Development of Ultra-Performance Liquid Chromatography–Mass Spectrometry Method for Simultaneous Determination of Three Cationic Dyes in Environmental Samples
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instrumentation
2.3. Preparation of Standard Solution
2.4. Sample Preparation
2.5. Development and Validation of UPLC-MS/MS and SPE Biosorbent Preparation
2.6. Solid-Phase Extraction Column Preparation and Experimental Conditions
3. Results and Discussion
3.1. Method Development and Optimization
3.2. Method Validation
3.2.1. Linearity and Range
3.2.2. Accuracy and Precision
3.2.3. Limit of Detection and Limit of Quantitation
3.2.4. Recovery Studies
3.2.5. Selectivity of the SPE material
3.3. Extraction Procedure and Characterization
3.3.1. Optimization of the Solid-Phase Extraction Method
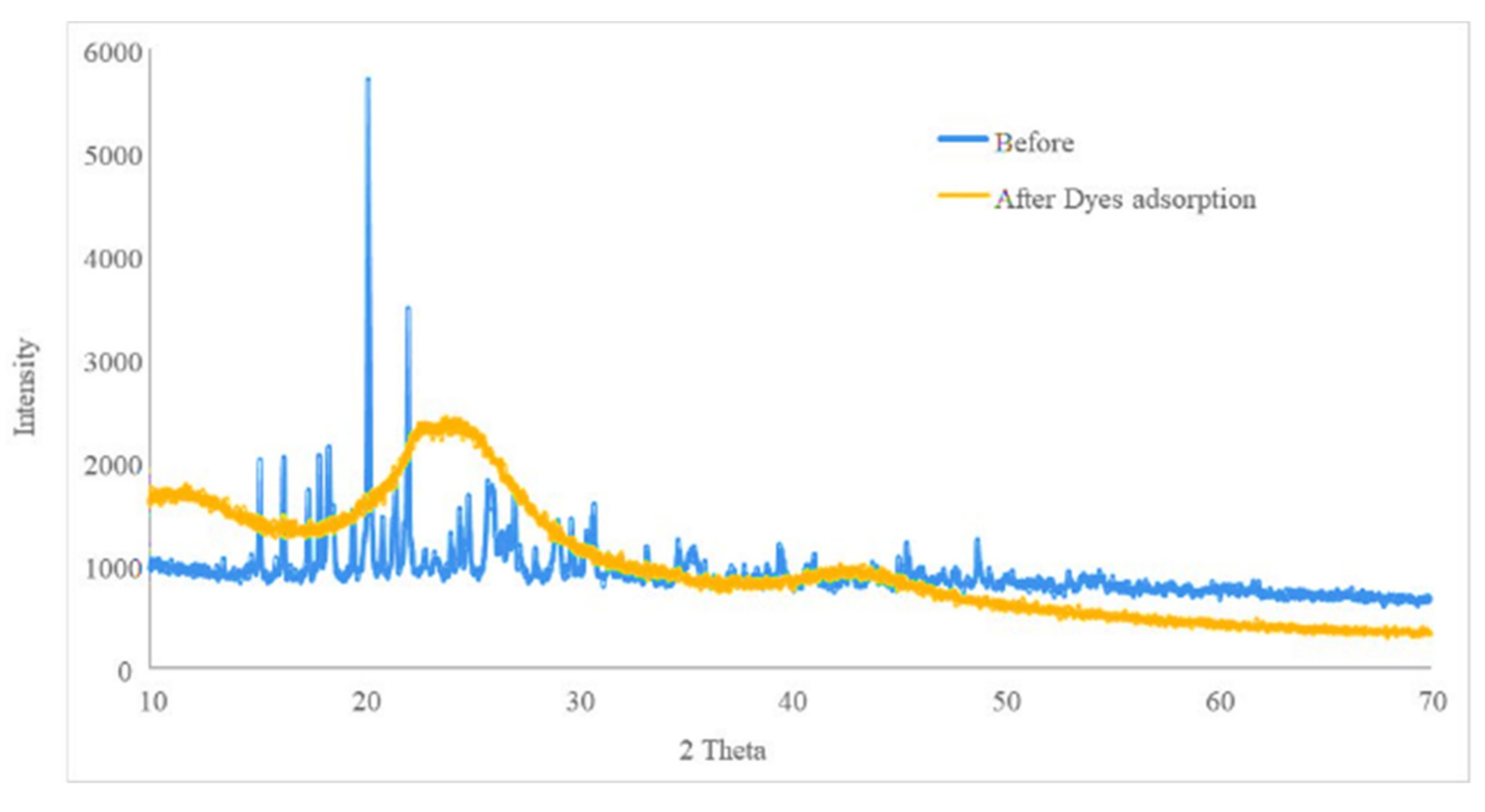
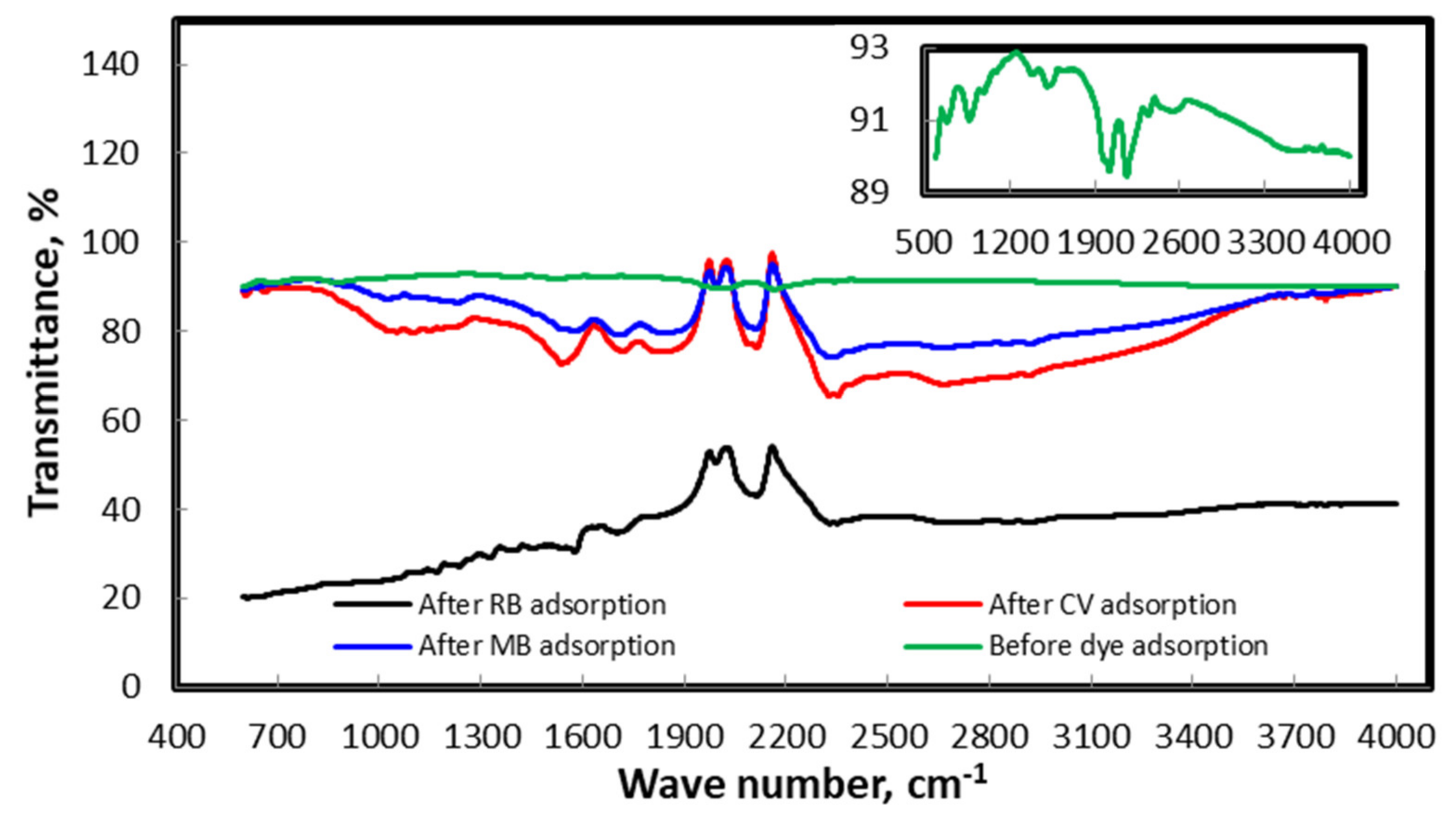
3.3.2. Characterization of the Biosorbent Post- and Pre-Extraction
3.4. Application of the Developed Method
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lo, J.C.; Darracq, M.A.; Clark, R.F. A Review of Methylene Blue Treatment for Cardiovascular Collapse. J. Emerg. Med. 2014, 46, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of Methylene Blue onto Low Cost Adsorbents: A Review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of Chitosan and Its Derivatives As Adsorbents for Dye Removal from Water and Wastewater: A Review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Smart, P.L.; Laidlaw, I.M.S. An Evaluation of Some Fluorescent Dyes for Water Tracing. Water Resour. Res. 1977, 13, 15–33. [Google Scholar] [CrossRef]
- Khan, M.A.; Siddiqui, M.R.; Otero, M.; Alshareef, S.A.; Rafatullah, M.; Momina. Removal of Rhodamine B from Water Using a Solvent Impregnated Polymeric Dowex 5WX8 Resin: Statistical Optimization and Batch Adsorption Studies. Polymers 2020, 12, 500. [Google Scholar] [CrossRef]
- Ji, R.-D.; Zhao, Z.; Yu, X.; Chen, M. Determination of Rhodamine B in Capsicol Using the First Derivative Absorption Spectrum. Optik 2019, 181, 796–801. [Google Scholar] [CrossRef]
- Respicio, N.C.; Heitz, J.R. Comparative Toxicity of Rhodamine B and Rhodamine 6G to the House Fly (Musca Domestica, L.). Bull. Environ. Contam. Toxicol. 1981, 27, 274–281. [Google Scholar] [CrossRef]
