Synthesis and Bioactivity of Thiosemicarbazones Containing Adamantane Skeletons
Abstract
1. Introduction
2. Results and Discussion
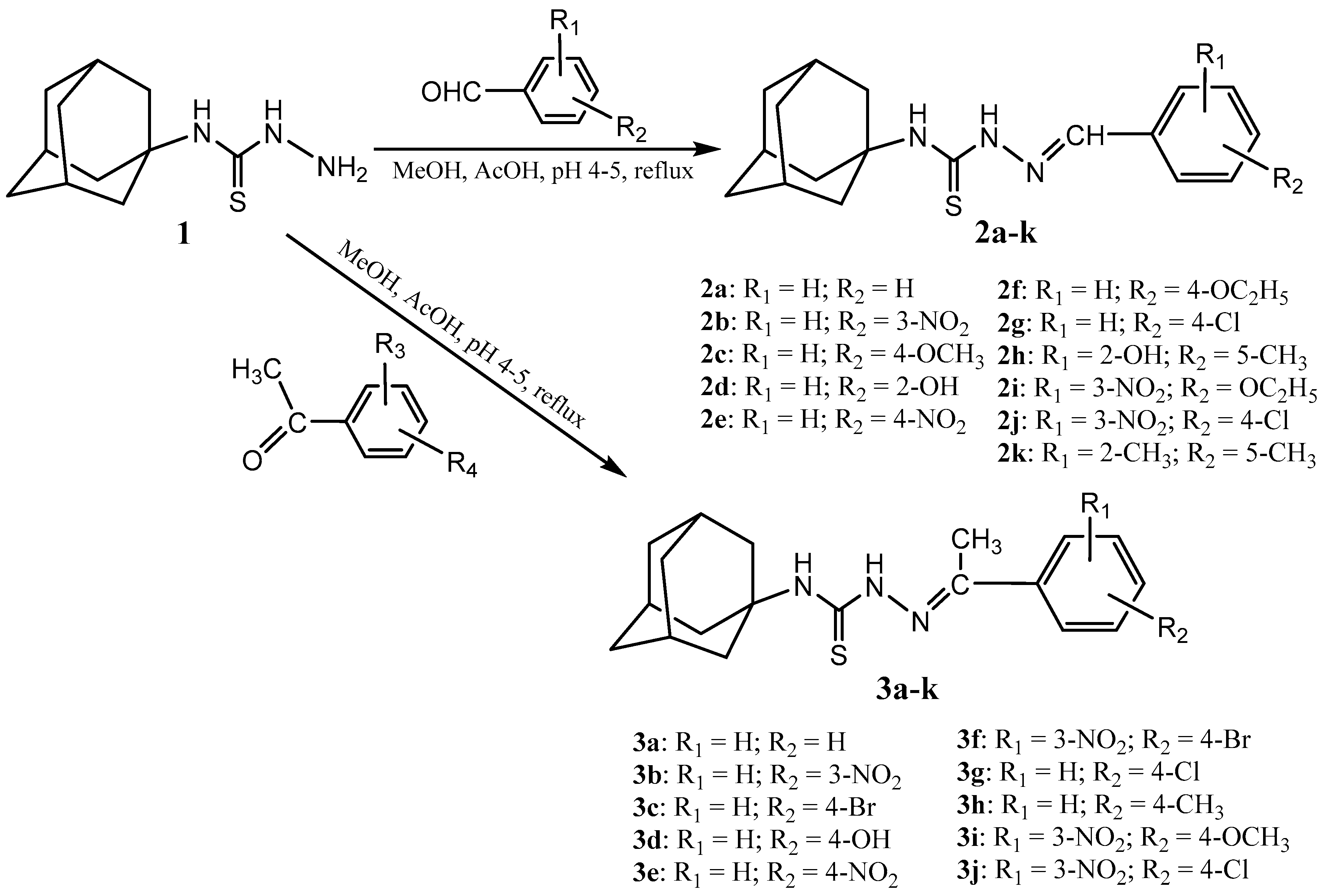
2.1. Chemistry
2.2. In Vitro Antimicrobial Activity
2.3. In Vitro Cytotoxicity
3. Experimental
3.1. General Information
3.2. Synthesis of Thiosemicarbazones 2a–k and 3a–j
3.3. Dertemination of Antimicrobial Activity by the Dilution Method
3.4. Determination of Cytotoxicity Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Haribabu, J.; Subhashree, G.R.; Saranya, S.; Gomathi, K.; Karvembu, R.; Gayathri, D. Isatin based thiosemicarbazone derivatives as potential bioactive agents: Anti-oxidant and molecular docking studies. J. Mol. Struct. 2016, 1110, 185–195. [Google Scholar] [CrossRef]
- Ghosh, S.; Misra, A.K.; Bhatia, G.; Khan, M.M.; Khanna, A.K. Syntheses and evaluation of glucosyl aryl thiosemicarbazide and glucosyl thiosemicarbazone derivatives as antioxidant and anti-dyslipidemic agents. Bioorg. Med. Chem. Lett. 2009, 19, 386–389. [Google Scholar] [CrossRef]
- Tenorio, R.P.; Carvalho, C.S.; Pessanha, C.S.; de Lima, J.G.; de Faria, A.R.; Alves, A.J.; de Melo, E.J.T.; Goes, A.J.S. Synthesis of thiosemicarbazone and 4-thiazolidinone derivatives and their in vitro anti-Toxoplasma gondii activity. Bioorg. Med. Chem. Lett. 2005, 15, 2575–2578. [Google Scholar] [CrossRef]
- Bharti, N.; Husain, K.; Garza, M.T.G.; Cruz-Vega, D.E.; Castro-Garza, J.; Mata-Cardenas, B.D.; Naqvi, F.; Azam, A. Synthesis and in vitro antiprotozoal activity of 5-nitrothiophene-2-carboxaldehyde thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 3475–3478. [Google Scholar] [CrossRef]
- de Oliveira, R.B.; de Souza-Fagundes, E.M.; Soares, R.P.P.; Andrade, A.A.; Krettli, A.U.; Zani, C.L. Synthesis and antimalarial activity of semicarbazone and thiosemicarbazone derivatives. Eur. J. Med. Chem. 2008, 43, 1983–1988. [Google Scholar] [CrossRef] [PubMed]
- Shailendra, N.S.; Bharti, N.; Garza, M.T.G.; Cruz-Vega, D.E.; Garza, J.C.; Saleem, K.; Naqvi, F.; Azam, A. Synthesis, characterisation and antiamoebic activity of new thiophene-2-carboxaldehyde thiosemicarbazone derivatives and their cyclooctadiene Ru (II) complexes. Bioorg. Med. Chem. Lett. 2001, 11, 2675–2678. [Google Scholar] [CrossRef]
- Dimmock, J.R.; McColl, J.M.; Wonko, S.L.; Thayer, R.S.; Hancock, D.S. Evaluation of the thiosemicarbazones of some aryl alkyl ketones and related compounds for anticonvulsant activities. Eur. J. Med. Chem. 1991, 26, 529–534. [Google Scholar] [CrossRef]
