Synthesis and Cytotoxic Activity of New Thiazolopyrimidine Sugar Hydrazones and Their Derived Acyclic Nucleoside Analogues
Abstract
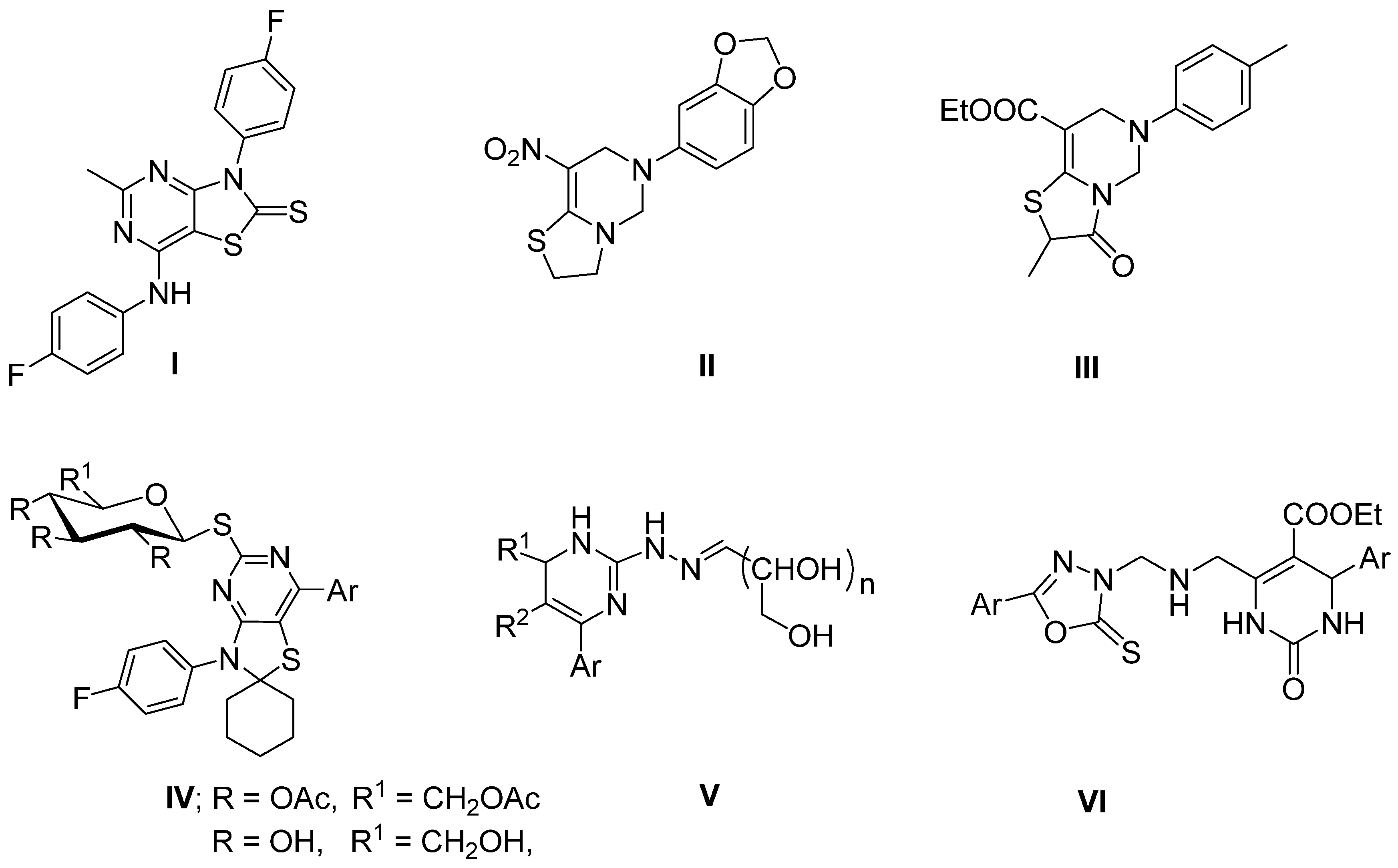
1. Introduction
2. Results and Discussion
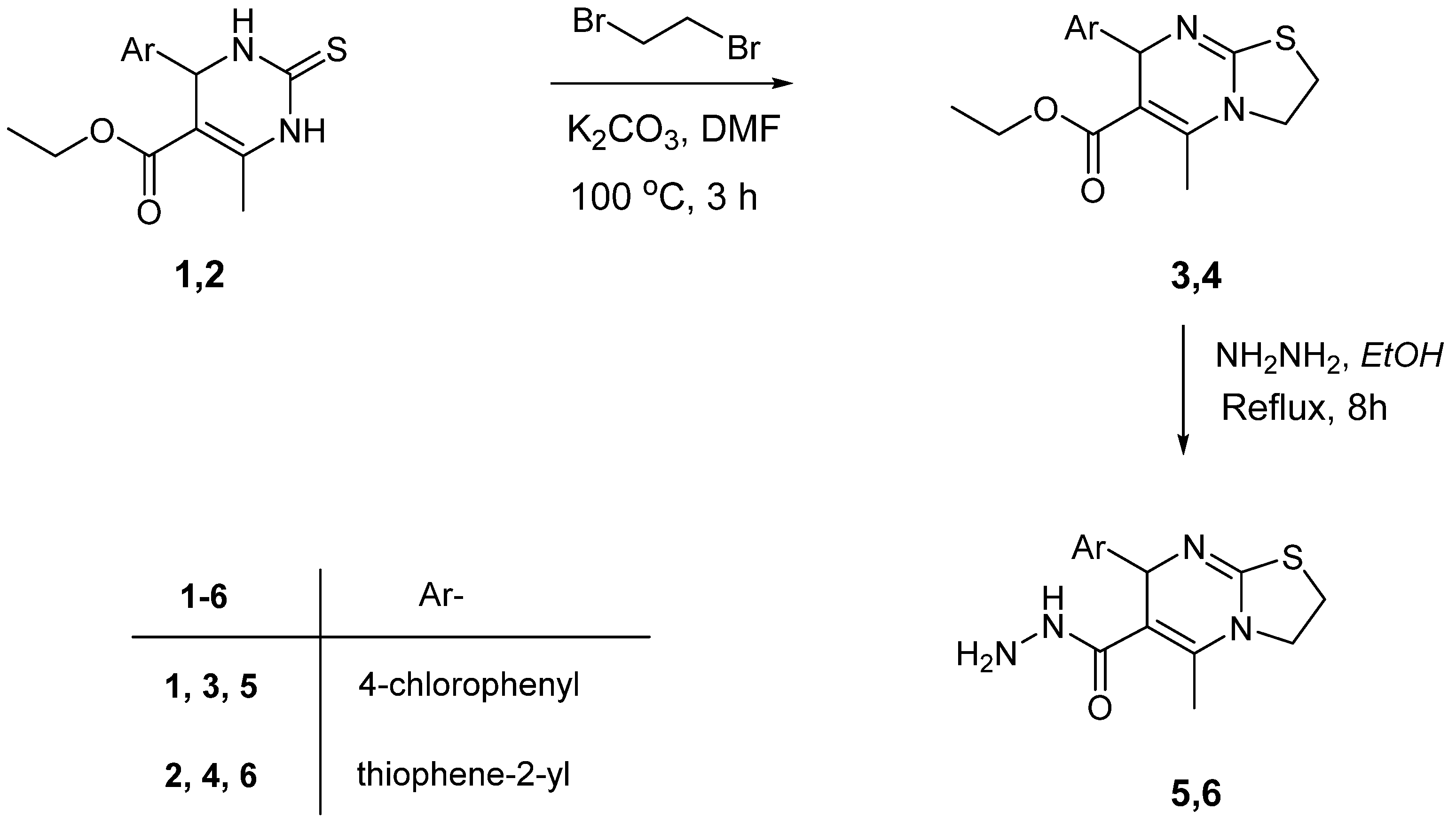
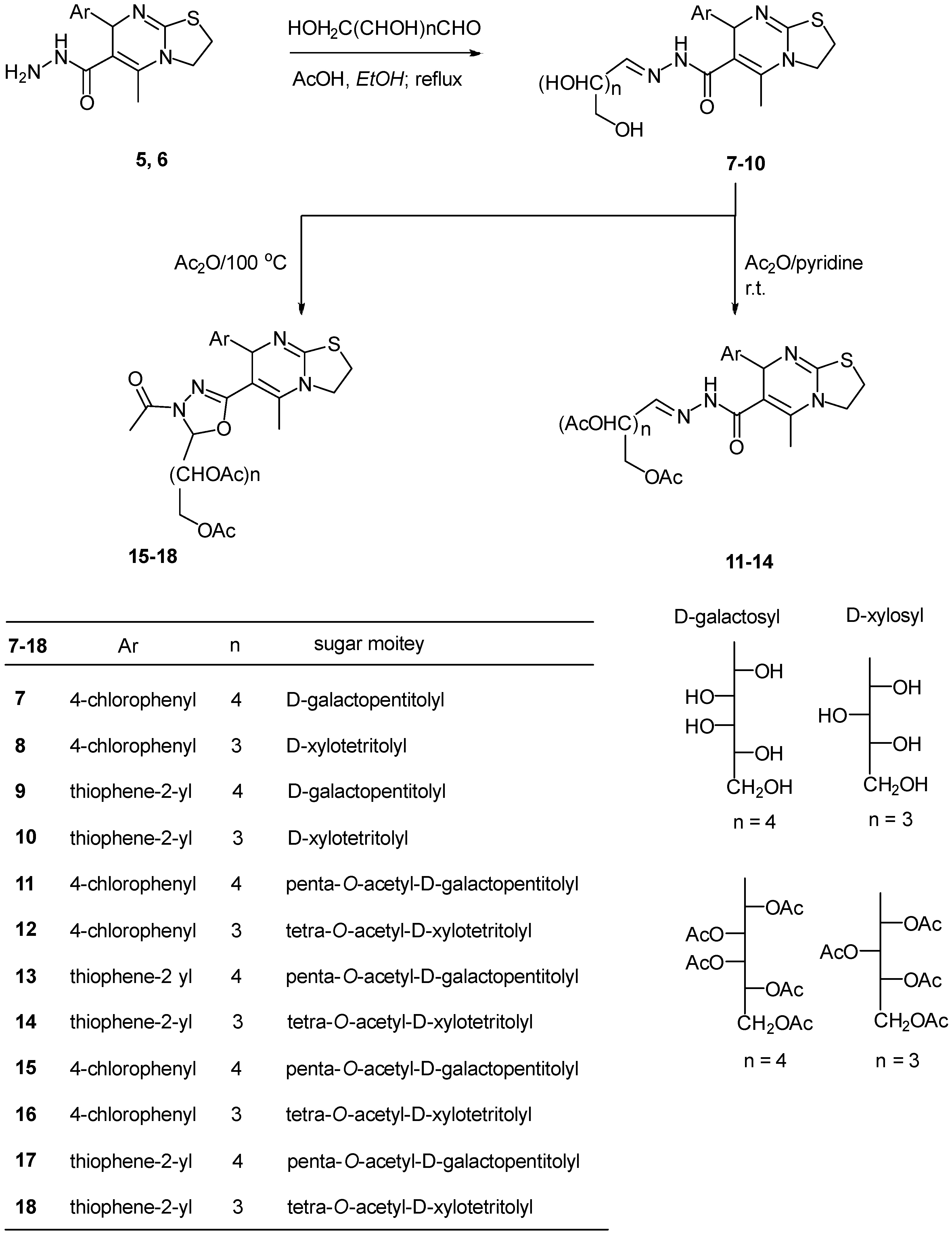
2.1. Chemistry
2.2. Cytotoxic Activity
3. Experimental
3.1. Synthesis
General Procedures
3.2. Ethyl 7-(Aryl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carboxylate (3, 4)
3.2.1. Ethyl 7-(4-Chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carboxylate (3)
3.2.2. Ethyl 5-Methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carboxylate (4)
3.3. 5-Methyl-7-(aryl)-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carbohydrazide (5, 6)
3.3.1. 7-(4-Chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carbohydrazide (5)
3.3.2. 5-Methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carbohydrazide (6)
3.4. Sugar-5-(aryl)-7-methyl-2,3-dihydro-5H-thiazolo[3,2-a]pyrimidine-6-carbohydrazone (7–10)
3.4.1. d-Galactose 7-(4-chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carbohydrazone (7)
3.4.2. d-Xylose 7-(4-chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-Carbohydrazone (8)
3.4.3. d-Galactose 5-methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carbohydrazone (9)
3.4.4. d-Xylose 5-methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-Carbohydrazone (10)
3.5. General Procedure for the Preparation of Compounds (11–14)
3.5.1. Penta-O-acetyl-d-galactopentitolyl-7-(4-chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidine-6-carbohydrazone (11)
3.5.2. Tetra-O-acetyl-d-xylotetritolyl-7-(4-chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]- pyrimidine-6-carbohydrazone (12)
3.5.3. Penta-O-acetyl-d-galactopentitolyl-5-methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]- pyrimidine-6-carbohydrazone (13)
3.5.4. Tetra-O-acetyl-d-xylotetritolyl-5-methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]- pyrimidine-6-carbohydrazone (14)
3.6. General Procedure for the Preparation of the Oxadiazoline Substituted Sugar Derivatives (15–18)
3.6.1. 1-(5-(7-(4-Chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidin-6-yl)-2-(penta-O-acetyl-d-galactopentitolyl)-1,3,4-oxadiazol-3(2H)-yl)ethan-1-one (15)