- Maley, A.M.; Arbiser, J.L. Gentian Violet: A 19th Century Drug Re-Emerges in the 21st Century. Exp. Dermatol. 2013, 22, 775–780. [Google Scholar] [CrossRef]
- Ahmad, A.; Lokhat, D.; Rafatullah, M.; Khatoon, A.; Setapar, S.H.M. Aloe Vera Biomass Containing Cellulosic Moieties Used As Sustainable Adsorbents for the Removal of Crystal Violet Dye from Aqueous Solution. Desalination Water Treat. 2019, 170, 337–348. [Google Scholar] [CrossRef]
- Ayed, L.; Cheriaa, J.; Laadhari, N.; Cheref, A.; Bakhrouf, A. Biodegradation of Crystal Violet by an IsolatedBacillus Sp. Ann. Microbiol. 2009, 59, 267–272. [Google Scholar] [CrossRef]
- Au, W.; Pathak, S.; Collie, C.J.; Hsu, T. Cytogenetic Toxicity of Gentian Violet and Crystal Violet on Mammalian Cells in Vitro. Mutat. Res. Toxicol. 1978, 58, 269–276. [Google Scholar] [CrossRef]
- Cheruiyot, G.K.; Wanyonyi, W.C.; Kiplimo, J.J.; Maina, E.N.; Joyce, K.J. Adsorption of Toxic Crystal Violet Dye Using Coffee Husks: Equilibrium, Kinetics and Thermodynamics Study. Sci. Afr. 2019, 5, e00116. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M.S. Recent Advances in New Generation Dye Removal Technologies: Novel Search for Approaches to Reprocess Wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Ahmad, T.; Danish, M.; Rafatullah, M.; Ghazali, A.; Sulaiman, O.; Hashim, R.; Ibrahim, M.N.M. The Use of Date Palm As a Potential Adsorbent for Wastewater Treatment: A Review. Environ. Sci. Pollut. Res. 2011, 19, 1464–1484. [Google Scholar] [CrossRef] [PubMed]
- Zeinali, N.; Ghaedi, M.; Shafie, G. Competitive Adsorption of Methylene Blue and Brilliant Green onto Graphite Oxide Nano Particle Following: Derivative Spectrophotometric and Principal Component-Artificial Neural Network Model Methods for Their Simultaneous Determination. J. Ind. Eng. Chem. 2014, 20, 3550–3558. [Google Scholar] [CrossRef]
- Kirsten, W.J.; Patel, V.J. On the Spectrophotometric Measurement of Methylene Blue. Microchem. J. 1972, 17, 277–284. [Google Scholar] [CrossRef]
- Razmara, R.S.; Daneshfar, A.; Sahrai, R. Determination of Methylene Blue and Sunset Yellow in Wastewater and Food Samples Using Salting-Out Assisted liquid–liquid Extraction. J. Ind. Eng. Chem. 2011, 17, 533–536. [Google Scholar] [CrossRef]
