Extraction and Physicochemical Characterization of Chitin Derived from the Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae)
Abstract
1. Introduction
2. Results and Discussion
2.1. Insect Source and Chitin Yield
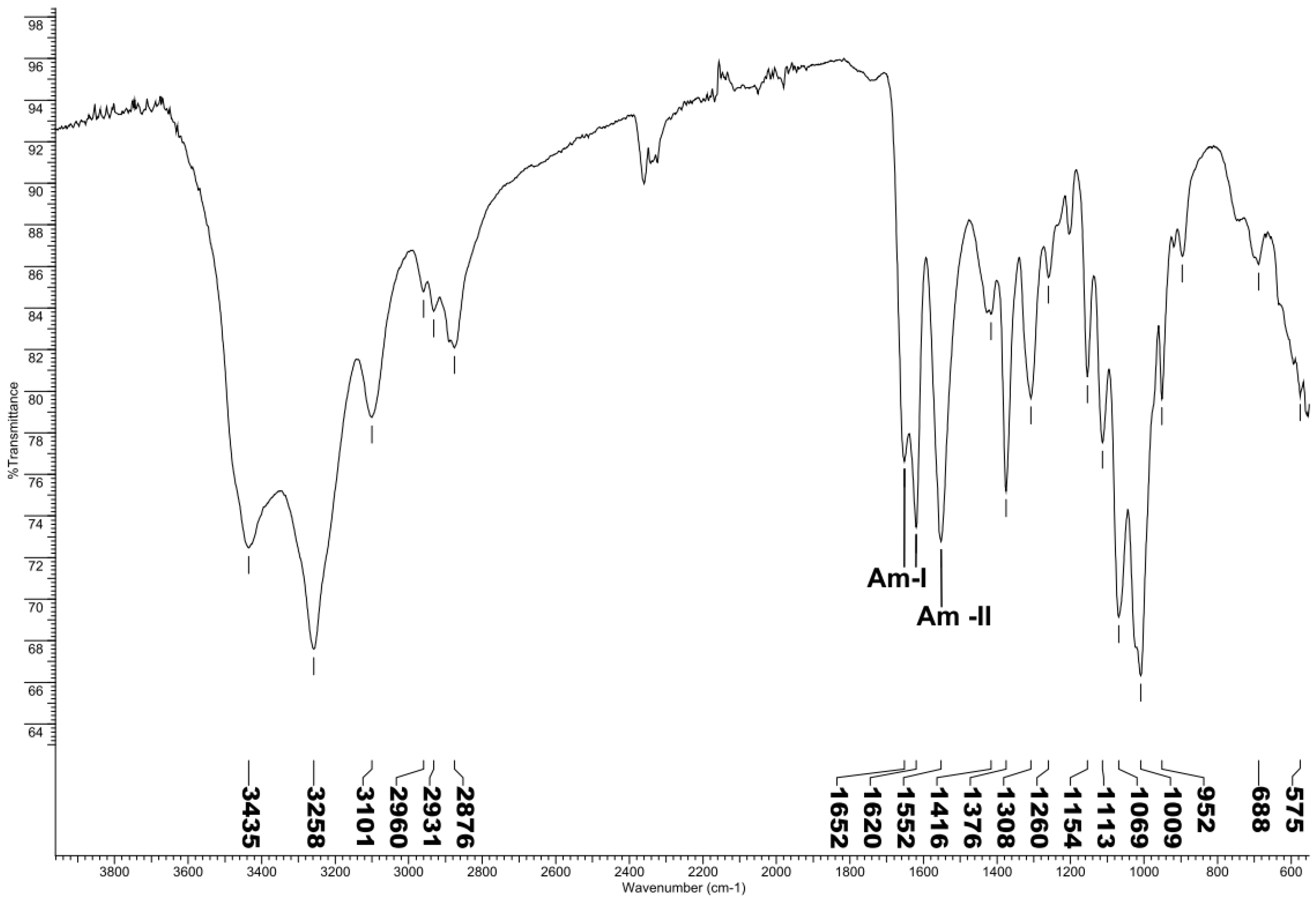
2.2. Spectroscopic Chitin Analysis
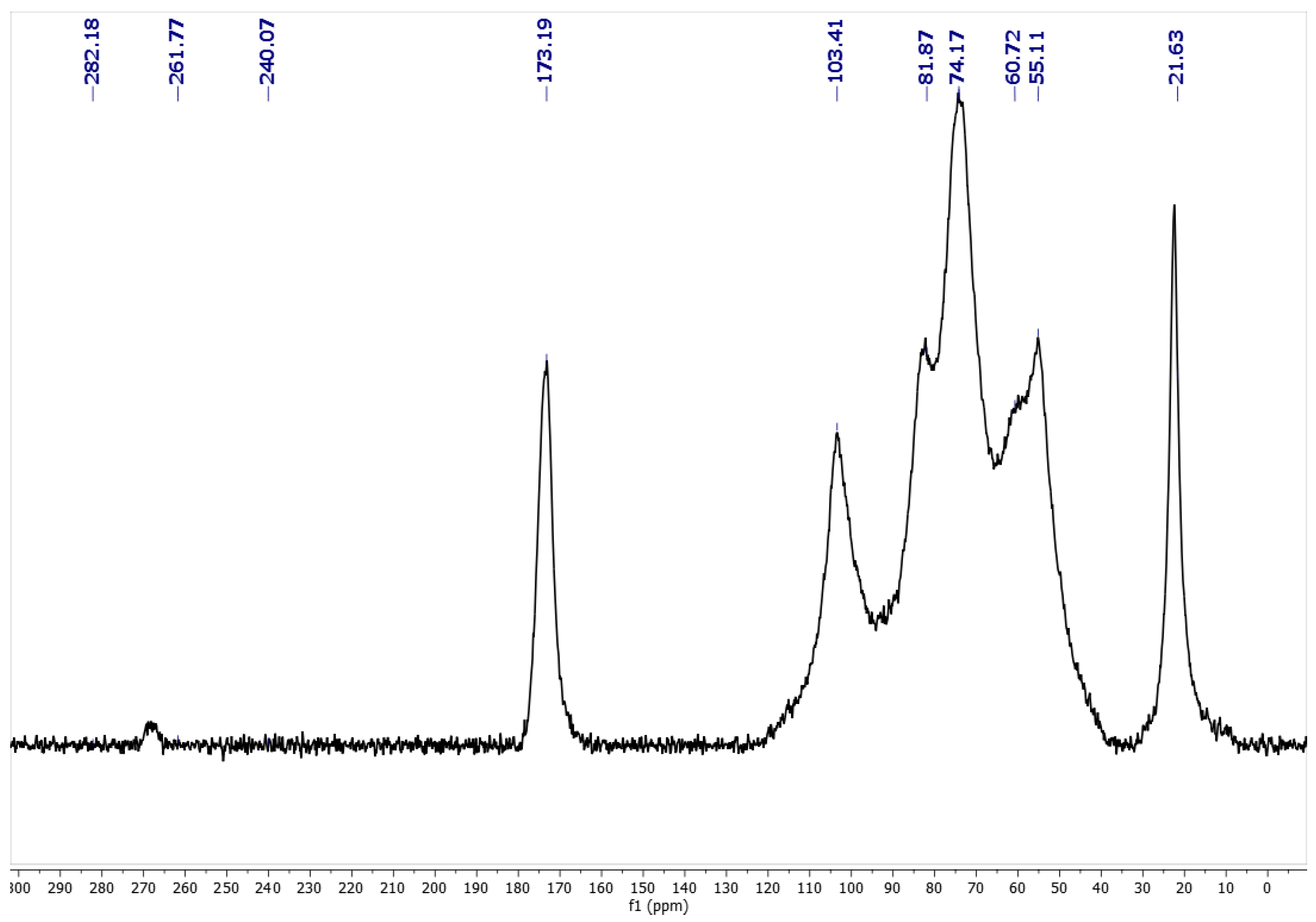
2.3. Elemental analysis (EA) and Degree of Acetylation (DA)
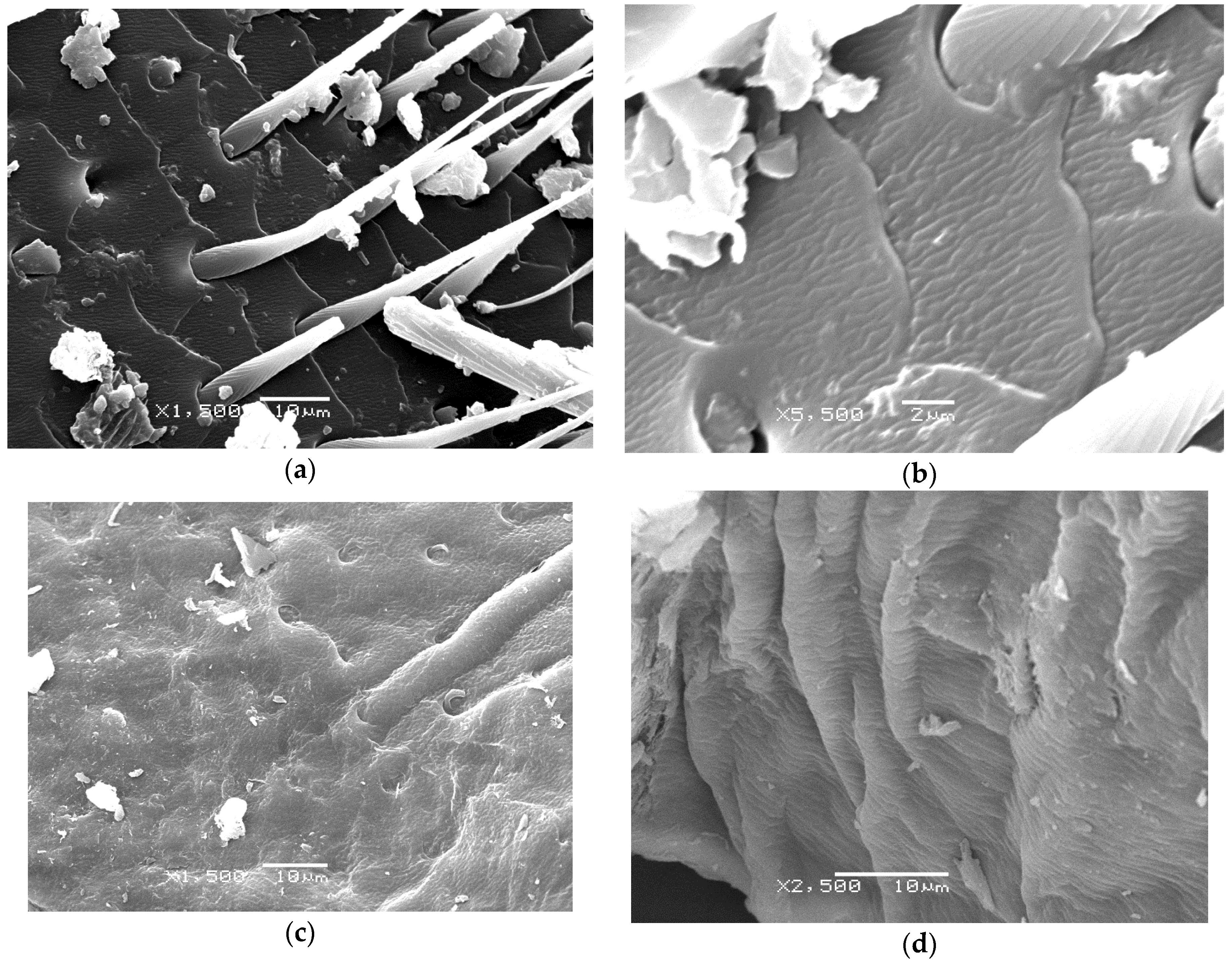
2.4. Scanning Electron Microscopy (SEM)
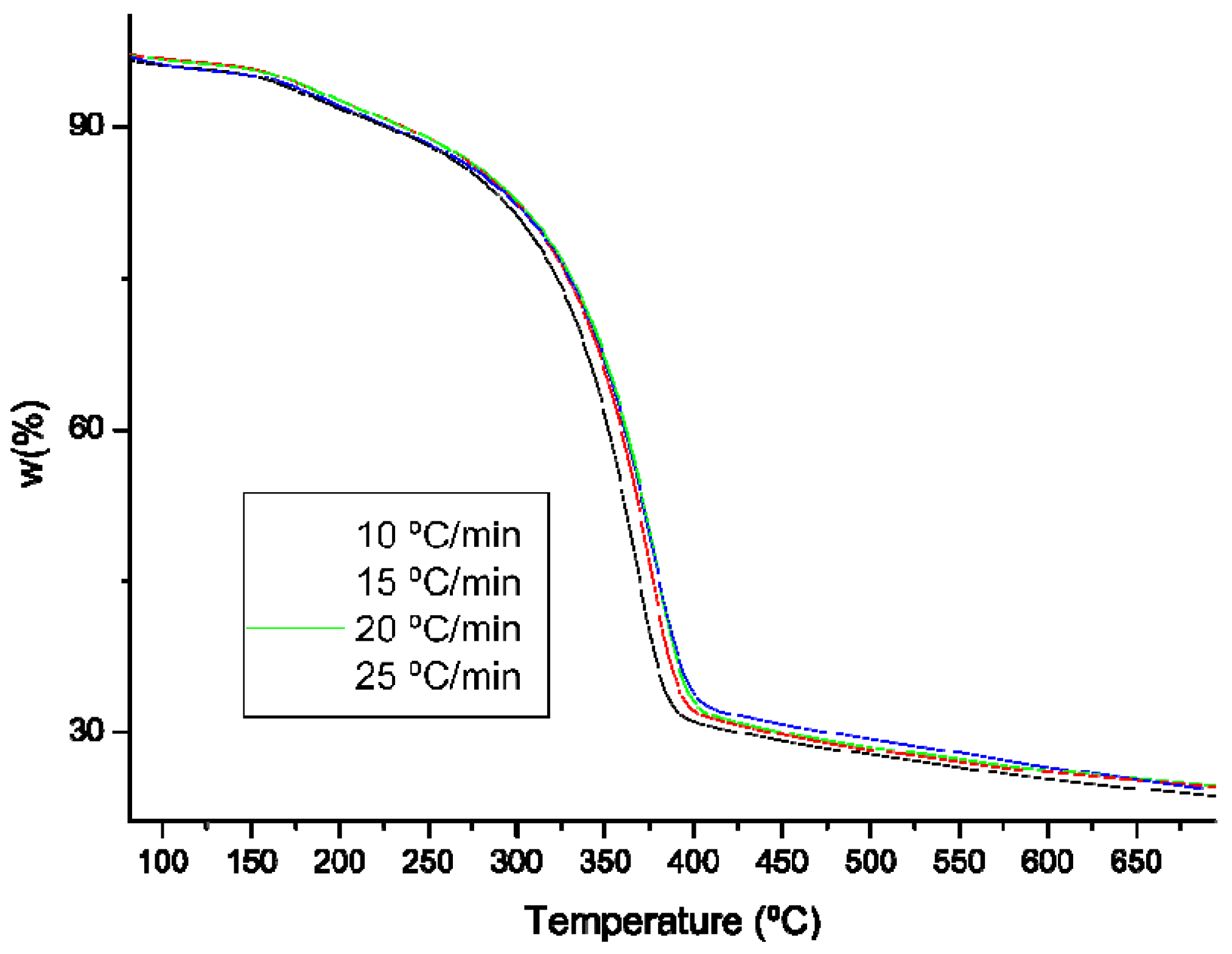
2.5. Thermogravimetric Analysis (TG)
2.6. Prediction of the Average Lifetime Considering the Reaction Mechanism
3. Materials and Methods
3.1. Sampling of Insects
3.2. Insects Pre-treatment
3.3. Extraction of Chitin
3.3.1. Demineralisation
3.3.2. Deproteinization
3.3.3. Decolourization
3.4. Chitin Obtained
3.5. Chitin Spectroscopic Characterisation
3.6. Solid-state NMR (ssNMR) Analysis
3.7. Chitin Thermogravimetric Analysis (TGA)
3.7.1. Kinetic Methods
3.7.2. Differential Method
3.7.3. Integral Methods
Flynn-Wall-Ozawa Method
Coats–Redfern Method
3.7.4. Prediction of the Average Lifetime Considering the Reaction Mechanism
3.8. Elemental Analysis
3.9. Degree of Acetylation (DA)
3.10. Scanning Eectron Microscopy (SEM)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rahman, M.; Halfar, J. First evidence of chitin in calcified coralline algae: New insights into the calcification process of Clathromorphum compactum. Sci. Rep. 2015, 4, 6162. [Google Scholar] [CrossRef]
- Rahman, M.A.; Halfar, J.; Adey, W.H.; Nash, M.; Paulo, C.; Dittrich, M. The role of chitin-rich skeletal organic matrix on the crystallization of calcium carbonate in the crustose coralline alga Leptophytum foecundum. Sci. Rep. 2019, 9, 11869. