Comparison of Volatiles in Different Jasmine Tea Grade Samples Using Electronic Nose and Automatic Thermal Desorption-Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Information
2.2. Chemicals
2.3. Automatic Thermal Desorption-Gas-Chromatography-Mass Spectrometry (ATD-GC-MS) Analysis
2.3.1. Extraction of Volatile Organic Compounds (VOC)
2.3.2. Thermal Desorption
2.3.3. Gas-Chromatography-Mass Spectrometry Analysis
2.3.4. Identification of Volatile Organic Compounds (VOC)
2.4. Electronic nose (E-Nose) Measurements
2.5. Statistical Analysis
3. Results and Discussion
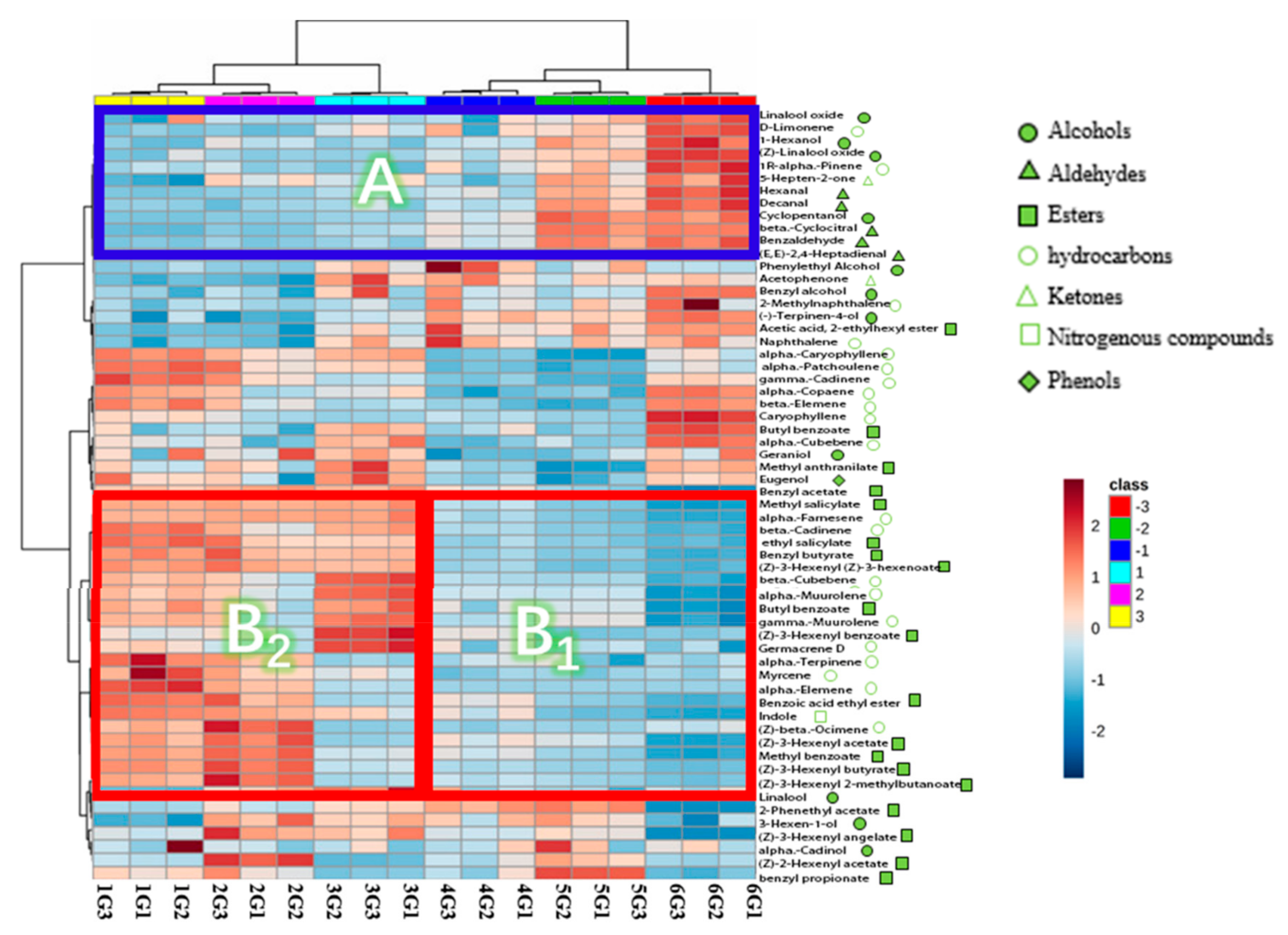
3.1. Identification of Volatile Organic Compounds in the Jasmine Tea by Automatic Thermal Desorption-Gas-Chromatography-Mass Spectrometry Spectrometry
3.1.1. Alcohols
3.1.2. Aldehydes
3.1.3. Esters
3.1.4. Hydrocarbons
3.1.5. Ketones
3.1.6. Nitrogen Compound
3.1.7. Phenols
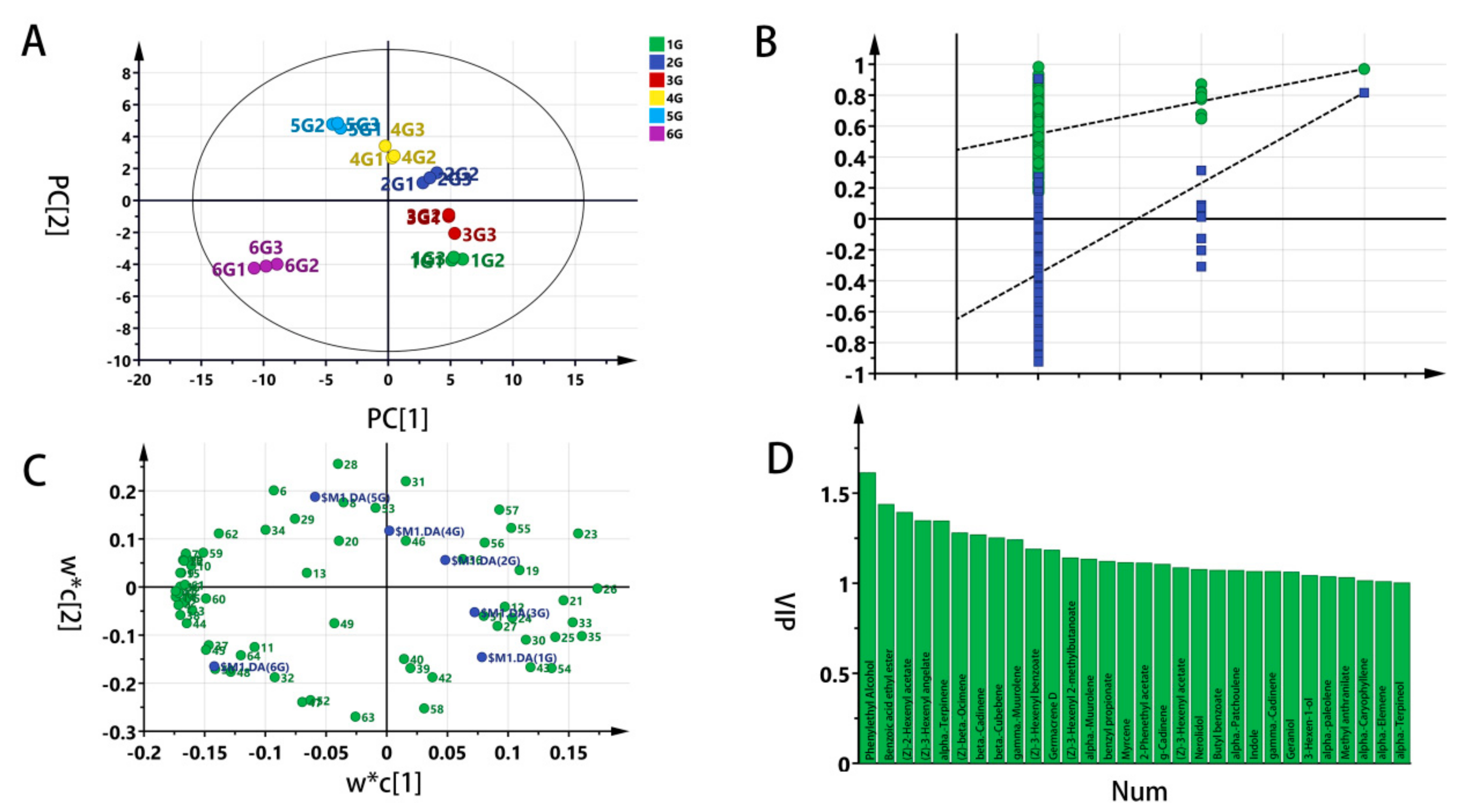
3.2. Multivariate Statistical Analysis of Identified Volatile Organic Compounds(VOC)
3.2.1. Hierarchical Clustering Analysis (HCA)
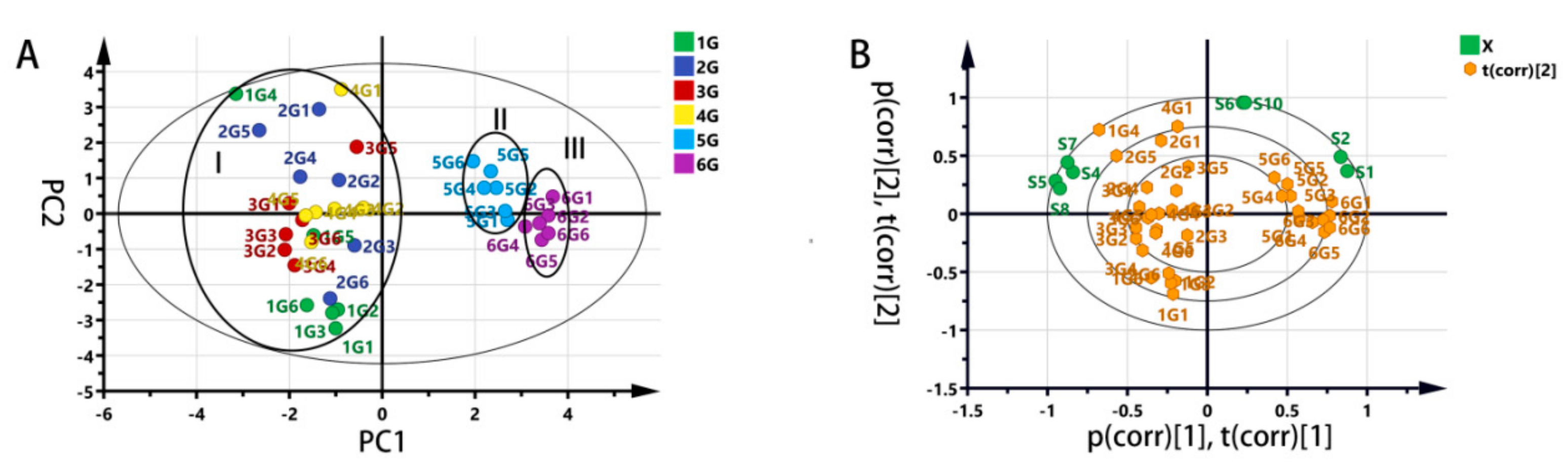
3.2.2. Partial Least Square- Discriminant Analysis (PLS-DA)
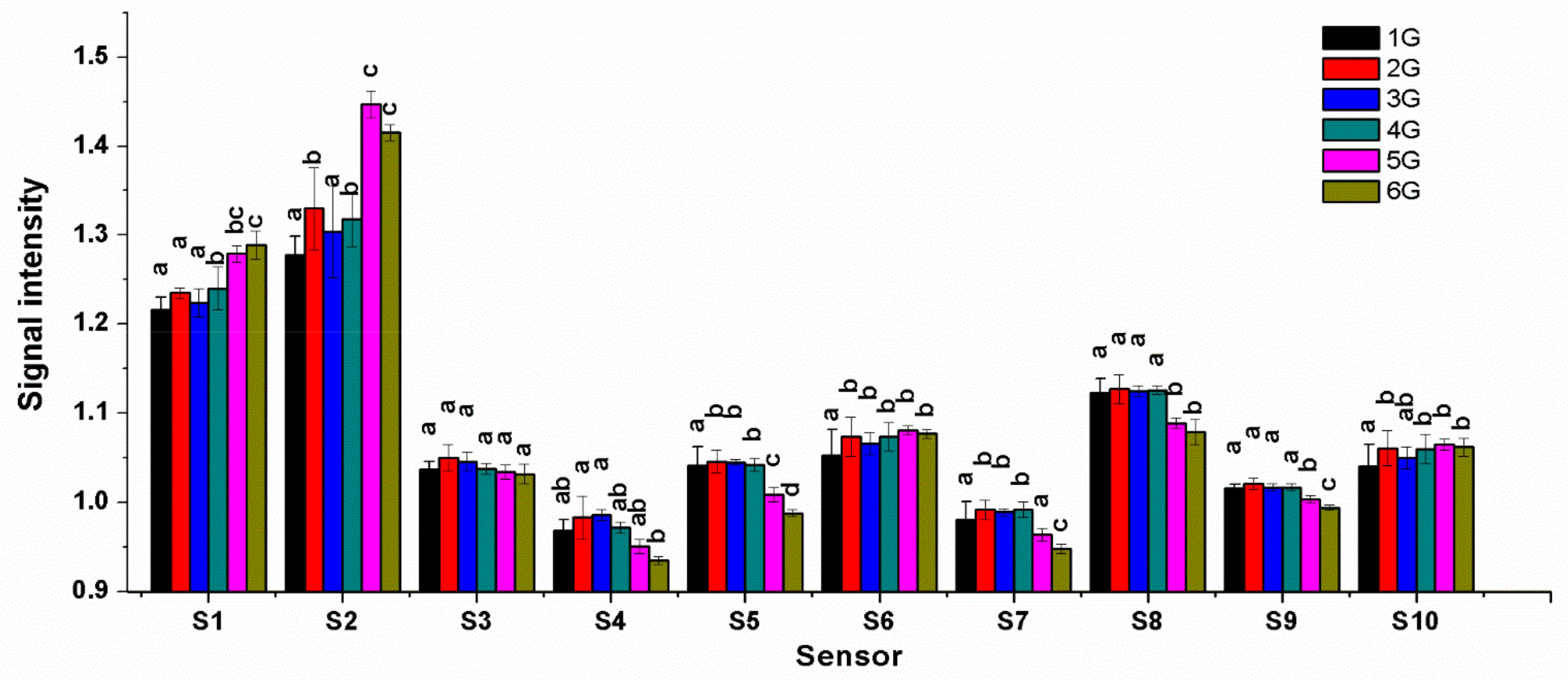
3.3. Response of Electronic Nose (E-Nose) Sensors to Volatile Organic Compounds (VOC) on Different Grades of Jasmine Tea
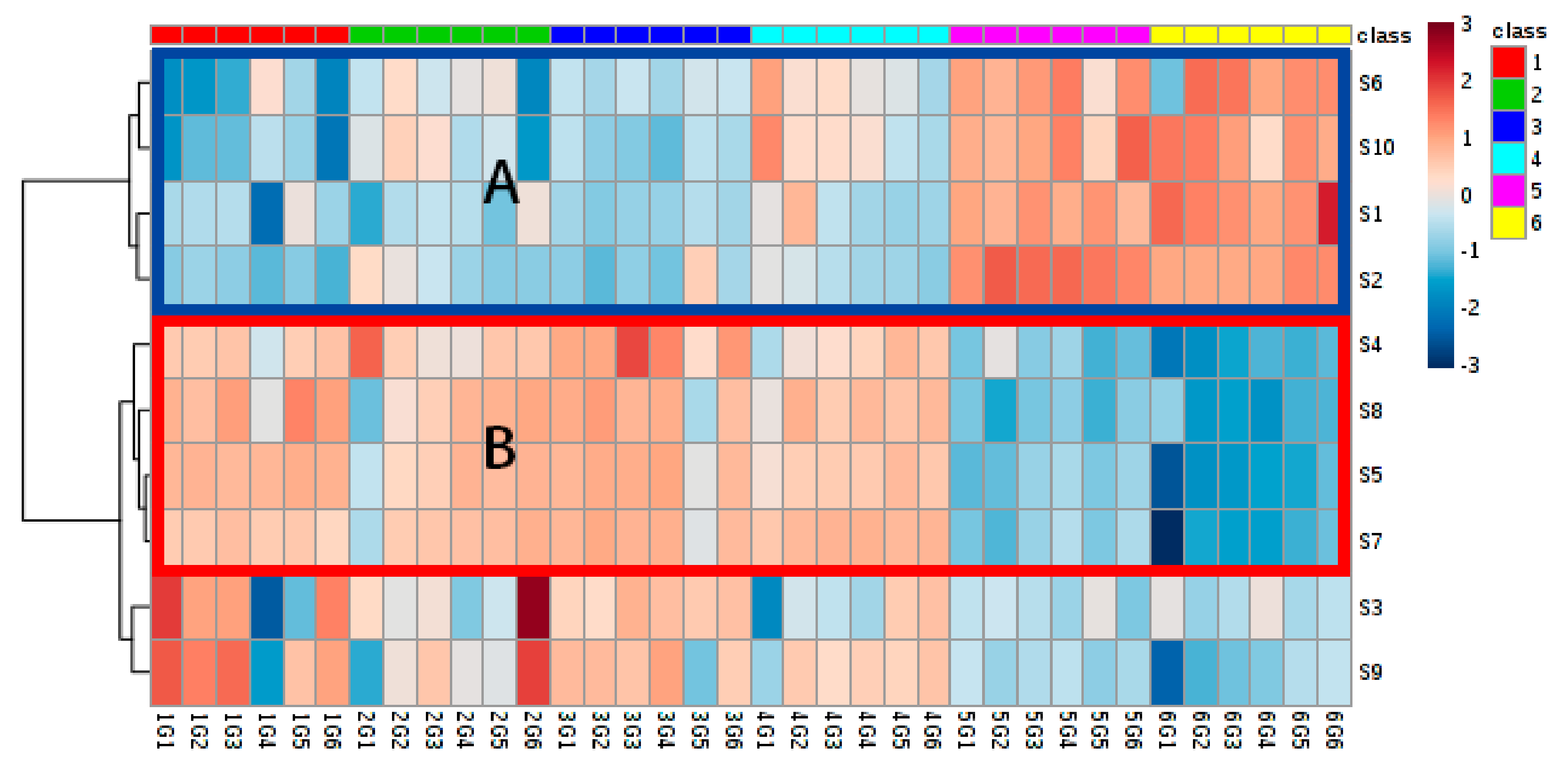
3.4. Multivariate Statistical Analysis of Electronic Nose (E-Nose) Sensor Response Signals
3.4.1. Hierarchical Clustering Analysis
3.4.2. Principal Component Analysis (PCA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and anti-aging potentials of essential oils from aromatic and medicinal plants. Front. Aging Neurosci. 2017, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent Advances in the Understanding of the Health Benefits and Molecular Mechanisms Associated with Green Tea Polyphenols. J. Agric. Food Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, Y.; Liu, B.; Chen, Z.; Zheng, J.; Guan, M.; Shi, H.; Wang, Y.; Yang, W. Changes in the volatiles, chemical components, and antioxidant activities of Chinese jasmine tea during the scenting processes. Int. J. Food Prop. 2017, 20, 681–693. [Google Scholar] [CrossRef]
- Li, H.; Luo, L.; Ma, M.; Zeng, L. Characterization of Volatile Compounds and Sensory Analysis of Jasmine Scented Black Tea Produced by Different Scenting Processes. J. Food Sci. 2018, 83, 2718–2732. [Google Scholar] [CrossRef] [PubMed]
- CSBTS (State Bureau of Technical Supervision). GB/T 34779-2017. Technique specification for processing of jasmine tea. 2017. [Google Scholar]
- CSBTS (State Bureau of Technical Supervision). GB/T 22292-2017. Jasmine tea. 2017. [Google Scholar]
- Yingbin, Z.; Xu, L.I.U.; Chengyin, L.U. Study on Primitive Morpheme in Sensory Terminology and Flavor Wheel Construction of Chinese Tea. J. Tea Sci. 2019, 39, 474–483. [Google Scholar]
