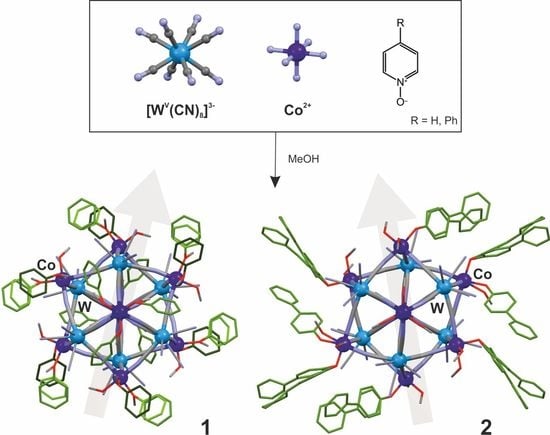
Structural Disorder in High-Spin {CoII9WV6} (Core)-[Pyridine N-Oxides] (Shell) Architectures
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural Studies
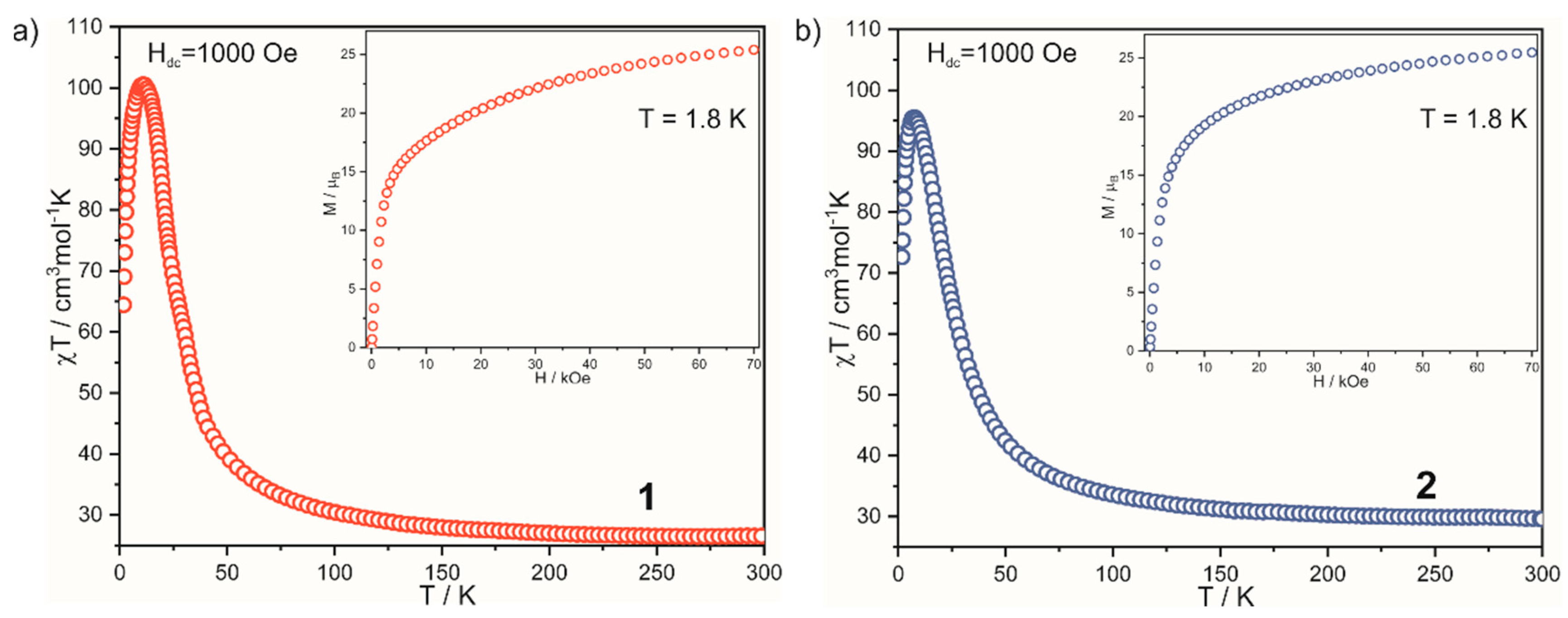
2.2. Magnetic Properties
3. Experimental
3.1. Reagents and Materials
3.2. X-ray Diffraction Analysis
3.3. Physical Techniques and Calculations
3.4. Synthetic Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dong, D.-P.; Liu, T.; Kanegawa, S.; Kang, S.; Sato, O.; He, C.; Duan, C.-Y. Photoswitchable dynamic magnetic relaxation in a well-isolated {Fe2Co} double-zigzag chain. Angew. Chem. Int. Ed. 2012, 51, 5119–5123. [Google Scholar] [CrossRef]
- Mondal, A.; Chamoreau, L.M.; Li, Y.; Journaux, Y.; Seuleiman, M.; Lescouëzec, R. W-Co discrete complex exhibiting photo- and thermo-induced magnetisation. Chem. Eur. J. 2013, 19, 7682–7685. [Google Scholar] [CrossRef]
- Daffé, N.; Jiménez, J.R.; Studniarek, M.; Benchohra, A.; Arrio, M.A.; Lescouëzec, R.; Dreiser, J. Direct observation of charge transfer and magnetism in Fe4Co4 cyanide-bridged molecular cubes. J. Phys. Chem. Lett. 2019, 10, 1799–1804. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Dolinar, B.S.; Liu, S.; Brown, A.J.; Zhang, X.; Wang, Z.X.; Dunbar, K.R. Enforcing Ising-like magnetic anisotropy via trigonal distortion in the design of a W(V)-Co(II) cyanide single-chain magnet. Chem. Sci. 2018, 9, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Nakabayashi, K.; Imoto, K.; Mlynarski, J.; Sieklucka, B.; Ohkoshi, S. Conjunction of chirality and slow magnetic relaxation in the supramolecular network constructed of crossed cyano-bridged CoII–WV molecular chains. J. Am. Chem. Soc. 2012, 134, 16151–16154. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.M.; Cao, F.; Li, J.; Yang, L.; Han, Y.; Zhang, X.L.; Wang, X.Y.; Song, Y. Single-chain magnets based on octacyanotungstate with the highest energy barriers for cyanide compounds. Sci. Rep. 2016, 6, 24372. [Google Scholar] [CrossRef]
- Pichon, C.; Suaud, N.; Duhayon, C.; Guihery, N.; Sutter, J.P. Cyano-bridged Fe(II)–Cr(III) single-chain magnet based on pentagonal bipyramid units: On the added value of aligned axial anisotropy. J. Am. Chem. Soc. 2018, 140, 7698–7704. [Google Scholar] [CrossRef]
- Venkatakrishnan, T.S.; Sahoo, S.; Brefuel, N.; Duhayon, C.; Paulsen, C.; Barra, A.L.; Ramasesha, S.; Sutter, J.P. Enhanced ion anisotropy by nonconventional coordination geometry: Single-chain magnet behavior for a [{FeIIL}2{NbIV(CN)8}] helical chain compound designed with heptacoordinate FeII. J. Am. Chem. Soc. 2010, 132, 6047–6056. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Southerland, H.I.; Avendano, C.; Prosvirin, A.; Sanders, C.; Wernsdorfer, W.; Pedersen, K.S.; Dreiser, J.; Clerac, R.; Nehrkorn, J.; et al. Cyanide single-molecule magnets exhibiting solvent dependent reversible “on” and “off” exchange bias behavior. J. Am. Chem. Soc. 2015, 137, 14406–14422. [Google Scholar] [CrossRef]