- Azarkohan, A.; Shemirani, F.; Alvand, M. Fast Analysis of Water Samples for Trace Amount of Crystal Violet Dye Based on Solid Phase Extraction Using Nanoporous SBA-3 Prior to Determination by Fiber Optic-Linear Array Detection Spectrophotometry. J. Chem. 2013, 2013, 1–8. [Google Scholar] [CrossRef]
- Ozkantar, N.; Soylak, M.; Tuzen, M. Spectrophotometric Detection of Rhodamine B in Tap Water, Lipstick, Rouge, and Nail Polish Samples After Supramolecular Solvent Microextraction. Turk. J. Chem. 2017, 41, 987–994. [Google Scholar] [CrossRef]
- Bişgin, A.T.; Surme, Y.; Uçan, M.; Narin, I. Separation, Preconcentration and Spectrophotometric Determination of Rhodamine B in Industrial, Cosmetic and Water Samples by Cloud Point and Solid Phase Extraction. J. Anal. Chem. 2018, 73, 452–458. [Google Scholar] [CrossRef]
- Manzo, V.; Navarro, O.; Honda, L.; Sánchez, K.; Toral, M.I.; Richter, P. Determination of Crystal Violet in Water by Direct Solid Phase Spectrophotometry After Rotating Disk Sorptive Extraction. Talanta 2013, 106, 305–308. [Google Scholar] [CrossRef]
- An, L.; Deng, J.; Zhou, L.; Li, H.; Chen, F.; Wang, H.; Liu, Y. Simultaneous Spectrophotometric Determination of Trace Amount of Malachite Green and Crystal Violet in Water After Cloud Point Extraction Using Partial Least Squares Regression. J. Hazard. Mater. 2010, 175, 883–888. [Google Scholar] [CrossRef]
- A Hassan, M.; El Jamal, M.M. Kinetic Study of the Electrochemical Oxidation of Methylene Blue with Pt Electrode. Port. Electrochimica Acta 2012, 30, 351–359. [Google Scholar] [CrossRef]
- Palma-Goyes, R.E.; Guzmán-Duque, F.L.; Peñuela, G.; González, I.; Nava, J.L.; Torres-Palma, R.A.; Palma, R.T. Electrochemical Degradation of Crystal Violet with BDD Electrodes: Effect of Electrochemical Parameters and Identification of Organic by-Products. Chemosphere 2010, 81, 26–32. [Google Scholar] [CrossRef]
- Yu, L.; Mao, Y.; Qu, L. Simple Voltammetric Determination of Rhodamine B by Using the Glassy Carbon Electrode in Fruit Juice and Preserved Fruit. Food Anal. Methods 2013, 6, 1665–1670. [Google Scholar] [CrossRef]
- Xie, J.; Peng, T.; Chen, D.-D.; Zhang, Q.-J.; Wang, G.-M.; Wang, X.; Guo, Q.; Jiang, F.; Chen, D.; Deng, J. Determination of Malachite Green, Crystal Violet and Their Leuco-Metabolites in Fish by HPLC–VIS Detection After Immunoaffinity Column Clean-up. J. Chromatogr. B 2013, 913–914, 123–128. [Google Scholar] [CrossRef]