- Shen, J.X.; Rana, M.M.; Liu, G.F.; Ling, T.J.; Gruber, M.Y.; Wei, S. Differential contribution of jasmine floral volatiles to the aroma of scented green tea. J. Food Qual. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Lin, J.; Chen, Y.; Zhang, P.; Ren, M.; Xu, H.; Wang, X. A novel quality evaluation index and strategies to identify scenting quality of jasmine tea based on headspace volatiles analysis. Food Sci. Biotechnol. 2013, 22, 331–340. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, Y.; Lu, J.; Zhang, L. Application of chemical composition and infusion colour difference analysis to quality estimation of jasmine-scented tea. Int. J. Food Sci. Technol. 2007, 42, 459–468. [Google Scholar] [CrossRef]
- Shi, H.; Zhang, M.; Adhikari, B. Advances of electronic nose and its application in fresh foods: A review. Crit. Rev. Food Sci. Nutr. 2018, 58, 2700–2710. [Google Scholar] [CrossRef]
- Wilson, A.D. Application of electronic-nose technologies and VOC-biomarkers for the noninvasive early diagnosis of gastrointestinal diseases. Sensors 2018, 18, 2613. [Google Scholar] [CrossRef]
- Capelli, L.; Sironi, S.; Del Rosso, R. Electronic noses for environmental monitoring applications. Sensors 2014, 14, 19979–20007. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Y.; Wang, J. Identification of tea storage times by linear discrimination analysis and back-propagation neural network techniques based on the eigenvalues of principal components analysis of E-nose sensor signals. Sensors 2009, 9, 8073–8082. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, K.; Tomizawa, Y.; Shimizu, N.; Ikeda, K.; Manome, Y. Improving the performance of an electronic nose by wine aroma training to distinguish between drip coffee and canned coffee. Sensors 2015, 15, 1354–1364. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lv, H.-P.; Zhu, Y.; Peng, Q.-H.; Zhang, Y.; Shao, C.-Y.; Yu, F.-L.; Lin, Z.; Tan, J.-F.; Dai, W.-D. Separation of aroma components in Xihu Longjing tea using simultaneous distillation extraction with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. Sep. Purif. Technol. 2016, 164, 146–154. [Google Scholar]
- Yang, T.; Zhu, Y.; Shao, C.Y.; Zhang, Y.; Shi, J.; Lv, H.P.; Lin, Z. Enantiomeric analysis of linalool in teas using headspace solid-phase microextraction with chiral gas chromatography. Ind. Crops Prod. 2016, 83, 17–23. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, H.; Chen, D.; Zhao, H.; Yan, F.; Li, X.; Chen, C.; Xu, Z.; Zhang, C.; Chen, X. Aroma characterization of Hanzhong black tea (Camellia sinensis) using solid phase extraction coupled with gas chromatography–mass spectrometry and olfactometry and sensory analysis. Food Chem. 2018, 274, 130–136. [Google Scholar]
- Kellogg, J.J.; Wallace, E.D.; Graf, T.N.H.; Oberlies, N.; B.Cech, N. Conventional and Accelerated-Solvent Extractions of Green Tea (Camellia sinensis) for Metabolomics-based Chemometrics. J. Pharm Biomed. Anal. 2017, 145, 604–610. [Google Scholar] [CrossRef]
- Gallego, E.; Folch, J.; Teixidor, P.; Roca, F.J.; Perales, J.F. Outdoor air monitoring: Performance evaluation of a gas sensor to assess episodic nuisance/odorous events using active multi-sorbent bed tube sampling coupled to TD-GC/MS analysis. Sci. Total Environ. 2019, 694, 133752. [Google Scholar] [CrossRef]
- Clément, M.; Arzel, S.; Le Bot, B.; Seux, R.; Millet, M. Adsorption/thermal desorption-GC/MS for the analysis of pesticides in the atmosphere. Chemosphere 2000, 40, 49–56. [Google Scholar] [CrossRef]
