Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity
Abstract
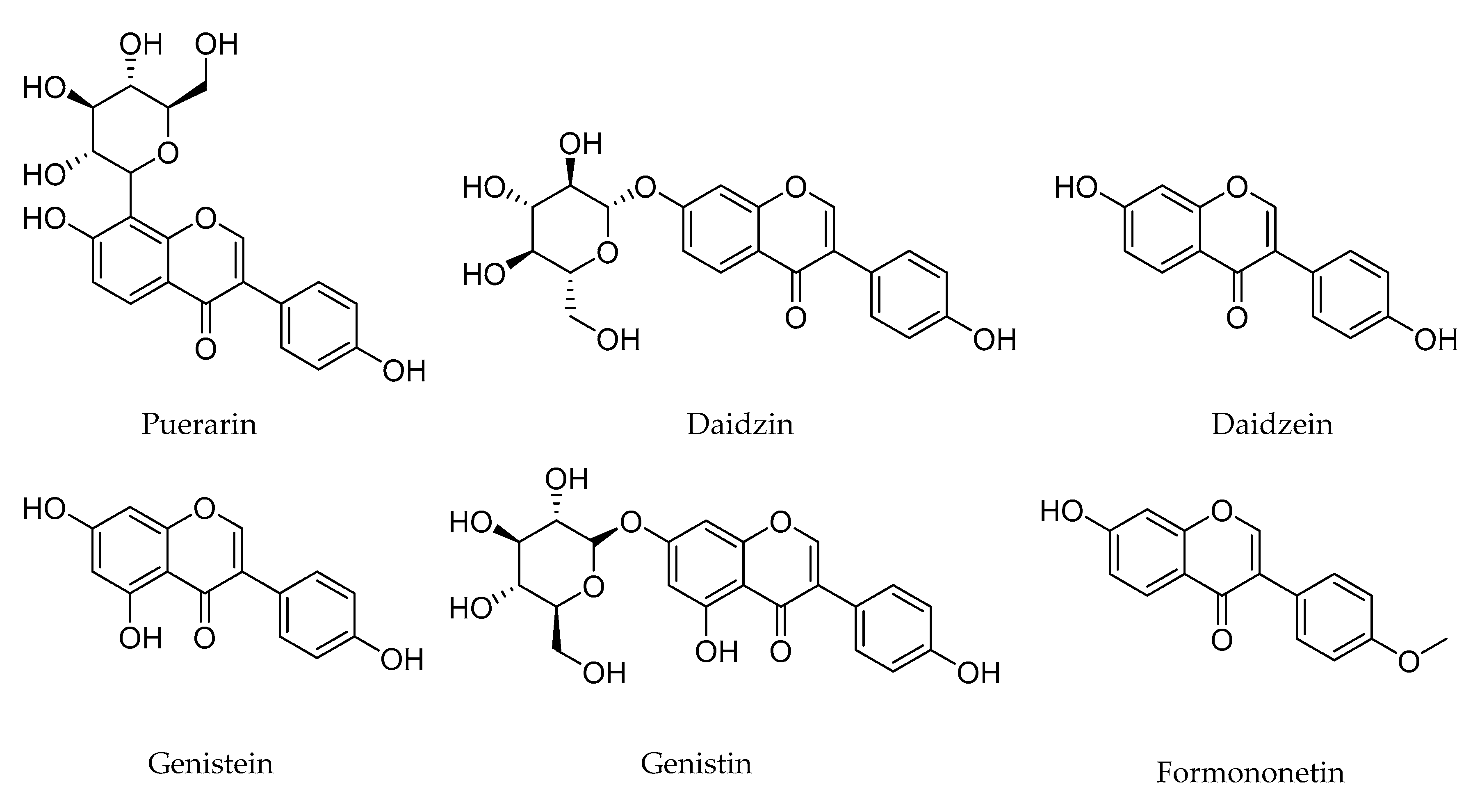
1. Introduction
2. Results and Discussion
2.1. Optimization of the Cloud-Point System
2.1.1. Effect of the Concentration of Triton X-100
2.1.2. Effect of Liquid-Solid Ratio
2.1.3. Effect of Equilibrium Temperature
2.1.4. Effect of Equilibrium Time
2.1.5. Effect of NaCl Addition
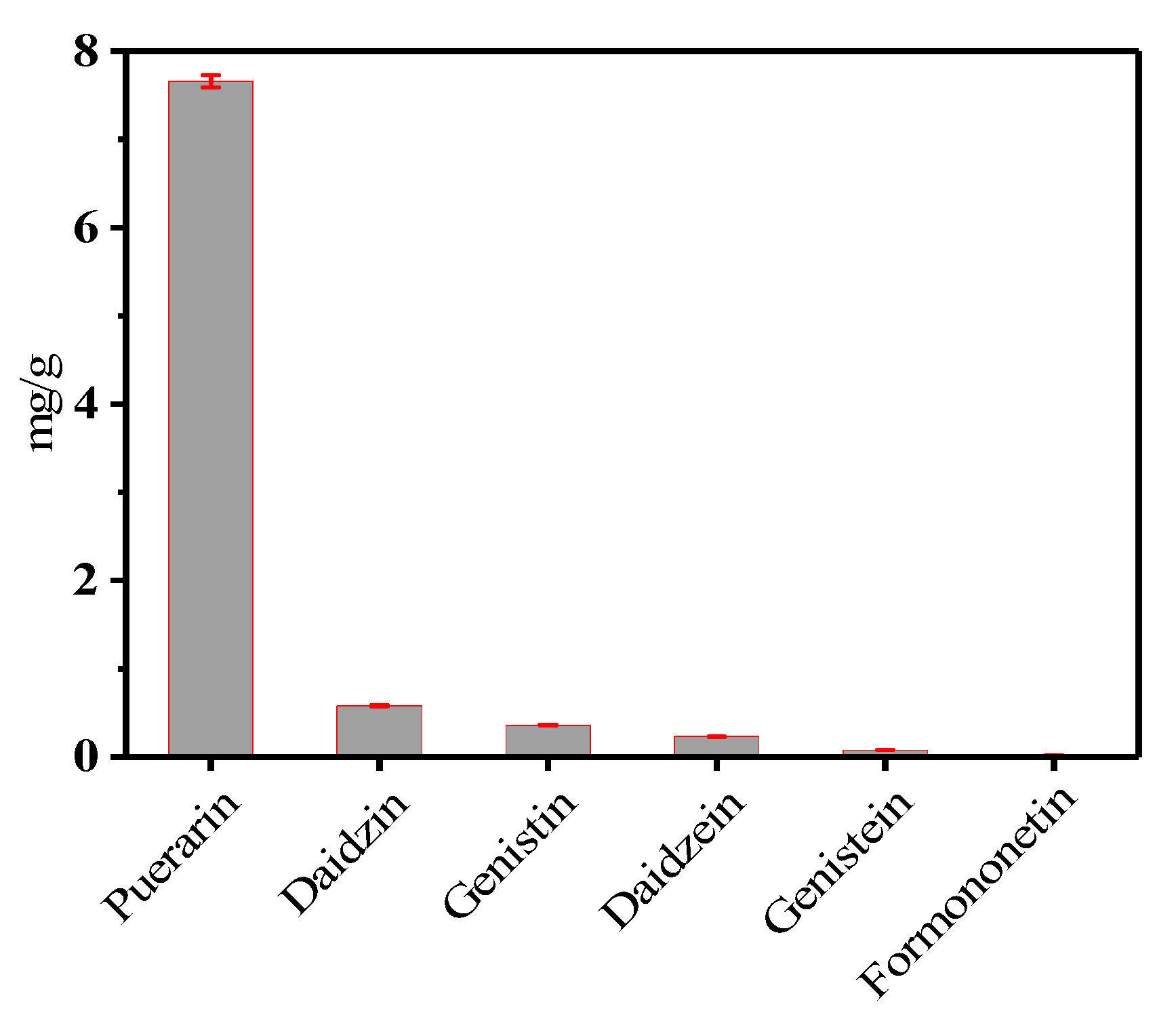
2.2. Optimization of Orthogonal Experiment Design
2.3. Calibration and Validation of the Analytical Method
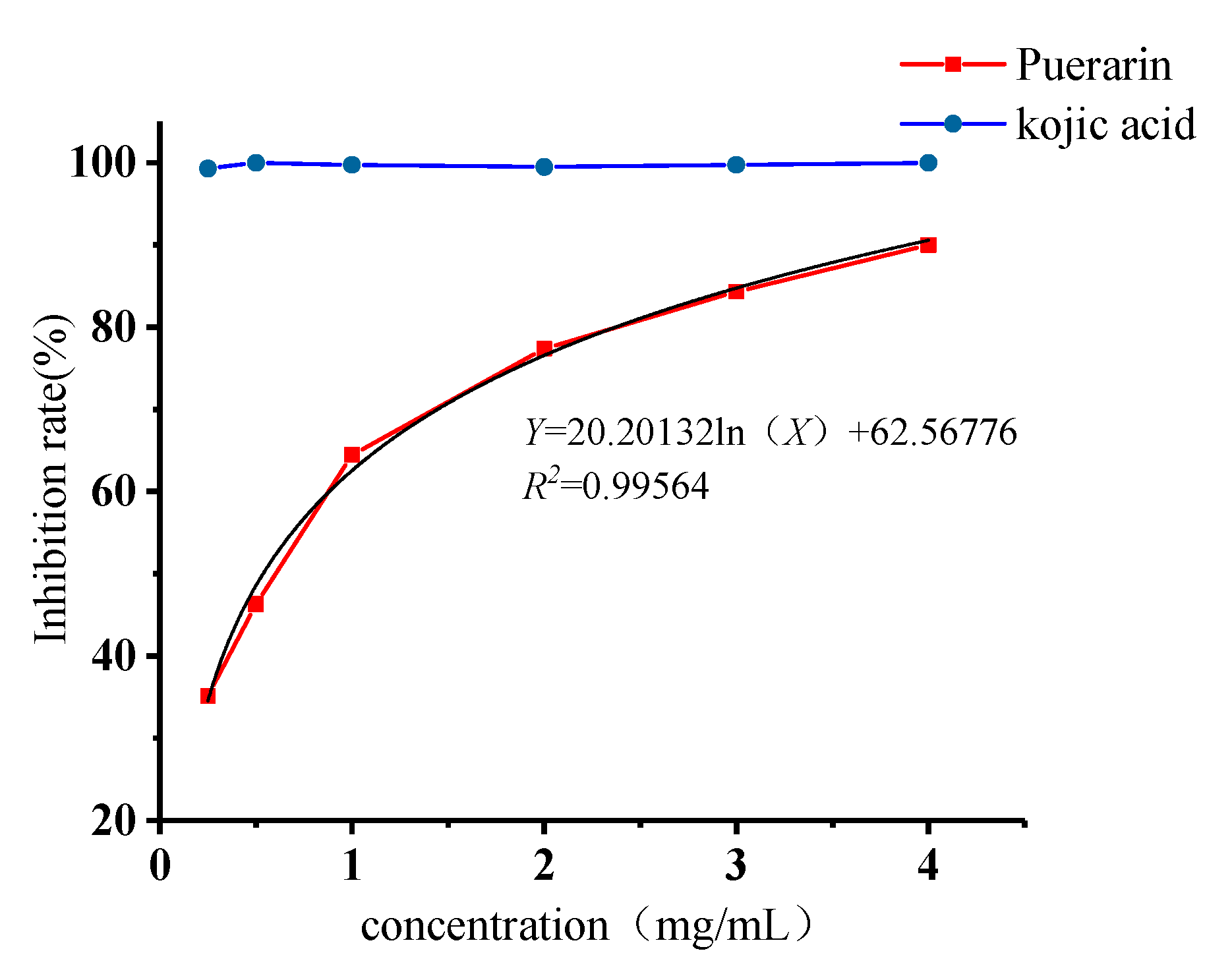
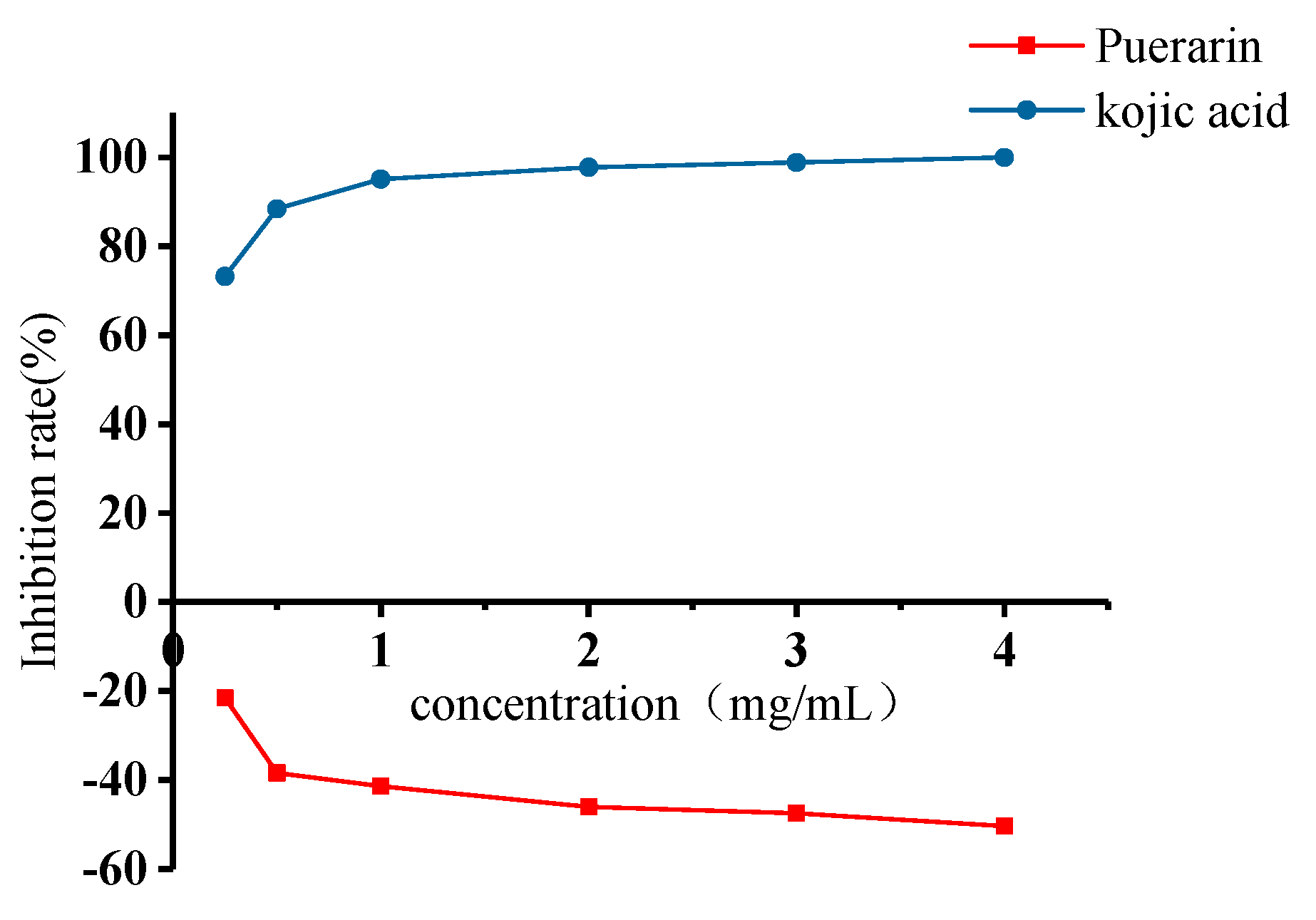
2.4. Inhibitory Effects of Puerarin on Mushroom Tyrosinase
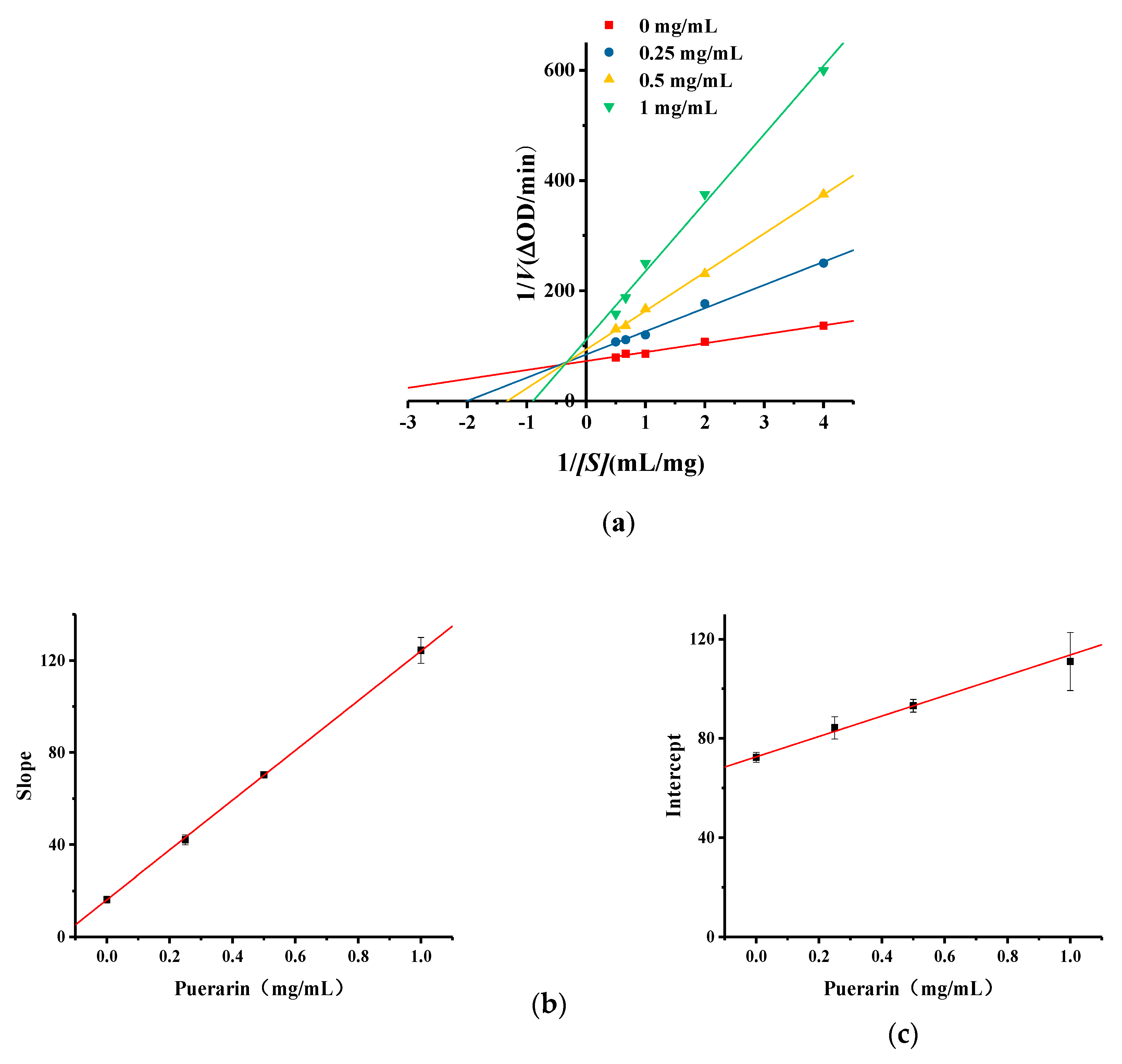
2.5. Kinetic Studies of Puerarin Inhibition of Monophenolase Activity
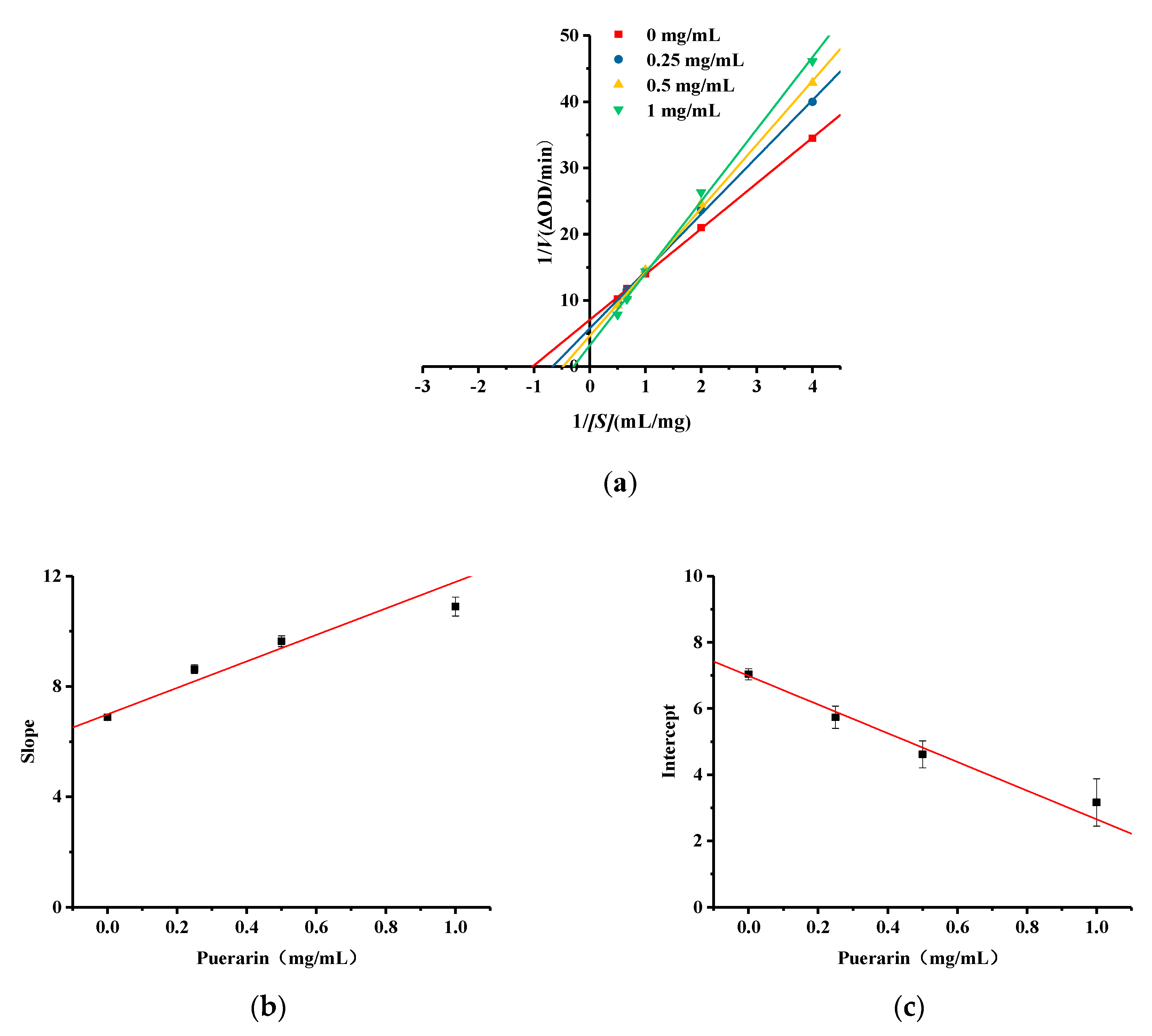
2.6. Kinetic Studies of Puerarin Inhibition of Diphenolase Activity
3. Experimental
3.1. Materials and Reagents
3.2. Preparation of Cloud-Point System and Extraction Strategy
3.3. Optimization of Orthogonal Experimental Design
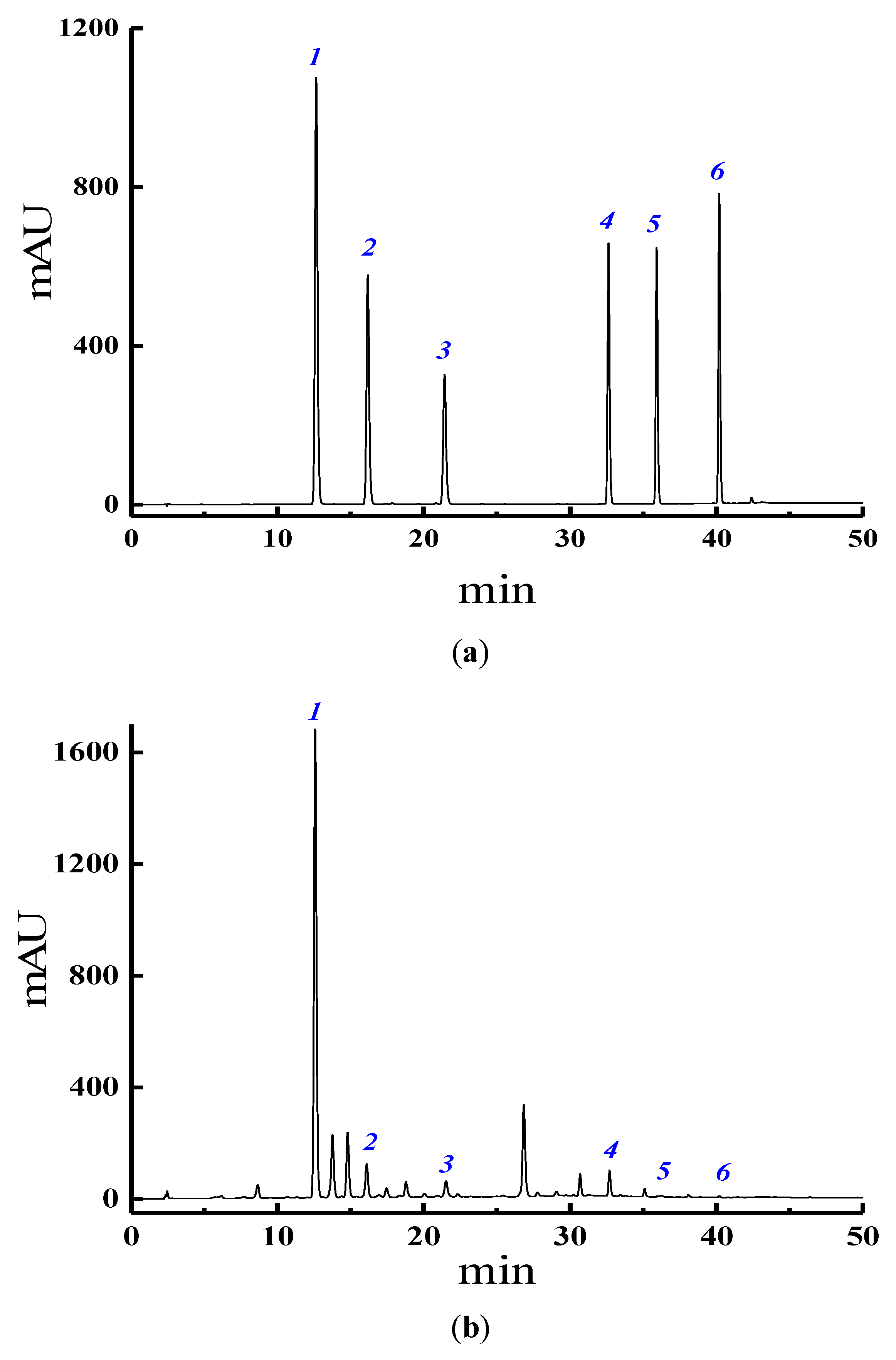
3.4. HPLC Analysis of Six Target Isoflavones
3.5. Calculations of Six Target Isoflavones Extraction Yield
3.6. HPLC Method Validation
3.7. Measurement of Tyrosinase Activity
3.7.1. Mushroom Tyrosinase Inhibition Assay
3.7.2. Kinetics of Puerarin Inhibiting Tyrosinase Monophenolase Catalytic Reaction