- Roybal, J.E.; Munns, R.K.; Hurlbut, J.A.; Shimoda, W. High-Performance Liquid Chromatography of Gentian Violet, Its Demethylated Metabolites, Leucogentian Violet and Methylene Blue With Electrochemical Detection. J. Chromatogr. A 1989, 467, 259–266. [Google Scholar] [CrossRef]
- Turnipseed, S.B.; Roybal, J.E.; Plakas, S.M.; Pfenning, A.P.; Hurlbut, J.A.; Long, A.R. Determination of methylene blue in channel catfish (Ictalurus Punctatus) tissue by liquid chromatography with visible detection. J. AOAC Int. 1997, 80, 31–36. [Google Scholar] [CrossRef]
- Lian, Z.; Wang, J. Determination of Crystal Violet in Seawater and Seafood Samples through off-Line Molecularly Imprinted SPE Followed by HPLC With Diode-Array Detection. J. Sep. Sci. 2013, 36, 980–985. [Google Scholar] [CrossRef]
- Khan, M.R.; Khan, M.A.; Alothman, Z.A.; Alsohaimi, I.H.; Naushad, M.; Al-Shaalan, N.H. Quantitative Determination of Methylene Blue in Environmental Samples by Solid-Phase Extraction and Ultra-Performance Liquid Chromatography-Tandem Mass Spectrometry: A Green Approach. RSC Adv. 2014, 4, 34037–34044. [Google Scholar] [CrossRef]
- Jia, W.; Chu, X.; Ling, Y.; Huang, J.; Lin, Y.; Chang, J. Simultaneous Determination of Dyes in Wines by HPLC Coupled to Quadrupole Orbitrap Mass Spectrometry. J. Sep. Sci. 2014, 37, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Ha, D.-J.; Koo, T.-S. Simultaneous Quantification of Methylene Blue and Its Major Metabolite, Azure B, in Plasma by LC-MS/MS and Its Application for a Pharmacokinetic Study. Biomed. Chromatogr. 2013, 28, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-Z.; Dai, L.; Wu, B.; Ding, T.; Zhu, J.-J.; Lin, H.; Chen, H.-L.; Shen, C.-Y.; Jiang, Y. Determination of Methylene Blue Residues in Aquatic Products by Liquid Chromatography-Tandem Mass Spectrometry. J. Sep. Sci. 2009, 32, 4193–4199. [Google Scholar] [CrossRef] [PubMed]
- Favaro, G.; Confortin, D.; Pastore, P.; Brustolon, M. Application of LC-MS and LC-MS-MS to the Analysis of Photo-Decomposed Crystal Violet in the Investigation of Cultural Heritage Materials Aging. J. Mass Spectrom. 2012, 47, 1660–1670. [Google Scholar] [CrossRef]