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from Basidiocarps of Cultivating Mushroom Pleurotus ostreatus: Isolation and Structural Characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chapter 25—Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources. Elsevier Sci. 2008, 10, 517–542. [Google Scholar]
- Duan, B.; Huang, Y.; Lu, A.; Zhang, L. Recent Advances in Chitin Based Materials Constructed via Physical Methods. Prog. Polym. Sci. 2018, 82, 1–33. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.; Chiellini, E.; Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef] [PubMed]
- Leke-Aladekoba, A. Comparison of Extraction Methods and Characterization of Chitin and Chitosan with Antimicrobial Properties from Black Soldier Fly (Hermatia Illucens) Meal. Ph.D. Thesis, Dalhousie University Halifax, Halifax, NS, Canada, November 2018. [Google Scholar]
- Tharanathan, R.N.; Kittur, F.S. Chitin—The undisputed biomolecule of great potential. Crit. Rev. Food Sci. Nutr. 2003, 43, 61–87. [Google Scholar] [CrossRef]
- Sultankulov, B.; Berillo, D.; Sultankulova, S.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Deka, S.R.; Sharma, A.K.; Kumar, P. Cationic polymers and their self-assembly for antibacterial applications. Curr. Top. Med. Chem. 2015, 15, 1179–1195. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Steven, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Laroche, C.; Delattre, C.; Mati-Baouche, N.; Salah, R.; Violeta Ursu, A.; Moulti-Mati, F.; Michaud, P.; Guillaume, P. Bioactivity of Chitosan and Its Derivatives. Curr. Org. Chem. 2018, 22, 641–647. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.; Donnarumma, G. Chitin Nanofibrils and Nanolignin as Functional Agents in Skin Regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef]
