Ammi Visnaga L., a Potential Medicinal Plant: A Review
Abstract
1. Introduction
1.1. Synonyms
1.2. Common Names
1.3. Morphology
1.4. Traditional Folk Medicinal Uses
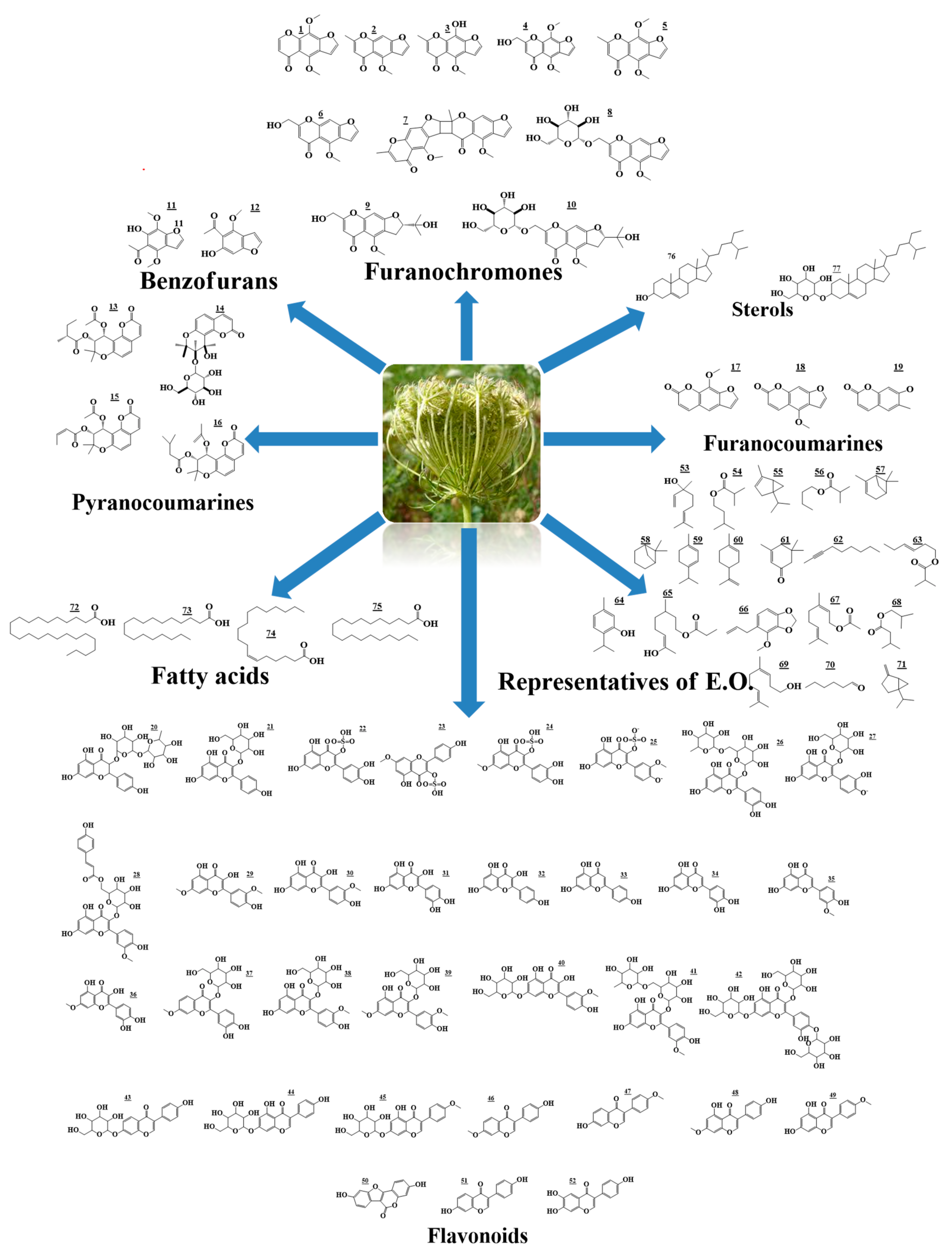
2. Chemical Review
2.1. γ-Pyrones and Coumarins
2.2. Phenolic Compounds
2.2.1. Flavonols
2.2.2. Isoflavones
2.2.3. Flavones as Apigenin, Luteolin and Chrysoeriol
2.3. Essential Oil
2.4. Sterols and Fatty Acids
| No. | Compound | Plant Origin | Plant Part | Reference |
|---|---|---|---|---|
| Furanochromones (γ-Pyrones) | ||||
| 1 | Khellin | Italy | Fruits | [11] |
| 2 | Visnagin | |||
| 3 | Khellinol | Egypt | Fruits | [16] |
| 4 | Ammiol | |||
| 5 | Visamminol | |||
| 6 | Khellol | |||
| 7 | Pimolin | Egypt | Fruits | [17] |
| 8 | Khellinin (khellol glycoside) | Turkey | Fruits | [13] |
| 9 | Cimifugin | Tunisia | Umbels | [10] |
| 10 | Prim-O-glucosyl cimifugin | |||
| Benzofurans | ||||
| 11 | Khellinone | Egypt | Fruits | [16] |
| 12 | Visnaginone | |||
| Pyranocoumarines | ||||
| 13 | Visnadin | Poland | Fruits | [19] |
| 14 | cis-khellactone-3′-β-d-glucopyranoside | Germany | Fruits | [18] |
| 15 | Samidin | Austria | Fruits | [31] |
| 16 | Dihydrosamidin | |||
| Furanocoumarins | ||||
| 17 | Xanthotoxin | Egypt | Fruits | [32] |
| 18 | Bergapten | |||
| 19 | Psoralen | |||
| Flavonoids | ||||
| 20 | Kaempferol-3-rutinoside | England | Leaf, flower and fruit | [21] |
| 21 | Kaempferol-3-glucoside | |||
| 22 | Quercetin3-sulfate | |||
| 23 | Rhamnocitrin-3-sulfate | |||
| 24 | Rhamnetin-3-sulfate | |||
| 25 | Isorhamnetin-3-sulfate | |||
| 26 | Quercetin-3-O-rutinoside | Algeria | Aerial parts | [22] |
| 27 | Quercetin-3-O-glucoside | |||
| 28 | Isorhamnetin 3-O-β-d-glucoside | Egypt | Fruits | [17] |
| 29 | Rhamnazin | Egypt | Fruits and aerial parts | [16] |
| 30 | Isorhamnetin | |||
| 31 | Quercetin | Iraq | Fruits and aerial parts | [33] |
| 32 | Kaempferol | Fruits | [33] | |
| 33 | Apigenin | |||
| 34 | Luteolin | |||
| 35 | Chrysoeriol | |||
| 36 | Rhamnetin | Algeria | Aerial parts | [22] |
| 37 | Rhamnetin-3-O-glucoside | |||
| 38 | Isorhamnetin-3-O-glucoside | |||
| 39 | Rhamnazin-3-glucoside | |||
| 40 | Isorhamnetin-7-O-glucoside | |||
| 41 | Isorhamnetin-3-O-rutinoside | |||