- Gallego, E.; Roca, F.J.; Perales, J.F.; Guardino, X.; Berenguer, M.J. VOCs and PAHs emissions from creosote-treated wood in a field storage area. Sci. Total Environ. 2008, 402, 130–138. [Google Scholar] [CrossRef]
- Ochiai, N.; Sasamoto, K.; Tsunokawa, J.; Hoffmann, A.; Okanoya, K.; MacNamara, K. Extension of a dynamic headspace multi-volatile method to milliliter injection volumes with full sample evaporation: Application to green tea. J. Chromatogr. A. 2015, 1421, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, P.; Chen, X.; Sun, Y.; Yue, C. Transcriptome and Metabolite Profiling Reveal Novel Insights into Volatile Heterosis in the Tea Plant (Camellia Sinensis). Molecules 2019, 24, 3380. [Google Scholar] [CrossRef] [PubMed]
- MEP (Ministry of Environmental Protection). HJ 644-2013. Ambient air-Determination of volatile organic compounds. 2013. [Google Scholar]
- Wang, C.; Zhang, C.; Kong, Y.; Peng, X.; Li, C.; Liu, S.; Du, L.; Xiao, D.; Xu, Y. A comparative study of volatile components in Dianhong teas from fresh leaves of four tea cultivars by using chromatography-mass spectrometry, multivariate data analysis, and descriptive sensory analysis. Food Res. Int. 2017, 100, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hou, Z.; Wang, D.; Ning, J.; Wei, S. Large scale preparation, stress analysis, and storage of headspace volatile condensates from Jasminum sambac fl owers. Food Chem. 2019, 286, 170–178. [Google Scholar] [CrossRef]
- Edris, A.E.; Chizzola, R.; Franz, C. Isolation and characterization of the volatile aroma compounds from the concrete headspace and the absolute of Jasminum sambac (L.) Ait. (Oleaceae) flowers grown in Egypt. Eur. Food Res. Technol. 2008, 226, 621–626. [Google Scholar] [CrossRef]
- Jiang, X.; Guo, Y.; Lai, L.; Liao, Z.; Qu, Y.; Diao, X. Application of electronic nose technology in aroma discrimination of special-typed jasmine tea. J. Food Saf. Qual. 2017, 8, 1–6. [Google Scholar]
- Yu, Y.; Lyu, S.; Chen, D.; Lin, Y.; Chen, J.; Chen, G.; Ye, N. Volatiles emitted at different flowering stages of Jasminum sambac & expression of genes related to α-farnesene biosynthesis. Molecules 2017, 22, 546. [Google Scholar]
- Zhu, Y.; Lv, H.P.; Shao, C.Y.; Kang, S.; Zhang, Y.; Guo, L.; Dai, W.D.; Tan, J.F.; Peng, Q.H.; Lin, Z. Identification of key odorants responsible for chestnut-like aroma quality of green teas. Food Res. Int. 2018, 108, 74–82. [Google Scholar] [CrossRef]
- Alasalvar, C.; Topal, B.; Serpen, A.; Bahar, B.; Pelvan, E.; Gökmen, V. Flavor characteristics of seven grades of black tea produced in Turkey. J. Agric. Food Chem. 2012, 60, 6323–6332. [Google Scholar] [CrossRef]
- Qi, D.; Miao, A.; Cao, J.; Wang, W.; Chen, W.; Pang, S.; He, X.; Ma, C. Study on the effects of rapid aging technology on the aroma quality of white tea using GC–MS combined with chemometrics: In comparison with natural aged and fresh white tea. Food Chem. 2018, 265, 189–199. [Google Scholar] [CrossRef]