- Ahsan, S.M.; Thomas, M.; Reddy, K.K.; Sooraparaju, S.G.; Asthana, A.; Bhatnagar, I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018, 110, 97–109. [Google Scholar] [CrossRef]
- Aguilar, A.; Zein, N.; Harmouch, E.; Hafdi, B.; Bornert, F.; Offner, D.; Clauss, F.; Fioretti, F.; Huck, O.; Benkirane-Jessel, N.; et al. Application of Chitosan in Bone and Dental Engineering. Molecules 2019, 24, 3009. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Abirami, R.G.; Rajan, D.K. Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish Shellfish. Immunol. 2019, 86, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Acosta, N.; Civera, C.; Elorza, B.; Mingo, J.; Castro, C.; Gandía, M.L.L.; Heras Caballero, A. Cosmetics and Cosmeceutical Applications of Chitin, Chitosan and Their Derivatives. Polymers 2018, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, H.; Qiao, J.; Yang, Y.; Wang, Y.; Liu, W.; Han, B. Potential Analysis and Preparation of Chitosan Oligosaccharides as Oral Nutritional Supplements of Cancer Adjuvant Therapy. Int. J. Mol. Sci. 2019, 20, 920. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, G.; Guan, F.; Liu, W. Application of Chitin/Chitosan and Their Derivatives in the Papermaking Industry. Polymers 2018, 10, 389. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef]
- Kaya, M.; Bağrıaçık, N.; Seyyar, O.; Baran, T. Comparison of chitin structures derived from three common wasp species (Vespa crabro Linnaeus, 1758, Vespa orientalis Linnaeus, 1771 and Vespula germanica (Fabricius, 1793)). Arch. Insect Biochem. Physiol. 2015, 89, 204–217. [Google Scholar] [CrossRef]