3.6.2. 1-(5-(7-(4-Chlorophenyl)-5-methyl-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidin-6-yl)-2-(tetra-O-acetyl-d-xylotetritolyl)-1,3,4-oxadiazol-3(2H)-yl)ethan-1-one (16)
3.6.3. 1-(5-(5-Methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidin-6-yl)-2-(penta-O-acetyl-d-galactopentitolyl)-1,3,4-oxadiazol-3(2H)-yl)ethan-1-one (17)
3.6.4. 1-(5-(5-Methyl-7-(thiophen-2-yl)-2,3-dihydro-7H-thiazolo[3,2-a]pyrimidin-6-yl)-2-(tetra-O-acetyl-d-xylotetritolyl)-1,3,4-oxadiazol-3(2H)-yl)ethan-1-one (18)
3.7. Materials of the Cell Lines Assay
3.7.1. Cell Culture, Maintenance, and Sub-Culture
3.7.2. Cell Proliferation by MTT Assay
3.7.3. IC50 Measurement
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Martino, J.K.; Boger, D.L. Glycinamide ribonucleotide transformylase (GAR TFase) as a target for cancer therapy. Drug Future 2008, 33, 969–979. [Google Scholar] [CrossRef]
- Curran, W.J. New chemotherapeutic agents: Update of major chemoradiation trials in solid tumors. Oncology 2002, 63, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.M.; Amr, A.E.G.; Alsharari, M.A.; Al-Qalawi, H.R.M.; Germoush, M.O.; Al-Omar, M.A. Anticancer Activities of Some New Synthesized Thiazolo[3,2-a]Pyrido[4,3-d]Pyrimidine Derivatives. Am. J. Biochem. Biotechnol. 2011, 7, 43–54. [Google Scholar] [CrossRef]
- Hammam, A.G.; El-Salam, O.I.A.; Mohamed, A.M.; Hafez, N.A. Novel fluoro substituted benzo[o]pyranwith anti-lung cancer activity. Ind. J. Chem. 2005, 44B, 1887–1893. [Google Scholar]
- Flefel, E.E.; Salama, M.A.; El-Shahat, M.; El-Hashash, M.A.; El-Farargy, A.F. A novel synthesis of some new pyrimidine and thiazolopyrimidine derivatives for anticancer evaluation. Phosphorus Sulfur Silicon Relat. Elem. 2007, 182, 1739–1756. [Google Scholar] [CrossRef]
- Cai, D.; Zhang, Z.-H.; Chen, Y.; Yan, X.-J.; Zou, L.-J.; Wang, Y.-X.; Liu, X.-Q. Synthesis, Antibacterial and Antitubercular Activities of Some 5H-Thiazolo[3,2-a]pyrimidin-5-ones and Sulfonic Acid Derivative. Molecules 2015, 20, 16419–16434. [Google Scholar] [CrossRef] [PubMed]
- Wichmann, J.; Adam, G.; Kolczewski, S.; Mutel, V.; Woltering, T. Structure-activity relationships of substituted 5H-thiazolo[3,2-a]pyrimidines as group 2 metabotropic glutamate receptor antagonists. Bioorg. Med. Chem. Lett. 1999, 9, 1573–1576. [Google Scholar] [CrossRef]
