(+)-Erythro-Δ8′-7S,8R-dihydroxy-3,3′,5′-trimethoxy-8-O-4′-neolignan, an Anti-Acne Component in Degreasing Myristica fragrans Houtt
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activity of MFE
2.2. NO Inhibitory Effects of MFE-M, MFE-H, and MFE-E
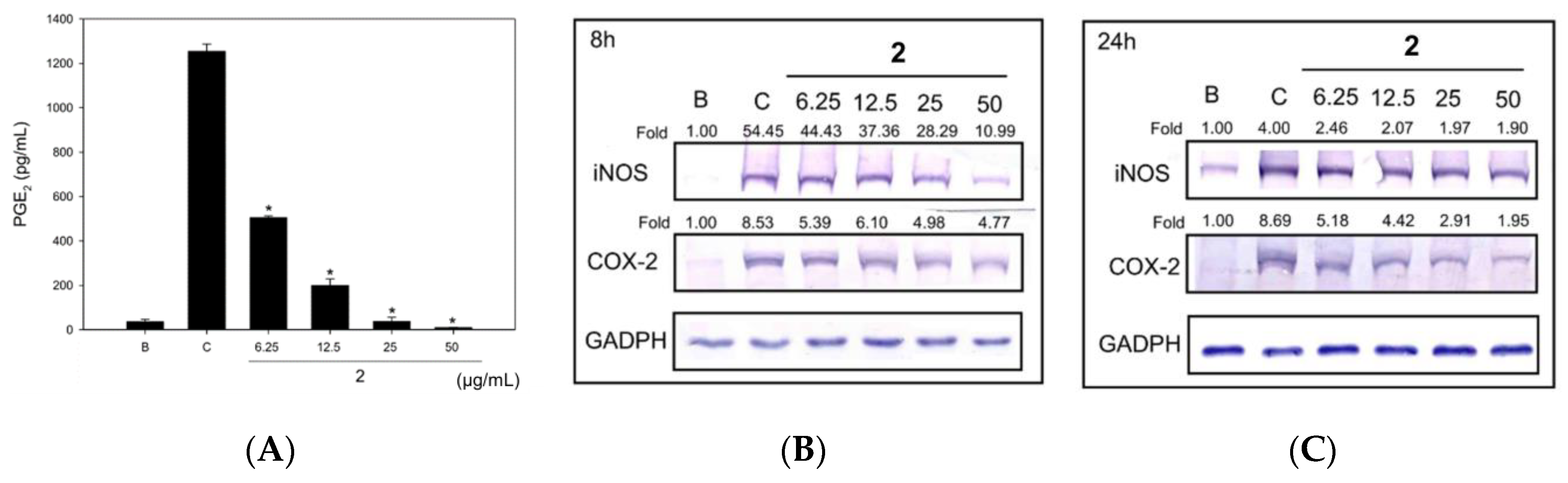
2.3. Neolignan Exerted Anti-Inflammatory Effects in RAW 264.7 Cells Treated with Heat-Killed C. acnes
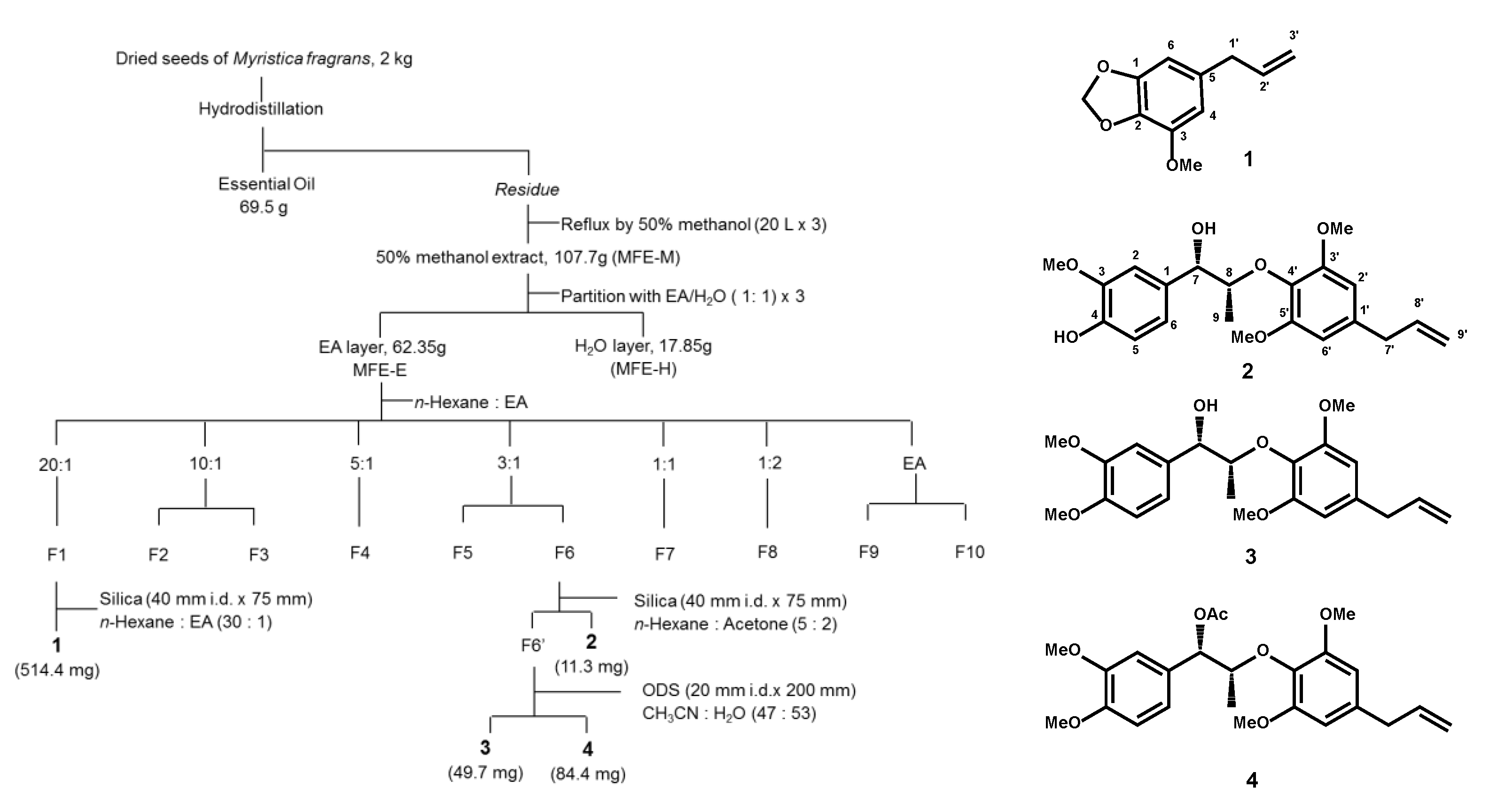
2.4. Quality Control of Myristicin and Safrole Characteristics
2.5. MFO and MFE-M Did Not Cause Skin Irritation
3. Discussion
4. Materials and Methods
4.1. General
4.2. Nuclear Magnetic Resonance (NMR) and Mass Spectrometry (MS) Instruments
4.3. Extraction and Isolation of M. fragrans
4.4. Antibacterial Activities against Cutibacterium Acnes and Staphylococcus aureus
4.5. Anti-Inflammatory Activity against LPS-Treated RAW 264.7 Cells
4.6. Anti-Inflammatory Activity against Heat-Killed C. acnes-Treated RAW 264.7 Cells
4.7. Quality Control of Myristicin and Safrole
4.8. Safety Control of Myristicin and Safrole
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Multiple activities of Punica granatum Linne against acne vulgaris. Int. J. Mol. Sci. 2017, 18, 141. [Google Scholar] [CrossRef] [PubMed]
- Chilicka, K.; Rogowska, A.M.; Szyguła, R.; Tarada, J. Examining quality of life after treatment with azelaic and pyruvic acid peels in women with acne vulgaris. Clin. Cosmet. Investig. Dermatol. 2020, 13, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Yaldiz, M.; Kara, A.; Güven, M.; Solak, B.; Kara, R.; Erdem, M.T. Assessment of auditory function and lipid levels in patients receiving oral isotretinoin (13-cis retinoid) therapy for acne vulgaris. Postepy. Dermatol. Alergol. 2020, 37, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Sonavane, G.S.; Sarveiya, V.P.; Kasture, V.S.; Kasture, S.B. Anxiogenic activity of Myristica fragrans seeds. Pharmacol. Biochem. Behav. 2002, 71, 239–244. [Google Scholar] [CrossRef]
- Chung, J.Y.; Choo, J.H.; Lee, M.H.; Hwang, J.K. Anticariogenic activity of macelignan isolated from Myristica fragrans (nutmeg) against Streptococcus mutans. Phytomedicine 2006, 13, 261–266. [Google Scholar] [CrossRef]