- Bal, T.R.; Anand, B.; Yogeeswari, P.; Sriram, D. Synthesis and evaluation of anti-HIV activity of isatin β-thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2005, 15, 4451–4455. [Google Scholar] [CrossRef] [PubMed]
- Finkielsztein, L.M.; Castro, E.F.; Fabián, L.E.; Moltrasio, G.Y.; Campos, R.H.; Cavallaro, L.V.; Moglioni, A.G. New 1-indanone thiosemicarbazone derivatives active against BVDV. Eur. J. Med. Chem. 2008, 43, 1767–1773. [Google Scholar] [CrossRef] [PubMed]
- Shipman, J.C.; Smith, S.H.; Drach, J.C.; Klayman, D.L. Antiviral activity of 2-acetylpyridine thiosemicarbazones against herpes simplex virus. Antimicrob. Agents Chemother. 1981, 19, 682. [Google Scholar] [CrossRef]
- Khan, S.A.; Kumar, P.; Joshi, R.; Iqbal, P.F.; Saleem, K. Synthesis and in vitro antibacterial activity of new steroidal thiosemicarbazone derivatives. Eur. J. Med. Chem. 2008, 43, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Kulandaivelu, U.; Padmini, V.G.; Suneetha, K.; Shireesha, B.; Vidyasagar, J.V.; Rao, T.R.; Basu, A.; Jayaprakash, V. Synthesis, antimicrobial and anticancer activity of new thiosemicarbazone derivatives. Arch. Pharm. 2011, 344, 84–90. [Google Scholar] [CrossRef]
- Sriram, D.; Yogeeswari, P.; Thirumurugan, R.; Pavana, R.K. Discovery of new antitubercular oxazolyl thiosemicarbazones. J. Med. Chem. 2006, 49, 3448–3450. [Google Scholar] [CrossRef] [PubMed]
- Altıntop, M.D.; Atlı, Ö.; Ilgın, S.; Demirel, R.; Özdemir, A.; Kaplancıklı, Z.A. Synthesis and biological evaluation of new naphthalene substituted thiosemicarbazone derivatives as potent antifungal and anticancer agents. Eur. J. Med. Chem. 2016, 108, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Degola, F.; Morcia, C.; Bisceglie, F.; Mussi, F.; Tumino, G.; Ghizzoni, R.; Pelosi, G.; Terzi, V.; Buschini, A.; Restivo, F.M.; et al. In vitro evaluation of the activity of thiosemicarbazone derivatives against mycotoxigenic fungi affecting cereals. Int. J. Food. Microbiol. 2015, 200, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Piantanida, I.; Cindric, M. Novel thiosemicarbazone derivatives as potential antitumor agents: synthesis, physicochemical and structural properties, DNA interactions and antiproliferative activity. Bioorg. Med. Chem. 2008, 16, 5189–5198. [Google Scholar]
- Feun, L.; Modiano, M.; Lee, K.; Mao, J.; Marini, A.; Savaraj, N.; Plezia, P.; Almassian, B.; Colacino, E.; Fischer, J. Phase I and pharmacokinetic study of 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP) using a single intravenous dose schedule. Cancer Chemother. Pharmacol. 2002, 50, 223–229. [Google Scholar] [CrossRef]