| 42 | Quercetin 7,3,3′-O-triglucoside | |||
| 43 | Daidzin | Czech Republic | Leaves and roots | [23] |
| 44 | Genistin | |||
| 45 | Sissotrin | |||
| 46 | Isoformononetin | |||
| 47 | Formononetin | |||
| 48 | Prunetin | |||
| 49 | Biochanin A | |||
| 50 | Coumestrol | |||
| 51 | Daidzein | |||
| 52 | 4′,6,7-Trihydroxyiso-flavone | |||
| Representatives of essential oil constituents | ||||
| 53 | Linalool | Tunisia | Fruits | [27] |
| 54 | Isoamyl-2-methylbutyrate | |||
| 55 | α-Thujene | |||
| 56 | Butyl isobutyrate | |||
| 57 | α-Pinène | Tunisia | Leaves, stems, flower buds, roots, umbels and fruits | [24,27] |
| 58 | β-Pinene | |||
| 59 | α-Terpinene | |||
| 60 | Limonene | |||
| 61 | α-Isophorone | Algeria | Fresh aerial parts | [26] |
| 62 | 2-Nonyne | |||
| 63 | Hexenyl isobutanoate | |||
| 64 | Thymol | |||
| 65 | Citronellyl propionate | |||
| 66 | Croweacin | |||
| 67 | Geranyl acetate | |||
| 68 | Isobutyl isovalerate | Algeria | Fruits and fresh aerial parts | [34] |
| 69 | Nerol | Fresh aerial parts | ||
| 70 | Hexanal | Algeria | Umbels | [28] |
| 71 | Sabinene | |||
| Fatty acids | ||||
| 72 | Tetracosanoic acid | Egypt | Aerial parts | [29] |
| 73 | Stearic acid | |||
| 74 | Petroselinic acid | Egypt | Fruits | [30] |
| 75 | Arachidic acid | |||
| Sterols | ||||
| 76 | β-sitosterol | Egypt | Aerial parts | [29] |
3. Pharmacological Review
3.1. Kidney Diseases
3.2. Antispasmodic and Vasodilating Effects
3.3. Antidiabetic Activities
3.4. Treatment of Vitiligo
3.5. Anti-inflammatory Effect
3.6. Antimicrobial Effect
3.7. Cytotoxic Activity
3.8. Antioxidant Activity
3.9. Hair Loss
3.10. Antimutagenic Effect
3.11. Cardiovascular Activity
3.12. Immunostimulatory Activity
3.13. Other Reported Activities on Human
3.14. Larvicidal and Insecticidal Activities
3.15. Herbicidal Activity
4. Standardization of A. visnaga Fruit
5. Methods for Analysis of γ-Pyrones (Khellin and Visnagin)
5.1. Spectrophotometric Method
5.2. Gas Chromatography
5.3. Capillary Electrophoresis
5.4. HPLC Methods
5.5. Supercritical Carbon Dioxide Fluid Chromatography
6. Pharmaceutical Products
- Kellagon® (capsules and effervescent sachets), produced by Arab Company for Pharmaceuticals and Medicinal Plants (Mepaco).