- Baba, R.; Amano, Y.; Wada, Y.; Kumazawa, K. Characterization of the Potent Odorants Contributing to the Characteristic Aroma of Matcha by Gas Chromatography-Olfactometry Techniques. J. Agric. Food Chem. 2017, 65, 2984–2989. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Song, S.; Cao, X. Discriminant analysis and similarity evaluation of gas chromatography-mass spectrometry fingerprints of aroma components in green tea grading. Chinese J. Chromatogr. 2019, 37, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.; Gulati, A. Fractionation and identification of minor and aroma-active constituents in Kangra orthodox black tea. Food Chem. 2015, 167, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Li, J.; Li, W.; Li, Y.; Li, T.; Xiao, D. Characterization of volatile compounds of pu-erh tea using solid-phase microextraction and simultaneous distillation-extraction coupled with gas chromatography-mass spectrometry. Food Res. Int. 2014, 57, 61–70. [Google Scholar] [CrossRef]
- Hu, C.J.; Li, D.; Ma, Y.X.; Zhang, W.; Lin, C.; Zheng, X.Q.; Liang, Y.R.; Lu, J.L. Formation mechanism of the oolong tea characteristic aroma during bruising and withering treatment. Food Chem. 2018, 269, 202–211. [Google Scholar] [CrossRef]
- Wang, W.; Jin, S.; Guo, Y. Exploration of a Method of Distinguishing Different Nongxiang Tieguanyin Tea Grades Based on Aroma Determined by GC-MS Combined with Chemometrics. Molecules 2019, 24, 1707. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Yin, H.; Deng, Y.; Jiang, Y.; Yuan, H.; Dong, C.; Li, J.; Hua, J.; Wang, J. Rapid profiling of volatile compounds in green teas using Micro-Chamber/Thermal Extractor combined with thermal desorption coupled to gas chromatography-mass spectrometry followed by multivariate statistical analysis. Lwt 2018, 96, 42–50. [Google Scholar] [CrossRef]
- Lv, S.; Wu, Y.; Wei, J.; Lian, M.; Wang, C.; Gao, X.; Meng, Q. Application of gas chromatography-mass spectrometry and chemometrics methods for assessing volatile profiles of Pu-erh tea with different processing methods and ageing years. RSC Adv. 2015, 5, 87806–87817. [Google Scholar] [CrossRef]
- Jumtee, K.; Komura, H.; Bamba, T.; Fukusaki, E. Predication of Japanese green tea (Sen-cha) ranking by volatile profiling using gas chromatography mass spectrometry and multivariate analysis. J. Biosci. Bioeng. 2011, 112, 252–255. [Google Scholar] [CrossRef]
Sample Availability: Samples of all compounds are not available from the authors. Because the experiment was done in February 2019, the samples have been deteriorated. |
| No. | Compound | CAS g | RT g | RI g | MS Fragments | MS g | ||
|---|---|---|---|---|---|---|---|---|
| 1 | Cyclopentanol | 96-41-3 | 5.004 | 788 | 57 | 44 | 41 | 83 |
| 2 | 1-Hexanol | 111-27-3 | 7.893 | 860 | 56 | 43 | 41 | 85 |
| 3 | Benzyl alcohol | 100-51-6 | 13.430 | 1036 | 79 | 108 | 107 | 95 |
| 4 | (Z)-Linalool oxide | 5989-33-3 | 14.665 | 1072 | 59 | 94 | 43 | 93 |
| 5 | (E)-Linalool oxide | 34,995-77-2 | 15.205 | 1088 | 59 | 94 | 43 | 97 |
| 6 | 3-Hexen-1-ol | 928-96-1 | 7.432 | 868 | 67 | 41 | 39 | 95 |
| 7 | Linalool | 78-70-6 | 15.775 | 1104 | 71 | 93 | 55 | 93 |
| 8 | Phenylethyl Alcohol | 60-12-8 | 16.050 | 1117 | 91 | 92 | 65 | 95 |
| 9 | (-)-Terpinen-4-ol | 20,126-76-5 | 18.265 | 1137 | 71 | 111 | 43 | 87 |
| 10 | α-Terpineol | 98-55-5 | 18.691 | 1143 | 59 | 93 | 121 | 88 |
| 11 | Geraniol | 106-24-1 | 20.455 | 1228 | 69 | 41 | 48 | 90 |
| 12 | Nerolidol | 7212-44-4 | 27.715 | 1564 | 41 | 69 | 43 | 84 |
| 13 | α-Cadinol | 481-34-5 | 28.526 | 1580 | 95 | 121 | 43 | 90 |