- Hu, K.; Yang, Z.H.; Pan, S.S.; Xu, H.J.; Ren, J. Synthesis and antitumor activity of liquiritigenin thiosemicarbazone derivatives. Eur J. Med. Chem. 2010, 45, 3453–3458. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Gu, W.; Shan, Y.; Liu, F.; Xu, X.; Yang, Y.Q.; Zhang, Q.J.; Zhang, Y.; Kuang, H.B.; Wang, Z.L. Design, synthesis and anticancer activity of novel nopinone-based thiosemicarbazone derivatives. Bioorg. Med. Chem. Lett. 2017, 27, 2360–2363. [Google Scholar] [CrossRef]
- de Oliveira, J.F.; Lima, T.S.; Vendramini-Costa, D.B.; de Lacerda Pedrosa, S.C.B.; Lafayette, E.A.; da Silva, R.M.F.; de Almeida, S.M.V.; de Moura, R.O.; Ruiz, A.L.T.G.; de Carvalho, J.E. Thiosemicarbazones and 4-thiazolidinones indole-based derivatives: Synthesis, evaluation of antiproliferative activity, cell death mechanisms and topoisomerase inhibition assay. Eur. J. Med. Chem. 2017, 136, 305–314. [Google Scholar] [CrossRef]
- Davies, W.L.; Grunert, R.R.; Haff, R.F.; McGahen, J.W.; Neumayer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral activity of 1-adamantanamine (amantadine). Science 1964, 144, 862–863. [Google Scholar] [CrossRef]
- Wendel, H.A.; Snyder, M.T.; Pell, S. Trial of amantadine in epidemic influenza. Clin. Pharmacol. Ther. 1966, 7, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Vernier, V.G.; Harmon, J.B.; Stump, J.M.; Lynes, T.E.; Marvel, J.P.; Smith, D.H. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol. Appl. Pharmacol. 1969, 15, 642–665. [Google Scholar] [CrossRef]
- Tilley, J.W.; Levitan, P.; Kramer, M.J. Adamantylthiourea derivatives as antiviral agents. J. Med. Chem. 1979, 22, 1009–1010. [Google Scholar] [CrossRef] [PubMed]
- Aigami, K.; Inamoto, Y.; Takaishi, N.; Hattori, K.; Takatsuki, A.; Tamura, G. Biologically active polycycloalkanes. 1. Antiviral adamantane derivatives. J. Med. Chem. 1975, 18, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Basarić, N.; Sohora, M.; Cindro, N.; Mlinarić-Majerski, K.; De Clercq, E.; Balzarini, J. Antiproliferative and antiviral activity of three libraries of adamantane derivatives. Arch. Pharm. 2014, 347, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.S.; El-Emam, A.A.; Gad, L.M.; Barghash, A.E.M. Synthesis, antimicrobial and antiviral testing of some new 1-adamantyl analogues. Saudi Pharm. J. 2010, 18, 123–128. [Google Scholar] [CrossRef]
- Göktaş, F.; Vanderlinden, E.; Naesens, L.; Cesur, N.; Cesur, Z. Microwave assisted synthesis and anti-influenza virus activity of 1-adamantyl substituted N-(1-thia-4-azaspiro[4.5]decan-4-yl)carboxamide derivatives. Bioorg. Med. Chem. 2012, 20, 7155–7159. [Google Scholar] [CrossRef]
