Screening for Sulfur Compounds by Molybdenum-Catalyzed Oxidation Combined with Liquid Chromatography-Mass Spectrometry
Abstract
1. Introduction
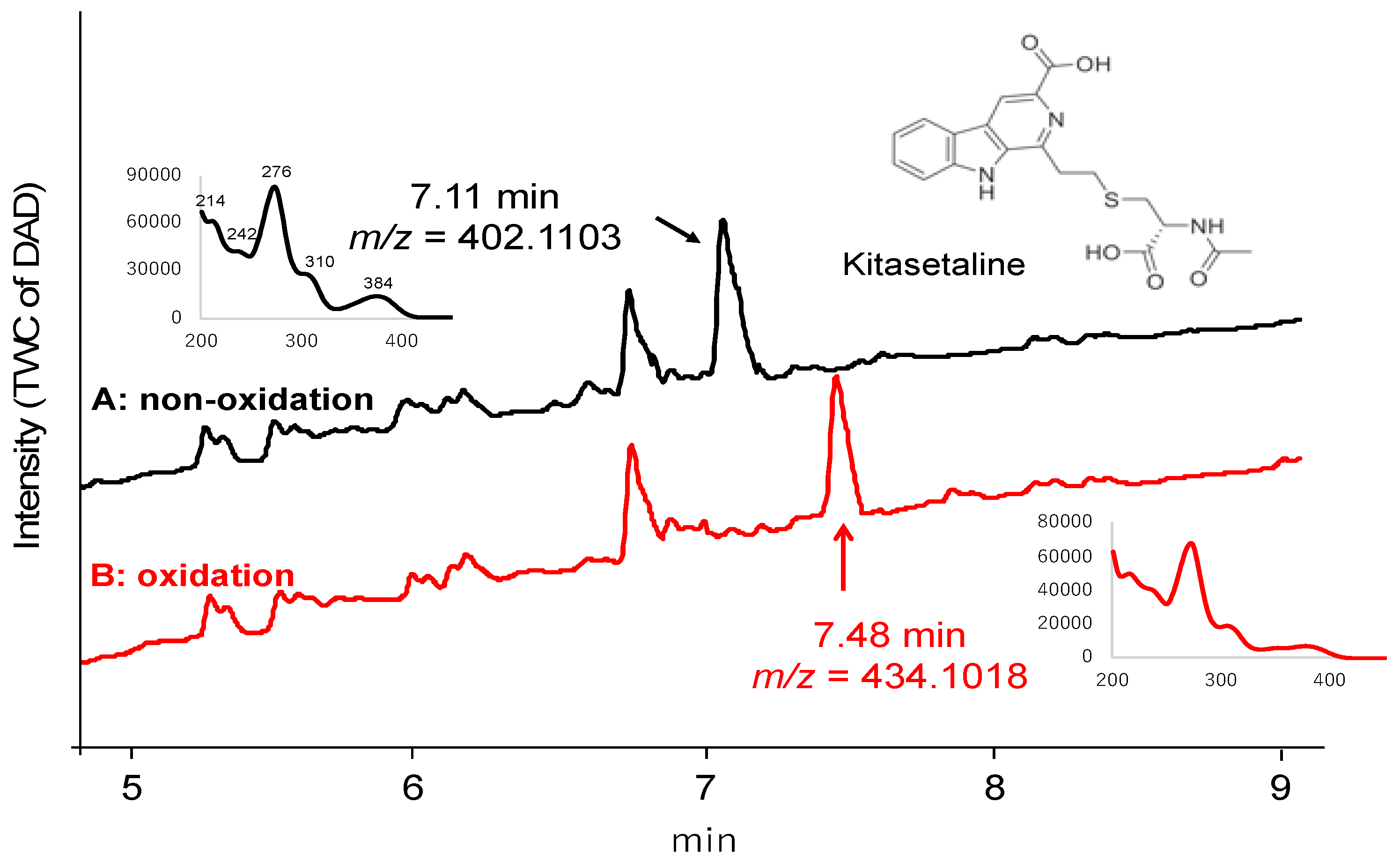
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Mo-Catalyzed Oxidation
3.3. Fermentation of Microbial Strains
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ōmura, S.; Iwai, Y.; Hirano, A.; Nakagawa, A.; Awaya, J.; Tsuchya, H.; Takahashi, Y.; Masuma, R. A new alkaloid AM-2282 of streptomyces origin. Taxonomy, fermentation, isolation and preliminary characterization. J. Antibiot. 1977, 30, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, T.; Nomoto, H.; Takahashi, I.; Kato, Y.; Morimoto, M.; Tomita, F. Staurosporine, a potent inhibitor of phospholipid/Ca++ dependent protein kinase. Biochem. Biophys. Res. Commun. 1986, 135, 397–402. [Google Scholar] [CrossRef]
- Nakano, H.; Kobayashi, E.; Takahashi, I.; Tamaoki, T.; Kuzuu, Y.; Iba, H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J. Antibiot. 1987, 40, 706–708. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Ōmura, S. Chemical biology of natural indolocarbazole products: 30 Years since the discovery of staurosporine. J. Antibiot. 2009, 62, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ōmura, S.; Asami, Y.; Crump, A. Staurosporine: New lease of life for parent compound of today’s novel and highly successful anti-cancer drugs. J. Antibiot. 2018, 71, 688–701. [Google Scholar] [CrossRef]
- Miller, E.L. The penicillins: A review and update. J. Midwifery Womens Health 2002, 47, 426–434. [Google Scholar] [CrossRef]
- Flynn, E.H.; McCormick, M.H.; Stamper, M.C.; DeValeria, H.; Godzeski, W. A new natural penicillin from Penicillium chrysogenum. J. Am. Chem. Soc. 1962, 84, 4594–4595. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Furumichi, M.; Tanabe, M.; Hirakawa, M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010, 38, D355–D360. [Google Scholar] [CrossRef]
- Hirano, A.; Iwai, Y.; Masuma, R.; Tei, K.; Ōmura, S. Neoxaline, a new alkaloid produced by aspergillus japonicus, production isolation and properties. J. Antibiot. 1979, 32, 781–785. [Google Scholar] [CrossRef]
- Ōmura, S.; Tanaka, H.; Awaya, Y.; Narimatsu, Y.; Konda, Y.; Hata, T. Pyrindicin, a new alkaloid from a streptomyces strain: Taxonomy, fermentation, isolation and biological activity. Agric. Biol. Chem. 1974, 38, 899–906. [Google Scholar] [CrossRef]