- Khellalgin® ampoules, produced by Misr Company.
- Glucolynamine®, produced by Memphis Company.
- Aesrutal® S tablets, produced by Steigerwald Company.
- Carduben® capsules, produced by Madaus Company.
- Khellangan N® tablets, produced by Ardeypharm Company.
- Steno-logs N® tablets, produced by Loges Company.
- Dr. Reckweg R66 arrythmin liquid, produced by Reckweg&Co.
- Ammi visnaga drops, produced by Biotron Laboratories.
- Ammi visnaga granules, produced by Biotron Laboratories.
- Ammi visnaga globules, produced by Biotron Laboratories.
- Vibeline®, produced by Promesa Company.
- United States of America
- Ammi visnaga Pellets, produced by Washington Homeopathic products.
- CL-N liquid, produced by Vitality Works, Inc.
- CN-Tone liquid, produced by Apotheca Company.
- Dr. Reckweg R66 arrythmin liquid, distrubed by Dr. Reckweg America Inc.
- GB-TONE liquid, produced by Apotheca Company.
- Liver Tonic II liquid, produced by Apotheca Company.
- Kellagon® capsules, produced by Mepaco Egypt and distributed by Sprint Pharma Company.
7. Dosage, Contraindications and Warnings
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Woodward, E.F. Botanical Drugs: A Brief Review of the Industry with Comments on Recent Developments. Econ. Bot. 1947, 1, 402–414. [Google Scholar] [CrossRef]
- Batanouny, K.H. Wild Medicinal Plants in Egypt: An Inventory to Support Conservation and Sustainable Use; Acad. of Scientific Research & Technology: Cairo, Egypt, 2001. [Google Scholar]
- Chevallier, A. The Encyclopedia of Medicinal Plants; Dorling Kindersley: London, UK, 1996. [Google Scholar]
- Boulos, L. Flora of Egypt; Al Hadara Publishing: Cairo, Egypt, 2000; Volume 2. [Google Scholar]
- Trinajstić, I. Supplements to the flora of island of Korčula (Croatia). Acta Bot. Croat. 1998, 57, 95–98. [Google Scholar]
- WHO. WHO Monographs on Selected Medicinal Plants; World Health Organization: Geneva, Switzerland, 2007; Volume 3. [Google Scholar]
- Rose, J.; Hulburd, J. The Aromatherapy Book: Applications and Inhalations; North Atlantic Books: Berkely, CA, USA, 1992. [Google Scholar]
- Farnsworth, N.R.; Krause, E.C.; Bolton, J.L.; Pauli, G.F.; van Breemen, R.B.; Graham, J.G. The University of Illinois at Chicago/National Institutes of Health Center for Botanical Dietary Supplements Research for Women’s Health: from plant to clinical use. Am. J. Clin. Nutr. 2008, 87, 504S–508S. [Google Scholar] [CrossRef] [PubMed]
- Miara, M.; Bendif, H.; Rebbas, K.; Rabah, B.; Hammou, M.; Maggi, F. Medicinal plants and their traditional uses in the highland region of Bordj Bou Arreridj (Northeast Algeria). J. Herb. Med. 2019, 16, 100262. [Google Scholar] [CrossRef]
- Sellami, H.K.; Napolitano, A.; Masullo, M.; Smiti, S.; Piacente, S.; Pizza, C. Influence of growing conditions on metabolite profile of Ammi visnaga umbels with special reference to bioactive furanochromones and pyranocoumarins. Phytochemistry 2013, 95, 197–206. [Google Scholar] [CrossRef]
- Franchi, G.G.; Ferri, S.; Bovalini, L.; Martelli, P. Ammi visnaga (L.) Lam.: Occurrence of Khellin and Visnagin in Primary Rib Channels and Endosperm, and Emptiness of Vittae, Revealed by U.V. Microscopy. Int. J. Crude Drug Res. 1987, 25, 137–144. [Google Scholar] [CrossRef]
- Mesbah, M.K. Determination of khellin and visnagin in Ammi visnaga fruits and in renal teas by high−performance liquid chromatography. Egypt. J. Pharm. Sci. 1992, 33, 897–904. [Google Scholar]
- Guenaydin, K.; Beyazit, N. The chemical investigations on the ripe fruits of Ammi visnaga (Lam.) Lamarck growing in Turkey. Nat. Prod. Res. 2004, 18, 169–175. [Google Scholar] [CrossRef]
- Zrira, S.; Elamrani, A.; Pellerin, P.; Bessiere, J.M.; Menut, C.; Benjilali, B. Isolation of Moroccan Ammi visnaga oil: comparison between hydrodistillation, steam distillation and supercritical fluid extraction. J. Essent. Oil-Bear. Plants 2008, 11, 30–35. [Google Scholar] [CrossRef]