| 14 | Hexanal | 66-25-1 | 5.780 | 806 | 44 | 56 | 41 | 96 |
| 15 | Benzaldehyde | 100-52-7 | 10.915 | 982 | 77 | 106 | 105 | 96 |
| 16 | (E,E)-2,4-Heptadienal | 4313-03-5 | 12.621 | 1011 | 81 | 110 | 41 | 90 |
| 17 | Decanal | 112-31-2 | 18.980 | 1204 | 43 | 41 | 57 | 96 |
| 18 | β-Cyclocitral | 432-25-7 | 19.437 | 1218 | 137 | 152 | 109 | 93 |
| 19 | (Z)-3-Hexenyl acetate | 3681-7-18 | 12.425 | 1002 | 43 | 67 | 82 | 95 |
| 20 | (Z)-2-Hexenyl acetate | 56,922-75-9 | 12.757 | 1005 | 43 | 67 | 82 | 87 |
| 21 | Methyl benzoate | 93-58-3 | 15.405 | 1060 | 105 | 77 | 136 | 98 |
| 22 | Acetic acid, 2-ethylhexyl ester | 103-09-3 | 17.130 | 1149 | 43 | 70 | 57 | 84 |
| 23 | Benzyl acetate | 140-11-4 | 17.750 | 1162 | 108 | 91 | 90 | 93 |
| 24 | Benzoic acid ethyl ester | 93-89-0 | 17.915 | 1171 | 105 | 77 | 122 | 88 |
| 25 | (Z)-3-Hexenyl butyrate | 16,491-36-4 | 18.342 | 1182 | 82 | 67 | 71 | 80 |
| 26 | Methyl salicylate | 119-36-8 | 18.635 | 1191 | 120 | 92 | 152 | 96 |
| 27 | (Z)-3-Hexenyl 2-methylbutanoate | 53,398-85-9 | 19.754 | 1226 | 67 | 82 | 57 | 80 |
| 28 | 2-Phenethyl acetate | 103-45-7 | 20.570 | 1249 | 104 | 43 | 91 | 93 |
| 29 | benzyl propionate | 122-63-4 | 20.675 | 1259 | 91 | 108 | 57 | 75 |
| 30 | ethyl salicylate | 118-61-6 | 21.161 | 1270 | 120 | 92 | 166 | 91 |
| 31 | (Z)-3-Hexenyl angelate | 84,060-80-0 | 23.486 | 1282 | 82 | 55 | 67 | 91 |
| 32 | Methyl anthranilate | 134-20-3 | 24.346 | 1343 | 119 | 92 | 151 | 96 |
| 33 | Benzyl butyrate | 103-37-7 | 24.497 | 1346 | 108 | 91 | 178 | 83 |
| 34 | Butyl benzoate | 136-60-7 | 25.261 | 1359 | 105 | 123 | 77 | 82 |
| 35 | (Z)-3-Hexenyl (Z)-3- hexenoate | 61,444-38-0 | 25.455 | 1388 | 82 | 67 | 69 | 92 |
| 36 | (Z)-3-Hexenyl benzoate | 25,152-85-6 | 27.825 | 1565 | 105 | 67 | 77 | 97 |
| 37 | Benzyl Benzoate | 120-51-4 | 29.302 | 1733 | 105 | 91 | 77 | 76 |
| 38 | α-Pinene | 7785-70-8 | 10.005 | 948 | 93 | 92 | 91 | 85 |
| 39 | Myrcene | 123-35-3 | 11.921 | 958 | 41 | 93 | 69 | 86 |
| 40 | α-Terpinene | 99-86-5 | 12.846 | 1016 | 121 | 93 | 136 | 90 |
| 41 | Limonene | 5989-27-5 | 13.261 | 1018 | 68 | 93 | 67 | 88 |
| 42 | (Z)-β-Ocimene | 13,877-91-3 | 13.845 | 1031 | 93 | 91 | 79 | 93 |
| 43 | α-Elemene | 20,307-84-0 | 24.097 | 1340 | 121 | 93 | 136 | 85 |
| 44 | α-Cubebene | 17,699-14-8 | 24.645 | 1351 | 161 | 105 | 119 | 93 |
| 45 | α-Copaene | 3856-25-5 | 25.365 | 1373 | 161 | 119 | 105 | 90 |
| 46 | Germacrene D | 23,986-74-5 | 25.530 | 1477 | 161 | 105 | 91 | 89 |
| 47 | γ-Cadinene | 39,029-41-9 | 25.600 | 1514 | 161 | 204 | 105 | 90 |
| 48 | β-Elemene | 515-13-9 | 25.626 | 1398 | 81 | 93 | 68 | 80 |
| 49 | α-Gurjunene | 489-40-7 | 25.960 | 1413 | 204 | 161 | 105 | 87 |
| 50 | Caryophyllene | 87-44-5 | 26.155 | 1494 | 93 | 133 | 91 | 82 |
| 51 | β-Cubebene | 13,744-15-5 | 26.306 | 1387 | 161 | 105 | 91 | 92 |
| 52 | α-Caryophyllene | 6753-98-6 | 26.660 | 1579 | 93 | 80 | 41 | 88 |
| 53 | γ-Muurolene | 3002-74-0 | 26.885 | 1435 | 161 | 105 | 119 | 88 |
| 54 | α-Farnesene | 502-6-4 | 27.205 | 1458 | 41 | 93 | 69 | 93 |
| 55 | β-Cadinene | 483-76-1 | 27.365 | 1469 | 161 | 134 | 119 | 90 |
| 56 | α-Muurolene | 10,208-80-7 | 27.545 | 1479 | 105 | 161 | 94 | 89 |
| 57 | α-Patchoulene | 560-32-7 | 27.976 | 1460 | 135 | 93 | 107 | 78 |