- Sajomsang, W.; Gonil, P. Preparation and characterization of α-chitin from cicada sloughs. Mater. Sci. Eng. C 2010, 30, 357–363. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Bulut, E.; Akyuz, B.; Zelencova, L.; Sofi, K. Fluctuation in physicochemical properties of chitins extracted from different body parts of honeybee. Carbohydr. Polym. 2015, 132, 9–16. [Google Scholar] [CrossRef]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in physicochemical properties of chitin at developmental stages (larvae, pupa and adult) of Vespa crabro (wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef]
- Abo Elsoud, M.M.; El Kady, E.M. Current trends in fungal biosynthesis of chitin and chitosan. Bull. Natl. Res. Cent. 2019, 43, 59. [Google Scholar] [CrossRef]
- Vasylchenko, O.; Abramova, M. Comparative analysis of sources for chitosan obtaining. Probl. Environ. Biotechnol. 2015, 1, 1–25. [Google Scholar] [CrossRef]
- Feás Sánchez, X.; Charles, R.J. Notes on the Nest Architecture and Colony Composition in Winter of the Yellow-Legged Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae), in Its Introduced Habitat in Galicia (NW Spain). Insects 2019, 10, 23737. [Google Scholar] [CrossRef]
- Feás, X. Vespa velutina Spreading from 2 Nests to 10’642 in Just 4 Years in Northern Spain. 2017. Available online: https://www.vespavelutina.co.uk/vespavelutinanews/asian-hornet-spreading-from-2-nests-to-10642-in-just-4-years-in-northern-spain (accessed on 25 April 2019).
- Feás, X. The Asian Hornet (Vespa velutina nigrithorax): An exotic predator in Europe. What does the future hold? In Proceedings of the 42th British Beekeepers Association (BBKA) Spring Convention, Newport Shropshire, UK, 4 April 2019. [Google Scholar]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Jang, M.-K.; Kong, B.-G.; Jeong, Y.-I.; Lee, C.H.; Nah, J.-W. Physicochemical Characterization of α-Chitin, β-Chitin, and γ-Chitin Separated from Natural Resources. J. Polym. Sci. Pol. Chem. 2004, 42, 3423–3432. [Google Scholar] [CrossRef]
