Thickness-Dependent NIR LSPR of Curved Ag/TiS2 Bilayer Film
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
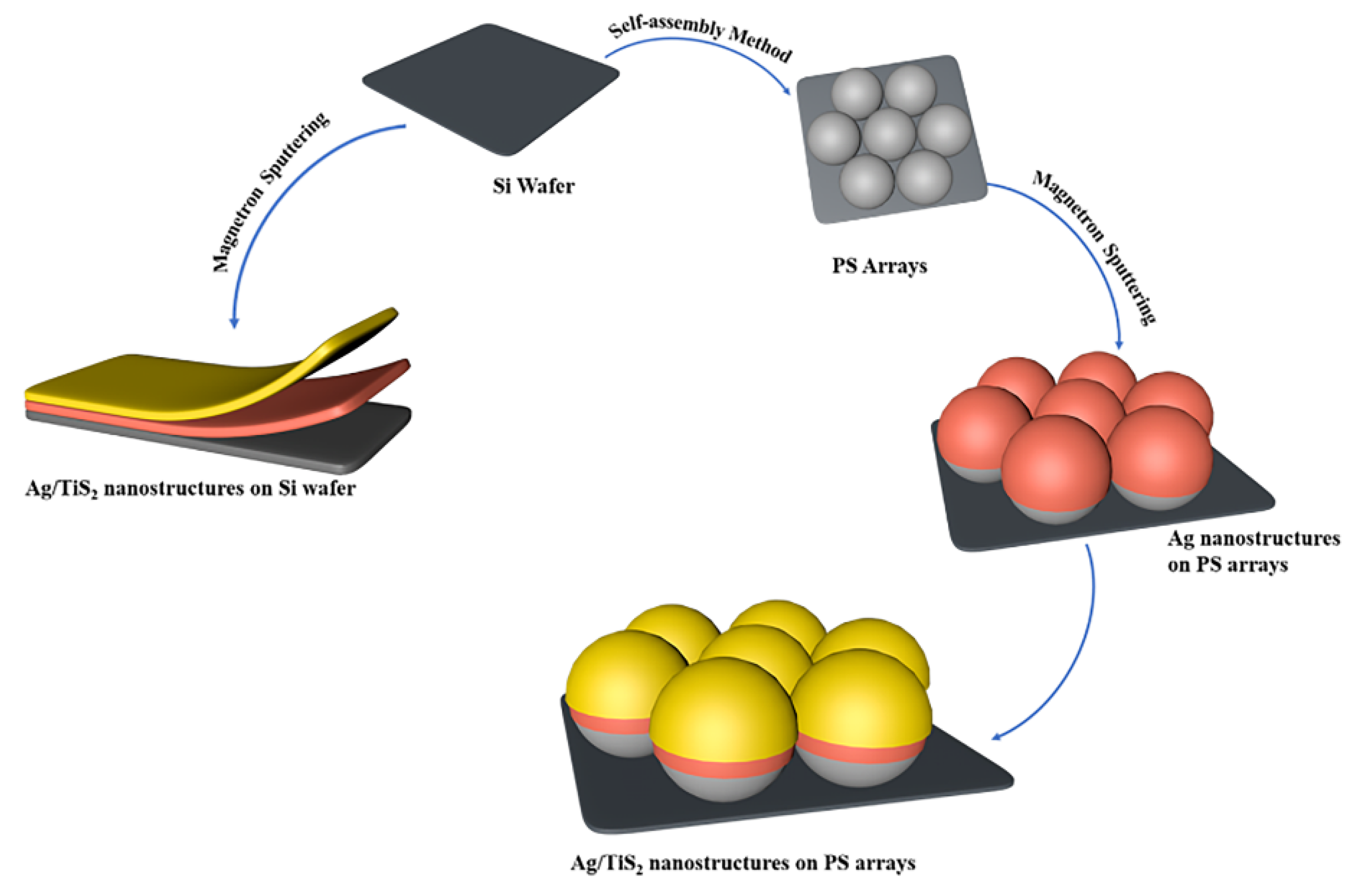
2.2. Preparation of Polystyrene (PS) Arrays
