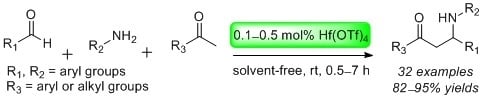
Hf(OTf)4 as a Highly Potent Catalyst for the Synthesis of Mannich Bases under Solvent-Free Conditions
Abstract
1. Introduction
2. Results and Discussion
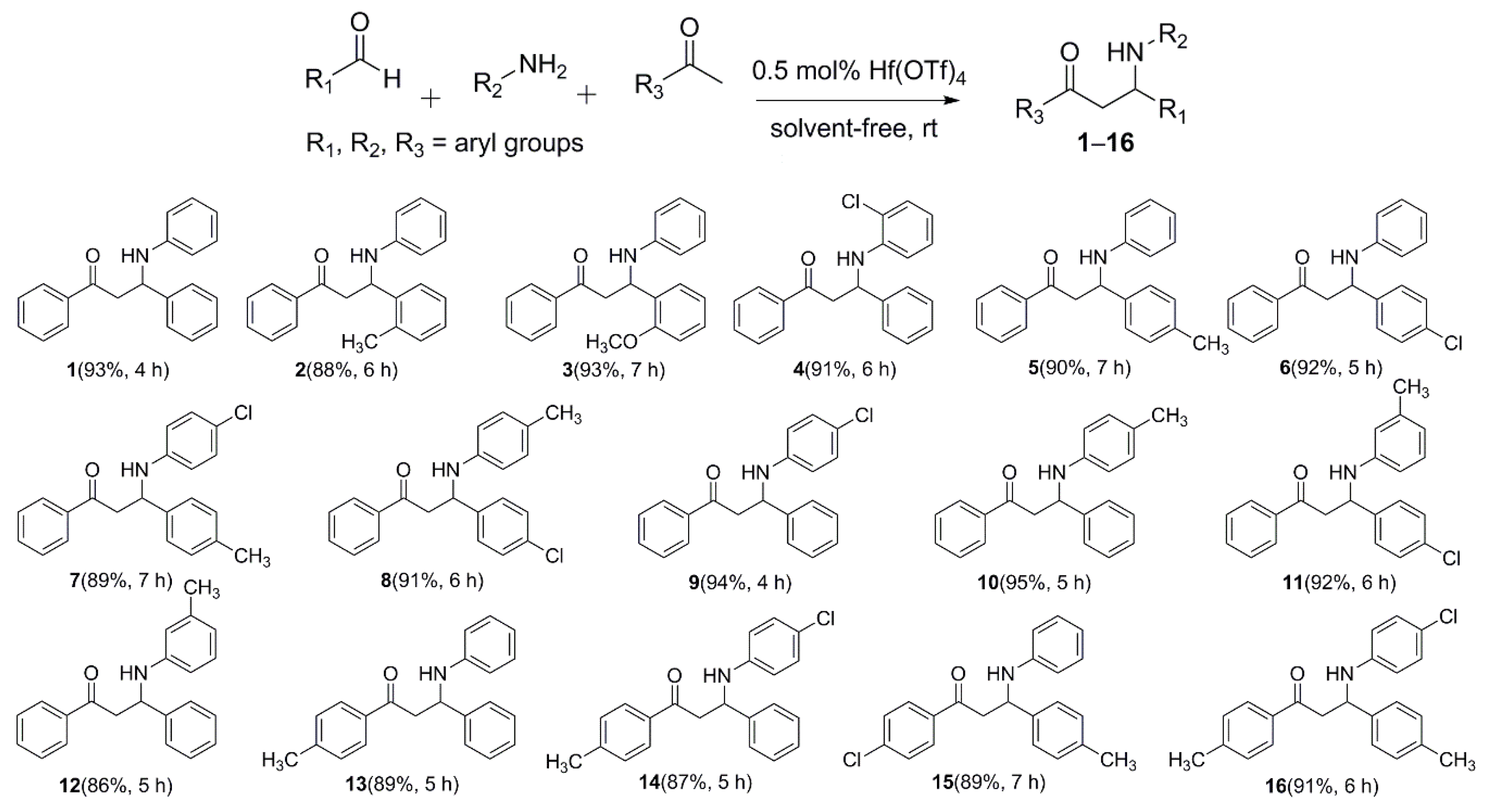
2.1. Aryl Ketone-Based Mannich Reaction Catalyzed by Hf(OTf)4
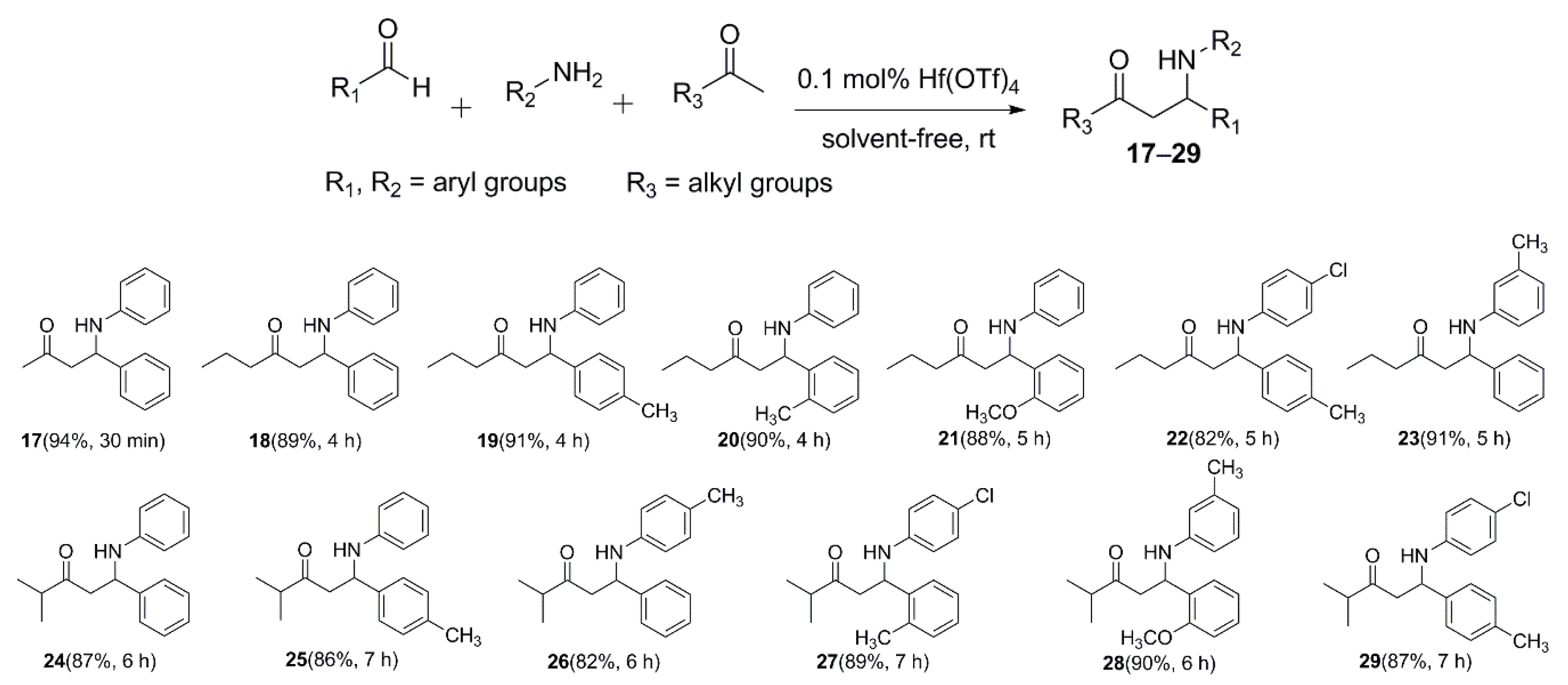
2.2. Alkyl Ketone-Based Mannich Reaction Catalyzed by Hf(OTf)4: Regioselectivity and Diastereoselectivity
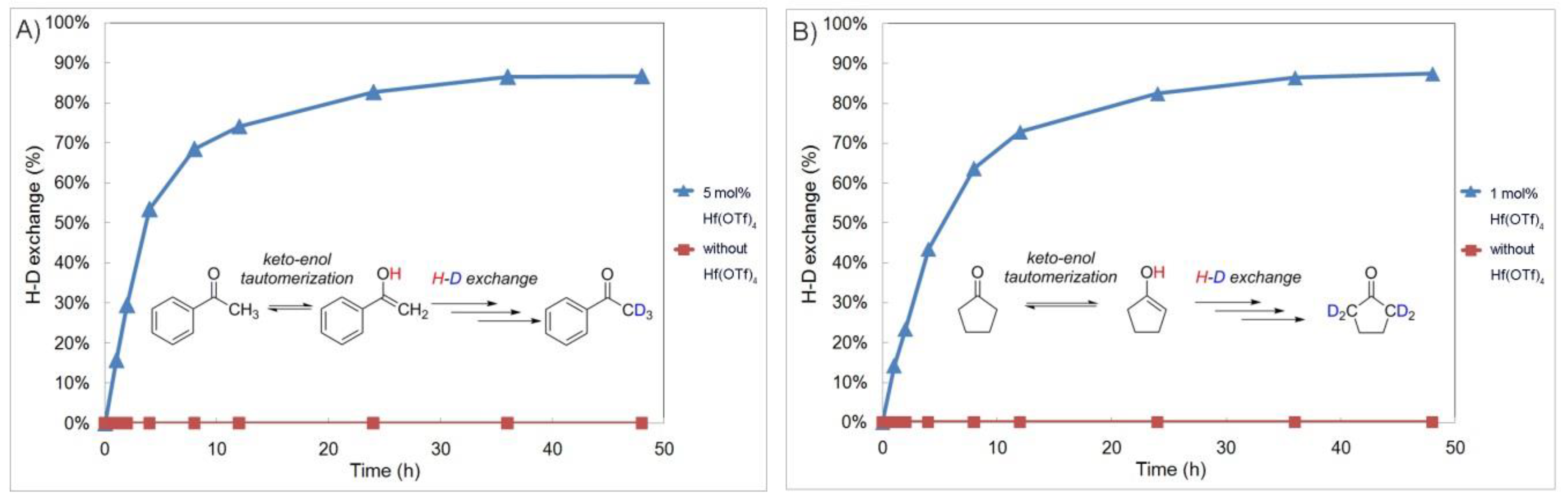
2.3. The Catalytic Role of Hf(OTf)4 on Keto-Enol Tautomerization
3. Materials and Methods
3.1. General Methods
3.2. General Synthetic Procedure and Characterization of Mannich Bases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Mannich, C. Eine synthese von β-ketonbasen. Arch. Pharm. 1917, 255, 261–276. [Google Scholar] [CrossRef]
- Arend, M.; Westermann, B.; Risch, N. Modern variants of the Mannich reaction. Angew. Chem. Int. Ed. 1998, 37, 1044–1070. [Google Scholar] [CrossRef]
- Kobayash, S.; Ishitani, H. Catalytic enantioselective addition to imines. Chem. Rev. 1999, 99, 1069–1094. [Google Scholar] [CrossRef] [PubMed]
- Allochio Filho, J.F.; Lemos, B.C.; De Souza, A.S.; Pinheiro, S.; Greco, S.J. Multicomponent Mannich reactions: General aspects, methodologies and applications. Tetrahedron 2017, 73, 6977–7004. [Google Scholar] [CrossRef]
- Paul, J.; Presset, M.; Le Gall, E. Multicomponent Mannich-like reactions of organometallic species. Eur. J. Org. Chem. 2017, 2386–2406. [Google Scholar] [CrossRef]
- Toure, B.B.; Hall, D.G. Natural product synthesis using multicomponent reaction strategies. Chem. Rev. 2009, 109, 4439–4486. [Google Scholar] [CrossRef]
- Roman, G. Mannich bases in medicinal chemistry and drug design. Eur. J. Med. Chem. 2015, 89, 743–816. [Google Scholar] [CrossRef]
- Subramaniapillai, S.G. Mannich reaction: A versatile and convenient approach to bioactive skeletons. J. Chem. Sci. 2013, 125, 467–482. [Google Scholar] [CrossRef]
- Yi, L.; Lei, H.S.; Zou, J.H.; Xu, X.J. The Mannich reaction between aromatic ketones, aromatic aldehydes and aromatic amines. Synthesis 1991, 717–718. [Google Scholar] [CrossRef]