- Cardenas, G.; Cabrera, G.; Taboada, E.; Miranda, S. Chitin characterization by SEM, FTIR, XRD, and 13C cross polarization/mass angle spinning NMR. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Muzzarelli, R. Native, Industrial, and Fossil Chitins; Jolles, P., Muzzarelli, R.A.A., Eds.; Chitin and Chitinases: Birkhauser, Switzerland, 1999; pp. 1–6. [Google Scholar]
- Feás, X.; Seijas, J.A.; Vázquez-Tato, M.P.; Regal, R.; Cepeda, A.; Fente, C. Syntheses of molecularly imprinted polymers: Molecular recognition of cyproheptadine using original print molecules and azatadine as dummy templates. Anal. Chim. Acta 2009, 631, 237–244. [Google Scholar] [CrossRef]
- Feás, X.; Ye, L.; Hosseini, S.V.; Fente, C.A.; Cepeda, A. Molecularly imprinted polyallylamine hydrogels: Another reassessment. Polym. Int. 2010, 59, 11–15. [Google Scholar] [CrossRef]
- Feás, X.; Fente, C.A.; Hosseini, S.V.; Seijas, J.A.; Vázquez, B.I.; Franco, C.M.; Cepeda, A. Use of acrylic acid in the synthesis of molecularly imprinted polymers for the analysis of cyproheptadine. Mater. Sci. Eng. C 2009, 29, 398–404. [Google Scholar] [CrossRef]
- Chandran, R.; Williams, L.; Hung, A.; Nowlin, K.; Lajeunesse, D. SEM characterization of anatomical variation in chitin organization in insect and arthropod cuticles. Micron 2016, 82, 74–85. [Google Scholar] [CrossRef]
- Mathot, V.B.F. Calorimetry and Thermal Analysis of Polymers; Hanser Publisher: Munich, Germany, 1994. [Google Scholar]
- Flynn, J.H. Degradation kinetics applied to lifetime predictions of polymers. Polym. Eng. Sci. 1980, 20, 675–677. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Chi-Tang Ho, C.-T. Volatile Compounds Generated from Thermal Degradation of N-Acetylglucosamine. J. Agric. Food Chem. 1998, 46, 3207–3209. [Google Scholar] [CrossRef]