3.7.3. Kinetics of Puerarin Activation of the Catalytic Tyrosinase Diphenolase Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Xu, J.; Liu, J.; Zhu, X.Q.; Yu, Y.Y.; Cao, S.W. Novel inhibitors of tyrosinase produced by the 4-substitution of TCT. Food Chem. 2016, 221, 1530–1538. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, J.Y.; Jenis, J.; Li, Z.P.; Ban, Y.J.; Baiseitova, A.; Park, K.H. Tyrosinase inhibitory study of flavonolignans from the seeds of Silybum marianum (Milk thistle). Bioorganic Med. Chem. 2019, 27, 2499–2507. [Google Scholar] [CrossRef]
- Quispe, Y.N.G.; Hwang, S.H.; Wang, Z.Q.; Lim, S.S. Screening of peruvian medicinal plants for tyrosinase inhibitory properties: Identification of tyrosinase inhibitors in Hypericum laricifolium Juss. Molecules 2017, 22, 402. [Google Scholar] [CrossRef]
- Kyoungseon, M.; Woo, P.G.; Je, Y.Y.; Lee, J.S. A perspective on the biotechnological applications of the versatile tyrosinase. Bioresour. Technol. 2019, 289, 121730. [Google Scholar]
- Hang, X.H.; Li, N.; Yang, B.L.; Zhang, F.C.; Li, Y.W. Research progress on mechanism of traditional chinese medicine in treating vitiligo. Glob. Tradit. Chin. Med. 2018, 11, 2047–2052. [Google Scholar]
