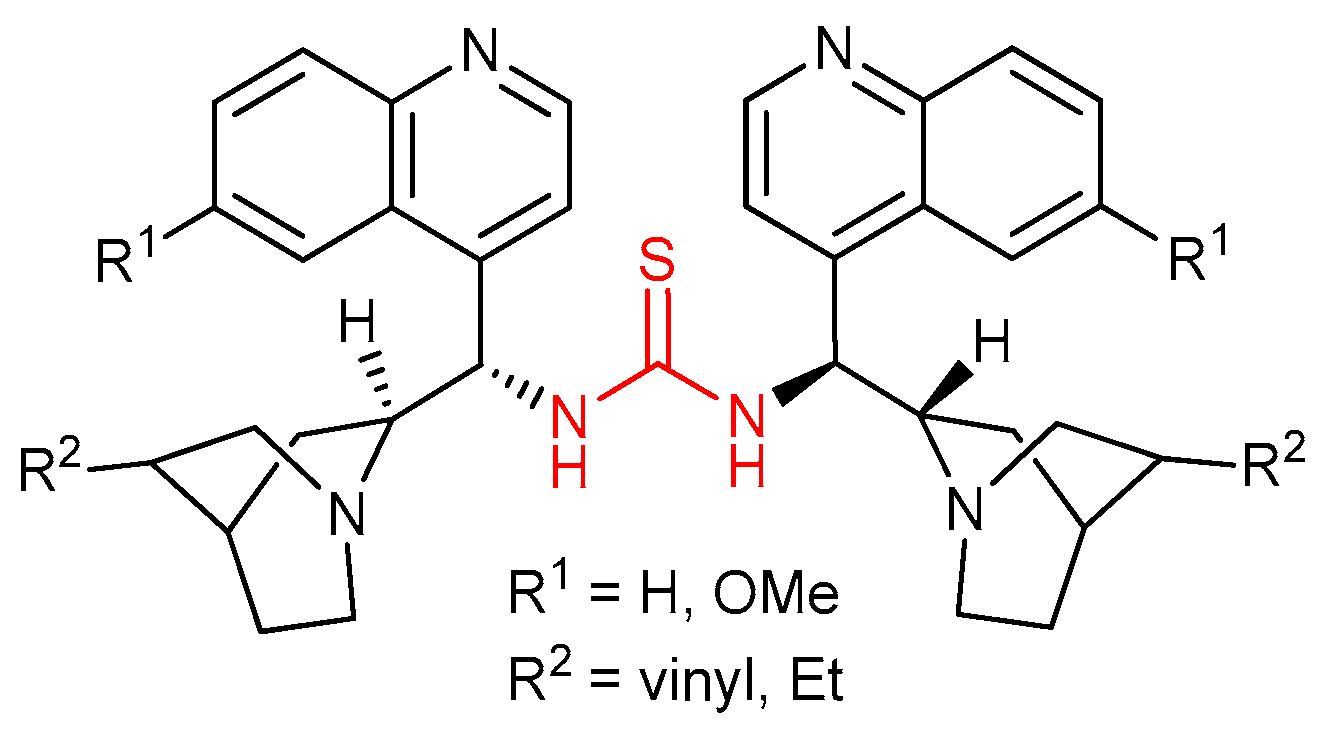

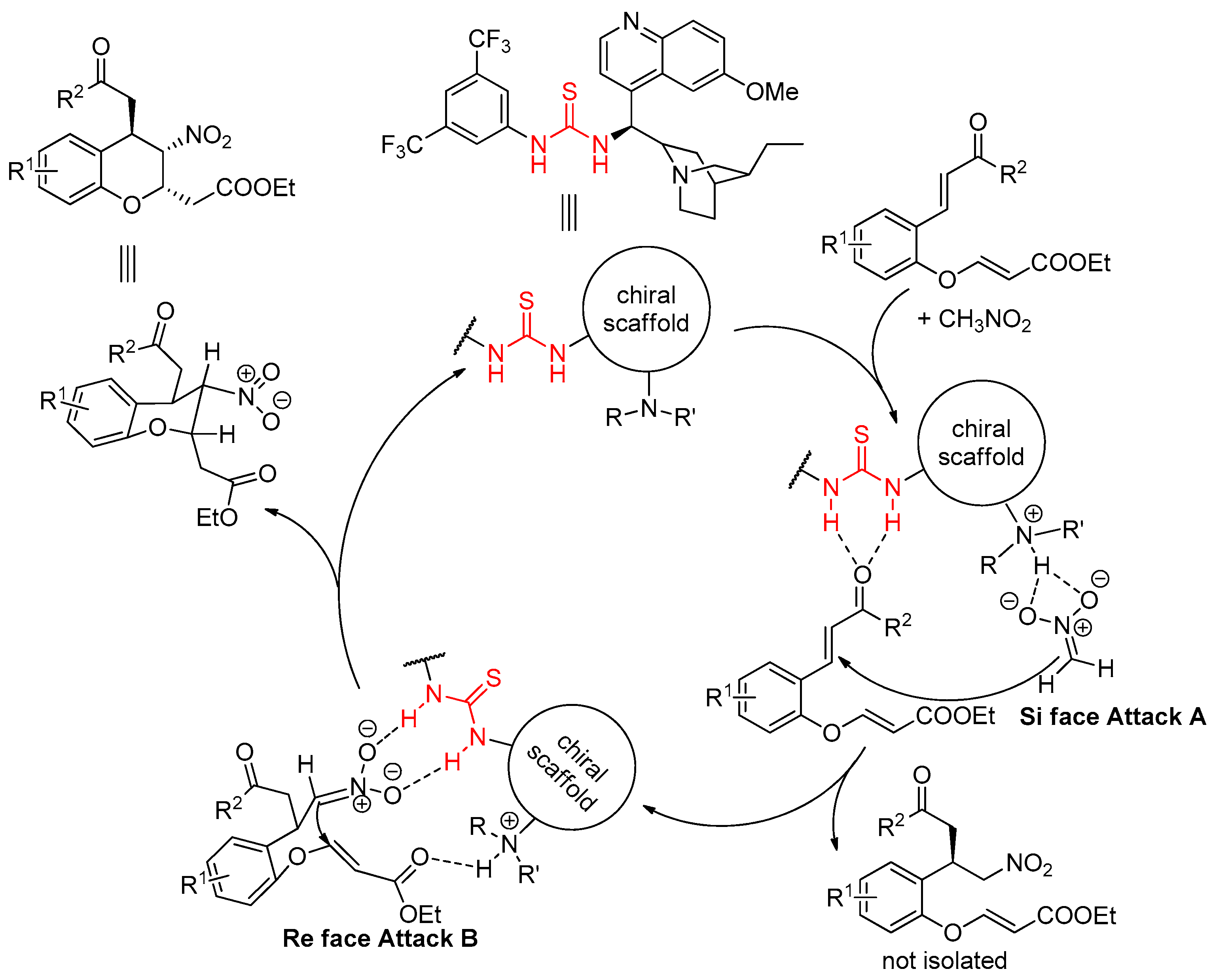
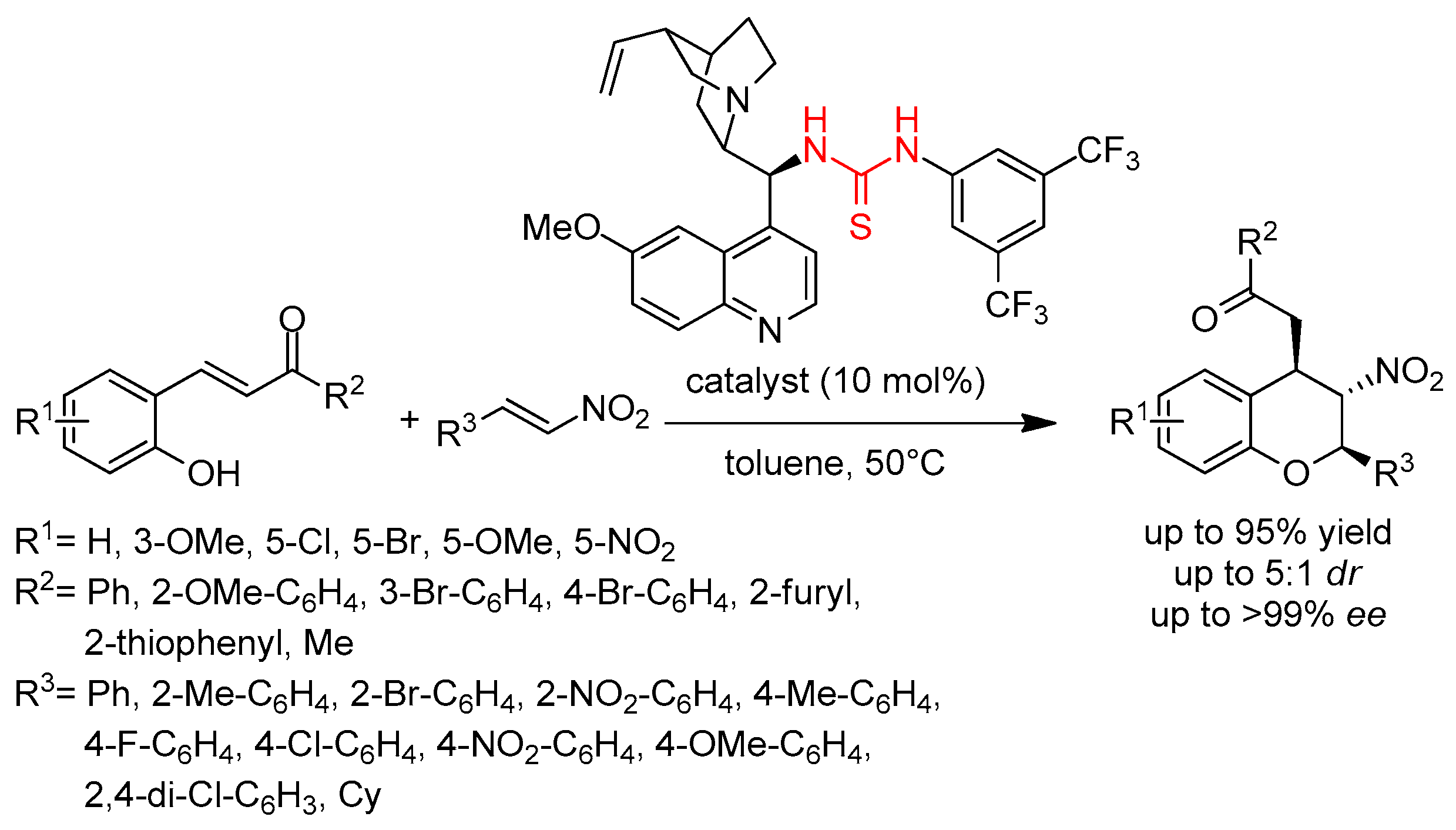
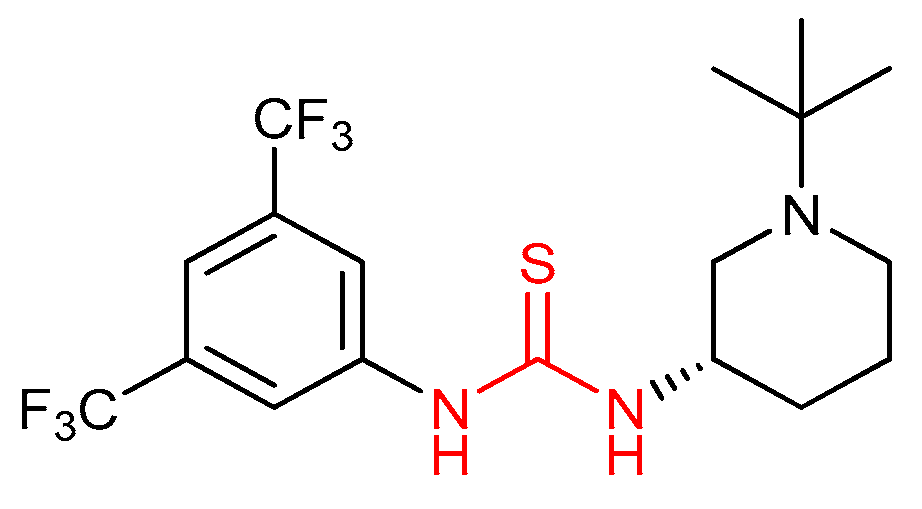

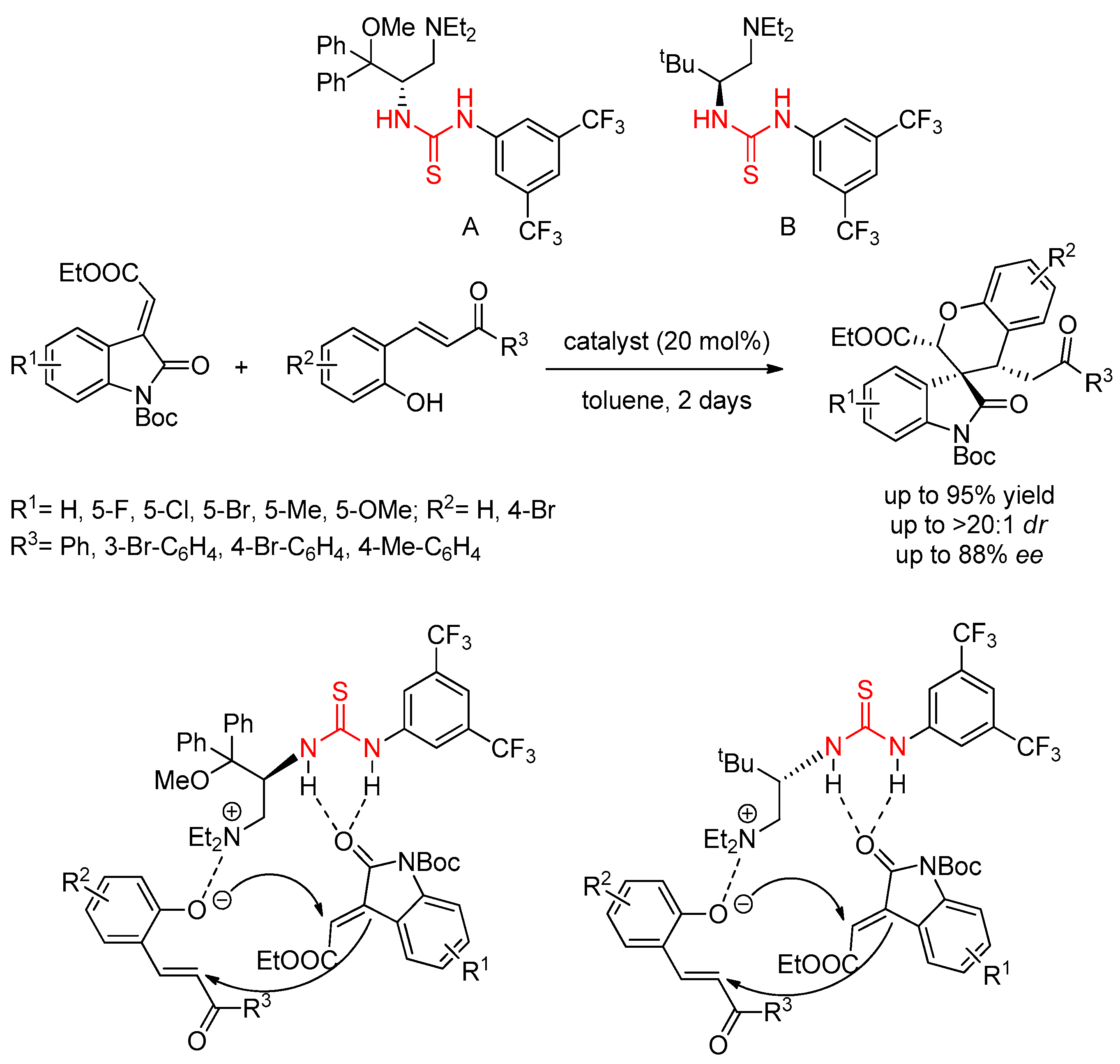
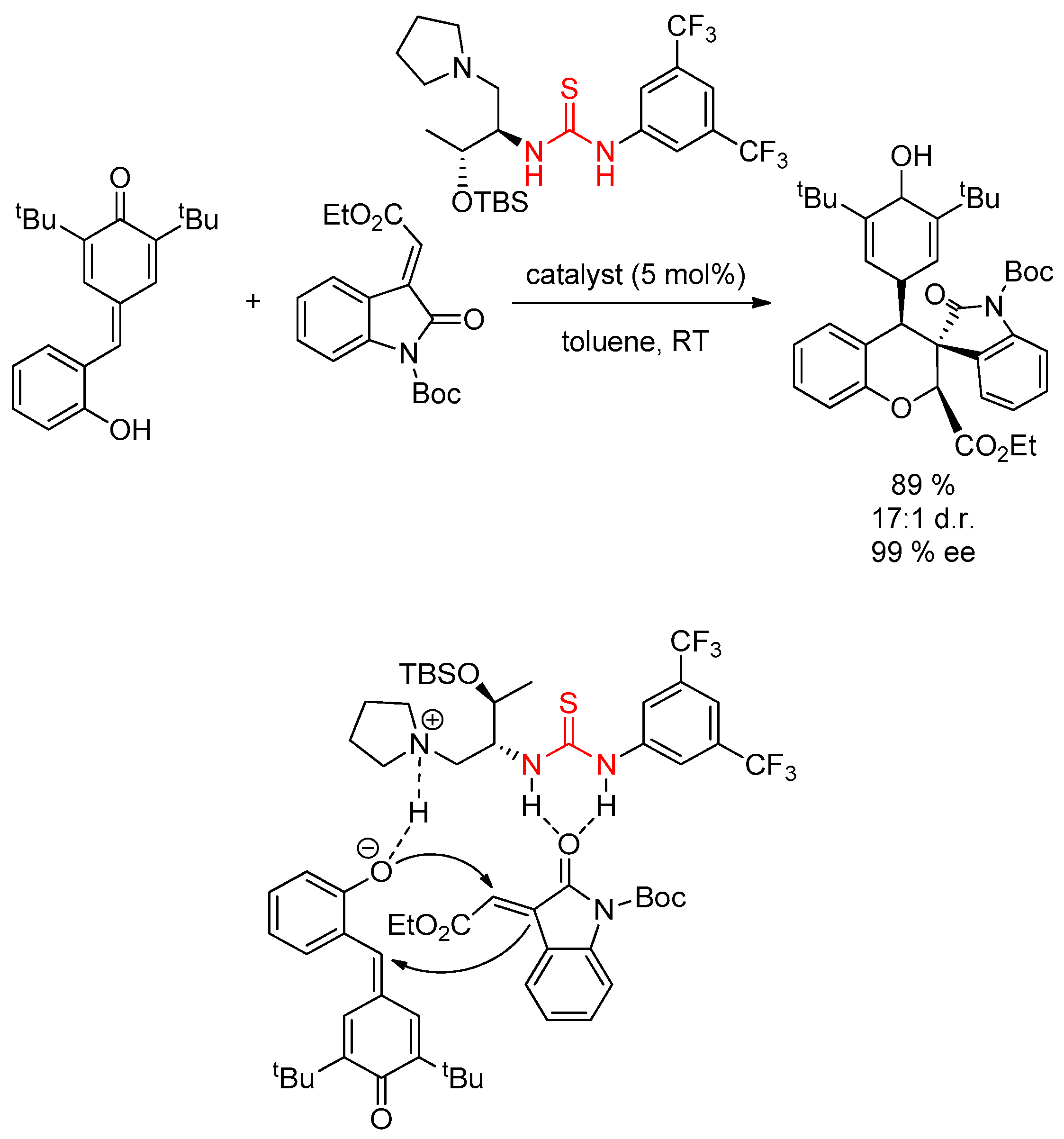