- Ascari, J.; Dracz, S.; Santos, F.A.; Lima, J.; Diniz, M.H.G.; Vargas, E.A. Validation of an LC-MS/MS Method for Malachite Green (MG), Leucomalachite Green (LMG), Crystal Violet (CV) and Leucocrystal Violet (LCV) Residues in Fish and Shrimp. Food Addit. Contam. Part A 2012, 29, 602–608. [Google Scholar] [CrossRef]
- Tatebe, C.; Zhong, X.; Ohtsuki, T.; Kubota, H.; Sato, K.; Akiyama, H. A Simple and Rapid Chromatographic Method to Determine Unauthorized Basic Colorants (rhodamine B, Auramine O, and Pararosaniline) in Processed Foods. Food Sci. Nutr. 2014, 2, 547–556. [Google Scholar] [CrossRef]
- Cheng, Y.-Y.; Tsai, T.-H. A Validated LC–MS/MS Determination Method for the Illegal Food Additive Rhodamine B: Applications of a Pharmacokinetic Study in Rats. J. Pharm. Biomed. Anal. 2016, 125, 394–399. [Google Scholar] [CrossRef]
- Wen, R.; Zeng, D.; Yang, Z.; Jiang, L.; Ma, M.; Chen, B.; Van Beek, T.A. Rapid Analysis of Illegal Cationic Dyes in Foods and Surface Waters Using High Temperature Direct Analysis in Real Time High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2018, 66, 7542–7549. [Google Scholar] [CrossRef]
- Li, J.; Ding, X.; Zheng, J.; Liu, D.; Guo, F.; Liu, H.; Zhang, Y. Determination of Synthetic Dyes in Bean And Meat Products by Liquid Chromatographywith Tandem Mass Spectrometry. J. Sep. Sci. 2014, 37, 2439–2445. [Google Scholar] [CrossRef]
- Sandra, K.; Dolashka-Angelova, P.; Devreese, B.; Van Beeumen, J.J. New Insights in Rapana Venosa Hemocyanin N-Glycosylation Resulting from on-Line Mass Spectrometric Analyses. Glycobiology 2006, 17, 141–156. [Google Scholar] [CrossRef]
- Khan, M.A.; Alothman, Z.A.; Kumar, M.; Ola, M.S.; Siddique, M.R. Biosorption Potential Assessment of Modified Pistachio Shell Waste for Methylene Blue: Thermodynamics and Kinetics Study. Desalination Water Treat. 2014, 56, 146–160. [Google Scholar] [CrossRef]
- Goksu, A.; Tanaydin, M.K. Adsorption of Hazardous Crystal Violet Dye by Almond Shells and Determination of Optimum Process Conditions by Taguchi Method. Desalination Water Treat. 2017, 88, 189–199. [Google Scholar] [CrossRef]
- Turan, N.G.; Mesci, B. Use of Pistachio Shells As an Adsorbent for the Removal of Zinc(II) Ion. CLEAN–Soil Air Water 2011, 39, 475–481. [Google Scholar] [CrossRef]