- Chai, W.M.; Huang, Q.; Lin, M.Z.; Ouyang, C.; Huang, W.Y.; Wang, Y.X.; Xu, K.L.; Feng, H.L. Condensed tannins from Longan bark as inhibitor of tyrosinase: Structure, activity, and mechanism. J. Agric. Food Chem. 2018, 66, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Sahin, S.C. The potential of Arthrospira platensis extract as a tyrosinase inhibitor for pharmaceutical or cosmetic applications. S. Afr. J. Bot. 2018, 119, 236–243. [Google Scholar] [CrossRef]
- Dehghania, Z.; Khoshneviszadeh, M.; Khoshneviszadeh, M.; Ranjbar, S. Veratric acid derivatives containing benzylidene-hydrazine moieties as promising tyrosinase inhibitors and free radical scavengers. Bioorganic Med. Chem. 2019, 27, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, X.; Tao, G.J.; Chen, J.; Zheng, Z.P. Investigating the inhibitory activity and mechanism differences between norartocarpetin and luteolin for tyrosinase: A combinatory kinetic study and computational simulation analysis. Food Chem. 2017, 223, 40–48. [Google Scholar] [CrossRef]
- Kubglomsong, S.; Theerakulkait, C.; Reed, R.L.; Yang, L.P.; Maier, C.S.; Stevens, J.F. Isolation and identification of tyrosinase inhibitory and copper-chelating peptides from hydrolyzed rice bran-derived albumin. J. Agric. Food Chem. 2018, 66, 8346–8354. [Google Scholar] [CrossRef]
- Wang, G.H.; Xia, Y.; Sui, W.J.; Si, C.L. Lignin as a novel tyrosinase inhibitor: Effects of sources and isolation processes. ACS Sustain. Chem. Eng. 2018, 6, 9510–9518. [Google Scholar] [CrossRef]