2.3. Preparation of SERS Active Substrates
2.4. Characterization of Substrates
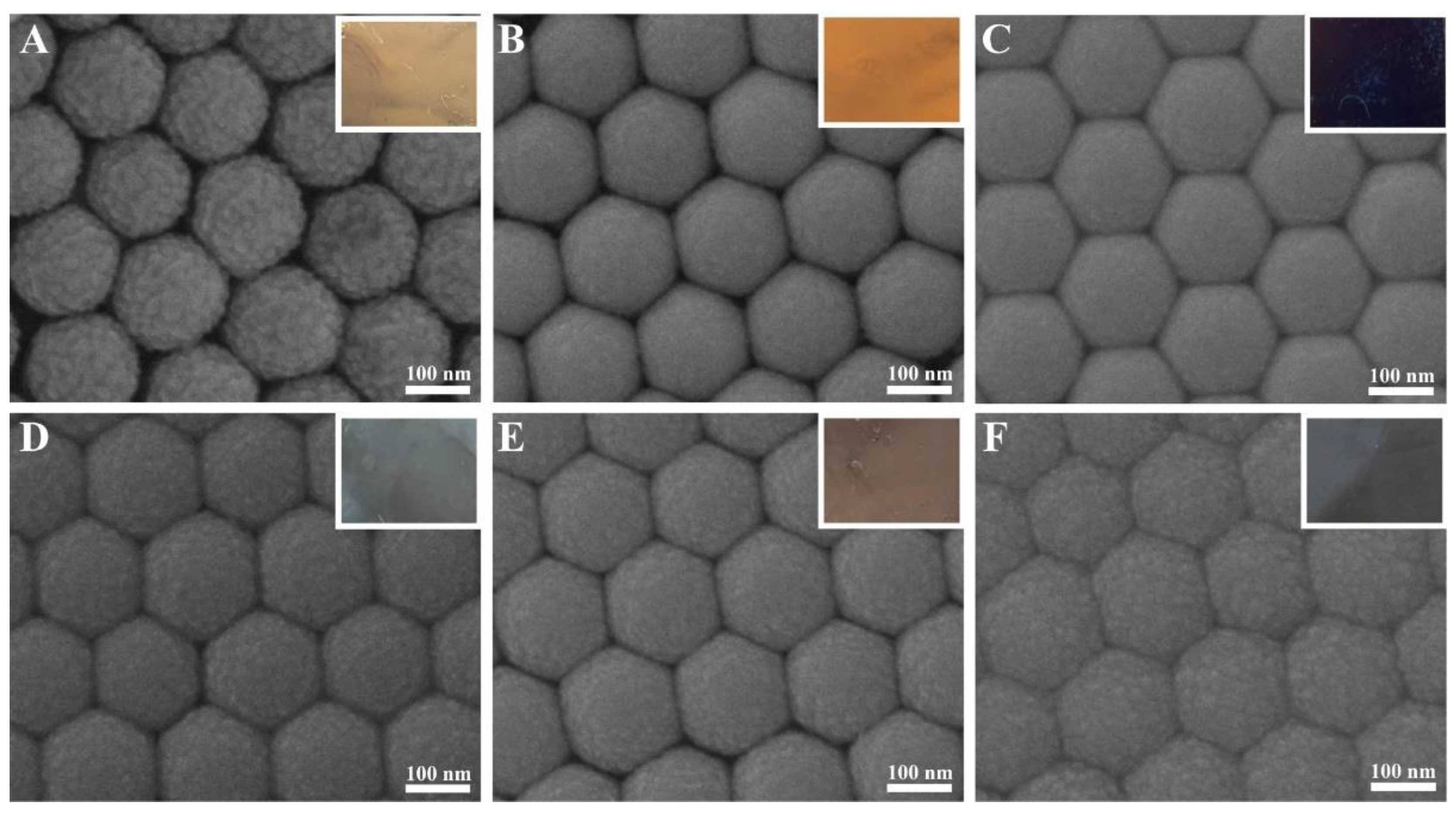