- Al-Omary, F.A.; Hassan, G.S.; El-Messery, S.M.; ElSubbagh, H.I. Substituted thiazoles V. Synthesis and antitumor activity of novel thiazolo[2,3-b]quinazoline and pyrido[4,3-d] thiazolo[3,2-a] pyrimidine analogues. Eur. J. Med. Chem. 2012, 47, 65–72. [Google Scholar] [CrossRef]
- Fatima, S.; Sharma, A.; Saxena, R.; Tripathi, R.; Shukla, S.K.; Pandey, S.K.; Tripathi, R.; Tripathi, R.P. One pot efficient diversity oriented synthesis of polyfunctional styryl thiazolopyrimidines and their bio-evaluation as antimalarial and anti-HIV agents. Eur. J. Med. Chem. 2012, 55, 195–204. [Google Scholar] [CrossRef]
- Yıldırım, A.B.; Mutlu, E.; Yıldırım, M. Cytotoxic Effects of Thiazolo[3,2-C]Pyrimidines Against Mcf-7 And Hepg2/C3a Carcinoma Cell Lines. Hacet. J. Biol. Chem. 2018, 46, 237–246. [Google Scholar] [CrossRef]
- Amr, A.-E.-G.; Maigali, S.S.; Abdulla, M.M. Synthesis, and analgesic and antiparkinsonian activities of thiopyrimidine, pyrane, pyrazoline, and thiazolopyrimidine derivatives from 2-chloro-6-ethoxy-4-acetylpyridine. Mon. Chem. 2008, 139, 1409–1415. [Google Scholar] [CrossRef]
- Branstetter, B.J.; Breitenbucher, J.G.; Lebsack, A.D.; Xiao, W. Thiazolopyrimidine Modulators of TRPV1. U.S. Patent WO 005303, 10 January 2008. [Google Scholar]
- Said, M.; Abouzid, K.; Mouneer, A.; Ahmedy, A.; Osman, A.-M. Synthesis and biological evaluation of new thiazolopyrimidines. Arch. Pharm. Res. 2004, 27, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Ackova, D.G.; Kotur-Stevuljevic, J.; Mishra, C.B.; Luthra, P.M.; Saso, L. Antioxidant Properties of Synthesized Bicyclic Thiazolopyrimidine Derivatives as Possible Therapeutic Agents. Appl. Sci. 2019, 9, 113. [Google Scholar] [CrossRef]
- Youssef, M.M.; Amin, M.A. Microwave Assisted Synthesis of Some New Thiazolopyrimidine, Thiazolodipyrimidine and Thiazolopyrimidothiazolopyrimidine Derivatives with Potential Antioxidant and Antimicrobial Activity. Molecules 2012, 17, 9652–9667. [Google Scholar] [CrossRef] [PubMed]
- Linder, W.; Brandes, W. Pesticidal Thiazolopyrimidine Derivatives. U.S. Patent 4,996,208, 26 February 1991. [Google Scholar]