- Kim, C.S.; Noh, S.G.; Park, Y.J.; Kang, D.W.; Chun, P.; Chung, H.Y.; Jung, H.J.; Moon, H.R. A potent tyrosinase inhibitor, (E)-3-(2,4-Dihydroxyphenyl)-1-(thiophen-2-yl)prop-2-en-1-one, with anti-melanogenesis properties in α-MSH and IBMX-induced B16F10 melanoma cells. Molecules 2018, 23, 2725. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Yang, J.; Lee, S.; Park, C.; Kang, D.W.; Akter, J.; Ullah, S.; Kim, Y.J.; Chun, P.; Moon, H.R. The tyrosinase inhibitory effects of isoxazolone derivatives with a (Z)-β-phenyl-α, β-unsaturated carbonyl scaffold. Bioorganic Med. Chem. 2018, 26, 3882–3889. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhu, J.; Xu, J.R.; Ji, D.D. Synthesis of N-hydroxycinnamoyl amide derivatives and evaluation of their anti-oxidative and anti-tyrosinase activities. Bioorganic Med. Chem. 2019, 27, 114918. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fan, L.P.; Duan, Z.H. Five individual polyphenols as tyrosinase inhibitors: Inhibitory activity, synergistic effect, action mechanism, and molecular docking. Food Chem. 2019, 297, 124910. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Bi, X.; Yu, B.; Chen, D. Isoflavones: Anti-inflammatory benefit and possible caveats. Nutrients 2016, 8, 361. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Naumovski, V.R.; Li, K.M.; Li, G.Q.; Chan, K. Differentiating Puerariae Lobatae Radix and Puerariae Thomsonii Radix using HPTLC coupled with multivariate classification analyses. J. Pharm. Biomed. Anal. 2014, 95, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Li, G.Q.; Li, K.M.; Razmovskinaumovski, V.; Chan, K. Kudzu root: Traditional uses and potential medicinal benefits in diabetes and cardiovascular diseases. J. Ethnopharmacol. 2011, 134, 584–607. [Google Scholar] [CrossRef]
- Feng, T.; Liu, F.; Sun, L.L.; Huo, H.N.; Ren, X.L.; Wang, M. Associated-extraction efficiency of six cyclodextrins on various flavonoids in Puerariae Lobatae Radix. Molecules 2019, 24, 93. [Google Scholar] [CrossRef]
- Guo, H.D.; Zhang, Q.F.; Chen, J.G.; Shangguang, X.C.; Guo, Y.X. Large scale purification of puerarin from Puerariae Lobatae Radix through resins adsorption and acid hydrolysis. J. Chromatogr. B 2015, 980, 11–15. [Google Scholar] [CrossRef]
- Watanabe, H.; Tanaka, H. A non-ionic surfactant as a new solvent for liquid—liquid extraction of zinc(II) with 1-(2-pyridylazo)-2-naphthol. Talanta 1978, 25, 585–589. [Google Scholar] [CrossRef]
- Han, Q.; Huo, Y.Y.; Yang, L.H.; Yang, X.H.; He, Y.P.; Wu, J.Y. Determination of trace nickel in water samples by graphite furnace atomic absorption spectrometry after mixed micelle-mediated cloud point extraction. Molecules 2018, 23, 2597. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Wu, W.; Ercal, N.; Ma, Y.F. Simultaneous determination of 3-nitro tyrosine, o-, m-, and p-tyrosine in urine samples by liquid chromatography-ultraviolet absorbance detection with pre-column cloud point extraction. J. Chromatogr. B 2004, 803, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Merino, F.; Rubio, S.; Perez-Bendito, D. Acid-induced cloud point extraction and preconcentration of polycyclic aromatic hydrocarbons from environmental solid samples. J. Chromatogr. A 2002, 962, 1–8. [Google Scholar] [CrossRef]
- Choi, M.P.K.; Chan, K.K.C.; Leung, H.W.; Huie, C.W. Pressurized liquid extraction of active ingredients (ginsenosides) from medicinal plants using nonionic surfactant solutions. J. Chromatogr. A 2003, 983, 153–162. [Google Scholar] [CrossRef]
- Campillo, N.; Marín, J.; Viñas, P.; Garrido, I.; Fenoll, J.; Hernández-Córdoba, M. Microwave assisted cloud point extraction for the determination of Vitamin K homologues in vegetables by liquid chromatography with tandem mass spectrometry. J. Agric. Food Chem. 2019, 67, 6658–6664. [Google Scholar] [CrossRef]
- Zeng, W.W.; Lai, L.S. Anti-melanization effects and inhibitory kinetics of tyrosinase of bird’s nest fern (Asplenium australasicum) frond extracts on melanoma and human skin. J. Biosci. Bioeng. 2019, 127, 738–743. [Google Scholar] [CrossRef]