- El-Emam, A.A.; Al-Deeb, O.A.; Al-Omar, M.; Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thiones. Bioorg. Med. Chem. 2004, 12, 5107–5113. [Google Scholar] [CrossRef]
- Orzeszko, A.; Kamińska, B.; Orzeszko, G.; Starościak, B.J. Synthesis and antimicrobial activity of new adamantane derivatives II. Il Farm. 2000, 55, 619–623. [Google Scholar] [CrossRef]
- Orzeszko, A.; Gralewska, R.; Starościak, B.J.; Kazimierczuk, Z. Synthesis and antimicrobial activity of new adamantane derivatives I. Acta Biochim. Pol. 2000, 47, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Wang, S.S.; Leeb, C.F.; Chung, M.A.; Chern, Y.T. In vitro antitumor and antimicrobial activities of N-substituents of maleimide by adamantane and diamantane. Chemotherapy 1997, 43, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Wahaibi, L.; Hassan, H.; Abo-Kamar, A.; Ghabbour, H.; El-Emam, A. Adamantane-isothiourea hybrid derivatives: Synthesis, characterization, in vitro antimicrobial, and in vivo hypoglycemic activities. Molecules 2017, 22, 710. [Google Scholar] [CrossRef] [PubMed]
- Balaji, G.L.; Sarveswari, S.; Vijayakumar, V. Synthesis of diversely substituted adamantanes as a new class of antimicrobial agent. Res. Chem. Intermed. 2015, 41, 6765–6776. [Google Scholar] [CrossRef]
- Al-Abdullah, E.; Al-Tuwaijri, H.; Hassan, H.; Al-Alshaikh, M.; Habib, E.; El-Emam, A. Synthesis, antimicrobial and hypoglycemic activities of novel N-(1-adamantyl) carbothioamide derivatives. Molecules 2015, 20, 8125–8143. [Google Scholar] [CrossRef] [PubMed]
- Tabbi, A.; Tebbani, D.; Caporale, A.; Saturnino, C.; Nabavi, S.F.; Giuseppe, P.; Arra, C.; Canturk, Z.; Turan-Zitouni, G.; Merazig, H. New Adamantyl Chalcones: Synthesis, Antimicrobial and Anticancer Activities. Curr. Top. Med. Chem. 2017, 17, 498–506. [Google Scholar] [CrossRef]
- Fesatidou, M.; Zagaliotis, P.; Camoutsis, C.; Petrou, A.; Eleftheriou, P.; Tratrat, C.; Haroun, M.; Geronikaki, A.; Ciric, A.; Sokovic, M. 5-Adamantan thiadiazole-based thiazolidinones as antimicrobial agents. Design, synthesis, molecular docking and evaluation. Bioorg. Med. Chem. 2018, 26, 4664–4676. [Google Scholar] [CrossRef]
- El-Emam, A.A.; Al-Tamimi, A.M.S.; Al-Omar, M.A.; Alrashood, K.A.; Habib, E.E. Synthesis and antimicrobial activity of novel 5-(1-adamantyl)-2-aminomethyl-4-substituted-1,2,4-triazoline-3-thiones. Eur. J. Med. Chem. 2013, 68, 96–102. [Google Scholar] [CrossRef]