- Duval, R.; Kolb, S.; Braud, E.; Genest, D.; Garbay, C. Rapid discovery of triazolobenzylidenethiazolopyrimidines (TBTP) as CDC25 phosphatase inhibitors by parallel click chemistry and in situ screening. J. Comb. Chem. 2009, 11, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Kolb, S.; Mondésert, O.; Goddard, M.L.; Jullien, D.; Villoutreix, B.O.; Ducommun, B.; Garbay, C.; Braud, E. Development of novel thiazolopyrimidines as CDC25B phosphatase inhibitors. ChemMedChem 2009, 4, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Mahgoub, M.Y.; Elmaghraby, A.M.; Harb, A.A.; da Silva, J.L.F.; Justino, G.C.; Marques, M.M. Synthesis, Crystal Structure, and Biological Evaluation of Fused Thiazolo[3,2-a]Pyrimidines as New Acetylcholinesterase Inhibitors. Molecules 2019, 24, 2306. [Google Scholar] [CrossRef]
- Liu, S.-J.; Yang, L.; Jin, Z.; Huang, E.F.; Wan, D.C.C.; Lin, H.-Q.; Hu, C. Design, synthesis, and biological evaluation of 7H-thiazolo[3,2-b]- 1,2,4-triazin-7-one derivatives as novel acetylcholinesterase inhibitors. Arkivoc 2009, 10, 333–348. [Google Scholar]
- Rashad, A.E.; Shamroukh, A.H.; Abdel-Megeid, R.E.; El-Sayed, W.A. Synthesis, reactions and antimicrobial evaluation of some polycondensedthieno-pyrimidine derivatives. Synth. Commun. 2010, 40, 1149–1160. [Google Scholar] [CrossRef]
- El-Emary, T.I.; Abdel-Mohsen, S.A. Synthesis and antimicrobial activity of some new 1,3-diphenylpyrazoles bearing pyrimidine, Pyrimidinethione, thiazolopyrimidine, triazolopyrimidine, thio- and alkylthiotriazolopyrimidinone moieties at the 4-position. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 2459–2474. [Google Scholar] [CrossRef]
- Maddila, S.; Damu, G.L.V.; Oseghe, E.O.; Abafe, O.A.; Venakata, R.C.; Lavanya, P. Synthesis and biological studies of novel biphenyl-3,5-dihydro-2H-thiazolo-pyrimidines derivatives. J. Korean Chem. Soc. 2012, 56, 334–340. [Google Scholar] [CrossRef]
- Khalilullah, H.; Ahsan, M.J.; Hedaitullah, M.; Khan, S.; Ahmad, B. 1,3,4-Oxadiazole: A Biologically Active Scaffold. Mini-Rev. Med. Chem. 2012, 12, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Kassem, A.F.; Nassar, I.F.; Abdel-Aal, M.T.; Awad, H.M.; El-Sayed, W.A. Synthesis and Anticancer Activity of New ((Furan-2-yl)-1,3,4-thiadiazolyl)-1,3,4-oxadiazole Acyclic Sugar Derivatives. Chem. Pharm. Bull. 2019, 67, 888–895. [Google Scholar] [CrossRef] [PubMed]