- Larik, F.A.; Saeed, A.; Channar, P.A.; Muqadar, U.; Abbas, Q.; Hassan, M.; Seo, S.Y.; Bolte, M. Design, synthesis, kinetic mechanism and molecular docking studies of novel 1-pentanoyl-3-arylthioureas as inhibitors of mushroom tyrosinase and free radical scavengers. Eur. J. Med. Chem. 2017, 141, 273–281. [Google Scholar] [CrossRef]
- Gou, L.; Lee, J.; Hao, H.; Park, Y.D.; Zhan, Y.; Lü, Z.R. The effect of oxaloacetic acid on tyrosinase activity and structure: Integration of inhibition kinetics with docking simulation. Int. J. Biol. Macromol. 2017, 101, 59–66. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
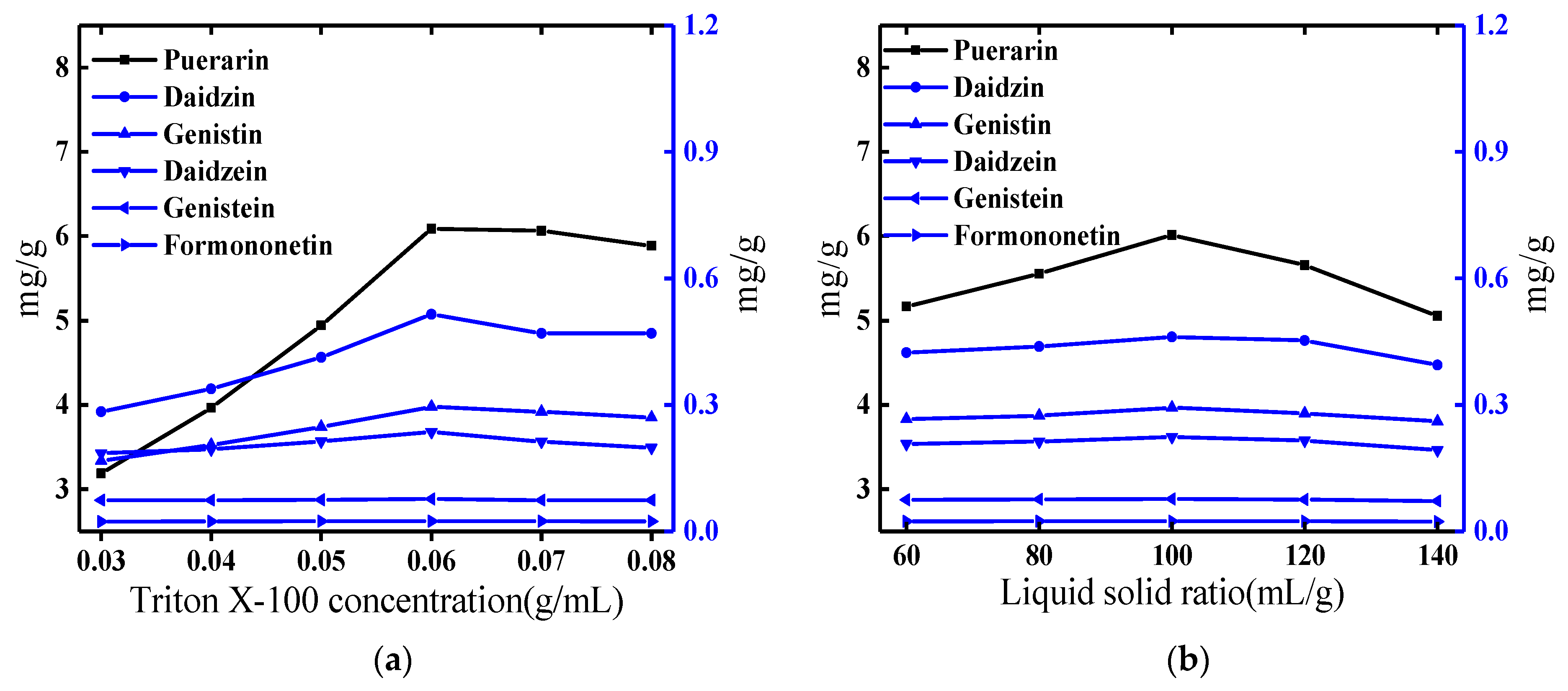
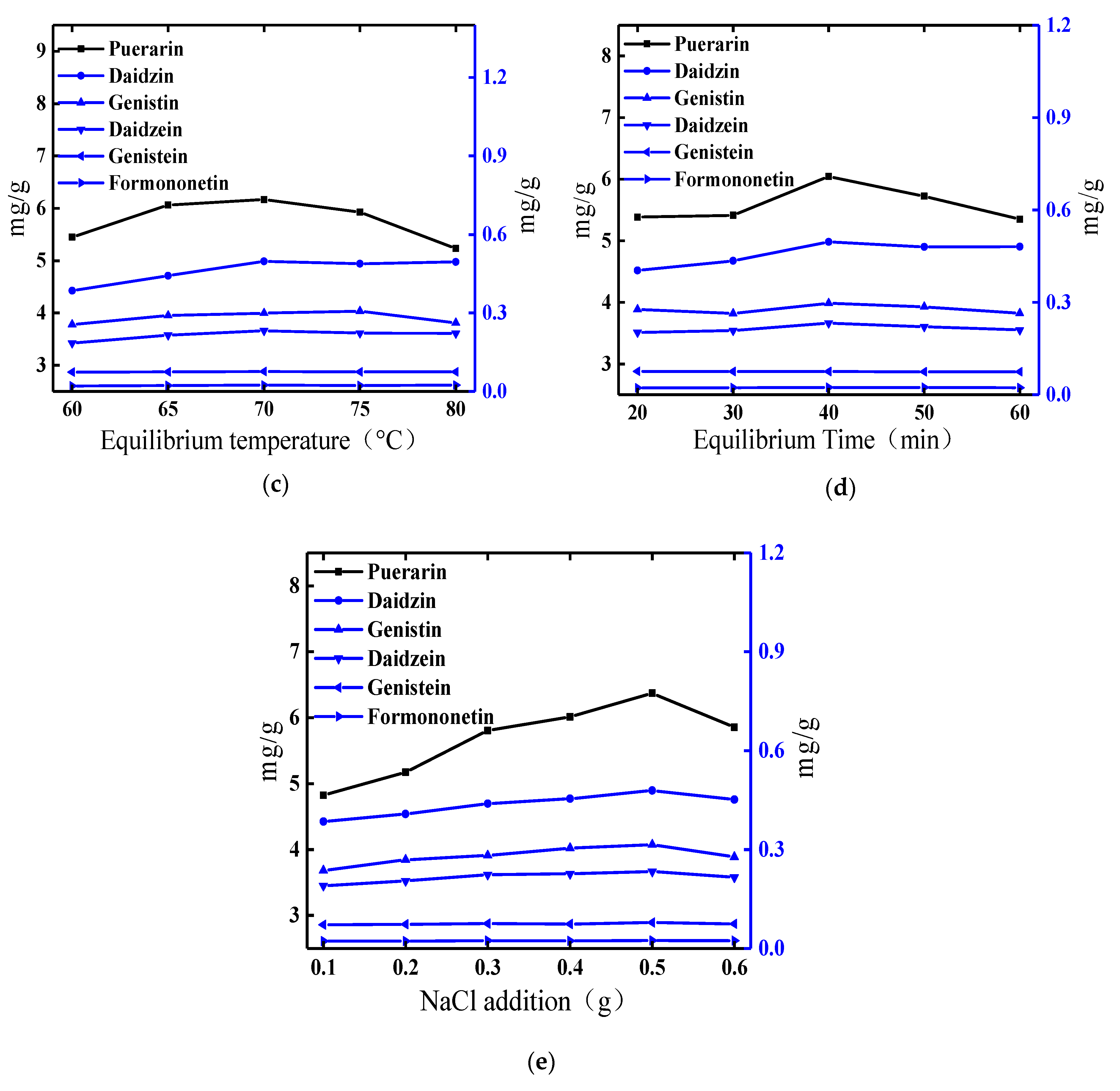
| Level | Triton X-100 Concentration (g/mL) | Liquid-Solid Ratio (mL/g) | NaCl Addition (g) | Equilibrium Time (min) |
|---|---|---|---|---|
| 1 | 0.05 | 80 | 0.4 | 30 |
| 2 | 0.06 | 100 | 0.5 | 40 |
| 3 | 0.07 | 120 | 0.6 | 50 |
| Experiment Number | A | B | C | D | Total Extraction mg/g |
|---|---|---|---|---|---|
| 1 | 2 | 3 | 3 | 1 | 6.92 |
| 2 | 2 | 1 | 2 | 2 | 7.77 |
| 3 | 3 | 3 | 1 | 2 | 7.03 |
| 4 | 3 | 1 | 3 | 3 | 8.47 |
| 5 | 3 | 2 | 2 | 1 | 7.60 |
| 6 | 1 | 1 | 1 | 1 | 5.58 |
| 7 | 2 | 2 | 1 | 3 | 6.74 |
| 8 | 1 | 3 | 2 | 3 | 6.29 |
| 9 | 1 | 2 | 3 | 2 | 6.85 |
| K1 | 18.72 | 21.82 | 19.34 | 20.10 | |
| K2 | 21.44 | 21.18 | 21.66 | 21.65 | |
| K3 | 23.10 | 20.24 | 22.24 | 21.50 | |
| R | 4.38 | 1.58 | 2.90 | 1.55 |