- Hashim, S.; Jan, A.; Marwat, K.B.; Khan, M.A. Phytochemistry and medicinal properties of Ammi visnaga (Apiacae). Pak. J. Bot. 2014, 46, 861–867. [Google Scholar]
- Abou-Mustafa, E.A.; Saleh, N.A.; Elgamal, M.H.; Shalaby, N.M.; Duddeck, H. A Further Contribution to the gamma-Pyrone Constituents of Ammi visnaga Fruits. Planta Med. 1990, 56, 134. [Google Scholar] [CrossRef] [PubMed]
- Elgamal, M.H.A.; Shalaby, N.M.M.; El-Hagrassy, A.M.; Toth, G.; Simon, A.; Duddeck, H. A further contribution to some gamma-pyrone constituents of Ammi visnaga. Fitoterapia 1998, 69, 549–550. [Google Scholar]
- Sonnenberg, H.; Kaloga, M.; Eisenbach, N.; Froemming, K.K. Isolation and characterization of an angular-type dihydropyranocoumarine glycoside from the fruits of Ammi visnaga (L.) Lam. (Apiaceae). Z. Fur Nat. 1995, 50, 729–731. [Google Scholar]
- Zgorka, G.; Dragan, T.; Glowniak, K.; Basiura, E. Determination of furanochromones and pyranocoumarins in drugs and Ammi visnaga fruits by combined solid-phase extraction-high-performance liquid chromatography and thin-layer chromatography-high-performance liquid chromatography. J. Chromatogr. A 1998, 797, 305–309. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Rezaee, M.B.; Sakuda, S. Natural Aflatoxin Inhibitors from Medicinal Plants. In Mycotoxins in Food, Feed and Bioweapons; Rai, M., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 329–352. [Google Scholar]
- Harborne, J.B.; King, L. Flavonoid sulphates in the Umbelliferae. Biochem. Syst. Ecol. 1976, 4, 111–115. [Google Scholar] [CrossRef]
- Bencheraiet, R.; Kherrab, H.; Kabouche, A.; Kabouche, Z.; Jay, M. Flavonols and antioxidant activity of Ammi visnaga L. (Apiaceae). Rec. Nat. Prod. 2011, 5, 52–55. [Google Scholar]
- Abdulmanea, K.; Prokudina, E.A.; Lanková, P.; Vaníčková, L.; Koblovská, R.; Zelený, V.; Lapčík, O. Immunochemical and HPLC identification of isoflavonoids in the Apiaceae family. Biochem. Syst. Ecol. 2012, 45, 237–243. [Google Scholar] [CrossRef]
- Abdul-Jalil, T.Z.; Saour, K.; Nasser, A.A. Phytochemical Study of some Flavonoids Present in the Fruits of Two Ammi L. Species Wildly Grown in Iraq. Iraqi J. Pharma. Sci. 2010, 19, 48–57. [Google Scholar]
- Sellami, H.; Flamini, G.; Cioni, P.; Smiti, S. Composition of the essential oils in various organs at different developmental stages of Ammi visnaga (L.) Lam. from Tunisia. Chem. Biodivers. 2011, 8, 1990–2004. [Google Scholar] [CrossRef]
- Soro, K.N.; Sabri, L.; Amalich, S.; Khabbal, Y.; Zair, T. Chemical composition of Moroccan Ammi visnaga L. (Lam.) and antibacterial activity of its essential oil against extended-spectrum beta-lactamase–producing and not producing bacteria. Phytotherapie 2015, 13, 168–175. [Google Scholar] [CrossRef]
- Khalfallah, A.; Labed, A.; Semra, Z.; Al kaki, B.; Kabouche, A.; Touzani, R.; Kabouche, Z. Antibacterial activity and chemical composition of the essential oil of Ammi visnaga L. (Apiaceae) from Constantine, Algeria. Inter. J. Med. Aromat. Plants 2011, 1, 302–305. [Google Scholar]
- Khadhri, A.; ElMokni, R.; Mguis, K.; Ouerfelli, I.; Araujo, M. Variability of two essential oils of Ammi visnaga (L.) Lam. a traditional Tunisian medicinal plant. J. Med. Plants Res. 2011, 5, 5079–5082. [Google Scholar]
- Keddad, A.; Baaliouamer, A.; Hazzit, M. Chemical Composition and Antioxidant Activity of Essential Oils from Umbels of Algerian Ammi visnaga (L.). J. Essent. Oil-Bear. Plants 2016, 19, 1243–1250. [Google Scholar] [CrossRef]
- Ashour, A.; El Sharkawy, S.; Amer, M.; Abdelbar, F.; Kondo, R. Melanin Biosynthesis Inhibitory Activity of Compounds Isolated from Unused Parts of Ammi visinaga. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 40–43. [Google Scholar]
- Nguyen, T.; Aparicio, M.; Saleh, M. Accurate mass GC/LC-quadrupole time of flight mass spectrometry analysis of fatty acids and triacylglycerols of spicy fruits from the Apiaceae Family. Molecules 2015, 20, 21421–21432. [Google Scholar] [CrossRef] [PubMed]