- Nassar, I.F.; El kady, D.S.; Awad, H.M.; El-Sayed, W.A. Design, Synthesis, and Anticancer Activity of New Oxadiazolyl-Linked and Thiazolyl-Linked Benzimidazole Arylidines, Thioglycoside, and Acyclic Analogs. J. Heterocycl. Chem. 2019, 56, 1086–1100. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Khalaf, H.S.; Osman, D.A.A.; Abbas, H.S.; Ali, M.M. Synthesis and Anticancer Activity of New Pyrazolyl and Oxadiazolyl Glycosides Based on Theinopyrimidine Nucleus and Their Acyclic Analogs. Acta Polonea Pharm. Drug Res. 2017, 74, 1739–1751. [Google Scholar]
- Gamal El-Din, M.M.; El-Gamal, M.I.; Abdel-Maksoud, M.S.; Yoo, K.H.; Oh, C.H. Synthesis and in vitro antiproliferative activity of new 1,3,4-oxadiazole derivatives possessing sulfonamide moiety. Eur. J. Med. Chem. 2015, 90, 45–52. [Google Scholar] [CrossRef]
- El-Sadek, M.M.; Hassan, S.Y.; Abd El-Dayem, N.S.; Yacout, G.A. 5-(5-Aryl-1,3,4-oxadiazole-2-carbonyl)furan-3-carboxylate and New Cyclic C-Glycoside Analogues from Carbohydrate Precursors with MAO-B, Antimicrobial and Antifungal Activities. Molecules 2012, 17, 7010–7027. [Google Scholar] [CrossRef]
- Glomb, T.; Szymankiewicz, K.; Swiatek, P. Anti-Cancer Activity of Derivatives of 1,3,4-Oxadiazole. Molecules 2018, 23, 336. [Google Scholar] [CrossRef]
- El Sayed, W.A.; Abbas, H.-A.S.; Ewais, A.M. C-Furyl glycosides, III: Synthesis and antimicrobial evaluation of C-furyl glycosides bearing substituted 1,3,4-oxadiazoles and their sugar hydrazone derivatives. J. Heterocycl. Chem. 2011, 48, 1006–1013. [Google Scholar] [CrossRef]
- Kahl, D.; Hutchings, K.; Lisabeth, E.M.; Larsen, S.D. 5-Aryl-1,3,4-oxadiazol-2-ylthioalkanoic Acids: A Highly Potent New Class of Inhibitors of Rho/Myocardin-Related Transcription Factor (MRTF)/Serum Reponse Factor (SRF)-Mediated Gene Transcription as Potential Antifibrotic Agents for Scleroderma. J. Med. Chem. 2019, 62, 4350–4369. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; El-Sofany, W.I.; Hussein, H.A.; Fathi, N.M. Synthesis and Anticancer Activity of New [(Indolyl)pyrazolyl]-1,3,4-oxadiazole Thioglycosides and Acyclic Nucleoside Analogs. Nucleosides Nucleotides Nucleic Acids 2017, 36, 474–495. [Google Scholar] [CrossRef] [PubMed]