- Wu, Y.S.; Cai, J.W.; Hu, Z.Y.; Lin, G.X. A new class of metal-free catalysts for direct diastereo- and regioselective Mannich reactions in aqueous media. Tetrahedron Lett. 2004, 45, 8949–8952. [Google Scholar] [CrossRef]
- Bigdeli, M.A.; Nemati, F.; Mahdavinia, G.H. HClO4–SiO2 catalyzed stereoselective synthesis of β-amino ketones via a direct Mannich-type reaction. Tetrahedron Lett. 2007, 48, 6801–6804. [Google Scholar] [CrossRef]
- Iimura, S.; Nobuton, D.; Manabe, K.; Kobayashi, S. Mannich-type reactions in water using a hydrophobic polymer-supported sulfonic acid catalyst. Chem. Commun. 2003, 14, 1644–1645. [Google Scholar] [CrossRef]
- Azizi, B.; Torkiyan, L.; Saidi, M.R. Highly efficient one-pot three-component Mannich reaction in water catalyzed by heteropoly acids. Org. Lett. 2006, 8, 2079–2082. [Google Scholar] [CrossRef] [PubMed]
- Manabe, K.; Kobayashi, S. Mannich-type reactions of aldehydes, amines, and ketones in a colloidal dispersion system created by a Brønsted acid-surfactant-combined catalyst in water. Org. Lett. 1999, 1, 1965–1967. [Google Scholar] [CrossRef]
- Alvim, H.G.O.; Bataglion, G.A.; Ramos, L.M.; De Oliveira, A.L.; De Oliveira, H.C.B.; Eberlin, M.N.; De Macedo, J.L.; Da Silva, W.A. Task-specific ionic liquid incorporating anionic heteropolyacid-catalyzed Hantzsch and Mannich multicomponent reactions. Ionic liquid effect probed by ESI-MS(/MS). Tetrahedron 2014, 70, 3306–3313. [Google Scholar] [CrossRef]
- Yi, W.B.; Cai, C. Mannich-type reactions of aromatic aldehydes, anilines, and methyl ketones in fluorous biphase systems created by rare earth (III) perfluorooctane sulfonates catalysts in fluorous media. J. Fluor. Chem. 2006, 127, 1515–1521. [Google Scholar] [CrossRef]
- Eftekhari-Sis, B.; Abdollahifar, A.; Hashemi, M.M.; Zirak, M. Stereoselective synthesis of β-amino ketones via direct Mannich-type reactions, catalyzed with ZrOCl2·8H2O under solvent-free conditions. Eur. J. Org. Chem. 2006, 5152–5157. [Google Scholar] [CrossRef]