- Visinescu, D.; Alexandru, M.G.; Madalan, A.M.; Pichon, C.; Duhayon, C.; Sutter, J.P.; Andruh, M. Magneto-structural variety of new 3d–4f–4 (5) d heterotrimetallic complexes. Dalton Trans. 2015, 44, 16713–16727. [Google Scholar] [CrossRef]
- Kumar, K.; Stefańczyk, O.; Chorazy, S.; Nakabayashi, K.; Sieklucka, B.; Ohkoshi, S. Effect of noble metals on luminescence and single-molecule magnet behavior in the cyanido-bridged Ln–Ag and Ln–Au (Ln = Dy, Yb, Er) complexes. Inorg. Chem. 2019, 58, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, P.; Ren, X.M.; Shen, X.F.; Li, Y.Z.; You, X.Z. Octacyanometallate-based single-molecule magnets: CoII9WV6 (M = W, Mo). J. Am. Chem. Soc. 2005, 127, 3708–3709. [Google Scholar] [CrossRef] [PubMed]
- Chorazy, S.; Hoczek, A.; Kubicki, M.; Tokoro, H.; Ohkoshi, S.; Sieklucka, B.; Podgajny, R. The solvent effect on the structural and magnetic features of bidentate ligand-capped {CoII9[WV(CN)8]6} single-molecule magnets. Cryst. Eng. Comm. 2016, 18, 1495–1504. [Google Scholar] [CrossRef]
- Chorazy, S.; Rams, M.; Hoczek, A.; Czarnecki, B.; Sieklucka, B.; Ohkoshi, S.; Podgajny, R. Structural anisotropy of cyanido-bridged {Co II9 W V6} single-molecule magnets induced by bidentate ligands: Towards the rational enhancement of an energy barrier. Chem. Commun. 2016, 52, 4772–4775. [Google Scholar] [CrossRef]
- Chorazy, S.; Reczyński, M.; Podgajny, R.; Nogas, W.; Buda, S.; Rams, M.; Nitek, W.; Nowicka, B.; Mlynarski, J.; Ohkoshi, S.; et al. Implementation of chirality into high-spin ferromagnetic CoII9WV6 and NiII9WV6 cyanido-bridged clusters. Cryst. Growth Des. 2015, 15, 3573–3581. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Augustyniak, K.; Chorazy, S.; Nowicka, B.; Pinkowicz, D.; Kozieł, M.; Muziol, T.; Podgajny, R. Cyanido-bridged clusters with remote N-oxide groups for branched multimetallic systems. Cryst. Growth Des. 2018, 18, 4766–4776. [Google Scholar] [CrossRef]
- Chorazy, S.; Podgajny, R.; Nitek, W.; Rams, M.; Ohkoshi, S.; Sieklucka, B. Supramolecular chains and coordination nanowires constructed of high-spin CoII9W V6 clusters and 4,4′-bpdo linkers. Cryst. Growth Des. 2013, 13, 3036–3045. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Nowicka, B.; Chorazy, S.; Reczynski, M.; Sieklucka, B. Magnetic clusters based on octacyanidometallates. Inorg. Chem. Front. 2015, 2, 10–27. [Google Scholar] [CrossRef]