- Miyano, R.; Matsuo, H.; Mokudai, T.; Higo, M.; Nonaka, K.; Niwano, Y.; Shiomi, K.; Takahashi, Y.; Ōmura, S.; Nakashima, T. Trichothioneic acid, a new antioxidant compound produced by the fungal strain Trichoderma virens FKI-7573. J. Biosci. Bioeng. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Nakabayashi, R.; Sawada, Y.; Yamada, Y.; Suzuki, M.; Yokota-Hirai, M.; Sakurai, T.; Saito, K. Combination of liquid chromatography–fourier transform ion cyclotron resonance-mass spectrometry with 13C-labeling for chemical assignment of sulfur-containing metabolites in onion bulbs. Anal. Chem. 2013, 85, 1310–1315. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhou, Z.; Guo, D. A strategy for fast screening and identification of sulfur derivatives in medicinal pueraria species based on the fine isotopic pattern filtering method using ultra-high-resolution mass spectrometry. Anal. Chim. Acta 2015, 894, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.M.; Masuyama, Y. Chemoselectivity in molybdenum catalyzed alcohol and aldehyde oxidations. Tetrahedron Lett. 1984, 25, 173–176. [Google Scholar] [CrossRef]
- Trost, B.M.; Amans, D.; Seganish, W.M.; Chung, C.K. Evaluating transition-metal-catalyzed transformations for the synthesis of laulimalide. J. Am. Chem. Soc. 2009, 131, 17087–17089. [Google Scholar] [CrossRef]
- Jeyakumar, K.; Chakravarthy, R.D.; Chand, D.K. Simple and efficient method for the oxidation of sufides to sulfones using hydrogen peroxide and a Mo(VI) based catalyst. Catal. Commun. 2009, 10, 1948–1951. [Google Scholar] [CrossRef]
- Kajula, M.; Ward, J.M.; Turpeinen, A.; Tejesvi, M.V.; Hokkanen, J.; Tolonen, A.; Häkkänen, H.; Picart, P.; Ihalanen, J.; Sahl, H.-G.; et al. Bridged epipolythiodiketopiperazines from Penicillium raciborskii, an endophytic fungus of rhododendron Tomentosum harmaja. J. Nat. Prod. 2016, 79, 685–690. [Google Scholar] [CrossRef]
- Matsuo, H.; Nakanishi, J.; Noguchi, Y.; Kitagawa, K.; Shigemura, K.; Sunazuka, T.; Ōmura, S.; Takahashi, Y.; Nakashima, T. Nanaomycin K, a new epithelial-mesenchymal transition inhibitor produced by the actinomycete “Streptomyces rosa subsp. notoensis” OS-3966. J. Biosci. Bioeng. 2019, in press. [Google Scholar] [CrossRef]
- Ōmura, S.; Fujimoto, T.; Otoguro, K.; Matsuzaki, K.; Moriguchi, R.; Tanaka, H.; Sasaki, Y. Lactacystin, a novel microbial metabolite, induces neuritogenesis of neurobrastoma cells. J. Antibiot. 1991, 44, 113–116. [Google Scholar] [CrossRef]
- Ōmura, S.; Matsuzaki, K.; Fujimoto, T.; Kosuge, K.; Furuya, T.; Fujita, S.; Nakagawa, A. Structure of lactacystin, a new metabolite which induces differentiation of neurobrastoma cells. J. Antibiot. 1991, 44, 117–118. [Google Scholar] [CrossRef]
- Tian, Y.; Qin, X.; Lin, X.; Kaliyaperumal, K.; Zhou, X.; Liu, J.; Ju, Z.; Tu, Z.; Liu, Y. Sydoxanthone C and acremolin B produced by deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01. J. Antibiot. 2015, 68, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Mizushina, Y.; Takeuchi, T.; Kamisuki, S.; Kuriyama, I.; Sugawara, F.; Yoshida, H. Phenol Compounds and Their Use for Pharmaceutical Compositions, DNA Polymerase Inhibitors, Anticancer or Anti-Inflammatory Agents, and Food Compositions. Japan Patent JP2013-194048A, 23 March 2012. [Google Scholar]