- Winderl, B.; Schwaiger, S.; Ganzera, M. Fast and improved separation of major coumarins in Ammi visnaga (L.) Lam. by supercritical fluid chromatography. J. Sep. Sci. 2016, 39, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Mabrouk, S.S.; El-Shayeb, N.M.A. Inhibition of aflatoxin production in Aspergillus flavus by natural coumarins and chromones. World J. Microbiol. Biotechnol. 1992, 8, 60–62. [Google Scholar] [CrossRef] [PubMed]
- Feirouz, B.; Salima, K. Antibacterial activity and chemical composition of Ammi visnaga l. Essential oil collected from Boumerdes (Algeria) during three periods of the plant growth. J. Essent. Oil-Bear. Plants 2014, 17, 1317–1328. [Google Scholar] [CrossRef]
- Sayed, M.D. Traditional medicine in health care. J. Ethnopharmacol. 1980, 2, 19–22. [Google Scholar] [CrossRef]
- Al-douri, N.A. A Survey of Medicinal Plants and Their Traditional Uses in Iraq. Pharm. Biol. 2000, 38, 74–79. [Google Scholar] [CrossRef]
- Said, O.; Khalil, K.; Fulder, S.; Azaizeh, H. Ethnopharmacological survey of medicinal herbs in Israel, the Golan Heights and the West Bank region. J. Ethnopharmacol. 2002, 83, 251–265. [Google Scholar] [CrossRef]
- Azaizeh, H.; Saad, B.; Khalil, K.; Said, O. The State of the Art of Traditional Arab Herbal Medicine in the Eastern Region of the Mediterranean: A Review. Evid. -Based Complementary Altern. Med. 2006, 3, 229–235. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Zaid, A.; Al-Ramahi, R.; Alqub, M.A.; Hussein, F.; Hamdan, Z.; Mustafa, M.; Qneibi, M.; Ali, I. Ethnopharmacological survey of medicinal plants practiced by traditional healers and herbalists for treatment of some urological diseases in the West Bank/Palestine. BMC Complementary Altern. Med. 2017, 17, 255. [Google Scholar] [CrossRef]
- Khan, Z.A.; Assiri, A.M.; Al-Afghani, H.M.A.; Maghrabi, T.M.A. Inhibition of oxalate nephrolithiasis with Ammi visnaga (AI-Khillah). Int. Urol. Nephrol. 2002, 33, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Vanachayangkul, P. Ammi visnaga L. for the Prevention of Urolithiasis. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2008. [Google Scholar]
- Vanachayangkul, P.; Byer, K.; Khan, S.; Butterweck, V. An aqueous extract of Ammi visnaga fruits and its constituents khellin and visnagin prevent cell damage caused by oxalate in renal epithelial cells. Phytomedicine 2010, 17, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Charafi, S.; Kzaiber, F.; Hafid, A.; Berkani, M.; Oussama, A. Study of Ammi visnaga Lam on oxalocalcic crystallization. Glob. J. Tradit. Med. Sys. 2012, 1, 7–12. [Google Scholar]
- Nagal, A.; Singla, R.K. Herbal Resources with Antiurolithiatic Effects: A Review. Indo Glob. J. Pharm. Sci. 2013, 3, 6–14. [Google Scholar]
- Nirumand, M.C.; Hajialyani, M.; Rahimi, R.; Farzaei, M.H.; Zingue, S.; Nabavi, S.M.; Bishayee, A. Dietary Plants for the Prevention and Management of Kidney Stones: Preclinical and Clinical Evidence and Molecular Mechanisms. Int. J. Mol. Sci. 2018, 19, 765. [Google Scholar] [CrossRef]
- Kilicaslan, I.; Coskun, S. Spontaneous stone passage: Is it Ammi visnaga effect? Urol. Res. 2012, 40, 799–800. [Google Scholar] [CrossRef]
- Khater, S.I.; Kandil, S.A.; Hussien, H. Preparation of radioiodinated khellin for the urinary tract imaging. J. Radioanal. Nucl. Chem. 2013, 295, 1939–1944. [Google Scholar] [CrossRef]
- Haug, K.G. Exploring the effects of Ammi visnaga L. on Nephrolithiasis Prevention: In vivo Pharmacokinetic and Pharmacodynamic Evaluation of Ammi visnaga L. Extract and Visnagin. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2013. [Google Scholar]
- Bhagavathula, A.S.; MahmoudAlKhatib, A.J.; Elnour, A.A.; AlKalbani, N.M.S.; Shehab, A. Ammi visnaga in treatment of urolithiasis and hypertriglyceridemia. Pharmacogn. Res. 2015, 7, 397–400. [Google Scholar]