- Liberka, M.; Kobylarczyk, J.; Muziol, T.M.; Ohkoshi, S.; Chorazy, S.; Podgajny, R. A heterotrimetallic synthetic approach in versatile functionalization of nanosized {MxCu13–xW7}3+ and {M1Cu8W6} (M = Co, Ni, Mn, Fe) metal—Cyanide magnetic clusters. Inorg. Chem. Front. 2019, 6, 3104–3118. [Google Scholar] [CrossRef]
- Kobylarczyk, J.; Liberka, M.; Konieczny, P.; Baran, S.; Kubicki, M.; Korzeniak, T.; Podgajny, R. Bulky ligands shape the separation between the large spin carriers to condition field-induced slow magnetic relaxation. Dalton Trans. 2020, 49, 300–311. [Google Scholar] [CrossRef]
- Szklarzewicz, J.; Matoga, D.; Lewiński, K. Photocatalytical decomposition of hydrazine in K4[Mo(CN)8] solution: X-ray crystal structure of (PPh4)2[Mo(CN)4O(NH3)]·2H2O. Inorg. Chim. Acta 2007, 360, 2002–2008. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0-new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Dimagnetic corrections and pascal’s constants. J. Chem. Educ. 2008, 85, 532. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE v. 2.1. Program. for the Calculation of Continuous Shape Measures of Polygonal and Polyhedral Molecular Fragments; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Nakabayashi, K.; Chorazy, S.; Komine, M.; Miyamoto, Y.; Takahashi, D.; Sieklucka, B.; Ohkoshi, S. Magnetic Lotus root based on a cyanido-bridged Co–W metal assembly. Cryst. Growth Des. 2017, 17, 4511–4515. [Google Scholar] [CrossRef]
- Podgajny, R.; Chorazy, S.; Nitek, W.; Rams, M.; Balanda, M.; Sieklucka, B. {MnII9WV6}n Nanowires Organized into Three-Dimensional Hybrid Network of I1O2 Topology. Cryst. Growth Des. 2010, 10, 4693–4696. [Google Scholar] [CrossRef]
- Podgajny, R.; Pinkowicz, D.; Czarnecki, B.; Koziel, M.; Chorazy, S.; Wis, M.; Nitek, W.; Rams, M.; Sieklucka, B. Role of Pyrazine-N,N′-dioxide in [W(CN)8]n−-Based Hybrid Networks: Anion−π Interactions. Cryst. Growth Des. 2014, 14, 4030–4040. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.C.; Wang, H.S.; Kang, L.C.; Zhou, H.B.; Song, Y.; You, X.Z. Eicosanuclear cluster [Cu13W7] of copper-octacyanotungstate bimetallic assembly: Synthesis, Structure, and magnetic properties. Inorg. Chem. 2010, 49, 3101–3103. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Wei, R.M.; Sheng, Z.H.; Wang, P.; Song, Y. electrochemical synthesis and magnetic properties of [Cu9W6]: The ultimate member of the quindecanuclear octacyanometallate-based transition-metal cluster? Inorg. Chem. 2015, 54, 11049–11051. [Google Scholar] [CrossRef]