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. The structure of beauvericin: A new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969, 49, 4255–4258. [Google Scholar] [CrossRef]
- Kuramoto, M.; Yamada, K.; Shikano, M.; Yazawa, K.; Arimoto, H.; Okamura, T.; Uemura, D. Tanzawaic acids A, B, C, and D: Inhibitors of superoxide anion production from Penicillium citrinum. Chem. Lett. 1997, 26, 885–886. [Google Scholar] [CrossRef]
- Aroonsri, A.; Kitani, S.; Ikeda, H.; Nihira, T. Kitasetaline, a novel β-carboline alkaloid from Kitasatospora setae NBRC 14216T. J. Biosci. Bioeng. 2012, 114, 56–58. [Google Scholar] [CrossRef]
Sample Availability: Not available. |

| Peak No. | Compound | Retention Time (min) | m/z [M + H]+ | Structure |
|---|---|---|---|---|
| 1, 1′ | Tanzawaic acid B | 11.56 | 295 | 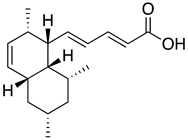 |
| 2, 2′ | Beauvericin | 11.16 | 784 | 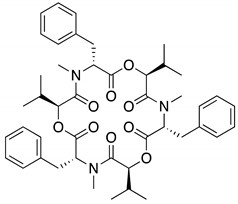 |
| 3 | SF-227 | 9.63 | 297 | 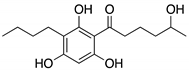 |
| 4, 4′ | Acremolin B | 7.10 | 246 | 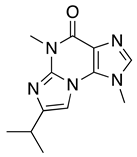 |
| 5 | Outovirin A | 6.52 | 481 | 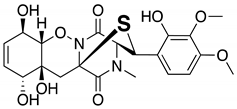 |
| 6 | Nanaomycin K | 6.45 | 548 | 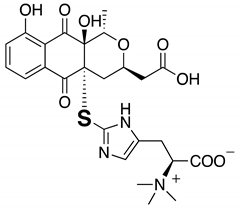 |
| 6b | Sulfonyl nanaomycin K | 6.21 | 580 | 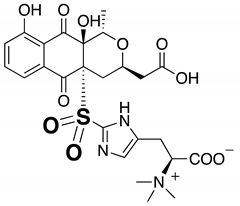 |
| 5a | Sulfinyl outovirin A | 5.72 | 497 |  |
| 6a | Sulfinyl nanaomycin K | 5.62 | 564 |  |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuo, H.; Hanamure, Y.; Miyano, R.; Takahashi, Y.; Ōmura, S.; Nakashima, T. Screening for Sulfur Compounds by Molybdenum-Catalyzed Oxidation Combined with Liquid Chromatography-Mass Spectrometry. Molecules 2020, 25, 240. https://doi.org/10.3390/molecules25020240
Matsuo H, Hanamure Y, Miyano R, Takahashi Y, Ōmura S, Nakashima T. Screening for Sulfur Compounds by Molybdenum-Catalyzed Oxidation Combined with Liquid Chromatography-Mass Spectrometry. Molecules. 2020; 25(2):240. https://doi.org/10.3390/molecules25020240
Chicago/Turabian StyleMatsuo, Hirotaka, Yu Hanamure, Rei Miyano, Yōko Takahashi, Satoshi Ōmura, and Takuji Nakashima. 2020. "Screening for Sulfur Compounds by Molybdenum-Catalyzed Oxidation Combined with Liquid Chromatography-Mass Spectrometry" Molecules 25, no. 2: 240. https://doi.org/10.3390/molecules25020240
APA StyleMatsuo, H., Hanamure, Y., Miyano, R., Takahashi, Y., Ōmura, S., & Nakashima, T. (2020). Screening for Sulfur Compounds by Molybdenum-Catalyzed Oxidation Combined with Liquid Chromatography-Mass Spectrometry. Molecules, 25(2), 240. https://doi.org/10.3390/molecules25020240