- Armagan, B.; Toprak, F. Using Pistachio Shell for Remazol Red Removal from Aqueous Solutions: Equilibrium, Kinetics and Thermodynamics. Desalination Water Treat. 2014, 56, 136–145. [Google Scholar] [CrossRef]
- Khan, M.A.; Otero, M.; Kazi, M.; Alqadami, A.A.; Wabaidur, S.M.; Siddiqui, M.R.; Alothman, Z.A.; Sumbul, S. Unary and Binary Adsorption Studies of Lead and Malachite Green onto a Nanomagnetic Copper ferrite/Drumstick Pod Biomass Composite. J. Hazard. Mater. 2019, 365, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Alqadami, A.A.; Otero, M.; Siddiqui, M.R.; Alothman, Z.A.; Alsohaimi, I.; Rafatullah, M.; Hamedelniel, A.E. Heteroatom-Doped Magnetic Hydrochar to Remove Post-Transition and Transition Metals from Water: Synthesis, Characterization, and Adsorption Studies. Chemosphere 2019, 218, 1089–1099. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Hai-Peng, Y.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of Cellulose Nanofibers from Wood Using High-Intensity Ultrasonication Combined With Chemical Pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Li, R.; Fei, J.; Cai, Y.; Li, Y.; Feng, J.; Yao, J. Cellulose Whiskers Extracted from Mulberry: A Novel Biomass Production. Carbohydr. Polym. 2009, 76, 94–99. [Google Scholar] [CrossRef]
- Alemdar, A.; Sain, M. Isolation and Characterization of Nanofibers from Agriculturalresidues-Wheat Straw and Soy Hulls. Bioresour. Technol. 2008, 99, 1664–1671. [Google Scholar] [CrossRef]
- Peters, B. Prediction of Pyrolysis of Pistachio Shells Based on Its Components Hemicellulose, Cellulose and Lignin. Fuel Process. Technol. 2011, 92, 1993–1998. [Google Scholar] [CrossRef]
- Sun, R.; Tomkinson, J.; Sun, X.-F.; Wang, N. Fractional Isolation and Physico-Chemical Characterization of Alkali-Soluble Lignins from Fast-Growing Poplar Wood. Polymer 2000, 41, 8409–8417. [Google Scholar] [CrossRef]

| S. No | MS Parameter | Value | Analyzer Parameter | Value |
|---|---|---|---|---|
| 1 | MS scan Range | 205–512 | LM1 Resolution | 9.24 |
| 2 | Capillary (kV) | 3.60 | HM 1 Resolution | 15.00 |
| 3 | Cone (V) | 45.00 | Ion Energy | 0.26 |
| 4 | Extractor (V) | 3.00 | MSMS Mode entrance | 1.0 |
| 5 | RF (V) | 0.10 | MSMS Collision Energy | 40 |
| 6 | Source Temperature | 150 | MSMS Exit mode | 0.50 |
| 7 | Desolvation temperature (°C) | 350 | LM 2 Resolution | 10.59 |