| Source of Variation | Sum of Squares | Variance | Mean Square | F Value | p Value |
|---|---|---|---|---|---|
| A | 9.756 | 2 | 4.878 | 125.146 | <0.01 |
| B | 1.262 | 2 | 0.631 | 16.184 | <0.01 |
| C | 4.712 | 2 | 2.356 | 60.446 | <0.01 |
| D | 1.463 | 2 | 0.731 | 18.760 | <0.01 |
| Error | 0.702 | 18 | |||
| Total | 1351.454 | 27 |
| Name | A1 | A2 | A3 | A4 |
|---|---|---|---|---|
| PBS/μL | 80 | 160 | 0 | 80 |
| Substrate/μL | 80 | 80 | 80 | 80 |
| Sample/μL | 0 | 0 | 80 | 80 |
| Tyrosinase/μL | 80 | 0 | 80 | 0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, L.; Song, K.; Zhang, Q.; Guo, J.; Huang, J. Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity. Molecules 2020, 25, 344. https://doi.org/10.3390/molecules25020344
Qu L, Song K, Zhang Q, Guo J, Huang J. Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity. Molecules. 2020; 25(2):344. https://doi.org/10.3390/molecules25020344
Chicago/Turabian StyleQu, Limin, Ke Song, Qi Zhang, Jie Guo, and Juan Huang. 2020. "Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity" Molecules 25, no. 2: 344. https://doi.org/10.3390/molecules25020344
APA StyleQu, L., Song, K., Zhang, Q., Guo, J., & Huang, J. (2020). Simultaneous Determination of Six Isoflavones from Puerariae Lobatae Radix by CPE-HPLC and Effect of Puerarin on Tyrosinase Activity. Molecules, 25(2), 344. https://doi.org/10.3390/molecules25020344