- El Ashry, E.S.H.; El Kilany, Y. Acyclonucleosides: Part 1. Seco-Nucleosides. Adv. Heterocycl. Chem. 1996, 67, 391–438. [Google Scholar]
- El Ashry, E.S.H.; El Kilany, Y. Acyclonucleosides: Part 3. tri-, tetra-, and pentaseco-Nucleosides. Adv. Heterocycl. Chem. 1998, 69, 129–215. [Google Scholar]
- Kassem, A.F.; Abbas, E.M.H.; El-Kady, D.S.; Awad, H.M.; El-Sayed, W.A. Synthesis, Docking Studies and Anticancer Activity of New Tetrazolyl- and (Triazolyl)thiazole Glycosides and Acyclic Analogs. Mini-Rev. Med. Chem. 2019, 19, 933–948. [Google Scholar] [CrossRef]
- Abdel Rahman, A.A.H.; Nassar, I.F.; Shaban, A.K.F.; EL-Kady, D.S.; Awad, H.M.; El Sayed, W.A. Synthesis, Docking Studies into CDK-2 and Anticancer Activity of New Derivatives Based Pyrimidine Scaffold and Their Derived Glycosides. Mini-Rev. Med. Chem. 2019, 19, 1093–1110. [Google Scholar] [CrossRef]
- Abdel-Aal, M.T.; El-Sayed, W.A.; El-Kosy, S.M.; El-Ashry, E.S.H. Synthesis and Antiviral Evaluation of Novel 5-(N-Aryl-aminomethyl-1,3,4-oxadiazol-2-yl)hydrazines and their Sugars, 1,2,4-triazoles, tetrazoles and pyrazolyl Derivatives. Arch. Pharm. Chem. Life Sci. 2008, 341, 307–313. [Google Scholar] [CrossRef]
- Yousif, M.N.M.; El-Sayed, W.A.; Abbas, H.S.; Awad, H.M.; Yousif, N.M. Anticancer Activity of New Substituted Pyrimidines, Their Thioglycosides and Thiazolopyrimidine Derivatives. J. Appl. Pharm. Sci. 2017, 7, 021–032. [Google Scholar]
- Iftikhar, F.; Yaqoob, F.; Tabassum, N.; Jan, M.S.; Sadiq, A.; Tahir, S.; Batool, T.; Niaz, B.; Ansari, F.L.; Choudhary, M.I.; et al. Design, Synthesis, in-Vitro Thymidine Phosphorylase Inhibition, In-Vivo Antiangiogenic and in-Silico Studies of C-6 Substituted Dihydropyrimidines. Bioorg. Chem. 2018, 80, 99–111. [Google Scholar] [CrossRef]
- Flefel, E.M.; El-Sayed, W.A.; El-Sofany, W.; Mohamed, A.M.; Awad, H.M. Synthesis and Anticancer Activity of New 1-Thia-4-azaspiro[4.5]decane, Their Derived Thiazolopyrimidine and 1,3,4-Thiadiazole Thioglycosides. Molecules 2017, 22, 170. [Google Scholar] [CrossRef]