| 8 | Cone gas flow (L/H) | 0 | HM 2 resolution | 15.00 |
| 9 | Desolvation gas flow (L/h) | 600 | Ion energy 2 | 1.0 |
| 10 | Collision gas flow (mL/min) | 0.12 | Gain | 1.0 |
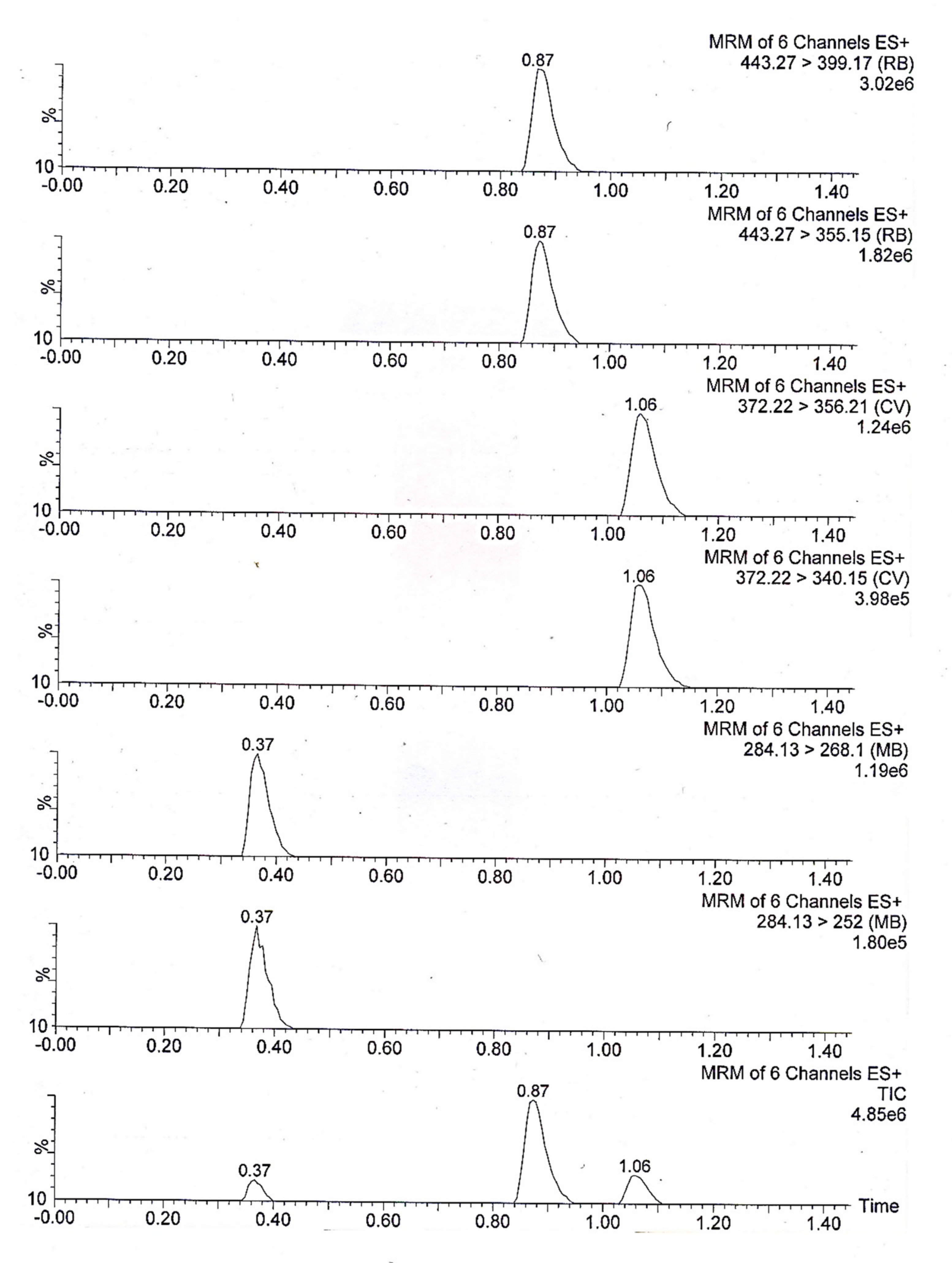
| Analyte | Molecular Formula | Retention Time | Precursor Ion | Molecular Transition | |
|---|---|---|---|---|---|
| Qualitative | Quantitative | ||||
| Methylene Blue | C16H18ClN3S | 0.37 | 284.13 | 252 | 268.1 |
| Rhodamine B | C28H31ClN2O3 | 0.87 | 443.27 | 355.16 | 399.17 |
| Crystal Violet | C25H30ClN3 | 1.06 | 372.22 | 340.15 | 356.21 |
| Precision | Intra-day Precision | Inter-day Precision | |||||
|---|---|---|---|---|---|---|---|
| Taken ng mL−1 | Found ng mL−1 | RSD % | Recovery % | Found ng mL−1 | RSD % | Recovery % | |
| 32 | 31.90 | 1.40 | 99.68 | 32.11 | 1.76 | 100.34 | |
| Methylene Blue | 125 | 123.72 | 1.26 | 98.97 | 123.44 | 1.60 | 98.75 |
| 500 | 500.36 | 1.68 | 100.07 | 494.88 | 1.99 | 98.98 | |
| 1000 | 987.21 | 1.82 | 98.72 | 1001.708 | 2.02 | 100.17 | |
| 32 | 31.37 | 1.47 | 98.02 | 32.09 | 1.99 | 100.27 | |
| Rhodamine B | 125 | 124.42 | 1.49 | 99.54 | 125.57 | 2.25 | 100.46 |
| 500 | 493.54 | 1.92 | 98.71 | 498.80 | 2.10 | 99.76 | |
| 1000 | 1003.65 | 1.33 | 100.37 | 999.94 | 1.99 | 99.99 | |
| Crystal Violet | 32 | 31.50 | 1.61 | 98.43 | 32.14 | 1.99 | 100.44 |
| 125 | 124.97 | 1.85 | 99.97 | 124.62 | 2.56 | 99.67 | |
| 500 | 499.75 | 1.74 | 99.95 | 508.51 | 2.19 | 101.70 | |
| 1000 | 996.11 | 1.45 | 99.61 | 1001.24 | 2.76 | 100.12 | |
| Methylene Blue | Rhodamine B | Crystal Violet | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Spiked (ng mL−1) | Found (ng mL−1) | RSD (%) | Recovery (%) | Spiked (ng mL−1) | Found | RSD (%) | Recovery (%) | Spiked (ng mL−1) | Found (ng mL−1) | RSD (%) | Recovery (%) |
| 64 | 62.79 | 1.56 | 98.11 | 64 | 62.93 | 0.66 | 98.33 | 64 | 63.58 | 2.30 | 99.34 |
| 125 | 123.25 | 1.34 | 98.60 | 125 | 123.94 | 1.89 | 99.15 | 125 | 124.41 | 2.82 | 99.53 |
| 250 | 248.09 | 2.59 | 99.23 | 250 | 248.64 | 2.15 | 99.45 | 250 | 248.61 | 1.77 | 99.44 |
| 1000 | 990.97 | 2.21 | 99.09 | 1000 | 989.15 | 1.39 | 98.91 | 1000 | 995.54 | 0.67 | 99.55 |
| Flow Rate | Concentration of Eluent | % of MB Found in Eluate | % of RB Found in Eluate | % of CV Found in Eluate |
|---|---|---|---|---|