- Yang, Y.Y.; Shou, W.G.; Wang, Y.G. Synthesis of β-amino carbonyl compounds via a Zn(OTf)2-catalyzed cascade reaction of anilines with aromatic aldehydes and carbonyl compounds. Tetrahedron 2006, 62, 10079–10086. [Google Scholar] [CrossRef]
- Yang, R.W.; Li, B.G.; Huang, T.K.; Shi, L.; Lu, X.X. NbCl5-Catalyzed one-pot Mannich-type reaction: Three component synthesis of β-amino carbonyl compounds. Tetrahedron Lett. 2007, 48, 2071–2073. [Google Scholar]
- Wang, M.; Song, Z.G.; Wan, X.; Zhao, S. SnCl2-catalyzed three-component one-pot Mannich-type reaction: Efficient synthesis of β-aminocarbonyl compounds. Monatsh. Chem. 2009, 140, 1205–1208. [Google Scholar] [CrossRef]
- Wang, M.; Song, Z.G.; Liang, Y. SnCl4 5H2O-Catalyzed synthesis of β-amino carbonyl compounds via a direct Mannich-type reaction. Prep. Biochem. Biotechnol. 2011, 41, 1–6. [Google Scholar] [CrossRef]
- Li, H.; Zeng, H.Y.; Shao, H.W. Bismuth(III) chloride-catalyzed one-pot Mannich reaction: Three-component synthesis of β-amino carbonyl compounds. Tetrahedron Lett. 2009, 50, 6858–6860. [Google Scholar] [CrossRef]
- Dai, Y.; Li, B.D.; Quan, H.D.; Lu, C.X. CeCl3 7H2O as an efficient catalyst for one-pot synthesis of β-amino ketones by three-component Mannich reaction. Chin. Chem. Lett. 2010, 21, 31–34. [Google Scholar] [CrossRef]
- Kidwai, M.; Bhatnagar, D.; Kumar Mishra, N.; Bansal, V. CAN catalyzed synthesis of β-amino carbonyl compounds via Mannich reaction in PEG. Catal. Commun. 2008, 9, 2547–2549. [Google Scholar] [CrossRef]
- Kureshy, R.I.; Agrawal, S.; Saravanan, S.; Khan, N.H.; Shah, A.K.; Abdi, S.H.R.; Bajaj, H.C.; Suresh, E. Direct Mannich reaction mediated by Fe(Cp)2PF6 under solvent-free conditions. Tetrahedron Lett. 2010, 51, 489–494. [Google Scholar] [CrossRef]
- Zhang, G.L.; Huang, Z.H.; Zou, J.P. Ga(OTf)3-catalyzed Three-component Mannich reaction in water promoted by ultrasound irradiation. Chin. J. Chem. 2009, 27, 1967–1974. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Luo, Y.L.; Wang, J.; Jian, Y.J.; Sun, H.M.; Zhang, G.F.; Zhang, W.Q.; Gao, Z.W. Solvent strategy for unleashing Lewis acidity of titanocene dichloride for rapid Mannich reactions. RSC Adv. 2016, 6, 15298–15303. [Google Scholar] [CrossRef]