- Wunderlich, B. Thermal Analysis; Academic Press Inc.: Cambridge, MA, USA, 1990. [Google Scholar]
- Flynn, J.H. A critique of lifetime prediction of polymers by thermal analysis. Therm. Anal. 1995, 44, 499–512. [Google Scholar] [CrossRef]
- Liaw, D.J.; Shen, W.C. Curing of acrylated epoxy resin based on bisphenol-S. Polym. Eng. Sci. 1994, 34, 1297–1303. [Google Scholar] [CrossRef]
- Brown, M.E. Introduction to Thermal Analysis. In Technique and Applications; Chapman and Hall: London, UK, 1988. [Google Scholar]
- Dollimore, D.; Gamlen, G.A.; Taylor, T.J. The effect of a pre-exponential function A = AnTn on rising temperature kinetic parameter calculations. Thermochim. Acta 1982, 54, 181–186. [Google Scholar] [CrossRef]
- Nuñez, L.; Lópex, F.F.; Grueiro, L.F.; Rodríguez, J.A. Activation energies and rate constants for an epoxy/cure agent reaction. Variation in peak exotherm temperature. J. Therm. Anal. Calorim. 1996, 47, 743–750. [Google Scholar] [CrossRef]
- Criado, J.M.; Málek, J.; Ortega, A. Applicability of the master plots in kinetic analysis of non-isothermal data. Thermochim. Acta 1989, 147, 377–385. [Google Scholar] [CrossRef]
- Kissinger, H.E. Reaction Kinetics in Differential Thermal Analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Nat. Bur. Stand. A Phys. Chem. 1966, 70A, 487–523. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Brown, M.E.; Galwey, A.K. Reaction in the Solid State, Comprehensive Chemical Kinetics; Elsevier: Amsterdam, The Netherlands, 1980; Volume 22. [Google Scholar]
- Kassman, A.J. Evaluation and optimization of integral methods for the analysis of thermogravimetric data. Thermochim. Acta 1985, 84, 89–99. [Google Scholar] [CrossRef]