| 58 | Naphthalene | 91-20-3 | 18.381 | 1231 | 128 | 129 | 127 | 97 |
| 59 | 2-Methylnaphthalene | 91-57-6 | 22.350 | 1345 | 142 | 141 | 115 | 86 |
| 60 | 5-Hepten-2-one | 110-93-0 | 11.735 | 938 | 43 | 41 | 69 | 93 |
| 61 | Acetophenone | 98-86-2 | 14.436 | 1068 | 105 | 77 | 51 | 97 |
| 62 | Indole | 120-72-9 | 22.107 | 1340 | 117 | 90 | 89 | 97 |
| 63 | Eugenol | 97-53-0 | 24.712 | 1392 | 164 | 103 | 77 | 88 |
| No. | MIf | Compound | Average Relative Content (×10 μg/g) | |||||
|---|---|---|---|---|---|---|---|---|
| 1Ge | 2Ge | 3Ge | 4Ge | 5Ge | 6Ge | |||
| 1 | MS,RI | Cyclopentanol | 0.04 ± 0.00b | 0.25 ± 0.02b | 0.13 ± 0.03b | 0.59 ± 0.15ab | 0.89 ± 0.25a | 1.02 ± 0.22a |
| 2 | MS,RI | 1-Hexanol | 0.04 ± 0.00b | 0.06 ± 0.01b | 0.11 ± 0.02b | 0.10 ± 0.03b | 0.09 ± 0.02b | 0.23 ± 0.08a |
| 3 | MS,RI | Benzyl alcohol | 5.38 ± 0.73b | 37.76 ± 19.39a | 6.69 ± 1.09b | 12.27 ± 4.05b | 4.71 ± 1.73b | 9.14 ± 1.41b |
| 4 | MS,RI | (Z)-Linalool oxide | 0.22 ± 0.04a | 0.32 ± 0.01a | 0.27 ± 0.05a | 0.36 ± 0.09a | 0.30 ± 0.11a | 0.50 ± 0.09a |
| 5 | MS,S,RI | (E)-Linalool oxide | 0.71 ± 0.12a | 1.09 ± 0.05a | 0.89 ± 0.16a | 0.84 ± 0.20a | 0.61 ± 0.23a | 0.73 ± 0.12a |
| 6 | MS,RI | 3-Hexen-1-ol | 2.82 ± 0.26b | 5.95 ± 0.72ab | 6.32 ± 1.43a | 5.15 ± 1.30ab | 2.92 ± 0.88b | 2.23 ± 0.52b |
| 7 | MS,S,RI | Linalool | 15.50 ± 1.04b | 37.60 ± 1.03a | 26.65 ± 5.61ab | 24.19 ± 6.96ab | 10.76 ± 3.19b | 13.84 ± 2.73b |
| 8 | MS,RI | Phenylethyl Alcohol | 0.04 ± 0.02b | 0.80 ± 0.36a | 0.02 ± 0.01b | 0.79 ± 0.28a | 0.18 ± 0.12ab | 0.08 ± 0.02b |
| 9 | MS,RI | (-)-Terpinen-4-ol | 0.01 ± 0.00b | 0.05 ± 0.02a | 0.01 ± 0.00b | 0.03 ± 0.01ab | 0.02 ± 0.01b | 0.02 ± 0.00ab |
| 10 | MS,RI | α-Terpineol | 0.12 ± 0.00ab | 0.21 ± 0.01a | 0.11 ± 0.02ab | 0.21 ± 0.06a | 0.09 ± 0.03b | 0.11 ± 0.01ab |
| 11 | MS,RI | Geraniol | 0.27 ± 0.05ab | 0.45 ± 0.03a | 0.41 ± 0.13ab | 0.19 ± 0.06b | 0.15 ± 0.06b | 0.21 ± 0.05ab |
| 12 | MS,RI | Nerolidol | 0.14 ± 0.00b | 0.30 ± 0.01a | 0.12 ± 0.02b | 0.11 ± 0.03b | 0.03 ± 0.01c | 0.04 ± 0.01c |
| 13 | MS,RI | α-Cadinol | 0.01 ± 0.00a | 0.04 ± 0.02a | 0.01 ± 0.00a | 0.01 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a |
| 14 | MS,RI | Hexanal | 0.05 ± 0.00b | 0.21 ± 0.03ab | 0.09 ± 0.02b | 0.20 ± 0.07ab | 0.20 ± 0.05ab | 0.36 ± 0.07a |
| 15 | MS,RI | Benzaldehyde | 0.25 ± 0.02b | 0.59 ± 0.11ab | 0.36 ± 0.08ab | 0.58 ± 0.16ab | 0.56 ± 0.16ab | 0.74 ± 0.15a |
| 16 | MS,RI | (E,E)-2,4-Heptadienal | 0.05 ± 0.00b | 0.09 ± 0.00b | 0.09 ± 0.02b | 0.20 ± 0.05b | 0.40 ± 0.12ab | 0.60 ± 0.12a |
| 17 | MS,RI | Decanal | 0.17 ± 0.01b | 0.34 ± 0.09b | 0.21 ± 0.07b | 0.34 ± 0.09b | 0.43 ± 0.10ab | 0.70 ± 0.14a |
| 18 | MS,RI | β-Cyclocitral | 0.08 ± 0.00b | 0.20 ± 0.02ab | 0.13 ± 0.03ab | 0.24 ± 0.07ab | 0.23 ± 0.07ab | 0.26 ± 0.05a |
| 19 | MS,RI | (Z)-3-Hexenyl acetate | 3.07 ± 0.30a | 1.47 ± 0.16ab | 4.93 ± 1.10a | 2.44 ± 0.63ab | 1.07 ± 0.30b | 0.23 ± 0.05b |
| 20 | MS,RI | (Z)-2-Hexenyl acetate | 0.03 ± 0.00b | 0.02 ± 0.00b | 0.09 ± 0.02a | 0.04 ± 0.01b | 0.03 ± 0.01b | 0.02 ± 0.00b |
| 21 | MS,RI | Methyl benzoate | 11.17 ± 0.90ab | 8.46 ± 0.57b | 16.96 ± 3.46a | 8.02 ± 2.14b | 2.65 ± 0.79bc | 0.92 ± 0.22c |