- Abdel–Aal, E.A.; Yassin, A.M.K.; El-Shahat, M.F. Inhibition of nucleation and crystallisation of kidney stone (calcium oxalate monohydrate) using Ammi visnaga (Khella) plant extract. Int. J. Nano Biomaterial. 2016, 6, 110–126. [Google Scholar]
- Balandrin, M.F.; Kinghorn, A.D.; Farnsworth, N.R. Plant-Derived Natural Products in Drug Discovery and Development. In Human Medicinal Agents from Plants; American Chemical Society: Washington, DC, USA, 1993; Volume 534, pp. 2–12. [Google Scholar]
- Al-Snafi, A.E. A Review of Medicinal Plants with Broncho-Dilatory Effect-Part 1. Sch. Acad. J. Pharm. 2016, 5, 297–304. [Google Scholar] [CrossRef]
- Rauwald, H.W.; Brehm, O.; Odenthal, K.P. The involvement of a Ca2+ channel blocking mode of action in the pharmacology of Ammi visnaga fruits. Planta Med. 1994, 60, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Duarte, J.; Vallejo, I.; Perez-Vizcaino, F.; Jimenez, R.; Zarzuelo, A.; Tamargo, J. Effects of visnadin on rat isolated vascular smooth muscles. Planta Med. 1997, 63, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on Natural Coumarin Lead Compounds for Their Pharmacological Activity. BioMed Res. Inter. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Ubeda, A.; Tejerina, T.; Tamargo, J.; Villar, A. Effects of khellin on contractile responses and 45Ca2+ movements in rat isolated aorta. J. Pharm. Pharmacol. 1991, 43, 46–48. [Google Scholar] [CrossRef]
- Campos-Toimil, M.; Orallo, F.; Santana, L.; Uriarte, E. Synthesis and Vasorelaxant Activity of New Coumarin and Furocoumarin Derivatives. Bioorg. Med. Chem. Lett. 2002, 12, 783–786. [Google Scholar] [CrossRef]
- Tripathi, Y.; Pandey, A. Bioprospecting of Phytodiversity for New Therapeutic Products:Trends, Potential and Challenges. Org. Med. Chem. 2017, 2, 1–7. [Google Scholar]
- Duarte, J.; Perez-Vizcaino, F.; Torres, A.I.; Zarzuelo, A.; Jimenez, J.; Tamargo, J. Vasodilator effects of visnagin in isolated rat vascular smooth muscle. Eur. J. Pharm. 1995, 286, 115–122. [Google Scholar] [CrossRef]
- Duarte, J.; Lugnier, C.; Torres, A.I.; Pérez-Vizcaino, F.; Zarzuelo, A.; Tamargo, J. Effects of Visnagin on Cyclic Nucleotide Phosphodiesterases and Their Role in its Inhibitory Effects on Vascular Smooth Muscle Contraction. Gen. Pharm. Vasc. Sys. 1999, 32, 71–74. [Google Scholar] [CrossRef]
- Duarte, J.; Torres, A.I.; Zarzuelo, A. Cardiovascular Effects of Visnagin on Rats. Planta Med. 2000, 66, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Jarald, E.; Joshi, S.B.; Jain, D.C. Diabetes VS Herbal Medicines. Iran. J. Pharm. Ther. 2008, 7, 97–100. [Google Scholar]
- Dalila, B.; Smahane, B.; Abderrahman, A.; Mohamed, G.; Latifa, E. Ethnopharmacological Study of anti–diabetic medicinal plants used in the Middle-Atlas region of Morocco (Sefrou region). Int. J. Pharma. Res. Health Sci. 2014, 2, 75–79. [Google Scholar]
- Yaniv, Z.; Dafni, A.; Friedman, J.; Palevitch, D. Plants used for the treatment of diabetes in Israel. J. Ethnopharma. 1987, 19, 145–151. [Google Scholar] [CrossRef]
- Hassan, J.; Mhamed, M.; Mohammed, E. Hypoglycemic effect of aqueous extract of Ammi visnaga in normal and streptozotocin-induced diabetic rats. J. Herb. Pharma. 2002, 2, 19–29. [Google Scholar]
- Bnouham, M.; Mekhfi, H.; Abdelkhaleq, L.; Ziyyat, A. Ethnopharmacology Forum Medicinal Plants used in the treatment of diabetes in Morocco. Int. J. Diabetes Metab. 2002, 10, 33–50. [Google Scholar]
- Orecchia, G.; Sangalli, M.; Gazzaniga, A.; Giordano, F. Topical photochemotherapy of vitiligo with a new khellin formulation. J. Dermatol. Treat. 1998, 9, 65–69. [Google Scholar] [CrossRef]
- Hofer, A.; Kerl, H.; Wolf, P. Long-term results in the treatment of vitiligo with oral khellin plus UVA. Eur. J. Dermatol. 2001, 11, 225–229. [Google Scholar]