- El-Emam, A.A.; Alrashood, K.A.; Al-Omar, M.A.; Al-Tamimi, A.M.S. Synthesis and antimicrobial activity of N’-heteroarylidene-1-adamantylcarbohydrazides and (+/-)-2-(1-adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules 2012, 17, 3475–3483. [Google Scholar] [CrossRef]
- Aguiar, D.F.; Dutra, L.L.A.; Dantas, W.M.; Camelo de Carvalho, G.G.; Gonçalves Lemes, R.P.; do Ó Pessoa, C.; Koscky Paier, C.R.; Barros Araujo, P.L.; Araujo, E.S.; Pena, L.J. Synthesis, Antitumor and Cytotoxic Activity of New Adamantyl O-Acylamidoximes and 3-Aryl-5-Adamantane-1, 2, 4-Oxadiazole Derivatives. Chem. Sel. 2019, 4, 9112–9118. [Google Scholar] [CrossRef]
- Anusha, S.; Mohan, C.D.; Ananda, H.; Baburajeev, C.P.; Rangappa, S.; Mathai, J.; Fuchs, J.E.; Li, F.; Shanmugam, M.K.; Bender, A.; et al. Adamantyl-tethered-biphenylic compounds induce apoptosis in cancer cells by targeting Bcl homologs. Bioorg. Med. Chem. Lett. 2016, 26, 1056–1060. [Google Scholar] [CrossRef] [PubMed]
- Fytas, C.; Zoidis, G.; Tsotinis, A.; Fytas, G.; Khan, M.A.; Akhtar, S.; Rahman, K.M.; Thurston, D.E. Novel 1-(2-aryl-2-adamantyl)piperazine derivatives with antiproliferative activity. Eur. J. Med. Chem. 2015, 93, 281–290. [Google Scholar] [CrossRef]
- Cincinelli, R.; Musso, L.; Giannini, G.; Zuco, V.; De Cesare, M.; Zunino, F.; Dallavalle, S. Influence of the adamantyl moiety on the activity of biphenylacrylohydroxamic acid-based HDAC inhibitors. Eur. J. Med. Chem. 2014, 79, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Sun, J.; Wang, S.S.; Bu, W.; Yao, M.N.; Gao, K.; Song, Y.; Zhao, J.Y.; Lu, C.T.; Zhang, E.H.; et al. Synthesis, crystal structure, superoxide scavenging activity, anticancer and docking studies of novel adamantyl nitroxide derivatives. J. Mol. Struct. 2016, 1108, 611–617. [Google Scholar] [CrossRef]
- Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Hydrazide-Hydrazones with the 1-Adamantyl-Carbonyl Moiety. Molecules 2019, 24, 4000. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Reis, C.M.; Pereira, D.S.; Paiva, R.O.; Kneipp, L.F.; Echevarria, A. Microwave-assisted synthesis of new N1, N4-substituted thiosemicarbazones. Molecules 2011, 16, 10668–10684. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound are available from the authors. |
| Component | R1 | R2 | Yield (%) | m.p (°C) | Molecular Formulae (Molecular Weight) | Rf |
|---|---|---|---|---|---|---|
| 2a | H | H | 97.0 | 210.1–212.2 | C18H23N3S (313.46) | 0.46 |
| 2b | H | 3-NO2 | 92.7 | 244.2–246.1 | C18H22N4O2S (358.46) | 0.38 |
| 2c | H | 4-OCH3 | 95.7 | 224.5–227.7 | C19H25N3OS (343.49) | 0.50 |
| 2d | H | 2-OH | 95.7 | 203.8–205.6 | C18H23N3OS (329.46) | 0.46 |