| 0.5 mL min−1 | 500 ng mL−1 | Below LOQ | Below LOQ | Below LOQ |
| 0.75 mL min−1 | 500 ng mL−1 | Below LOQ | Below LOQ | Below LOQ |
| 1.0 mL min−1 | 500 ng mL−1 | 11.3% | 14.6% | 12.9% |
| 2.0 mL min−1 | 500 ng mL−1 | 40.1% | 54.6% | 49.8% |
| 3.0 mL min−1 | 500 ng mL−1 | 70.1% | 80.2% | 75.9% |
| S. No | Type of Sample | Methylene Blue (ng mL−1) | Rhodamine B (ng mL−1) | Crystal Violet (ng mL−1) |
|---|---|---|---|---|
| 01 | Laundry sample 1 | 390 | 320 | 270 |
| 02 | Laundry sample 2 | 430 | 410 | 380 |
| 03 | Textile dyeing unit waste sample 1 | 5560 | 1030 | 3890 |
| 04 | Textile dyeing unit waste sample 2 | 4020 | 1920 | 4360 |
| 05 | Printing press | 1930 | ND * | 2090 |
| 06 | Domestic supplies | ND * | ND * | ND * |
| 07 | Bottled drinking water | ND * | ND * | ND * |
| 08 | Irrigation supply water | ND * | ND * | ND * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hakami, A.A.H.; Wabaidur, S.M.; Ali Khan, M.; Abdullah Alothman, Z.; Rafatullah, M.; Siddiqui, M.R. Development of Ultra-Performance Liquid Chromatography–Mass Spectrometry Method for Simultaneous Determination of Three Cationic Dyes in Environmental Samples. Molecules 2020, 25, 4564. https://doi.org/10.3390/molecules25194564
Hakami AAH, Wabaidur SM, Ali Khan M, Abdullah Alothman Z, Rafatullah M, Siddiqui MR. Development of Ultra-Performance Liquid Chromatography–Mass Spectrometry Method for Simultaneous Determination of Three Cationic Dyes in Environmental Samples. Molecules. 2020; 25(19):4564. https://doi.org/10.3390/molecules25194564
Chicago/Turabian StyleHakami, Afnan Ali Hussain, Saikh Mohammad Wabaidur, Moonis Ali Khan, Zeid Abdullah Alothman, Mohd. Rafatullah, and Masoom Raza Siddiqui. 2020. "Development of Ultra-Performance Liquid Chromatography–Mass Spectrometry Method for Simultaneous Determination of Three Cationic Dyes in Environmental Samples" Molecules 25, no. 19: 4564. https://doi.org/10.3390/molecules25194564
APA StyleHakami, A. A. H., Wabaidur, S. M., Ali Khan, M., Abdullah Alothman, Z., Rafatullah, M., & Siddiqui, M. R. (2020). Development of Ultra-Performance Liquid Chromatography–Mass Spectrometry Method for Simultaneous Determination of Three Cationic Dyes in Environmental Samples. Molecules, 25(19), 4564. https://doi.org/10.3390/molecules25194564