- Duan, L.; Tao, H.W.; Hao, X.J.; Gu, Q.Q.; Zhu, W.M. Cytotoxic and antioxidative phenolic compounds from the traditional Chinese medicinal plant, Myristica fragrans. Planta Med. 2009, 75, 1241–1245. [Google Scholar] [CrossRef]
- Dorman, H.J.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Matulyte, I.; Jekabsone, A.; Jankauskaite, L.; Zavistanaviciute, P.; Sakiene, V.; Bartkiene, E.; Ruzauskas, M.; Kopustinskiene, D.M.; Santini, A.; Bernatoniene, J. The essential oil and hydrolats from Myristica fragrans Seeds with magnesium aluminometasilicate as excipient: Antioxidant, antibacterial, and anti-inflammatory activity. Foods 2020, 9, 37. [Google Scholar] [CrossRef]
- Hu, L.; Wu, F.; He, J.; Zhong, L.; Song, Y.; Shao, H. Cytotoxicity of safrole in HepaRG cells: Studies on the role of CYP1A2-mediated ortho-quinone metabolic activation. Xenobiotica 2019, 49, 1504–1515. [Google Scholar] [CrossRef]
- Liu, T.Y.; Chen, C.C.; Chen, C.L.; Chi, C.W. Safrole-induced oxidative damage in the liver of Sprague-Dawley rats. Food Chem. Toxicol. 1999, 37, 697–702. [Google Scholar] [CrossRef]
- McKenna, A.; Nordt, S.P.; Ryan, J. Acute nutmeg poisoning. Eur. J. Emerg. Med. 2004, 11, 240–241. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Kijima, A.; Suzuki, Y.; Hibi, D.; Inoue, T.; Ishii, Y.; Nohmi, T.; Nishikawa, A.; Ogawa, K.; Umemura, T. Comprehensive toxicity study of safrole using a medium-term animal model with gpt delta rats. Toxicology 2011, 290, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.H. Studies on several naturally occurring lignans. Gaoxiong Yi Xue Ke Xue Za Zhi 1989, 5, 621–624. [Google Scholar] [PubMed]
- Ginting, B.; Saidi, N.; Murniana, P.; Mustanir, Z.; Maulidna, B.; Simanjuntak, P. Lignan compound isolated from n-Hexane extract myristica fragrans Houtt root as antioxidant and antitumor activities against MCF-7 cell lines data. Data Brief. 2020, 31, 105997. [Google Scholar] [CrossRef]
- Zhang, C.R.; Jayashre, E.; Kumar, P.S.; Nair, M.G. Antioxidant and antiinflammatory compounds in nutmeg (Myristica fragrans) pericarp as determined by in vitro assays. Nat. Prod. Commun. 2015, 10, 1399–1402. [Google Scholar]