- De Leeuw, J.; Van der Beek, N.; Maierhofer, G.; Neugebauer, W.D. A case study to evaluate the treatment of vitiligo with khellin encapsulated in L-phenylalanin stabilized phosphatidylcholine liposomes in combination with ultraviolet light therapy. Eur. J. Dermatol. 2003, 13, 474–477. [Google Scholar]
- De Leeuw, J.; Assen, Y.J.; van der Beek, N.; Bjerring, P.; Martino Neumann, H.A. Treatment of vitiligo with khellin liposomes, ultraviolet light and blister roof transplantation. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, J.; Park, S.; Sim, Y.; Kim, S.; Ha, T.; Suh, H. Anti-inflammatory effect of visnagin in lipopolysaccharide-stimulated BV-2 microglial cells. Arch. Pharma. Res. 2010, 33, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lee, J.; Park, S.; Sim, Y.; Jung, J.; Won, M.; Kim, S.; Suh, H. Neuroprotective effect of visnagin on kainic acid-induced neuronal cell death in the mice hippocampus. Korean J. Physiol. Pharma. 2010, 14, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.M.; Jaffer, H.J.; Alnaib, A.; Naji, A. Antimicrobial Activity of Sesquiterpene Lactone and Alkaloid Fractions from Iraqi-Plants. Int. J. Crude Drug Res. 1988, 26, 185–188. [Google Scholar] [CrossRef]
- Grange, J.M.; Davey, R.W. Detection of antituberculous activity in plant extracts. J. Appl. Bacteriol. 1990, 68, 587–591. [Google Scholar] [CrossRef]
- Mahmoud, A.L.E. Inhibition of growth and aflatoxin biosynthesis of Aspergillus flavus by extracts of some Egyptian plants. Lett. Appl. Microbiol. 1999, 29, 334–336. [Google Scholar] [CrossRef]
- Semyari, H.; Owlia, P.; Farhadi, S.; Tabrizi, S. Evaluation of antimicrobial effect of “Ammi visnaga” against oral streptococci. J. Microbiol. Antimicrob. 2011, 3, 126–129. [Google Scholar]
- Ghareeb, A.M.; Zedan, T.H.; Gharb, L.A. Antibacterial and antifungal activities of Ammi visnaga extracts against pathogenic microorganisms. Iraqi J. Sci. 2011, 52, 30–36. [Google Scholar]
- Satrani, B.; Farah, A.; Fechtal, M.; Talbi, M.; Bouamrani, M.L. Chemical composition and antibacterial and antimycotic activities of the essential oil of Ammi visnaga (L.) Lam. from Morocco. Acta Bot. Gall. 2004, 151, 65–71. [Google Scholar] [CrossRef]
- Rasooli, I.; Taghizadeh, M.; Astaneh, S.D.A.; Rezaei, M.B.; Jaimand, K. Phytobiological properties of Ammi visnaga L. and Lavandula angustifolia Mill. essential oils. Int. J. Essent. Oil Ther. 2007, 1, 72–78. [Google Scholar]
- Cordero, C.P.; Gómez-González, S.; León-Acosta, C.J.; Morantes-Medina, S.J.; Aristizabal, F.A. Cytotoxic activity of five compounds isolated from Colombian plants. Fitoterapia 2004, 75, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Monem, A.; Maged, A.; Zeinab, E.; Fawkia, A. Biological Activity of the Egyptian Medicinal Plants: Part 4 Cytotoxicity of 50 Egyptian Plants and Spices Against Hepatocellular Carcinoma. Am. J. Ethnomed. 2014, 1, 56–63. [Google Scholar]
- Pakfetrat, H.; Nemati, N.; Shiravi, A. Cytotoxicity effects OF Ammi visnaga extract on Hela and MCF7 cancer cell lines. J. Anim. Bio. 2015, 7, 25–33. [Google Scholar]
- Beltagy, A.M.; Beltagy, D.M. Chemical composition of Ammi visnaga L. and new cytotoxic activity of its constituents Khellin and Visnagin. J. Pharma. Sci. Res. 2015, 7, 285–291. [Google Scholar]
- Curri, S.B.; Bombardelli, E. Pharmaceutical compositions having activity on the cutaneous microcirculation. US patent 5,176,919, 1 May 1993. [Google Scholar]
- Schimmer, O.; Rauch, P. Inhibition of metabolic activation of the promutagens, benzo[a]pyrene, 2–aminofluorene and 2-aminoanthracene by furanochromones in Salmonella typhimurium. Mutagenesis 1998, 13, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, A.; Aarab, L.; Abdel-Sattar, E. Screening of immunomodulatory activity of total and protein extracts of some Moroccan medicinal plants. Toxicol. Ind. Health 2013, 29, 245–253. [Google Scholar] [CrossRef]
- Gouda, Y.G.; Hamam, F.S.; AlRemawi, M.M.; Abdallah, M.A.; AlAbbadi, M.A.; AlShehary, S.M.; AlGohary, R.T.; Mohamed, K.M. Study of analgesia caused by some commonly used herbs in the kingdom of Saudi Arabia. Ann. Biol. Sci. 2014, 2, 48–55. [Google Scholar]