- Zhang, X.W.; Yin, S.F.; Qiu, R.H.; Xia, J.; Dai, W.L.; Yu, Z.Y.; Au, C.T.; Wong, W.F. Synthesis and structure of an air-stable hypervalent organobismuth (III) perfluorooctanesulfonate and its use as high-efficiency catalyst for Mannich-type reactions in water. J. Organomet. Chem. 2009, 694, 3559–3564. [Google Scholar] [CrossRef]
- Xia, J.; Qiu, R.H.; Yin, S.F.; Zhang, X.W.; Luo, S.L.; Au, C.T.; Xia, K.; Wong, W.Y. Synthesis and structure of an air-stable organoantimony complex and its use as a catalyst for direct diastereoselective Mannich reactions in water. J. Organomet. Chem. 2010, 695, 1487–1492. [Google Scholar] [CrossRef]
- Qiu, R.H.; Xu, X.H.; Peng, L.F.; Zhao, Y.L.; Li, N.B.; Yin, S.F. Strong Lewis acids of air-stable metallocene bis(perfluorooctanesulfonate)s as high-efficiency catalysts for carbonyl group transformation reactions. Chem. Eur. J. 2012, 18, 6172–6182. [Google Scholar] [CrossRef]
- Zhang, X.H.; Xu, X.H.; Li, N.B.; Liang, Z.W.; Tang, Z.L. Air-stable μ2-hydroxyl bridged cationic binuclear complexes of zirconocene perfluorooctanesulfonates: Their structures, characterization and application. Tetrahedron 2018, 74, 1926–1932. [Google Scholar] [CrossRef]
- Khan, A.T.; Parvin, T.; Choudhury, L.H. Bromodimethylsulfonium bromide catalyzed three-component Mannich-type reactions. Eur. J. Org. Chem. 2008, 5, 834–839. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Han, J.W.; Liu, Z.J. Diaryliodonium salts as efficient Lewis acid catalysts for direct three component Mannich reactions. RSC Adv. 2015, 32, 25485–25488. [Google Scholar] [CrossRef]
- Azizi, N.; Baghi, R.; Batebi, E.; Bolourtchian, S.M. Catalytic stereoselective Mannich reaction under solvent-free conditions. C. R. Chim. 2012, 15, 278–282. [Google Scholar] [CrossRef]
- Sawant, D.P.; Justus, J.; Balasubramanian, V.V.; Ariga, K.; Srinivasu, P.; Velmathi, S.; Halligudi, S.B.; Vinu, A. Heteropoly acid encapsulated SBA-15/TiO2 nanocomposites and their unusual performance in acid-catalyzed organic transformations. Chem. Eur. J. 2008, 14, 3200–3212. [Google Scholar] [CrossRef]