| 2e | H | 4-NO2 | 91.3 | 258.1–260.1 | C18H22N4O2S (358.46) | 0.43 |
| 2f | H | 4-OC2H5 | 95.6 | 232.2–233.6 | C20H27N3OS (357.52) | 0.54 |
| 2g | H | 4-Cl | 89.6 | 238.9–239.7 | C18H22ClN3S (347.91) | 0.68 |
| 2h | 2-OH | 5-CH3 | 91.0 | 241.6–242.5 | C19H25N3OS (343.49) | 0.54 |
| 2i | 3-NO2 | 4-OC2H5 | 61.2 | 218.7–220.7 | C20H26N4O3S (402.51) | 0.64 |
| 2j | 3-NO2 | 4-Cl | 78.5 | 252.8–254.0 | C18H21ClN4O2S (392.90) | 0.53 |
| 2k | 2-CH3 | 5-CH3 | 92.5 | 212.4–213.8 | C20H27N3S (341.52) | 0.68 |
| 3a | H | H | 91.8 | 231.2–232.7 | C19H25N3S (327.49) | 0.46 |
| 3b | H | 3-NO2 | 67.0 | 251.7–253.5 | C19H24N3O2S (372.49) | 0.47 |
| 3c | H | 4-Br | 65.5 | 240.9–242.9 | C19H24BrN3S (406.39) | 0.46 |
| 3d | H | 4-OH | 44.3 | 272.8–273.5 | C19H25N3OS (343.49) | 0.53 |
| 3e | H | 4-NO2 | 90.4 | 266.5–268.9 | C19H24N4O2S (372.49) | 0.50 |
| 3f | 3-NO2 | 4-Br | 17.5 | 224.5–225.3 | C19H23BrN4O2S (451.38) | 0.53 |
| 3g | H | 4-Cl | 94.0 | 235.0–236.3 | C19H24ClN3S (361.93) | 0.58 |
| 3h | H | 4-CH3 | 73.5 | 230.3–232.2 | C20H27N3S (341.52) | 0.36 |
| 3i | 3-NO2 | 4-OCH3 | 69.3 | 224.6–226.3 | C20H26N4O3S (402.51) | 0.45 |
| 3j | 3-NO2 | 4-Cl | 49.8 | 250.9–252.4 | C19H23ClN4O2S (406.93) | 0.54 |
| Comp. No. | MIC of Synthesized Compounds (μM) | ||||||
|---|---|---|---|---|---|---|---|
| Gram (+) | Gram (−) | Fungus | |||||
| EF | SA | BC | EC | PA | SE | CA | |
| 2a | 100 | 25 | 25 | - | - | - | 25 |
| 2b | 25 | 50 | 25 | - | - | - | 6.25 |
| 2c | 12.5 | 50 | 100 | - | - | - | 6.25 |
| 2d | 25 | - | 100 | - | 100 | - | 25 |
| 2e | 100 | 50 | 50 | - | - | - | 12.5 |
| 2f | 50 | 50 | 50 | - | - | - | 25 |
| 2g | 25 | 25 | 25 | - | - | - | 6.25 |
| 2h | 50 | 25 | 50 | - | 100 | - | 12.5 |
| 2i | 50 | 50 | 25 | - | - | - | 12.5 |
| 2j | 50 | 50 | 50 | - | - | - | 25 |
| 2k | 100 | 25 | 50 | - | - | - | 12.5 |
| 3a | 25 | 25 | 50 | - | - | - | 12.5 |
| 3b | 100 | 25 | 25 | - | - | - | 25 |
| 3c | 100 | 100 | 100 | - | - | - | 25 |
| 3d | 50 | 50 | 50 | - | - | - | 25 |
| 3e | 25 | 25 | 25 | - | - | - | 6.25 |
| 3f | 25 | 50 | 50 | - | - | - | 25 |
| 3g | 25 | 100 | 100 | - | - | - | 25 |
| 3h | 50 | 25 | 50 | - | - | - | 12.5 |
| 3i | 50 | 50 | 50 | - | - | - | 25 |
| 3j | 100 | 25 | 50 | - | - | - | 12.5 |
| STM | 350 | 350 | 175 | 44 | 350 | 175 | NT |
| CHM | NT | NT | NT | NT | NT | NT | 114 |
| Comp. No. | IC50 of Synthesized Compounds (μM) | ||||||
|---|---|---|---|---|---|---|---|
| Gram (+) | Gram (−) | Fungus | |||||
| EF | SA | BC | EC | PA | SE | CA | |
| 2a | 24.78 | 4.78 | 4.12 | - | - | - | 6.78 |
| 2b | 10.78 | 8.99 | 12.45 | - | - | - | 3.57 |