- Razzaghi-Abyaneh, M.; Yoshinari, T.; Shams-Ghahfarokhi, M.; Rezaee, M.B.; Nagasawa, H.; Sakuda, S. Dillapiol and Apiol as specific inhibitors of the biosynthesis of aflatoxin G1 in Aspergillus parasiticus. Biosci. Biotechnol. Biochem. 2007, 71, 2329–2332. [Google Scholar] [CrossRef]
- Kasahara, H.; Miyazawa, M.; Kameoka, H. Absolute configuration of 8-O-4’-neolignans from Myristica fragrans. Phytochemistry 1995, 40, 1515–1517. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Jafri, H.; Khan, M.S.A.; Ahmad, I. In vitro efficacy of eugenol in inhibiting single and mixed-biofilms of drug-resistant strains of Candida albicans and Streptococcus mutans. Phytomedicine 2019, 54, 206–213. [Google Scholar] [CrossRef]
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Kharshoum, R.M.; Abd El-Ghafar, O.A.; Farouk, H.O. Mitigation of rheumatic arthritis in a rat model via transdermal delivery of dapoxetine HCl amalgamated as a nanoplatform: In vitro and in vivo assessment. Int. J. Nanomed. 2020, 15, 1517–1535. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |


| Microorganisms | MIC µg/mL | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| Cutibacterium acnes | >100 | 6.25 | 100 | 25 |
| Staphylococcus aureus | 12.5 | 3.12 | 6.25 | 12.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-J.; Huang, C.-W.; Chen, L.-G.; Wang, C.-C. (+)-Erythro-Δ8′-7S,8R-dihydroxy-3,3′,5′-trimethoxy-8-O-4′-neolignan, an Anti-Acne Component in Degreasing Myristica fragrans Houtt. Molecules 2020, 25, 4563. https://doi.org/10.3390/molecules25194563
Lee C-J, Huang C-W, Chen L-G, Wang C-C. (+)-Erythro-Δ8′-7S,8R-dihydroxy-3,3′,5′-trimethoxy-8-O-4′-neolignan, an Anti-Acne Component in Degreasing Myristica fragrans Houtt. Molecules. 2020; 25(19):4563. https://doi.org/10.3390/molecules25194563
Chicago/Turabian StyleLee, Chia-Jung, Chun-Wei Huang, Lih-Geeng Chen, and Ching-Chiung Wang. 2020. "(+)-Erythro-Δ8′-7S,8R-dihydroxy-3,3′,5′-trimethoxy-8-O-4′-neolignan, an Anti-Acne Component in Degreasing Myristica fragrans Houtt" Molecules 25, no. 19: 4563. https://doi.org/10.3390/molecules25194563
APA StyleLee, C.-J., Huang, C.-W., Chen, L.-G., & Wang, C.-C. (2020). (+)-Erythro-Δ8′-7S,8R-dihydroxy-3,3′,5′-trimethoxy-8-O-4′-neolignan, an Anti-Acne Component in Degreasing Myristica fragrans Houtt. Molecules, 25(19), 4563. https://doi.org/10.3390/molecules25194563