- Wang, H.B.; Yao, N.; Wang, L.; Hu, Y.L. Brønsted–Lewis dual acidic ionic liquid immobilized on mesoporous silica materials as an efficient cooperative catalyst for Mannich reactions. New J. Chem. 2017, 41, 10528–10531. [Google Scholar] [CrossRef]
- Esfahani, F.K.; Zareyee, D.; Rad, A.S.; Taher-Bahrami, S. Sulfonic acid supported on magnetic nanoparticle as an eco-friendly, durable and robust catalyst for the synthesis of β-amino carbonyl compounds through solvent free Mannich reaction. Appl. Organometal. Chem. 2017, 31, E3865. [Google Scholar] [CrossRef]
- Nasresfahani, Z.; Kassaee, M.Z.; Nejati-Shendi, M.; Eidi, E.; Taheri, Q. Mesoporous silica nanoparticles (MSNs) as an efficient and reusable nanocatalyst for synthesis of β-amino ketones through one-pot three-component Mannich reactions. RSC Adv. 2016, 6, 32183–32188. [Google Scholar] [CrossRef]
- Pachamuthu, M.P.; Shanthi, K.; Luque, R.; Ramanathan, A. SnTUD-1: A solid acid catalyst for three component coupling reactions at room temperature. Green Chem. 2013, 15, 2158–2166. [Google Scholar] [CrossRef]
- Wang, Y.F.; Biradar, A.V.; Wang, G.; Sharma, K.K.; Duncan, C.T.; Rangan, S.; Asefa, T. Controlled synthesis of water-dispersible faceted crystalline copper nanoparticles and their catalytic properties. Chem. Eur. J. 2010, 16, 10735–10743. [Google Scholar] [CrossRef]
- Sharma, R.K.; Rawat, D.; Gaba, G. Inorganic–organic hybrid silica based tin(II) catalyst: Synthesis, characterization and application in one-pot three-component Mannich reaction. Catal. Commun. 2012, 19, 31–36. [Google Scholar] [CrossRef]
- Rajesh Krishnan, G.; Sreeraj, M.K.; Sreekumar, K. Modification of poly(vinyl chloride) with pendant metal complex for catalytic applications. C. R. Chim. 2013, 16, 736–741. [Google Scholar] [CrossRef]