| 22 | MS,RI | Acetic acid, 2-ethylhexyl ester | 0.03 ± 0.00b | 0.08 ± 0.02a | 0.03 ± 0.00b | 0.07 ± 0.02ab | 0.03 ± 0.01b | 0.04 ± 0.01ab |
| 23 | MS,RI | Benzyl acetate | 35.70 ± 2.87a | 57.80 ± 2.39a | 52.12 ± 10.66a | 37.08 ± 10.04a | 12.84 ± 3.90b | 4.24 ± 1.45b |
| 24 | MS,RI | Benzoic acid ethyl ester | 0.02 ± 0.00ab | 0.03 ± 0.00a | 0.02 ± 0.00ab | 0.01 ± 0.00ab | 0.01 ± 0.00b | 0.01 ± 0.00b |
| 25 | MS,RI | (Z)-3-Hexenyl butyrate | 0.41 ± 0.03b | 0.18 ± 0.05c | 0.63 ± 0.13a | 0.15 ± 0.04c | 0.06 ± 0.02c | 0.02 ± 0.00c |
| 26 | MS,S,RI | Methyl salicylate | 10.45 ± 0.72ab | 15.45 ± 1.13a | 13.25 ± 2.45a | 7.02 ± 1.86b | 2.58 ± 0.82bc | 1.11 ± 0.23c |
| 27 | MS,RI | (Z)-3-Hexenyl 2-methylbutanoate | 0.18 ± 0.01b | 0.13 ± 0.01bc | 0.30 ± 0.06a | 0.13 ± 0.03bc | 0.06 ± 0.02c | 0.05 ± 0.01c |
| 28 | MS,RI | 2-Phenethyl acetate | 0.29 ± 0.02ab | 0.60 ± 0.01a | 0.43 ± 0.07ab | 0.60 ± 0.16a | 0.35 ± 0.12ab | 0.12 ± 0.02b |
| 29 | MS,RI | Benzyl propionate | 0.02 ± 0.00ab | 0.03 ± 0.00ab | 0.03 ± 0.01a | 0.03 ± 0.01ab | 0.02 ± 0.01ab | 0.01 ± 0.00b |
| 30 | MS,RI | Ethyl salicylate | 0.08 ± 0.00a | 0.09 ± 0.00a | 0.08 ± 0.01a | 0.04 ± 0.01b | 0.02 ± 0.01b | 0.02 ± 0.00b |
| 31 | MS,RI | (Z)-3-Hexenyl angelate | 0.21 ± 0.01b | 0.41 ± 0.02a | 0.39 ± 0.07a | 0.27 ± 0.07ab | 0.16 ± 0.05b | 0.08 ± 0.01b |
| 32 | MS,RI | Methyl anthranilate | 4.84 ± 0.25b | 15.64 ± 3.15a | 6.38 ± 0.94b | 4.76 ± 1.35b | 1.39 ± 0.55b | 3.47 ± 1.20b |
| 33 | MS,RI | Benzyl butyrate | 0.06 ± 0.01a | 0.08 ± 0.00a | 0.08 ± 0.01a | 0.03 ± 0.01b | 0.02 ± 0.01b | 0.01 ± 0.00b |
| 34 | MS,RI | Butyl benzoate | 0.08 ± 0.00b | 0.13 ± 0.00a | 0.09 ± 0.02ab | 0.09 ± 0.02ab | 0.05 ± 0.02b | 0.04 ± 0.01b |
| 35 | MS,RI | (Z)-3-Hexenyl (Z)-3-hexenoate | 0.20 ± 0.01a | 0.26 ± 0.00a | 0.25 ± 0.04a | 0.09 ± 0.02b | 0.03 ± 0.01b | 0.02 ± 0.01b |
| 36 | MS,RI | (Z)-3-Hexenyl benzoate | 7.09 ± 0.19bc | 23.58 ± 0.95a | 8.62 ± 1.22b | 9.25 ± 2.63b | 2.51 ± 0.87c | 2.47 ± 0.61c |
| 37 | MS,RI | Benzyl Benzoate | 0.02 ± 0.00b | 0.05 ± 0.01a | 0.02 ± 0.00b | 0.03 ± 0.01b | 0.01 ± 0.00b | 0.03 ± 0.01ab |
| 38 | MS,RI | α-Pinene | 0.05 ± 0.01a | 0.07 ± 0.01a | 0.07 ± 0.01a | 0.08 ± 0.03a | 0.06 ± 0.01a | 0.11 ± 0.02a |
| 39 | MS,RI | Myrcene | 0.10 ± 0.02a | 0.07 ± 0.01ab | 0.09 ± 0.02ab | 0.05 ± 0.01b | 0.03 ± 0.01b | 0.03 ± 0.01b |
| 40 | MS,RI | α-Terpinene | 0.01 ± 0.00a | 0.01 ± 0.00ab | 0.01 ± 0.00a | 0.01 ± 0.00ab | 0.00 ± 0.00b | 0.00 ± 0.00b |
| 41 | MS,RI | Limonene | 0.11 ± 0.01b | 0.33 ± 0.10a | 0.14 ± 0.03b | 0.17 ± 0.05ab | 0.11 ± 0.04b | 0.15 ± 0.03ab |
| 42 | MS,RI | (Z)-β-Ocimene | 0.14 ± 0.01b | 0.06 ± 0.01c | 0.27 ± 0.05a | 0.06 ± 0.01c | 0.03 ± 0.01c | 0.06 ± 0.01c |
| 43 | MS,RI | α-Elemene | 0.04 ± 0.00a | 0.01 ± 0.00c | 0.03 ± 0.01b | N.D.f | N.D.f | N.D.f |
| 44 | MS,RI | α-Cubebene | 0.12 ± 0.01b | 0.20 ± 0.01a | 0.13 ± 0.03ab | 0.12 ± 0.03ab | 0.07 ± 0.02b | 0.12 ± 0.02b |
| 45 | MS,RI | α-Copaene | 0.32 ± 0.02ab | 0.40 ± 0.01a | 0.33 ± 0.06a | 0.27 ± 0.07ab | 0.15 ± 0.05b | 0.26 ± 0.05ab |
| 46 | MS,RI | Germacrene D | 0.01 ± 0.00b | 0.01 ± 0.00a | 0.01 ± 0.00b | 0.01 ± 0.00b | 0.00 ± 0.00b | 0.00 ± 0.00b |
| 47 | MS,RI | γ-Cadinene | 0.18 ± 0.01a | 0.15 ± 0.01ab | 0.18 ± 0.03a | 0.11 ± 0.03ab | 0.05 ± 0.02b | 0.10 ± 0.02b |