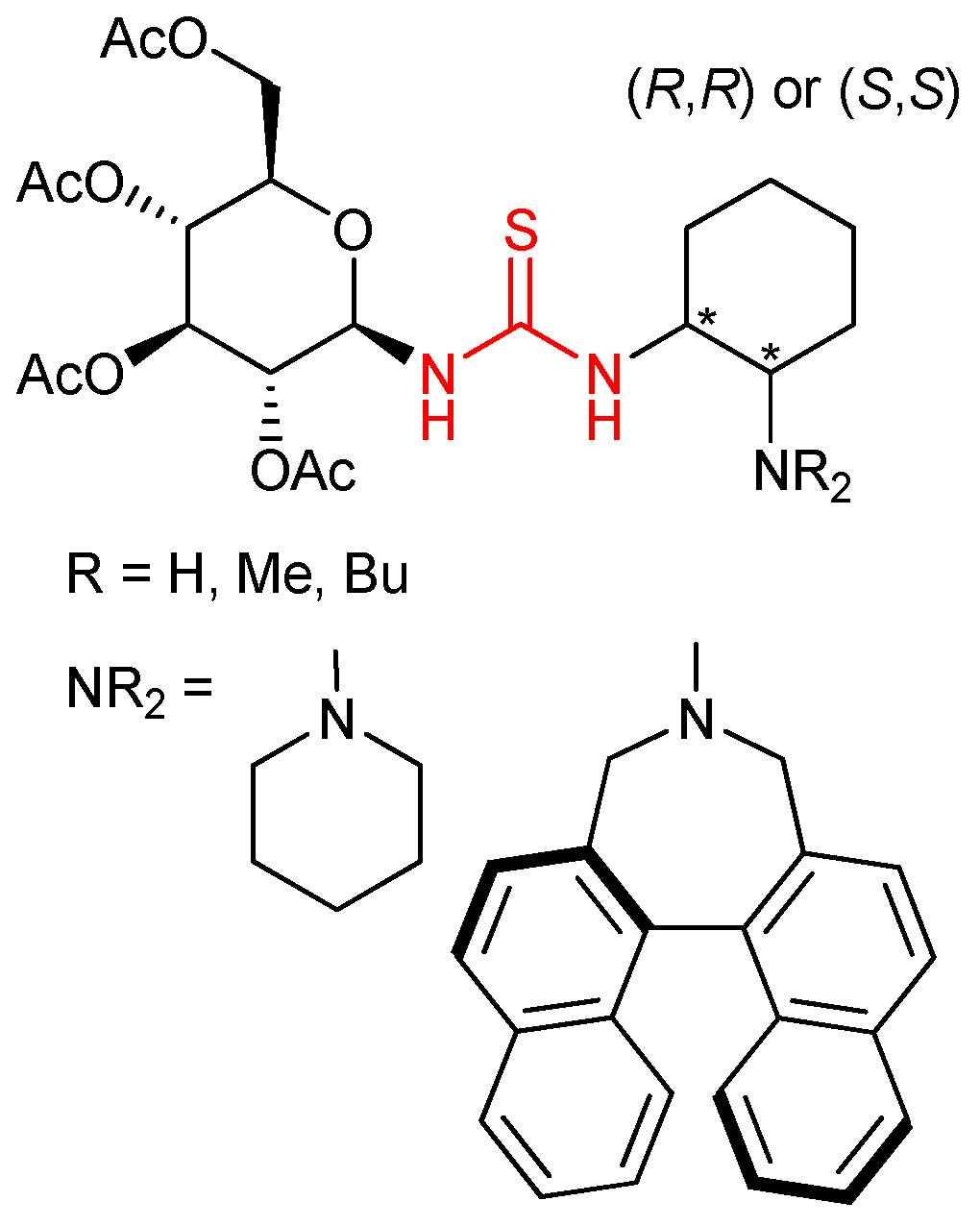
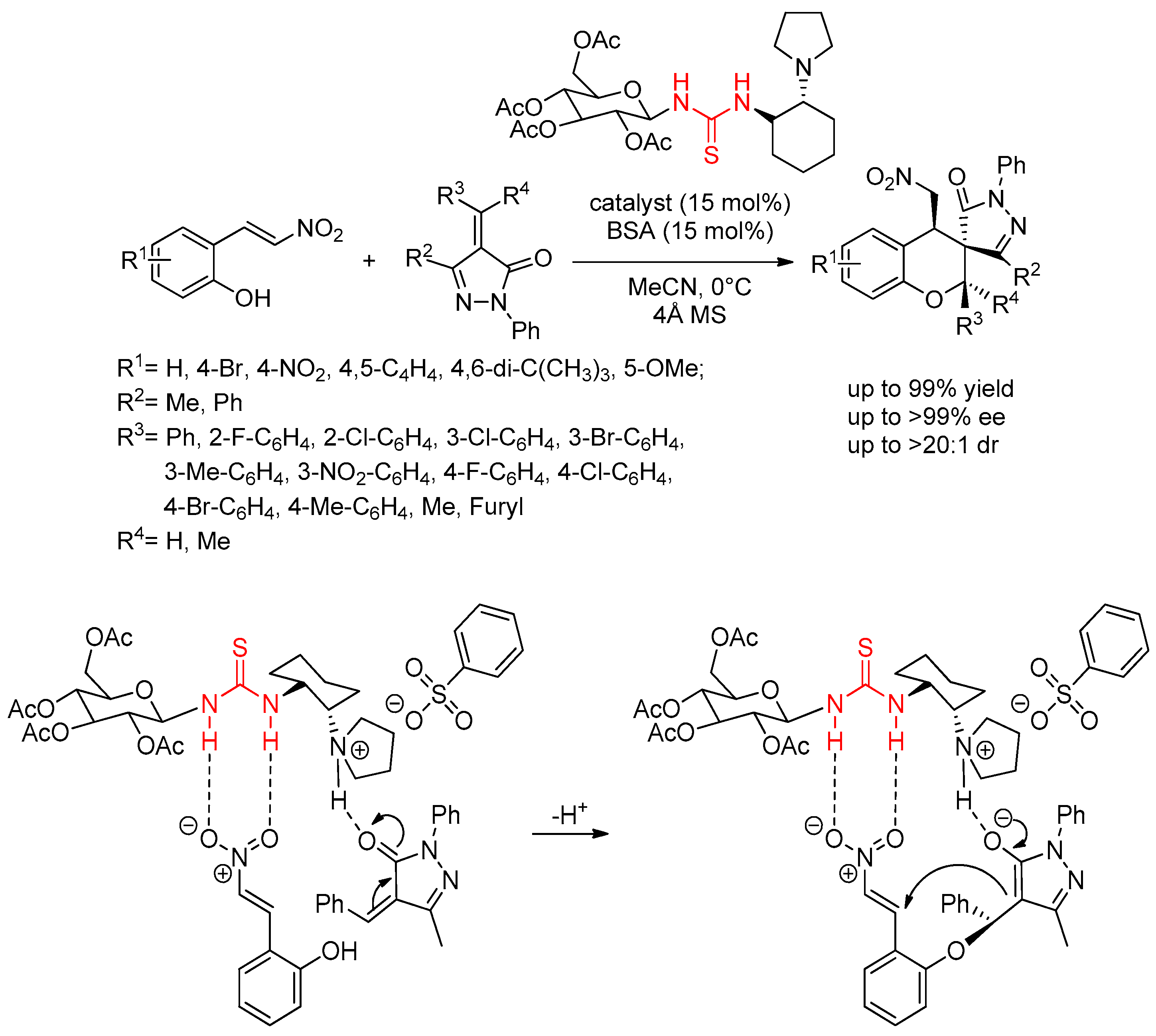
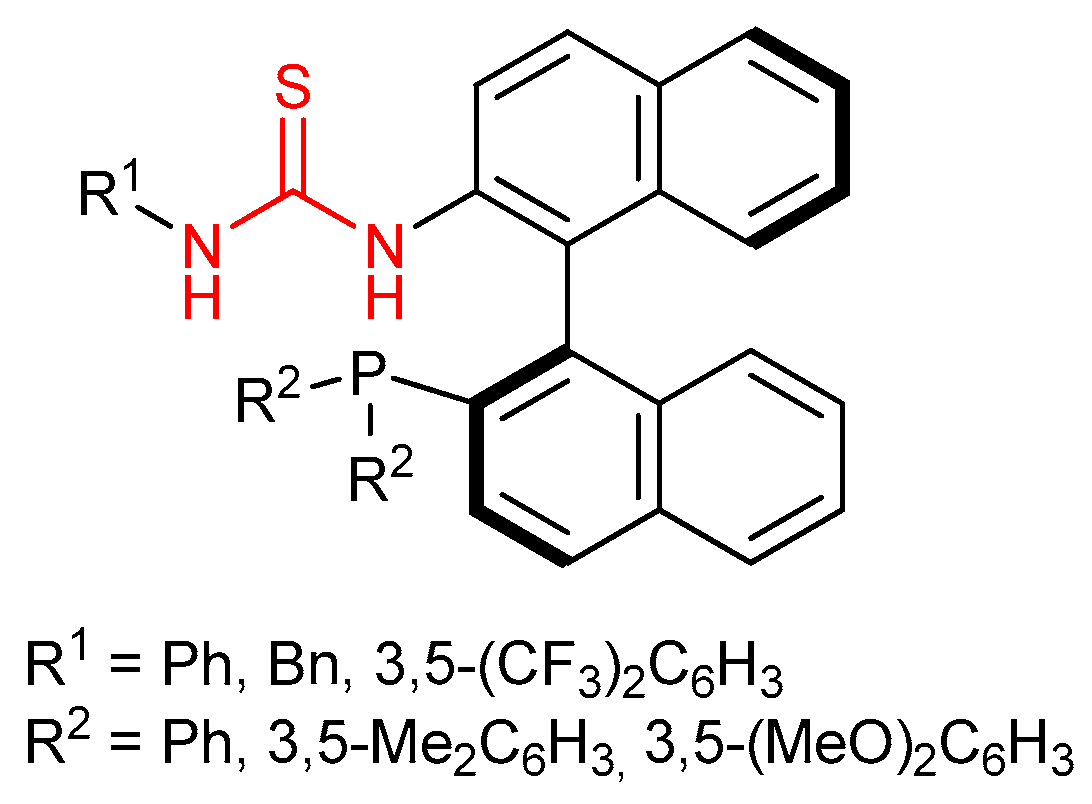
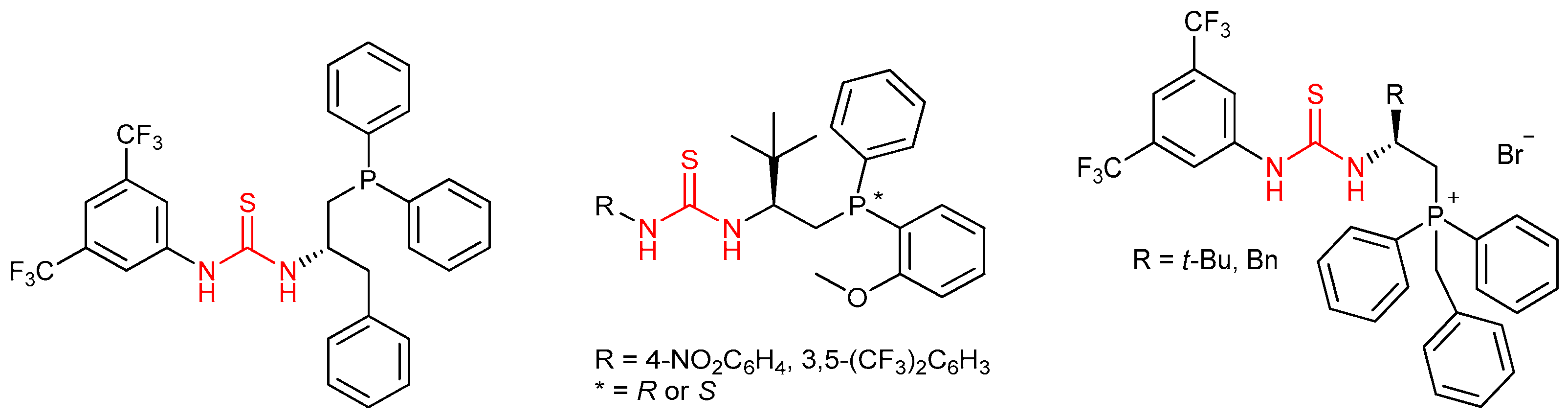
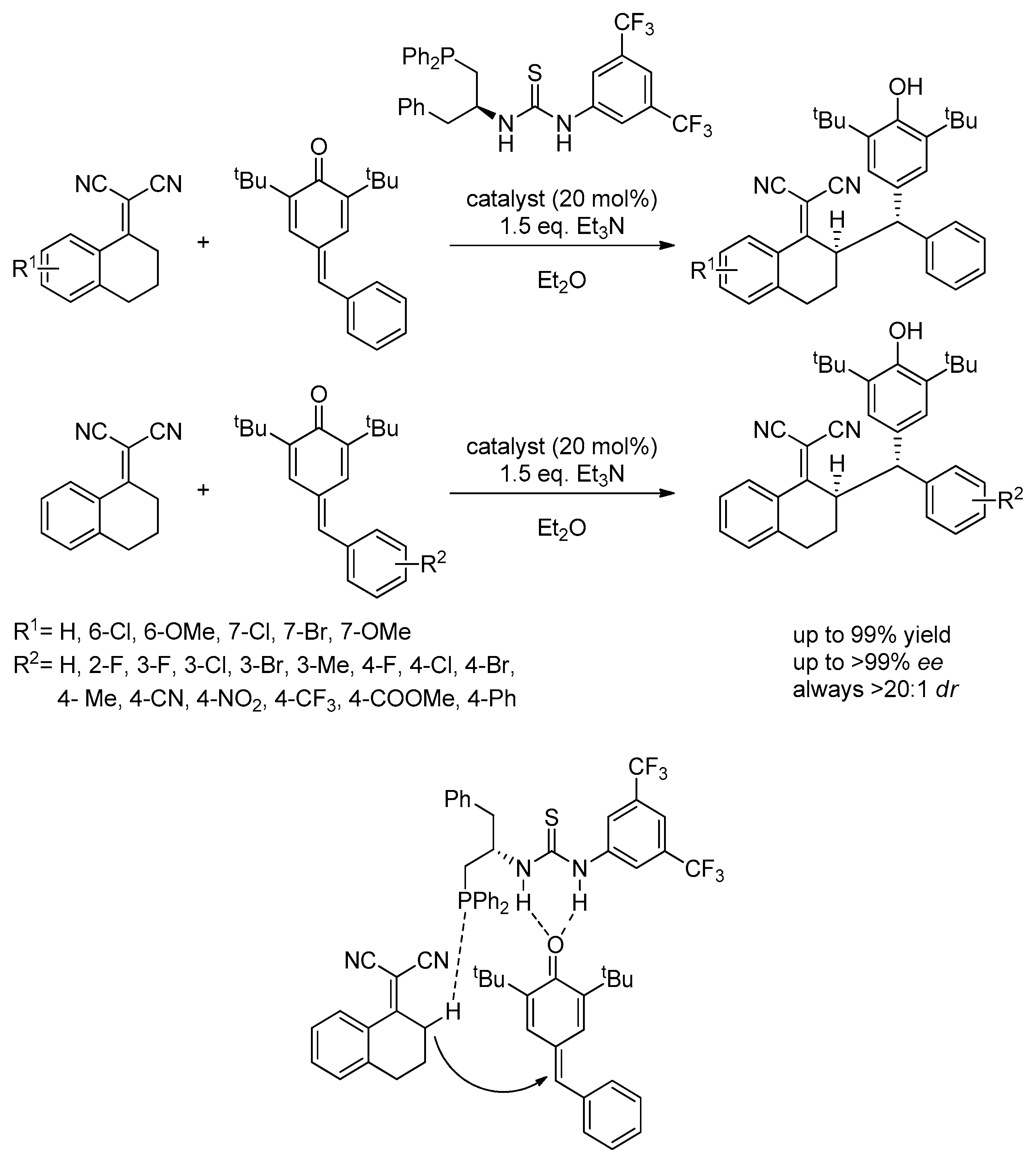
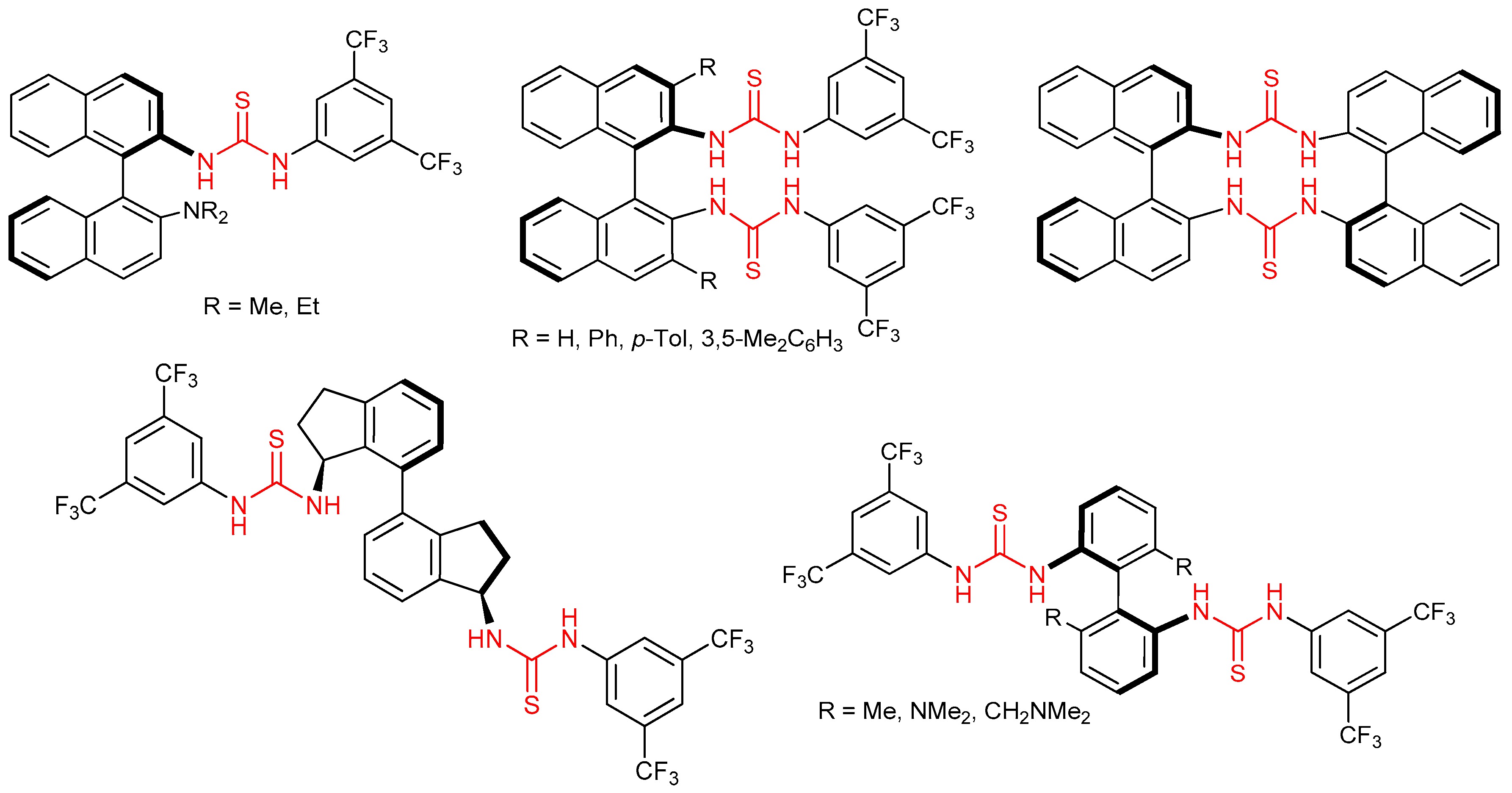