- Ishihara, K.; Ohara, S.; Yamamoto, H. Direct condensation of carboxylic acids with alcohols catalyzed by hafnium(IV) salts. Science 2000, 290, 1140–1142. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-C.; Gong, S.-S.; Zeng, D.-Y.; You, Y.-H.; Sun, Q. Highly efficient synthesis of α-aminophosphonates catalyzed by hafnium(IV) chloride. Tetrahedron Lett. 2016, 57, 1782–1785. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.-Z.; Zheng, X.-A.; Kong, R.; Gong, S.-S.; Sun, Q. Hafnium(IV) triflate as a potent catalyst for selective 1-O-deacetylation of peracetylated saccharides. Carbohyd. Res. 2018, 455, 114–118. [Google Scholar] [CrossRef]
- Kong, R.; Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Xie, Z.-B.; Gong, S.-S.; Sun, Q. Highly efficient synthesis of substituted 3,4-Dihydropyrimidin-2-(1H)-ones (DHPMs) catalyzed by Hf(OTf)4: Mechanistic insights into reaction pathways under metal Lewis acid catalysis and solvent-free conditions. Molecules 2019, 24, 364. [Google Scholar] [CrossRef]
- Barbero, M.; Cadamuro, S.; Dughera, S. Brønsted acid catalyzed enantio- and diastereoselective one-pot three component Mannich reaction. Tetrahedron Asymmetry 2015, 26, 1180–1188. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Y.; Jia, G.; Zhu, X.-Y.; Sun, H.-M.; Zhang, G.-F.; Zhang, W.-Q.; Gao, Z.-W. Salicylato titanocene complexes as cooperative organometallic Lewis acid and Brønsted acid catalysts for three-component Mannich reactions. Chem. Eur. J. 2014, 20, 8530–8535. [Google Scholar] [CrossRef]
- Rueping, M.; Sugiono, E.; Schoepke, F.R. Development of the first Brønsted acid assisted enantioselective Brønsted acid catalyzed direct Mannich reaction. Synlett 2007, 9, 1441–1445. [Google Scholar] [CrossRef]
- Zhou, J.-H. The Mannich reaction of dialkyl ketones, aromatic aldehydes and aromatic amines. Org. Prep. Proced. Int. 1996, 28, 618–622. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–32 are available from the authors. |
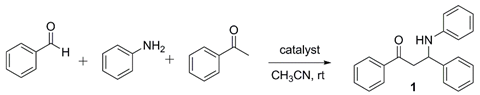
| Catalyst (5 mol%) | Reaction Time (h) | Yield of 1 (%) | |
|---|---|---|---|
| 1 | no | 24 | no reaction |
| 2 | ZrCl4 | 24 | 70 |
| 3 | ZrOCl2·8H2O | 24 | 71 |
| 4 | ZrCp2Cl2 | 12 | 73 |
| 5 | HfCl4 | 8 | 81 |