- Doyle, C.D. Series Approximations to the Equation of Thermogravimetric Data. Nature 1965, 207, 290–291. [Google Scholar] [CrossRef]
- Coats, A.W.; Redfern, J.P. Kinetic Parameters from Thermogravimetric Data. Nature 1964, 201, 68–69. [Google Scholar] [CrossRef]
- Kucukgulmez, A.; Celik, M.; Yanar, Y.; Sen, D.; Polat, H.; Kadak, A.E. Physicochemical characterization of chitosan extracted from Metapenaeus stebbingi shells. Food Chem. 2011, 126, 1144–1148. [Google Scholar] [CrossRef]
Sample Availability: Samples of the insects are available from the authors. |

| % C | % H | % N | % CHN | C/N | DA% | |
|---|---|---|---|---|---|---|
| Vespa velutina chitin | 43.47 | 6.94 | 6.85 | 57.26 | 6.35 | 95.44 |
| Theoretical value of chitin | 47.29 | 6.40 | 6.90 | 60.59 | 6.86 | 100 |
| Found-Theoretical | −3.82 | 0.54 | −0.05 | −3.33 | −0.51 | −4.56 |
| B (°C/min) | T (°C) |
|---|---|
| 5 | 363 |
| 10 | 367.8 |
| 15 | 369.3 |
| 20 | 376.3 |
| α | Ea(kJ/mol) |
|---|---|
| 5 | 117.8 |
| 10 | 118.9 |
| 15 | 114.1 |
| 20 | 105.5 |
| Solid State Process | Mechanism | 5 °C/min | 10 °C/min | 15 °C/min |
|---|---|---|---|---|
| Nucleation and growth (Avrami Equation (1)) | A2 | 35.2 | 53.6 | 44.7 |
| Nucleation and growth (Avrami Equation (2)) | A3 | 16.9 | 33.8 | 11.3 |
| Nucleation and growth (Avrami Equation (3)) | A4 | 6.5 | 17.4 | 5.9 |
| Phase boundary controlled reaction (one-dimensional movement) | R1/F0 | 67.1 | 53.7 | 73.6 |
| Phase boundary controlled reaction (contracting area) | R2 | 77.2 | 62.8 | 82.7 |
| Phase boundary controlled reaction (contracting volume) | R3 | 80.8 | 66.07 | 85.9 |
| One-dimensional diffusion | D1 | 187.7 | 161.8 | 181.5 |
| Two-dimensional diffusion | D2 | 200.9 | 173.7 | 193.4 |
| Three-dimensional diffusion (Jander eq.) | D3 | 215 | 186.6 | 202.4 |