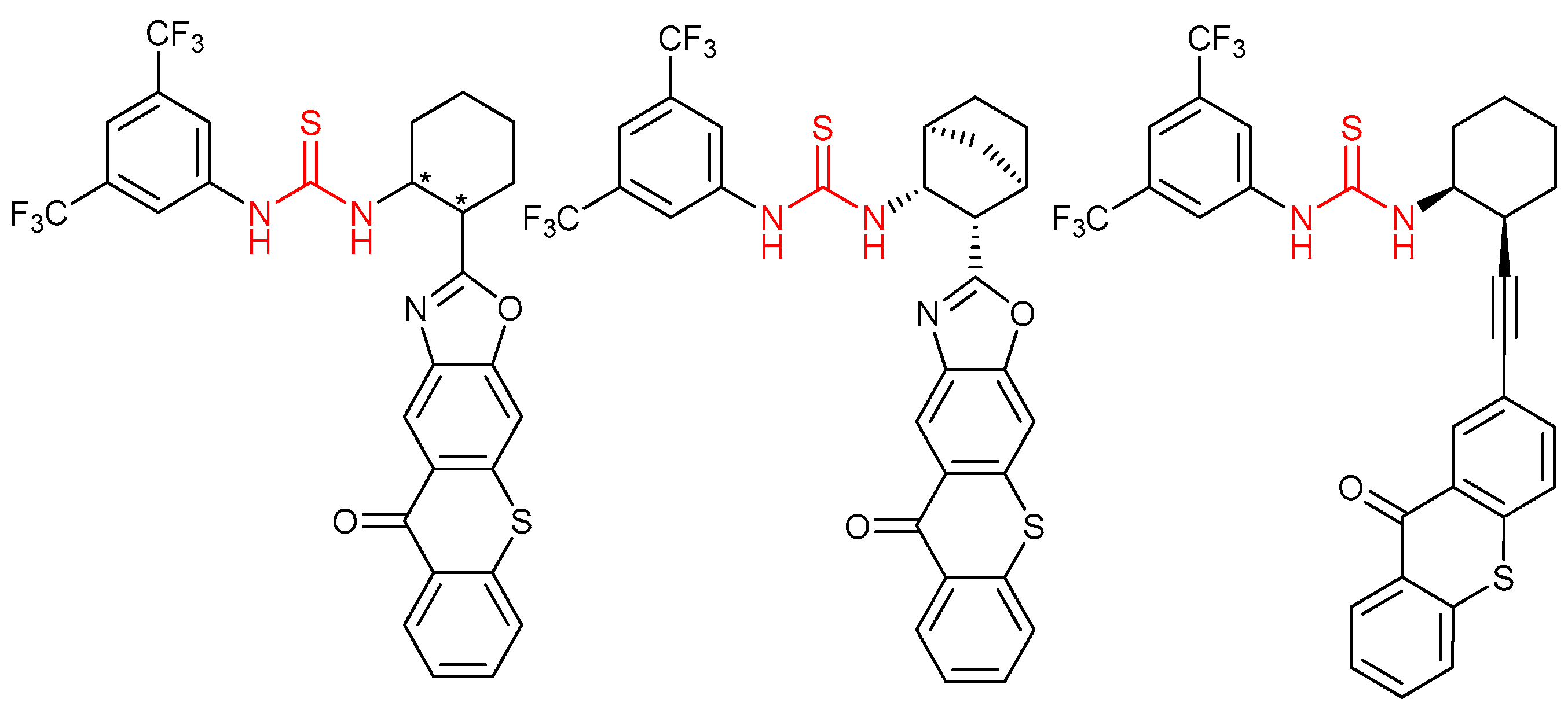
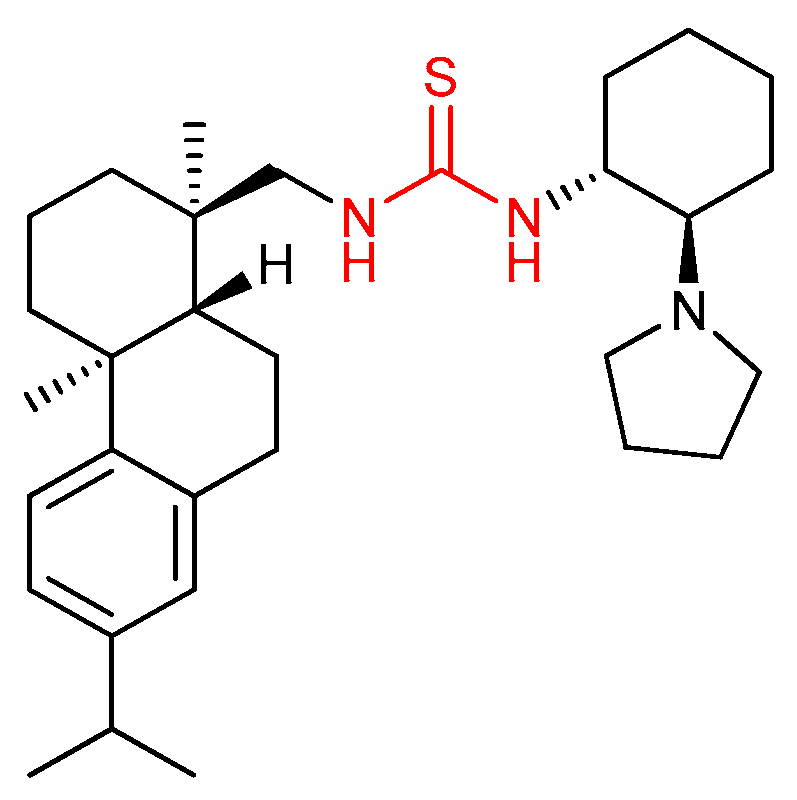

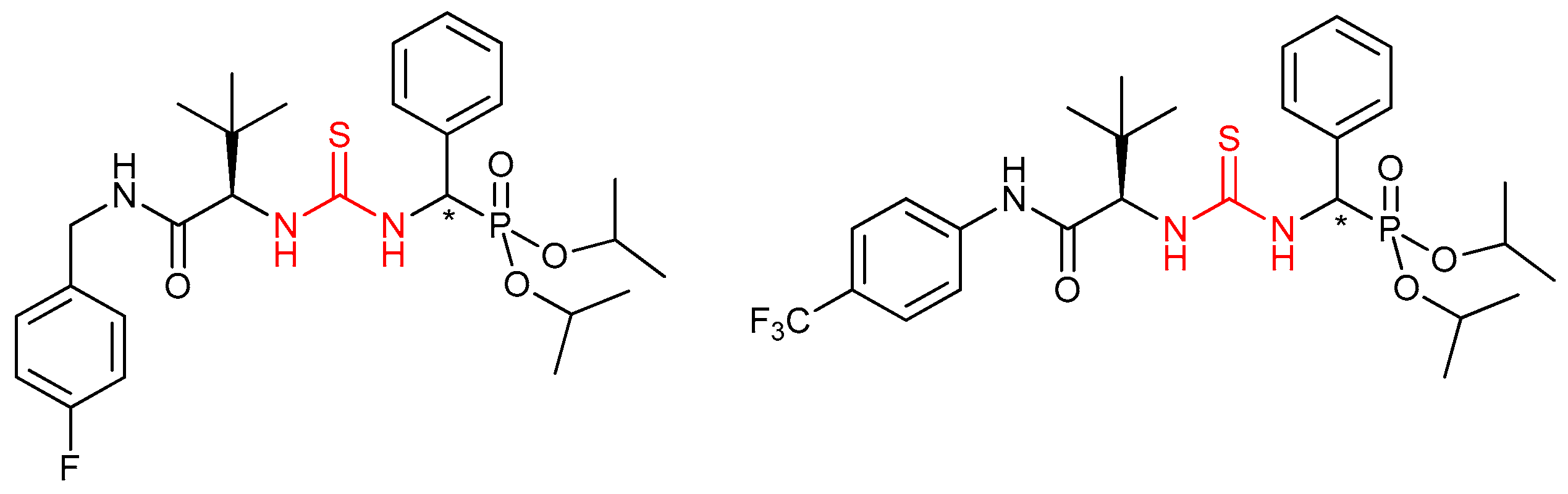
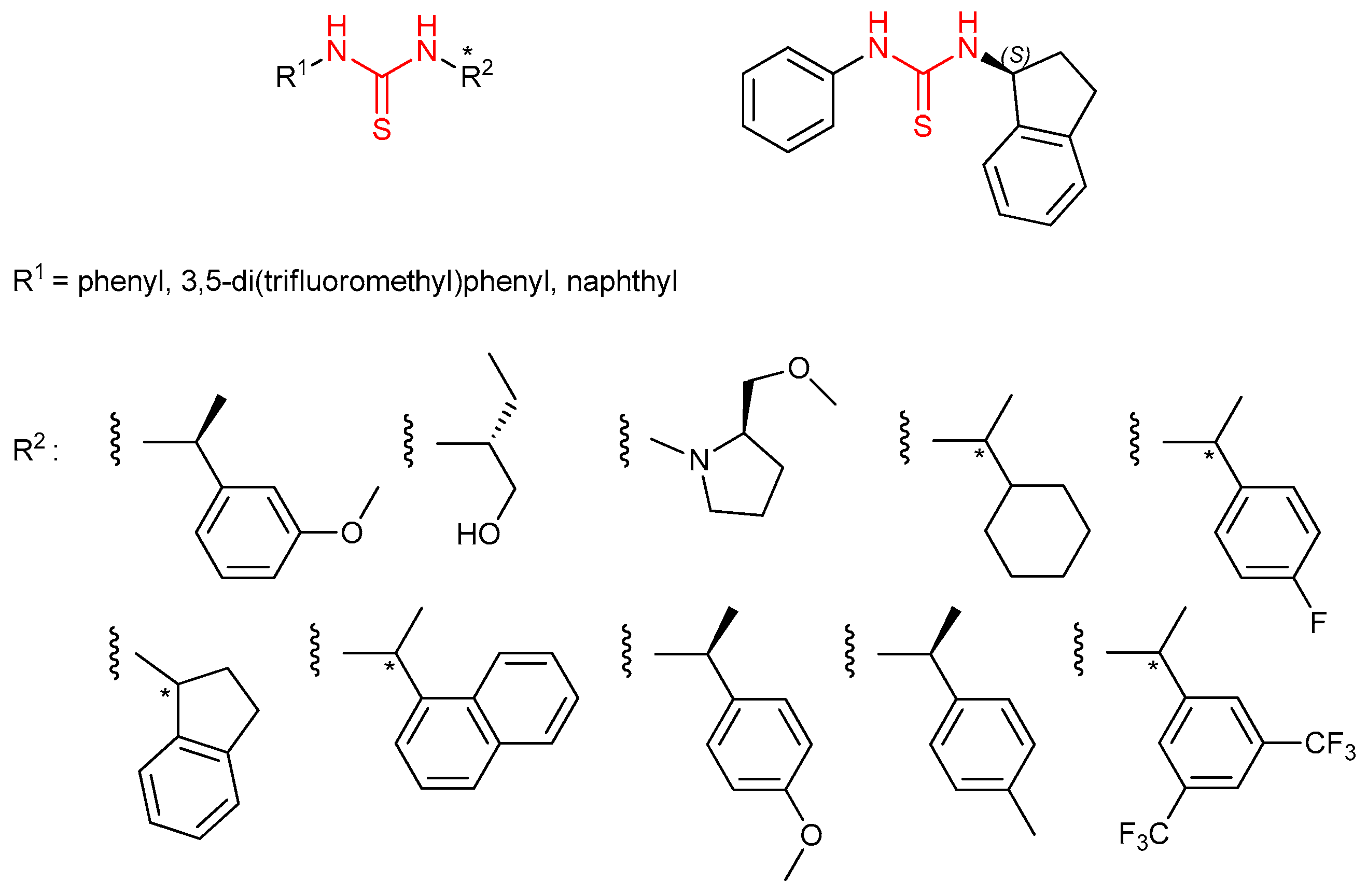
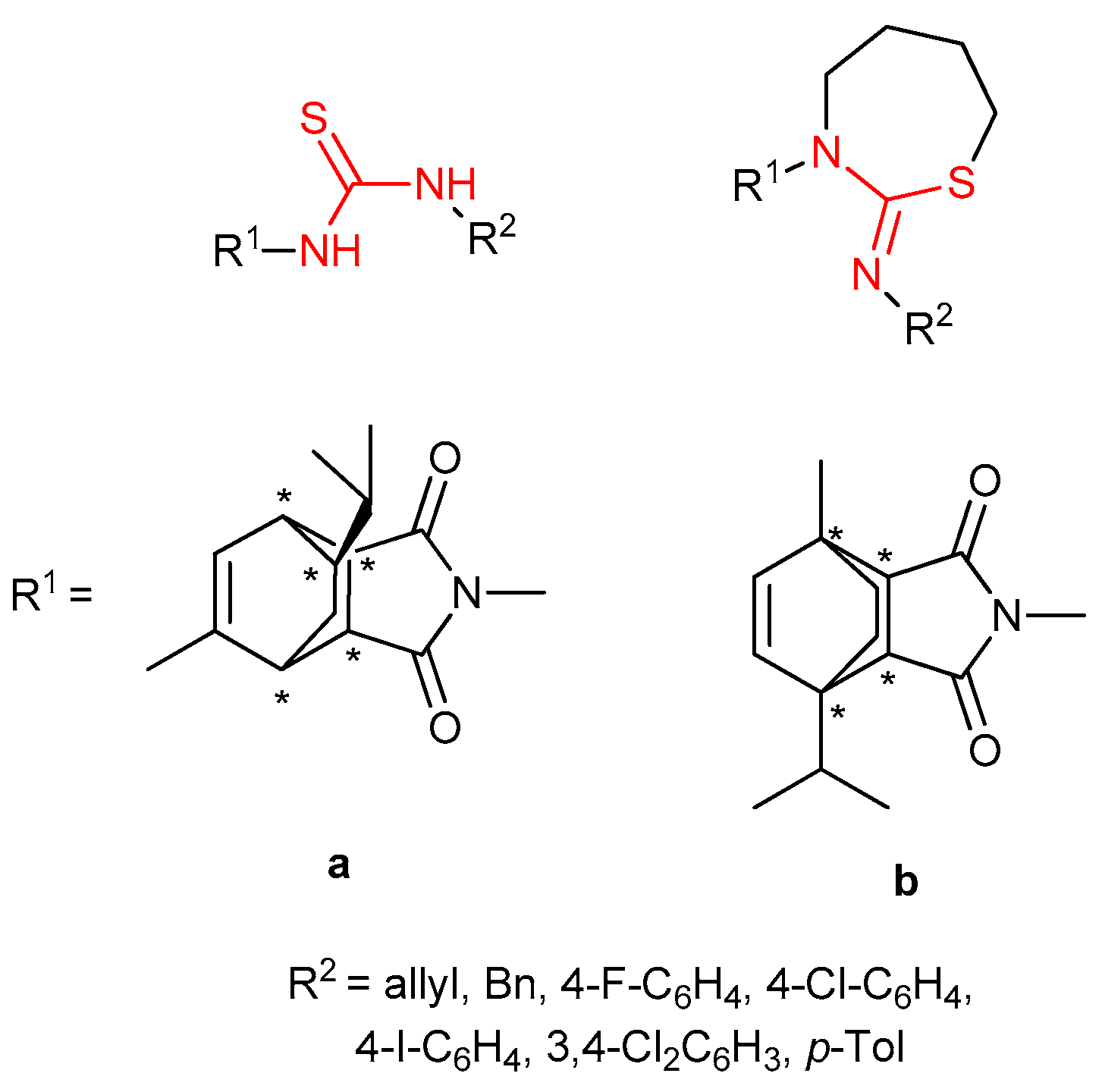
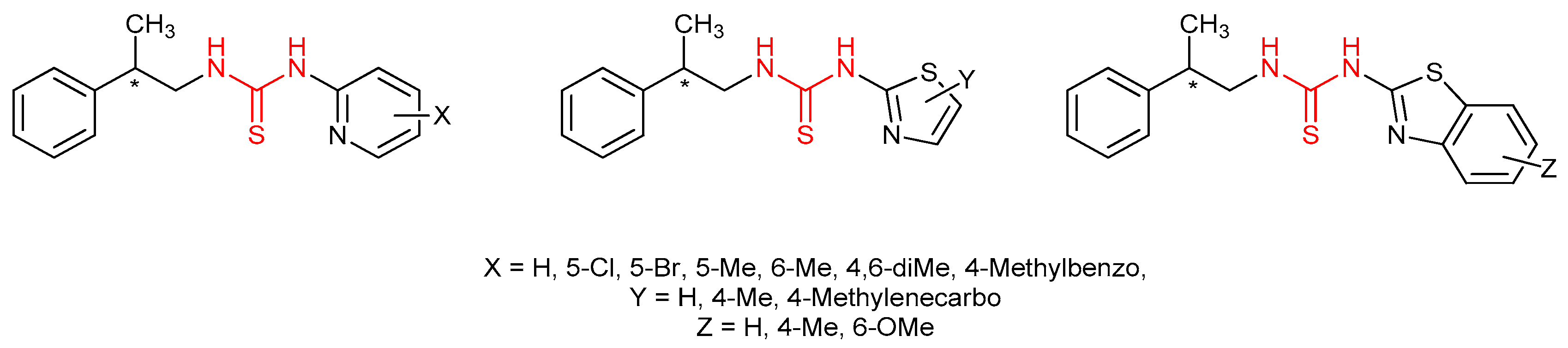