| 6 | Hf(OTf)4 | 6 | 89 |
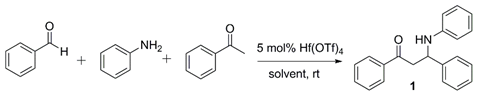
| Solvent | Reaction Time (h) | Yield of 1 (%) | |
|---|---|---|---|
| 1 | THF | 36 | 82 |
| 2 | DME | 36 | 80 |
| 3 | benzene | 24 | 73 |
| 4 | CH2Cl2 | 24 | 68 |
| 5 | CH3CN | 6 | 89 |
| 6 | EtOH | 5 | 94 |
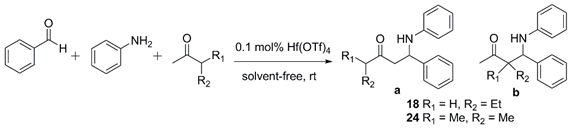
| Compd | Hf(OTf)4 (mol%) | Reaction Time (h) | Yield (%) | |
|---|---|---|---|---|
| 1 | 18 | - | 48 | 71 (a:b = 1:0.53) |
| 2 | 18 | 0.1 | 4 | 89 (a only) |
| 3 | 24 | - | 72 | 15 (a:b = 1:0.48) |
| 4 | 24 | 0.1 | 6 | 87 (a only) |
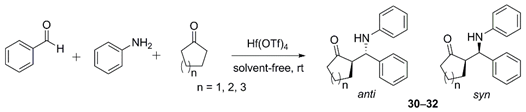
| Compd | n | Hf(OTf)4 (mol%) | Reaction Time (h) | Yield (%) | anti/syn | |
|---|---|---|---|---|---|---|
| 1 | 30 | 1 | - | 12 | - | - |
| 2 | 30 | 1 | 0.1 | 0.5 | 89 | 92:8 |
| 3 | 31 | 2 | - | 6 | 71 | 63:37 |
| 4 | 31 | 2 | 0.1 | 0.33 | 92 | 96:4 |
| 5 | 32 | 3 | - | 48 | 58 | 20:80 |
| 6 | 32 | 3 | 0.1 | 8 | 88 | 59:41 |
| 7 | 32 | 3 | 1 | 1 | 90 | 68:32 |
| 8 | 32 | 3 | 10 | 0.16 | 89 | 77:23 |
| 9 | 32 | 3 | 50 | 0.05 | 82 | 86:14 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.-B.; Wei, J.-Y.; Peng, X.-C.; Liu, R.; Gong, S.-S.; Sun, Q. Hf(OTf)4 as a Highly Potent Catalyst for the Synthesis of Mannich Bases under Solvent-Free Conditions. Molecules 2020, 25, 388. https://doi.org/10.3390/molecules25020388
Han S-B, Wei J-Y, Peng X-C, Liu R, Gong S-S, Sun Q. Hf(OTf)4 as a Highly Potent Catalyst for the Synthesis of Mannich Bases under Solvent-Free Conditions. Molecules. 2020; 25(2):388. https://doi.org/10.3390/molecules25020388
Chicago/Turabian StyleHan, Shuai-Bo, Jing-Ying Wei, Xiao-Chong Peng, Rong Liu, Shan-Shan Gong, and Qi Sun. 2020. "Hf(OTf)4 as a Highly Potent Catalyst for the Synthesis of Mannich Bases under Solvent-Free Conditions" Molecules 25, no. 2: 388. https://doi.org/10.3390/molecules25020388
APA StyleHan, S.-B., Wei, J.-Y., Peng, X.-C., Liu, R., Gong, S.-S., & Sun, Q. (2020). Hf(OTf)4 as a Highly Potent Catalyst for the Synthesis of Mannich Bases under Solvent-Free Conditions. Molecules, 25(2), 388. https://doi.org/10.3390/molecules25020388