| 2c | 5.68 | 9.66 | 8.24 | - | - | - | 3.45 |
| 2d | 4.89 | - | 25.22 | - | 24.67 | - | 5.35 |
| 2e | 25.89 | 6.78 | 6.09 | - | - | - | 5.56 |
| 2f | 12.78 | 7.88 | 7.82 | - | - | - | 6.35 |
| 2g | 6.78 | 7.89 | 6.88 | - | - | - | 3.24 |
| 2h | 11.67 | 6.24 | 7.56 | - | 27.45 | - | 4.57 |
| 2i | 12.78 | 12.56 | 12.11 | - | - | - | 3.57 |
| 2j | 6.88 | 22.67 | 22.12 | - | - | - | 4.34 |
| 2k | 47.89 | 6.45 | 8.49 | - | - | - | 5.68 |
| 3a | 6.34 | 6.99 | 12.33 | - | - | - | 3.67 |
| 3b | 25.89 | 8.99 | 9.91 | - | - | - | 7.89 |
| 3c | 28.99 | 50.22 | 40.45 | - | - | - | 5.67 |
| 3d | 17.89 | 21.45 | 25.89 | - | - | - | 6.78 |
| 3e | 4.67 | 9.23 | 10.11 | - | - | - | 3.22 |
| 3f | 13.57 | 21.44 | 11.88 | - | - | - | 3.67 |
| 3g | 4.78 | 35.67 | 32.11 | - | - | - | 5.34 |
| 3h | 12.56 | 7.88 | 9.85 | - | - | - | 6.79 |
| 3i | 12.57 | 15.67 | 25.62 | - | - | - | 7.89 |
| 3j | 35.46 | 6.46 | 7.49 | - | - | - | 4.67 |
| Comp. No. | Conc. | Hep3B | Hela | A549 | MCF-7 |
|---|---|---|---|---|---|
| 2a | 30 µM | 69.07 ± 1.37 | 71.58 ± 1.49 | 75.40 ± 1.50 | 58.80 ± 1.23 |
| 100 µM | 64.47 ± 0.86 | 60.07 ± 0.97 | 70.38 ± 0.94 | 49.35 ± 0.79 | |
| 2b | 30 µM | 76.33 ± 1.79 | 55.29 ± 1.10 | 83.32 ± 1.96 | 45.42 ± 0.91 |
| 100 µM | 70.72 ± 0.46 | 53.8 ± 1.41 | 77.2 ± 0.50 | 43.31 ± 2.63 | |
| 2c | 30 µM | 68.6 ± 2.74 | 72.09 ± 2.30 | 76.36 ± 1.82 | 59.22 ± 1.89 |
| 100 µM | 59.84 ± 2.20 | 59.67 ± 1.43 | 65.32 ± 2.40 | 49.02 ± 1.18 | |
| 2d | 30 µM | 19.34 ± 2.54 | 61.12 ± 1.91 | 21.11 ± 2.78 | 50.21 ± 1.57 |
| 100 µM | 16.82 ± 1.60 | 24.55 ± 1.85 | 18.37 ± 1.75 | 20.17 ± 1.52 | |
| 2e | 30 µM | 67.73 ± 1.34 | 72.77 ± 2.42 | 73.94 ± 1.46 | 59.79 ± 1.99 |
| 100 µM | 68.2 ± 0.63 | 61.01 ± 1.16 | 74.45 ± 0.69 | 50.12 ± 0.95 | |
| 2f | 30 µM | 63.5 ± 1.47 | 69.84 ± 1.85 | 69.32 ± 1.61 | 57.38 ± 1.52 |
| 100 µM | 54.53 ± 1.19 | 57.2 ± 2.90 | 59.53 ± 1.30 | 47.00 ± 2.38 | |
| 2g | 30 µM | 76.83 ± 2.31 | 71.65 ± 2.01 | 83.87 ± 2.52 | 42.50 ± 2.35 |
| 100 µM | 70.25 ± 0.41 | 47.9 ± 2.03 | 76.69 ± 0.44 | 39.35 ± 1.67 | |
| 2h | 30 µM | 23.71 ± 0.88 | 44.42 ± 2.35 | 25.88 ± 0.96 | 36.50 ± 1.93 |
| 100 µM | 21.86 ± 0.20 | 34.76 ± 1.36 | 23.86 ± 0.22 | 28.55 ± 1.12 | |
| 2i | 30 µM | 78.88 ± 2.63 | 64.37 ± 1.47 | 86.11 ± 2.88 | 52.89 ± 1.21 |
| 100 µM | 76.06 ± 0.27 | 61.4 ± 0.17 | 83.03 ± 0.29 | 50.45 ± 0.14 | |
| 2j | 30 µM | 65.01 ± 2.17 | 46.16 ± 0.38 | 70.97 ± 2.37 | 37.92 ± 0.31 |
| 100 µM | 62.83 ± 2.23 | 40.66 ± 1.04 | 68.59 ± 2.43 | 33.40 ± 0.86 | |
| 2k | 30 µM | 67.46 ± 1.69 | 69.88 ± 2.12 | 73.64 ± 1.84 | 57.41 ± 1.74 |