- Fahmy, H.T.Y.; Rostom, S.A.F.; Saudi, M.N.; Zjawiony, J.K.; Robins, D.J. Synthesis and in vitro evaluation of the anticancer activity of novel fluorinated thiazolo[4,5-d]pyrimidines. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 216–225. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Khalaf, H.S.; Mohamed, S.F.; Hssien, H.A.; Kutkat, O.M.; Amr, A.E.E. Synthesis and Antiviral Activity of 1,2,3-Triazole Glycosides Based Substituted Pyridine Via Click Cycloaddition. Russ. J. Gen. Chem. 2017, 87, 2444–2453. [Google Scholar] [CrossRef]
- Nassar, I.F.; El-Sayed, W.A.; Ragab, T.I.M.; Shalaby, A.S.G.; Mehany, A.B.M. Design, Synthesis of New Pyridine and Pyrimidine Sugar Compounds as Antagonists Targeting the ERα via Structure-Based Virtual Screening. Mini-Rev. Med. Chem. 2019, 19, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Alminderej, F.M.; Elganzory, H.H.; El-Bayaa, M.N.; Awad, H.M.; El-Sayed, W.A. Synthesis and Cytotoxic Activity of New 1,3,4-Thiadiazole Thioglycosides and 1,2,3-Triazolyl-1,3,4-Thiadiazole N-glycosides. Molecules 2019, 24, 3738. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, M.T.; El-Sayed, W.A.; El-Ashry, E.S.H. Synthesis and Antiviral Evaluation of Some Sugar Arylglycinoylhydrazones and Their Oxadiazoline Derivatives. Arch. Pharm. Chem. Life Sci. 2006, 339, 656–663. [Google Scholar] [CrossRef]
- Attri, P.; Bhatia, R.; Gaur, J.; Arora, B.; Gupta, A.; Kumar, N.; Choi, E.H. Triethylammonium acetate ionic liquid assisted one-pot synthesis of dihydropyrimidinones and evaluation of their antioxidant and antibacterial activities. Arab. J. Chem. 2017, 10, 206–214. [Google Scholar]
- Chen, L.; Jin, Y.; Fu, W.; Xiao, S.; Feng, C.; Fang, B.; Gu, Y.; Li, C.; Zhao, Y.; Liu, Z.; et al. Design, Synthesis, and Structure–Activity Relationship Analysis of Thiazolo[3,2-a]pyrimidine Derivatives with Anti-inflammatory Activity in Acute Lung Injury. ChemMedChem 2017, 12, 1022–1032. [Google Scholar] [CrossRef]
- Mobinikhaledi, A.; Forughifar, N.; Goodarzi, F. Synthesis of Some Bicyclic Thiazolopyrimidine Derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2003, 178, 2539–2543. [Google Scholar] [CrossRef]
- Abdel Rahman, M.M.; El Ashry, E.S.H.; Abdalla, A.A.; Rashed, N. C-(Polyacetoxy)alkyloxadiazolines and related compounds. Carbhydr. Res. 1979, 73, 103–111. [Google Scholar] [CrossRef]