- Maleck, M.; Dos Santos, F.C.C.; Serdeiro, M.T.; Guimaraes, A.E.; Ferreira, B.; Gunaydin, K.; de Almeida, A.P. Khellin: a furanochromone with toxicity against Oncopeltus fasciatus (Hemiptera) and Aedes aegypti (Diptera). J. Nat. Pharma. 2013, 4, 32–36. [Google Scholar] [CrossRef]
- Pavela, R. Acaricidal properties of extracts and major furanochromenes from the seeds of Ammi visnaga Linn. against Tetranychus urticae Koch. Ind. Crop. Prod. 2015, 67, 108–113. [Google Scholar] [CrossRef]
- Travaini, M.L.; Sosa, G.M.; Ceccarelli, E.A.; Walter, H.; Cantrell, C.L.; Carrillo, N.J.; Dayan, F.E.; Meepagala, K.M.; Duke, S.O. Khellin and Visnagin, Furanochromones from Ammi visnaga (L.) Lam., as Potential Bioherbicides. J. Agric. Food Chem. 2016, 64, 9475–9487. [Google Scholar] [CrossRef]
- Ghoneim, K.; Al-Daly, A.; Amer, M.; Mohammad, A.; Khadrawy, F.; Mahmoud, M.A. Effects of Ammi visnaga L. (Apiaceae) extracts on the main metabolites in haemolymph and fat bodies of Schistocerca gregaria (Forskal)(Orthoptera: Acrididae). J. Adv. Zool. 2014, 1, 11–23. [Google Scholar]
- Ghoneim, K.; Al-Daly, A.; Khadrawy, F.; Amer, M.; Mahmoud, M.A. Efficacy of the toothpick weed Ammi visnaga L. (Apiaceae) fruit extracts on transaminase activity in certain tissues of Schistocerca gregaria (Forskal)(Orthoptera: Acrididae). Int. J. Entomol. Res. 2016, 1, 19–28. [Google Scholar]
- Buriak, V.P.; Kurinna, N.V. Spectrophotometric determination of kellin and santonin. Farmatsevtychnyi Zhurnal 1971, 26, 39–42. [Google Scholar]
- Carlin, A.S.; Simmons, J.E.; el-Arini, S.K.; Shiu, G.K. Determination of khellin in serum by gas chromatography. J. Chromatogr. 1993, 614, 324–327. [Google Scholar] [CrossRef]
- Gunaydin, K.; Erim, F.B. Determination of khellin and visnagin in Ammi visnaga fruits by capillary electrophoresis. J. Chromatogr. A 2002, 954, 291–294. [Google Scholar] [CrossRef]
- Badr, J.M.; Hadad, G.M.; Nahriry, K.; Hassanean, H.A. Validated HPLC method for simultaneous estimation of khellol glucoside, khellin and visnagin in Ammi visnaga L. fruits and pharmaceutical preparations. Nat. Prod. Res. 2015, 29, 593–601. [Google Scholar] [CrossRef]
- El-Domiaty, M.M. Improved high-performance liquid chromatographic determination of khellin and visnagin in Ammi visnaga fruits and pharmaceutical formulations. J. Pharm. Sci. 1992, 81, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Alqasoumi, S.; Alam, P.; Anwer, M.; Abdel-Kader, M. Qualitative and quantitative analysis of khellin in Ammi visnaga fruits and pharmaceutical preparations using HPTLC and HPLC. J. Liq. Chromatogr. Relat. Tech. 2014, 37, 61–72. [Google Scholar] [CrossRef]
- Bishr, M.; El Degwy, M.; Amin, M.; Salama, O. HPLC Simultaneous Determination of Khellin and Visnagin in Ammi visnaga L. Fruits. J. Pharm. Bio. Sci. 2016, 11, 110–115. [Google Scholar]
- Bishr, M.; El-Degwy, M.; Abdel Hady, M.; Amin, M.; Salama, O. Supercritical fluid extraction of γ-Pyrones from Ammi visnaga L. fruits. Future J. Pharm. Sci. 2018, 4, 57–62. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, N.; Bishr, M.; Desouky, S.; Salama, O. Ammi Visnaga L., a Potential Medicinal Plant: A Review. Molecules 2020, 25, 301. https://doi.org/10.3390/molecules25020301
Khalil N, Bishr M, Desouky S, Salama O. Ammi Visnaga L., a Potential Medicinal Plant: A Review. Molecules. 2020; 25(2):301. https://doi.org/10.3390/molecules25020301
Chicago/Turabian StyleKhalil, Noha, Mokhtar Bishr, Samar Desouky, and Osama Salama. 2020. "Ammi Visnaga L., a Potential Medicinal Plant: A Review" Molecules 25, no. 2: 301. https://doi.org/10.3390/molecules25020301
APA StyleKhalil, N., Bishr, M., Desouky, S., & Salama, O. (2020). Ammi Visnaga L., a Potential Medicinal Plant: A Review. Molecules, 25(2), 301. https://doi.org/10.3390/molecules25020301