| Three-dimensional diffusion (Ginstling-Brounshtein eq.) | D4 | 205.6 | 177.9 | 197.6 |
| Random nucleation with one nucleus on the individual particle | F1 | 88 | 72.5 | 92.36 |
| Random nucleation with two nuclei on the individual particle | F2 | 111.3 | 93.5 | 113.3 |
| Random nucleation with three nuclei on the individual particle | F3 | 137.1 | 116.7 | 102.6 |
| T (°C) | Time (years) |
|---|---|
| 20 | 285 |
| 30 | 61 |
| 40 | 14 |
| 50 | 4 |
| 60 | 1 |
| 70 | 0.3 (110 days) |
| 80 | 0.1 (37 days) |
| 90 | 0.03 (12 days) |
| 100 | 0.01 (5 days) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feás, X.; Vázquez-Tato, M.P.; Seijas, J.A.; Pratima G. Nikalje, A.; Fraga-López, F. Extraction and Physicochemical Characterization of Chitin Derived from the Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae). Molecules 2020, 25, 384. https://doi.org/10.3390/molecules25020384
Feás X, Vázquez-Tato MP, Seijas JA, Pratima G. Nikalje A, Fraga-López F. Extraction and Physicochemical Characterization of Chitin Derived from the Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae). Molecules. 2020; 25(2):384. https://doi.org/10.3390/molecules25020384
Chicago/Turabian StyleFeás, Xesús, M. Pilar Vázquez-Tato, Julio A. Seijas, Anna Pratima G. Nikalje, and Francisco Fraga-López. 2020. "Extraction and Physicochemical Characterization of Chitin Derived from the Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae)" Molecules 25, no. 2: 384. https://doi.org/10.3390/molecules25020384
APA StyleFeás, X., Vázquez-Tato, M. P., Seijas, J. A., Pratima G. Nikalje, A., & Fraga-López, F. (2020). Extraction and Physicochemical Characterization of Chitin Derived from the Asian Hornet, Vespa velutina Lepeletier 1836 (Hym.: Vespidae). Molecules, 25(2), 384. https://doi.org/10.3390/molecules25020384