| 100 µM | 46.78 ± 0.21 | 55.18 ± 2.92 | 51.06 ± 0.23 | 45.33 ± 2.40 | |
| 3a | 30 µM | 72.06 ± 1.92 | 74.40 ± 1.07 | 78.67 ± 2.09 | 61.12 ± 0.88 |
| 100 µM | 67.93 ± 1.11 | 69.77 ± 0.35 | 74.16 ± 1.22 | 57.32 ± 0.29 | |
| 3b | 30 µM | 75.15 ± 0.36 | 70.17 ± 1.90 | 82.04 ± 0.40 | 57.64 ± 1.56 |
| 100 µM | 71.76 ± 0.48 | 68.65 ± 2.51 | 78.34 ± 0.52 | 56.40 ± 2.06 | |
| 3c | 30 µM | 85.76 ± 2.42 | 81.90 ± 2.11 | 93.62 ± 2.64 | 67.28 ± 1.74 |
| 100 µM | 68.37 ± 1.58 | 64.27 ± 2.47 | 74.63 ± 1.73 | 52.80 ± 2.03 | |
| 3d | 30 µM | 80.15 ± 1.68 | 81.46 ± 1.60 | 87.5 ± 1.83 | 66.92 ± 1.31 |
| 100 µM | 72.57 ± 1.83 | 73.28 ± 2.50 | 79.22 ± 2.00 | 60.20 ± 2.05 | |
| 3e | 30 µM | 67.02 ± 1.37 | 80.59 ± 1.39 | 73.17 ± 1.49 | 66.21 ± 1.14 |
| 100 µM | 53.79 ± 0.71 | 77.66 ± 0.29 | 58.72 ± 0.77 | 63.80 ± 0.24 | |
| 3f | 30 µM | 72.26 ± 1.01 | 77.05 ± 2.19 | 78.89 ± 1.11 | 63.30 ± 1.80 |
| 100 µM | 65.95 ± 0.25 | 67.78 ± 1.64 | 71.99 ± 0.28 | 55.68 ± 1.35 | |
| 3g | 30 µM | 80.42 ± 1.16 | 62.38 ± 0.71 | 87.79 ± 1.27 | 51.25 ± 0.58 |
| 100 µM | 70.65 ± 1.77 | 51.23 ± 0.49 | 77.13 ± 1.94 | 42.09 ± 0.40 | |
| 3h | 30 µM | 75.08 ± 1.11 | 81.90 ± 1.04 | 81.96 ± 1.21 | 67.28 ± 0.85 |
| 100 µM | 67.16 ± 2.57 | 75.92 ± 1.60 | 73.31 ± 2.81 | 62.37 ± 1.31 | |
| 3i | 30 µM | 78.34 ± 0.71 | 77.12 ± 2.03 | 85.52 ± 0.77 | 63.36 ± 1.67 |
| 100 µM | 74.04 ± 0.61 | 56.81 ± 1.81 | 80.83 ± 0.67 | 46.67 ± 1.49 | |
| 3j | 30 µM | 69.54 ± 2.39 | 87.18 ± 1.91 | 75.92 ± 2.61 | 71.62 ± 1.57 |
| 100 µM | 61.25 ± 2.24 | 74.51 ± 2.17 | 66.86 ± 2.45 | 61.21 ± 1.78 | |
| CPT * | 0.3 μM | 69.56 ± 1.27 | 57.06 ± 1.35 | 67.68 ± 1.88 | 56.68 ± 0.68 |
| 14.4 μM | 37.65 ± 1.21 | 18.61 ± 0.56 | 26.74 ± 2.16 | 28.89 ± 1.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, V.H.; Phan, T.P.D.; Phan, D.C.; Vu, B.D. Synthesis and Bioactivity of Thiosemicarbazones Containing Adamantane Skeletons. Molecules 2020, 25, 324. https://doi.org/10.3390/molecules25020324
Pham VH, Phan TPD, Phan DC, Vu BD. Synthesis and Bioactivity of Thiosemicarbazones Containing Adamantane Skeletons. Molecules. 2020; 25(2):324. https://doi.org/10.3390/molecules25020324
Chicago/Turabian StylePham, Van Hien, Thi Phuong Dung Phan, Dinh Chau Phan, and Binh Duong Vu. 2020. "Synthesis and Bioactivity of Thiosemicarbazones Containing Adamantane Skeletons" Molecules 25, no. 2: 324. https://doi.org/10.3390/molecules25020324
APA StylePham, V. H., Phan, T. P. D., Phan, D. C., & Vu, B. D. (2020). Synthesis and Bioactivity of Thiosemicarbazones Containing Adamantane Skeletons. Molecules, 25(2), 324. https://doi.org/10.3390/molecules25020324