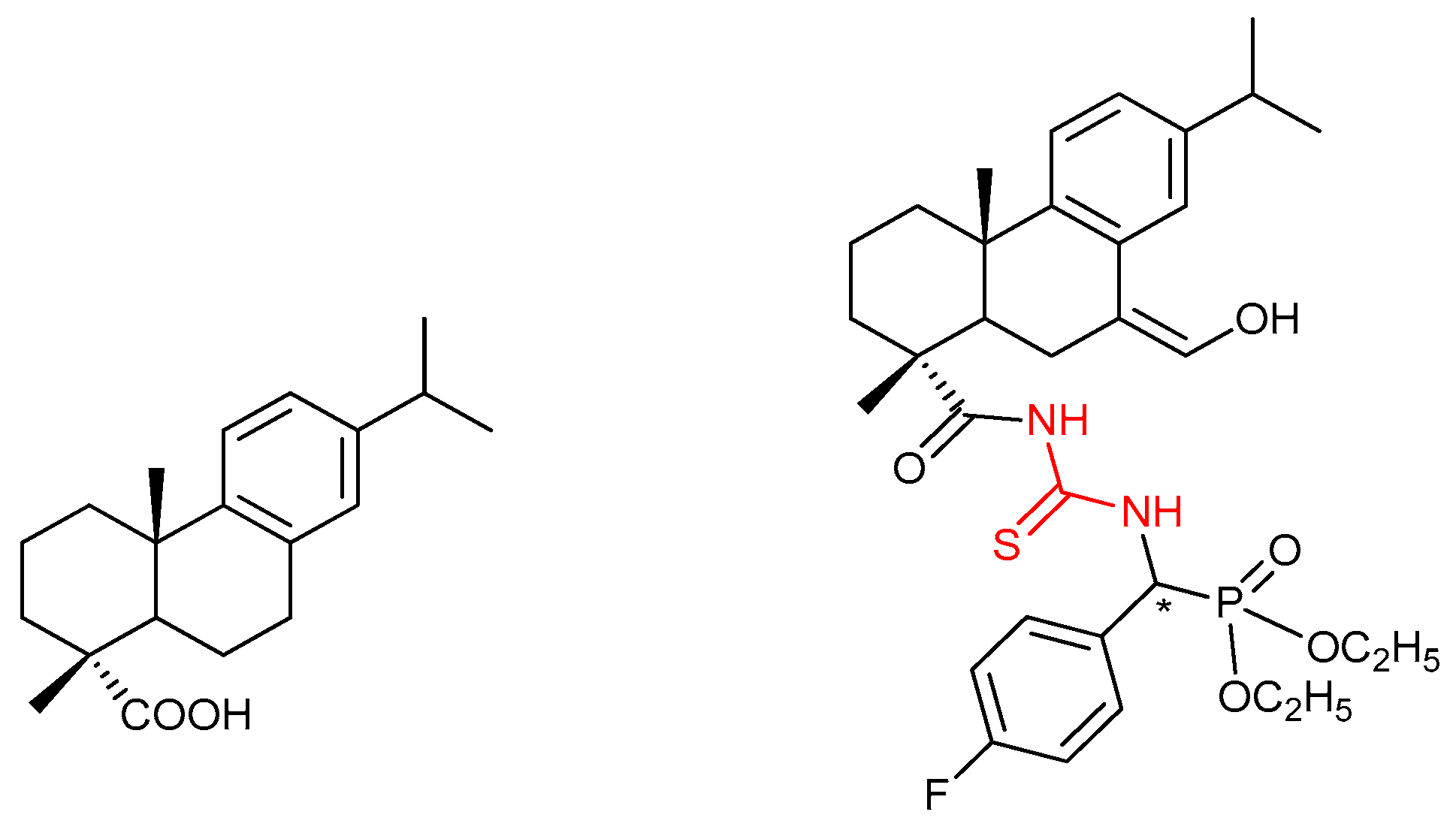
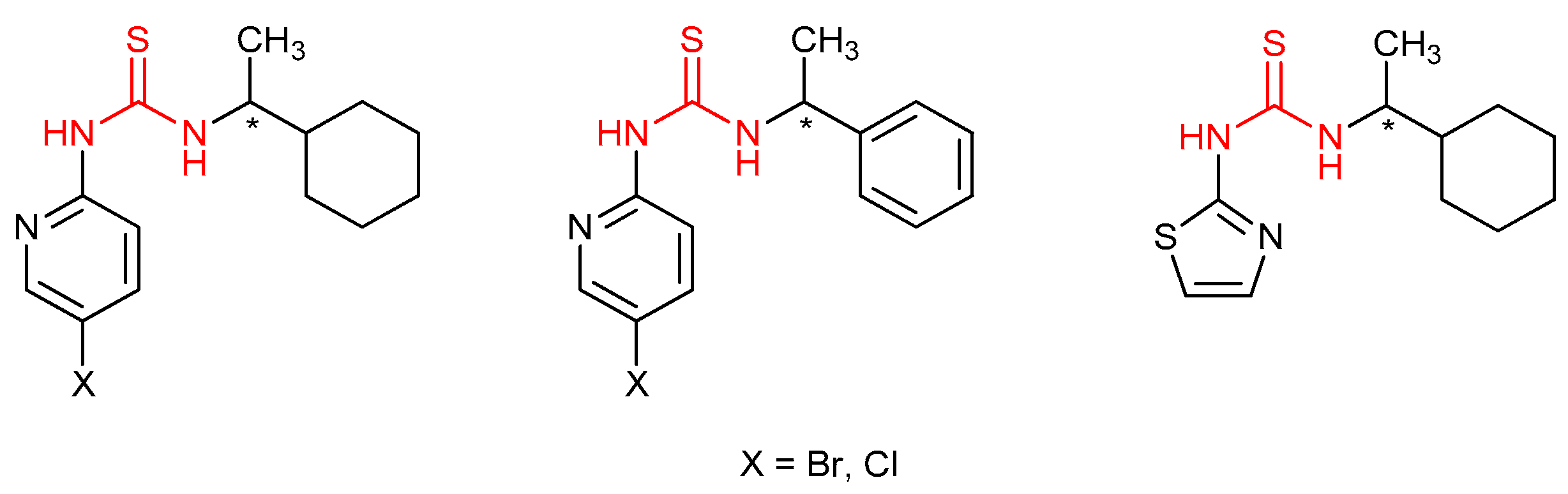
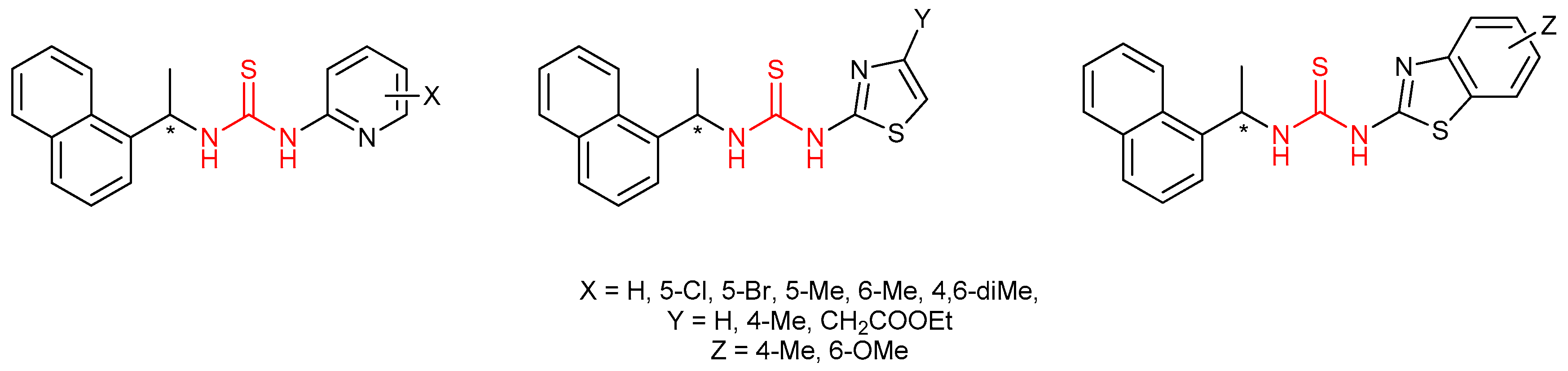
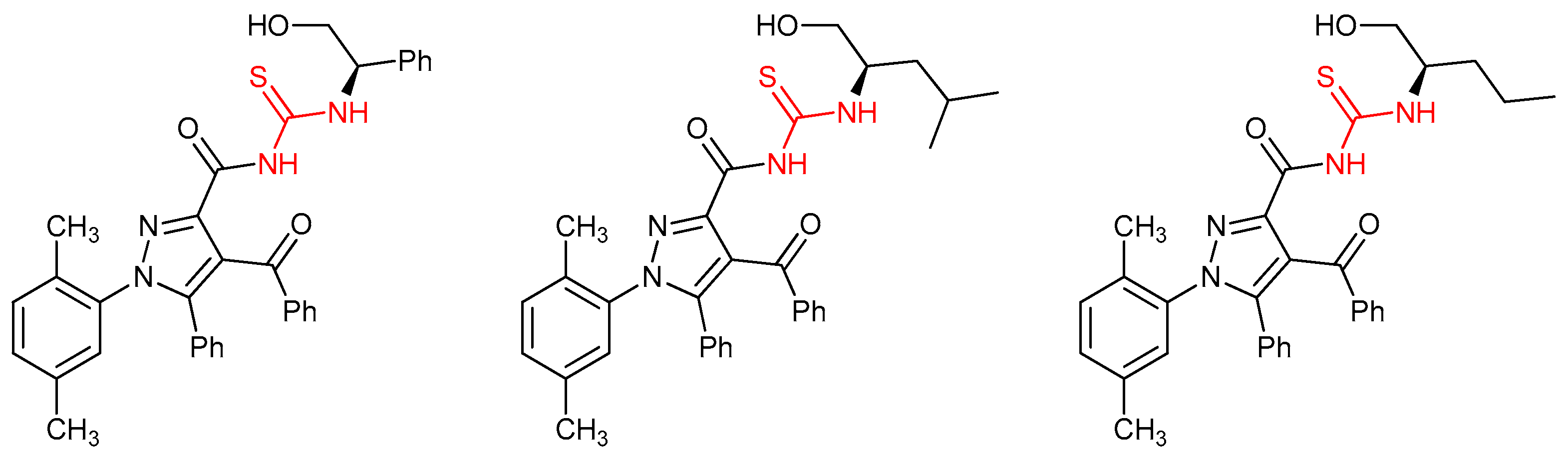
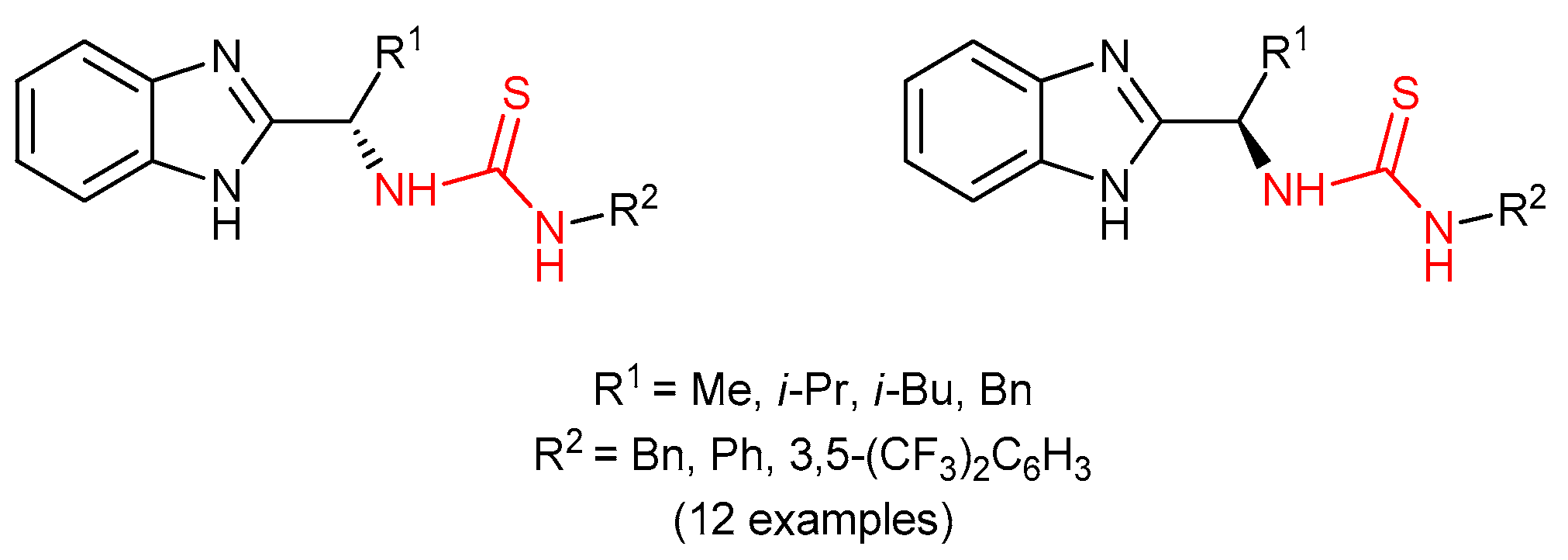
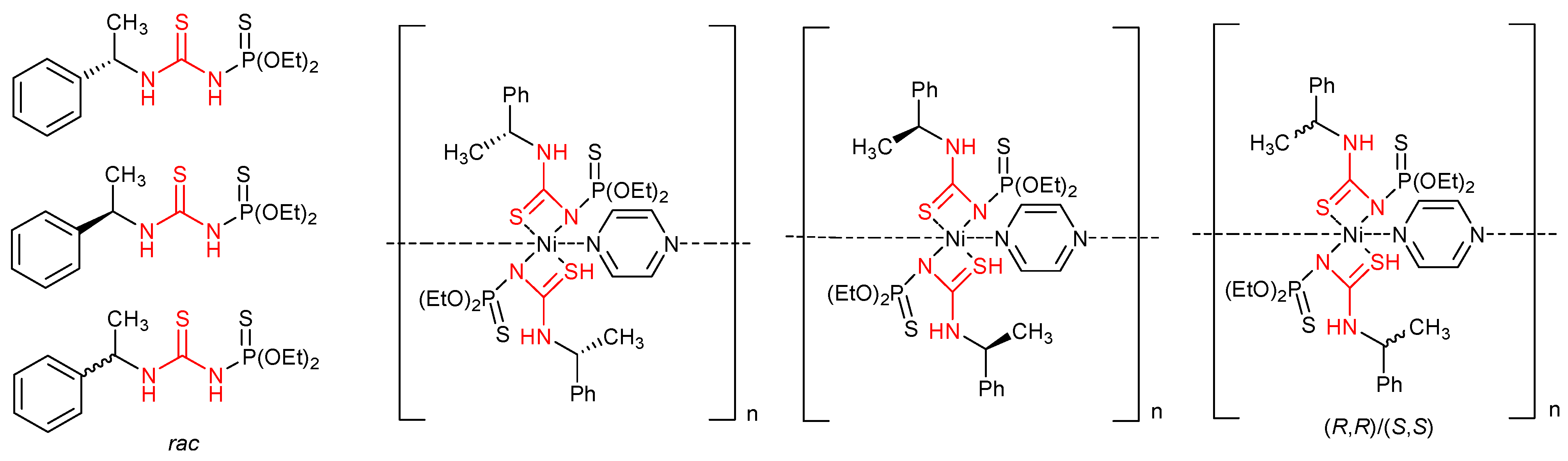
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Steppeler, F.; Iwan, D.; Wojaczyńska, E.; Wojaczyński, J. Chiral Thioureas—Preparation and Significance in Asymmetric Synthesis and Medicinal Chemistry. Molecules 2020, 25, 401. https://doi.org/10.3390/molecules25020401
Steppeler F, Iwan D, Wojaczyńska E, Wojaczyński J. Chiral Thioureas—Preparation and Significance in Asymmetric Synthesis and Medicinal Chemistry. Molecules. 2020; 25(2):401. https://doi.org/10.3390/molecules25020401
Chicago/Turabian StyleSteppeler, Franz, Dominika Iwan, Elżbieta Wojaczyńska, and Jacek Wojaczyński. 2020. "Chiral Thioureas—Preparation and Significance in Asymmetric Synthesis and Medicinal Chemistry" Molecules 25, no. 2: 401. https://doi.org/10.3390/molecules25020401
APA StyleSteppeler, F., Iwan, D., Wojaczyńska, E., & Wojaczyński, J. (2020). Chiral Thioureas—Preparation and Significance in Asymmetric Synthesis and Medicinal Chemistry. Molecules, 25(2), 401. https://doi.org/10.3390/molecules25020401