- Somogyi, L. Structure and reactions of L-rhamnose benzoylhydrazone tetra-acetates. Carbohydr. Res. 1978, 64, 289–292. [Google Scholar] [CrossRef]
- Van Meerloo, J.; Kaspers, G.J.; Cloos, J. Cell sensitivity assays: The MTT assay. J. Methods Mol. Biol. 2011, 731, 237–245. [Google Scholar]
Sample Availability: Samples of the synthesized compounds are available from the authors. |
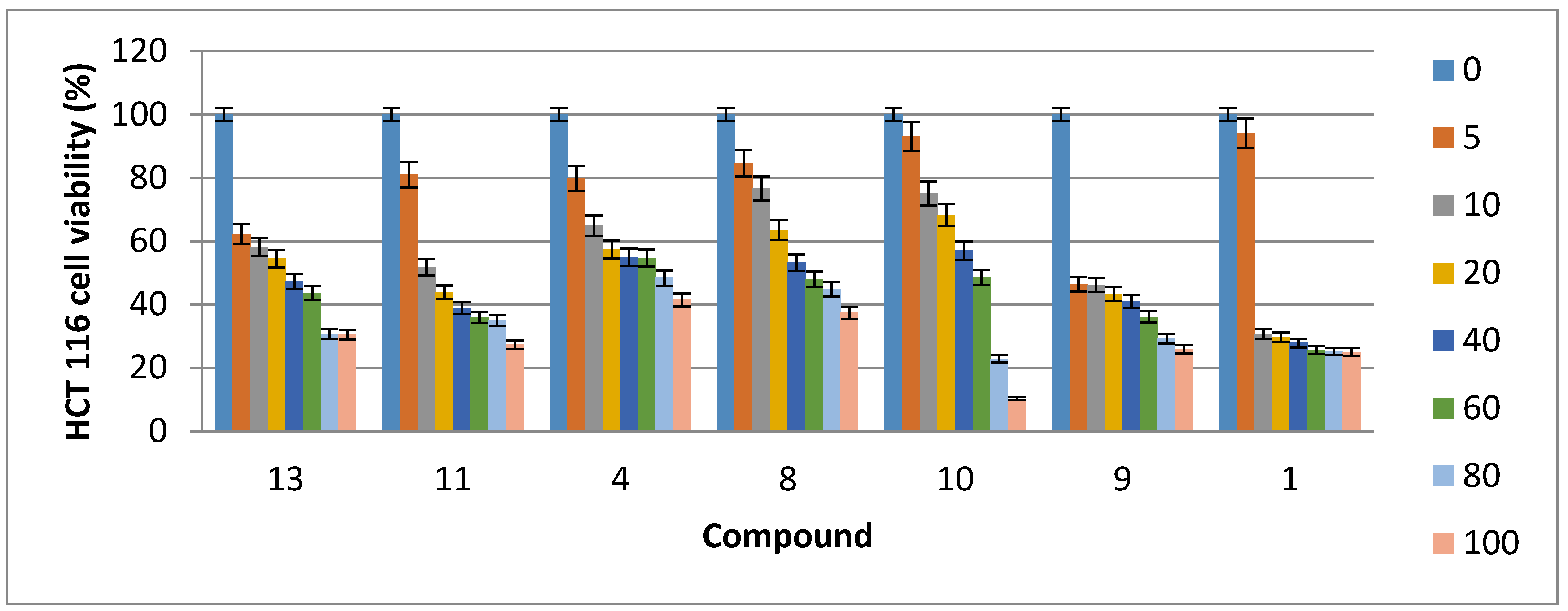
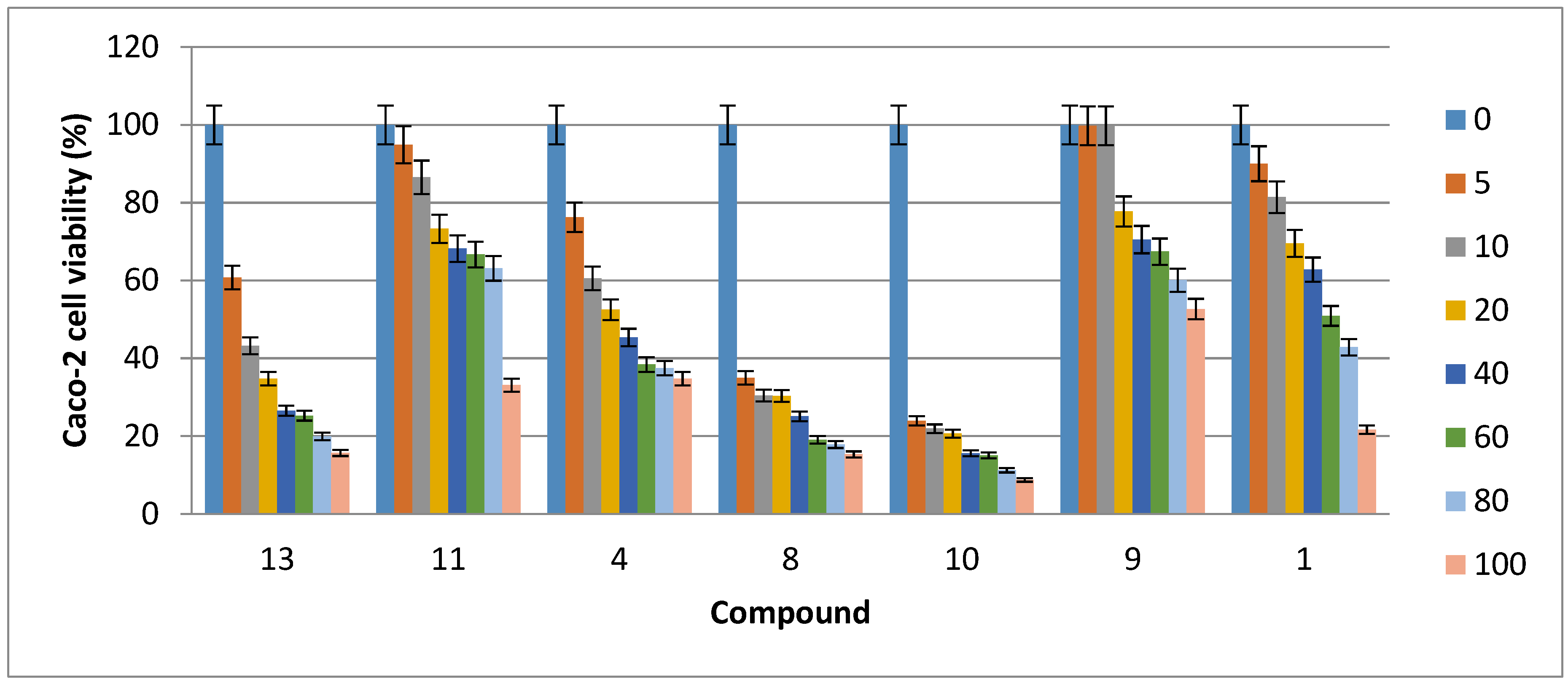
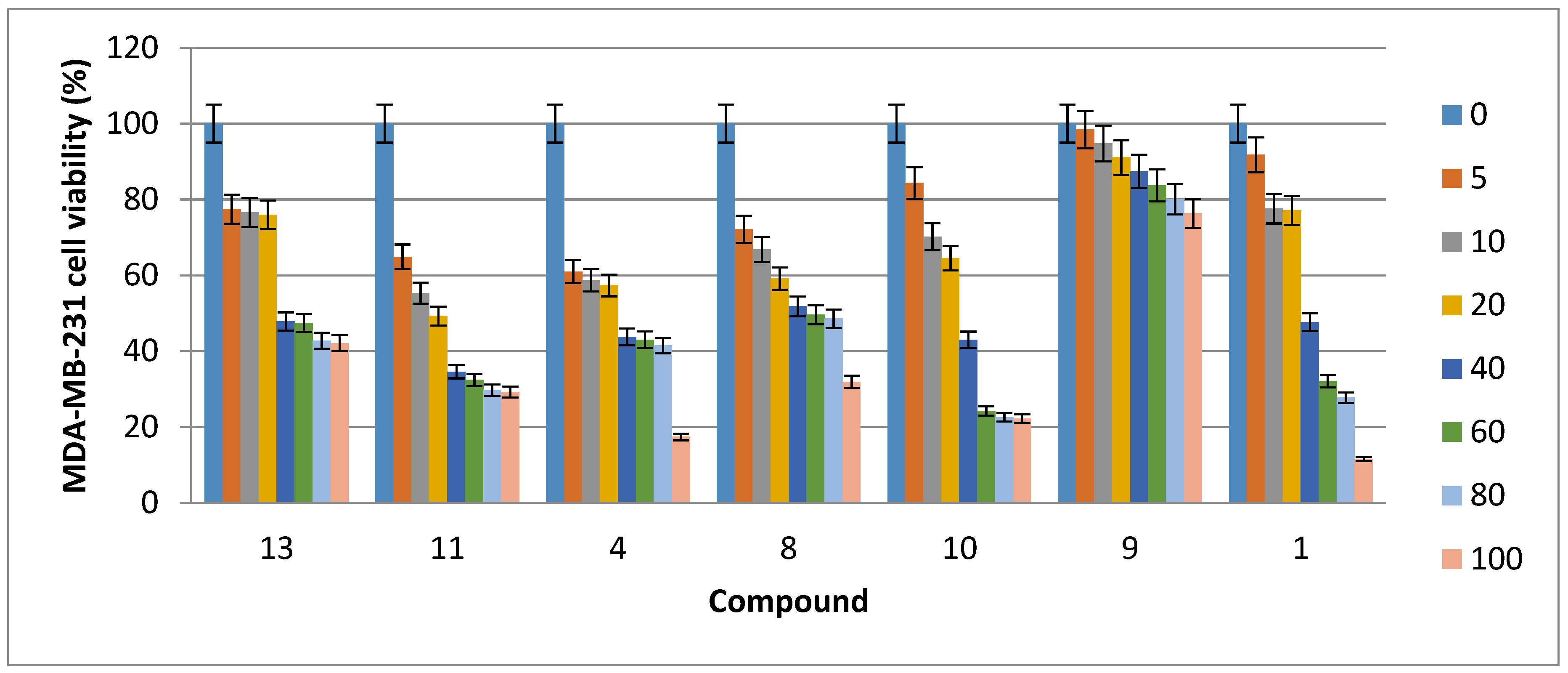
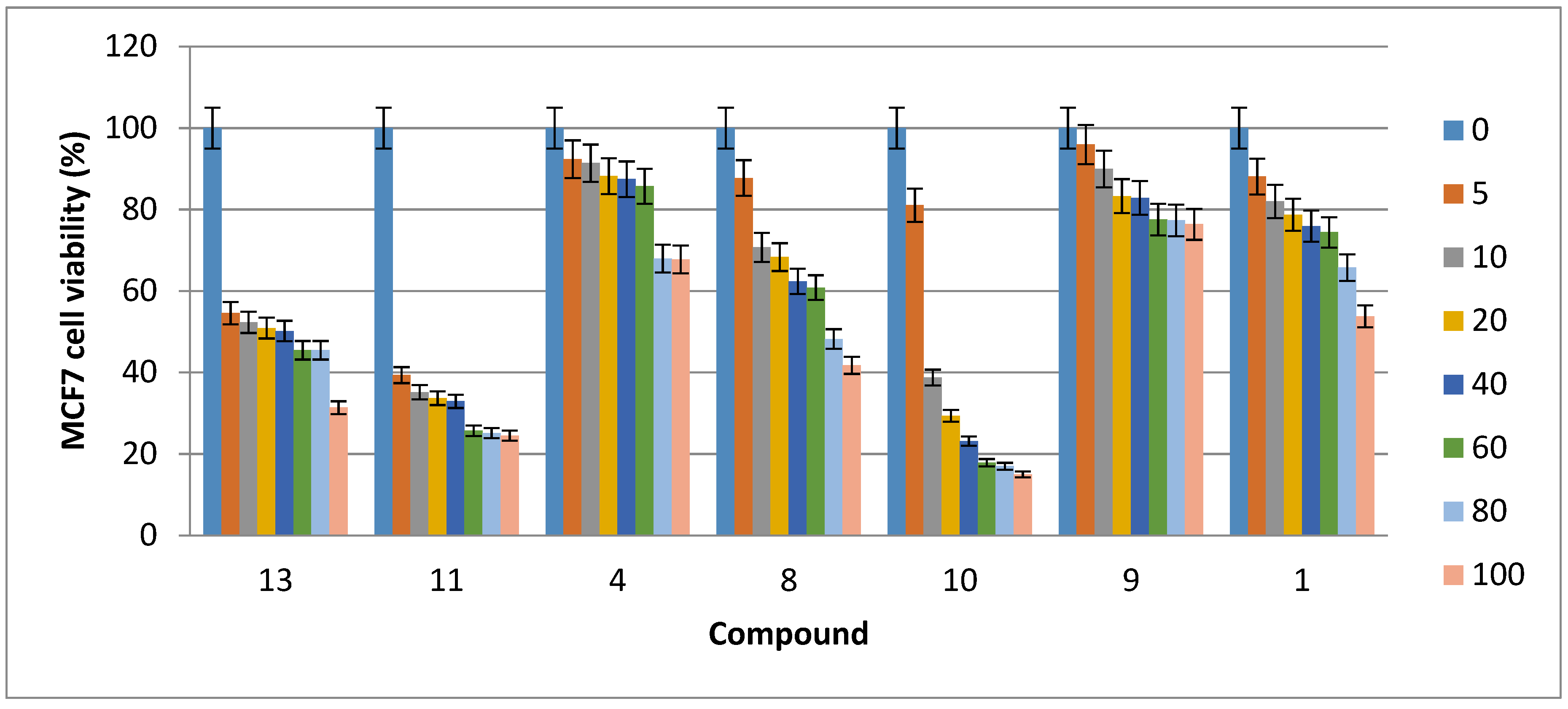
| Compound | HCT116 Cells | Caco-2 Cells | MDA-MB-231 Cells | MCF7 Cells |
|---|---|---|---|---|
| 1 | 25.28 | 58.31 | 40.78 | ND |
| 4 | 63.61 | 30.84 | 36.55 | ND |
| 8 | 66.75 | 9.63 | 46.99 | 69.90 |
| 9 | 27.95 | ND | ND | ND |
| 10 | 65.89 | 4.79 | 30.58 | 16.85 |
| 11 | 34.80 | 83.01 | 23.35 | 12.47 |
| 13 | 44.04 | 16.82 | 46.30 | 34.83 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basiony, E.A.; Hassan, A.A.; Al-Amshany, Z.M.; Abd-Rabou, A.A.; Abdel-Rahman, A.A.-H.; Hassan, N.A.; El-Sayed, W.A. Synthesis and Cytotoxic Activity of New Thiazolopyrimidine Sugar Hydrazones and Their Derived Acyclic Nucleoside Analogues. Molecules 2020, 25, 399. https://doi.org/10.3390/molecules25020399
Basiony EA, Hassan AA, Al-Amshany ZM, Abd-Rabou AA, Abdel-Rahman AA-H, Hassan NA, El-Sayed WA. Synthesis and Cytotoxic Activity of New Thiazolopyrimidine Sugar Hydrazones and Their Derived Acyclic Nucleoside Analogues. Molecules. 2020; 25(2):399. https://doi.org/10.3390/molecules25020399
Chicago/Turabian StyleBasiony, Ebtesam A., Allam A. Hassan, Zahra M. Al-Amshany, Ahmed A. Abd-Rabou, Adel A.-H. Abdel-Rahman, Nasser A. Hassan, and Wael A. El-Sayed. 2020. "Synthesis and Cytotoxic Activity of New Thiazolopyrimidine Sugar Hydrazones and Their Derived Acyclic Nucleoside Analogues" Molecules 25, no. 2: 399. https://doi.org/10.3390/molecules25020399
APA StyleBasiony, E. A., Hassan, A. A., Al-Amshany, Z. M., Abd-Rabou, A. A., Abdel-Rahman, A. A.-H., Hassan, N. A., & El-Sayed, W. A. (2020). Synthesis and Cytotoxic Activity of New Thiazolopyrimidine Sugar Hydrazones and Their Derived Acyclic Nucleoside Analogues. Molecules, 25(2), 399. https://doi.org/10.3390/molecules25020399