| 48 | MS,RI | β-Elemene | 0.10 ± 0.01a | 0.10 ± 0.00a | 0.10 ± 0.02a | 0.08 ± 0.02ab | 0.04 ± 0.02b | 0.08 ± 0.01ab |
| 49 | MS,RI | α-Gurjunene | 0.01 ± 0.00a | 0.02 ± 0.01a | 0.01 ± 0.00a | 0.00 ± 0.00a | 0.00 ± 0.00a | 0.01 ± 0.00a |
| 50 | MS,RI | Caryophyllene | 0.13 ± 0.01a | 0.11 ± 0.00ab | 0.12 ± 0.02ab | 0.11 ± 0.03ab | 0.05 ± 0.02b | 0.18 ± 0.03a |
| 51 | MS,RI | β-Cubebene | 0.31 ± 0.02ab | 0.41 ± 0.01a | 0.30 ± 0.05ab | 0.23 ± 0.06b | 0.10 ± 0.03b | 0.10 ± 0.02b |
| 52 | MS,RI | α-Caryophyllene | 0.37 ± 0.02ab | 0.51 ± 0.02a | 0.39 ± 0.07ab | 0.26 ± 0.07b | 0.12 ± 0.04b | 0.21 ± 0.03b |
| 53 | MS,RI | γ-Muurolene | 0.23 ± 0.02b | 0.41 ± 0.01a | 0.24 ± 0.05b | 0.22 ± 0.06b | 0.13 ± 0.04bc | 0.08 ± 0.01c |
| 54 | MS,RI | α-Farnesene | 6.56 ± 0.42bc | 12.54 ± 1.29a | 8.80 ± 1.59b | 4.10 ± 1.14c | 1.02 ± 0.14c | 1.62 ± 0.45c |
| 55 | MS,RI | β-Cadinene | 1.22 ± 0.06b | 2.39 ± 0.02a | 1.21 ± 0.20b | 1.02 ± 0.29bc | 0.51 ± 0.18c | 0.35 ± 0.04c |
| 56 | MS,RI | α-Muurolene | 0.16 ± 0.01b | 0.33 ± 0.00a | 0.16 ± 0.03b | 0.15 ± 0.04bc | 0.08 ± 0.03c | 0.03 ± 0.00c |
| 57 | MS,RI | α-Patchoulene | 0.05 ± 0.00a | 0.06 ± 0.00a | 0.05 ± 0.01a | 0.02 ± 0.01b | 0.01 ± 0.00b | 0.02 ± 0.00b |
| 58 | MS,RI | Naphthalene | 0.41 ± 0.02b | 1.41 ± 0.29a | 0.48 ± 0.07b | 1.15 ± 0.36ab | 0.44 ± 0.15b | 0.51 ± 0.04b |
| 69 | MS,RI | 2-Methylnaphthalene | 0.06 ± 0.00b | 0.15 ± 0.05a | 0.08 ± 0.01ab | 0.12 ± 0.04ab | 0.05 ± 0.02b | 0.07 ± 0.00ab |
| 60 | MS,RI | 6-Methyl-5-hepten-2-one | 0.71 ± 0.05b | 1.61 ± 0.12ab | 1.62 ± 0.35a | 1.12 ± 0.30ab | 0.93 ± 0.25ab | 1.38 ± 0.31ab |
| 61 | MS,RI | Acetophenone | 0.31 ± 0.01b | 1.48 ± 0.24a | 0.33 ± 0.05b | 1.03 ± 0.33ab | 0.40 ± 0.14b | 0.42 ± 0.04b |
| 62 | MS,RI | Indole | 7.20 ± 0.27ab | 14.19 ± 3.36ab | 1.78 ± 0.82b | 20.55 ± 11.49a | 1.45 ± 0.07b | 0.70 ± 0.24b |
| 63 | MS,RI | Eugenol | 0.12 ± 0.00b | 0.25 ± 0.04a | 0.11 ± 0.02b | 0.10 ± 0.03b | 0.05 ± 0.02b | 0.06 ± 0.02b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhao, F.; Wu, W.; Wang, P.; Ye, N. Comparison of Volatiles in Different Jasmine Tea Grade Samples Using Electronic Nose and Automatic Thermal Desorption-Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis. Molecules 2020, 25, 380. https://doi.org/10.3390/molecules25020380
Wang S, Zhao F, Wu W, Wang P, Ye N. Comparison of Volatiles in Different Jasmine Tea Grade Samples Using Electronic Nose and Automatic Thermal Desorption-Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis. Molecules. 2020; 25(2):380. https://doi.org/10.3390/molecules25020380
Chicago/Turabian StyleWang, Shuyan, Feng Zhao, Wenxi Wu, Pengjie Wang, and Naixing Ye. 2020. "Comparison of Volatiles in Different Jasmine Tea Grade Samples Using Electronic Nose and Automatic Thermal Desorption-Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis" Molecules 25, no. 2: 380. https://doi.org/10.3390/molecules25020380
APA StyleWang, S., Zhao, F., Wu, W., Wang, P., & Ye, N. (2020). Comparison of Volatiles in Different Jasmine Tea Grade Samples Using Electronic Nose and Automatic Thermal Desorption-Gas Chromatography-Mass Spectrometry Followed by Multivariate Statistical Analysis. Molecules, 25(2), 380. https://doi.org/10.3390/molecules25020380