- Chorazy, S.; Stanek, J.J.; Kobylarczyk, J.; Ohkoshi, S.; Sieklucka, B.; Podgajny, R. Modulation of the FeII spin crossover effect in the pentadecanuclear {Fe9[M(CN)8]6} (M = Re, W) clusters by facial coordination of tridentate polyamine ligands. Dalton Trans. 2017, 46, 8027–8036. [Google Scholar] [CrossRef]
- Bridonneau, N.; Chamoreau, L.M.; Gontard, G.; Cantin, J.L.; von Bardeleben, J.; Marvaud, V. A high-nuclearity metal-cyanide cluster [Mo6Cu14] with photomagnetic properties. Dalton Trans. 2016, 45, 9412–9418. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1 and 2 are available from the authors. |

| Compound | 1 | 2 |
|---|---|---|
| Formula | Co9W6C120H108N60O24 | Co9W6C183H159N58O34 |
| Formula weight/g·mol−1 | 4408.04 | 5348.14 |
| T/K | 100 | 100 |
| λ/Å | 0.71073 | 0.71073 |
| Crystal system | Trigonal | Triclinic |
| Space group | R | P |
| Unit cell | ||
| a/Å | 28.126(4) | 17.179(1) |
| b/Å | 28.126(4) | 17.513(1) |
| c/Å | 18.038(2) | 20.029(1) |
| α/deg | 90 | 83.770(1) |
| β/deg | 90 | 79.935(1) |
| γ/deg | 120 | 64.080(1) |
| V/Å3 | 12,358.0(30) | 5333.1(4) |
| Z | 3 | 1 |
| Calculated density/g·cm−1 | 1.777 | 1.665 |
| Absorption coefficient/cm−1 | 5.125 | 3.978 |
| F(000) | 6381 | 2622 |
| Crystal size/mm × mm × mm | 0.41 × 0.27 × 0.18 | 0.33 × 0.27 × 0.21 |
| θ range/deg | 2.48 to 25.38 | 2.30 to 25.39 |
| Limiting indices | −33 < h < 33 | −20 < h < 20 |
| −33 < k < 29 | −20 < k < 20 | |
| −21 < l < 21 | −23 < l < 23 | |
| Collected reflections | 5210 | 9900 |
| Symmetry independent reflections | 4818 | 18,710 |
| Rint | 0.1055 | 0.0372 |
| Completeness/% | 99.0 | 99.3 |
| Data/restrains/parameters | 4818/463/472 | 18710/926/1856 |
| GOF on F2 | 1.093 | 1.394 |
| Final R indices | R[F2 > 2σ(F2)] = 0.0675 | R[F2 > 2σ(F2)] = 0.0804 |
| wR(F2) = 0.1564 | wR(F2) = 0.1740 | |
| Largest diff peak and hole/e·Å3 | 1.851/−1.759 | 1.409/−2.372 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberka, M.; Kobylarczyk, J.; Podgajny, R. Structural Disorder in High-Spin {CoII9WV6} (Core)-[Pyridine N-Oxides] (Shell) Architectures. Molecules 2020, 25, 251. https://doi.org/10.3390/molecules25020251
Liberka M, Kobylarczyk J, Podgajny R. Structural Disorder in High-Spin {CoII9WV6} (Core)-[Pyridine N-Oxides] (Shell) Architectures. Molecules. 2020; 25(2):251. https://doi.org/10.3390/molecules25020251
Chicago/Turabian StyleLiberka, Michal, Jedrzej Kobylarczyk, and Robert Podgajny. 2020. "Structural Disorder in High-Spin {CoII9WV6} (Core)-[Pyridine N-Oxides] (Shell) Architectures" Molecules 25, no. 2: 251. https://doi.org/10.3390/molecules25020251
APA StyleLiberka, M., Kobylarczyk, J., & Podgajny, R. (2020). Structural Disorder in High-Spin {CoII9WV6} (Core)-[Pyridine N-Oxides] (Shell) Architectures. Molecules, 25(2), 251. https://doi.org/10.3390/molecules25020251