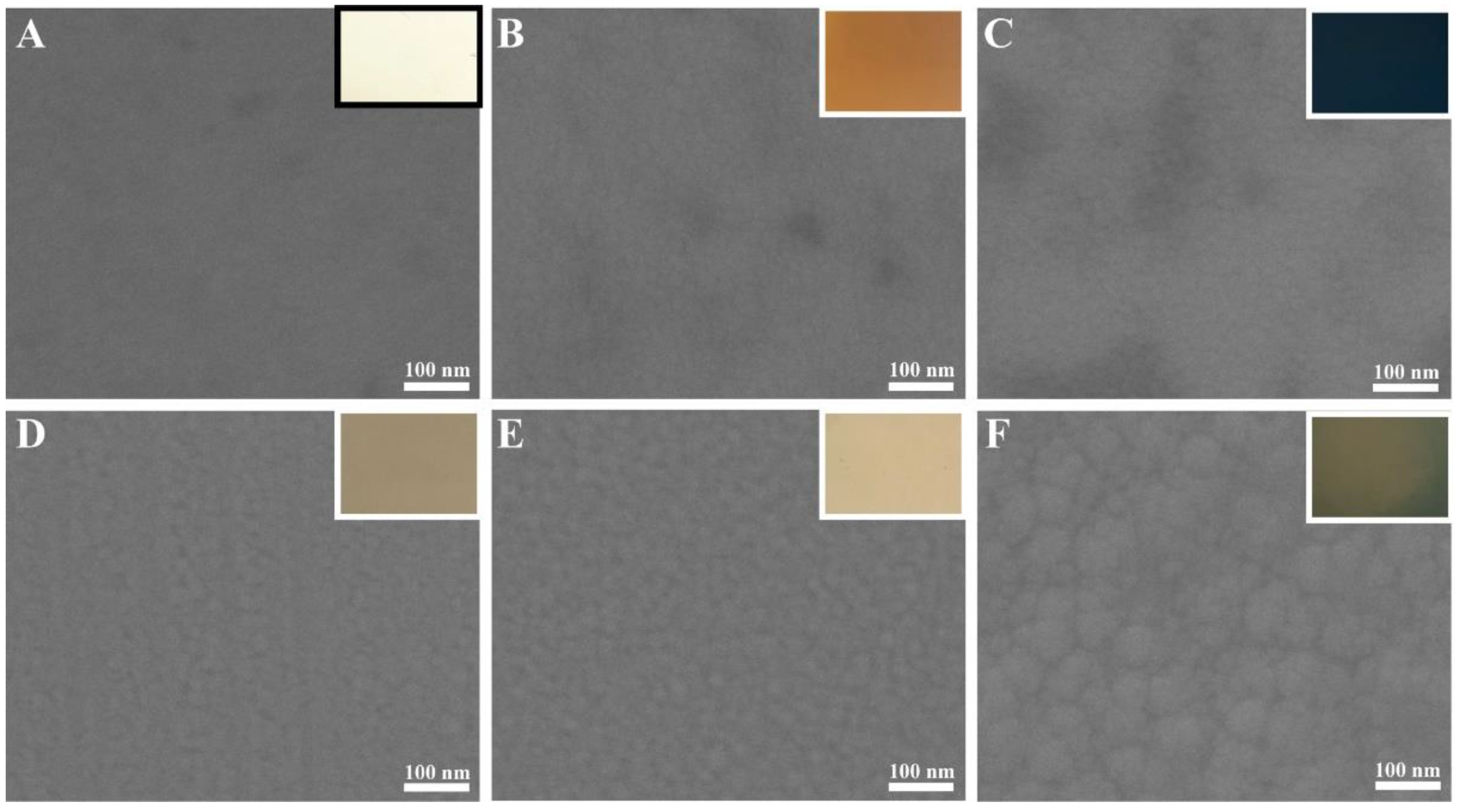
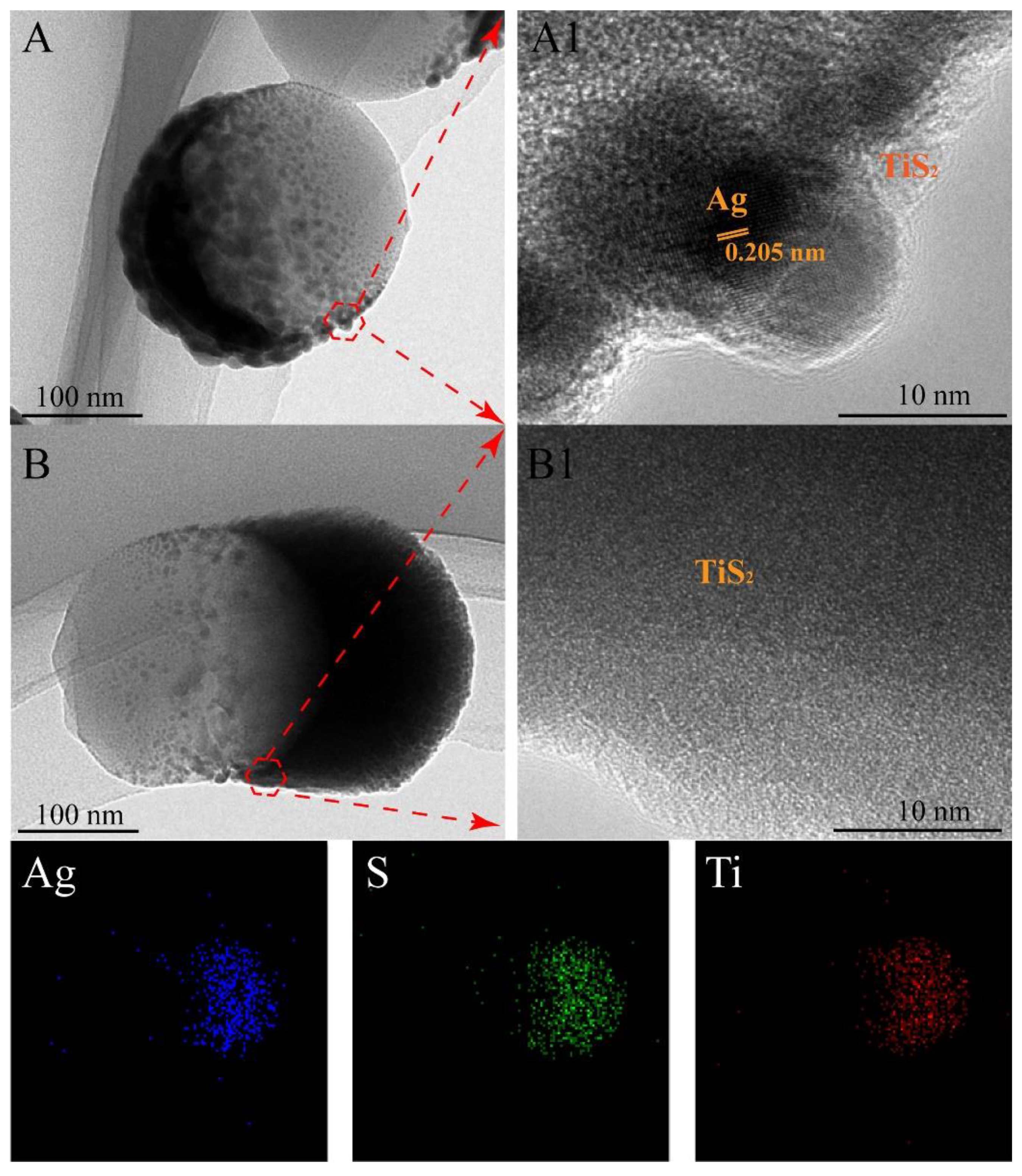
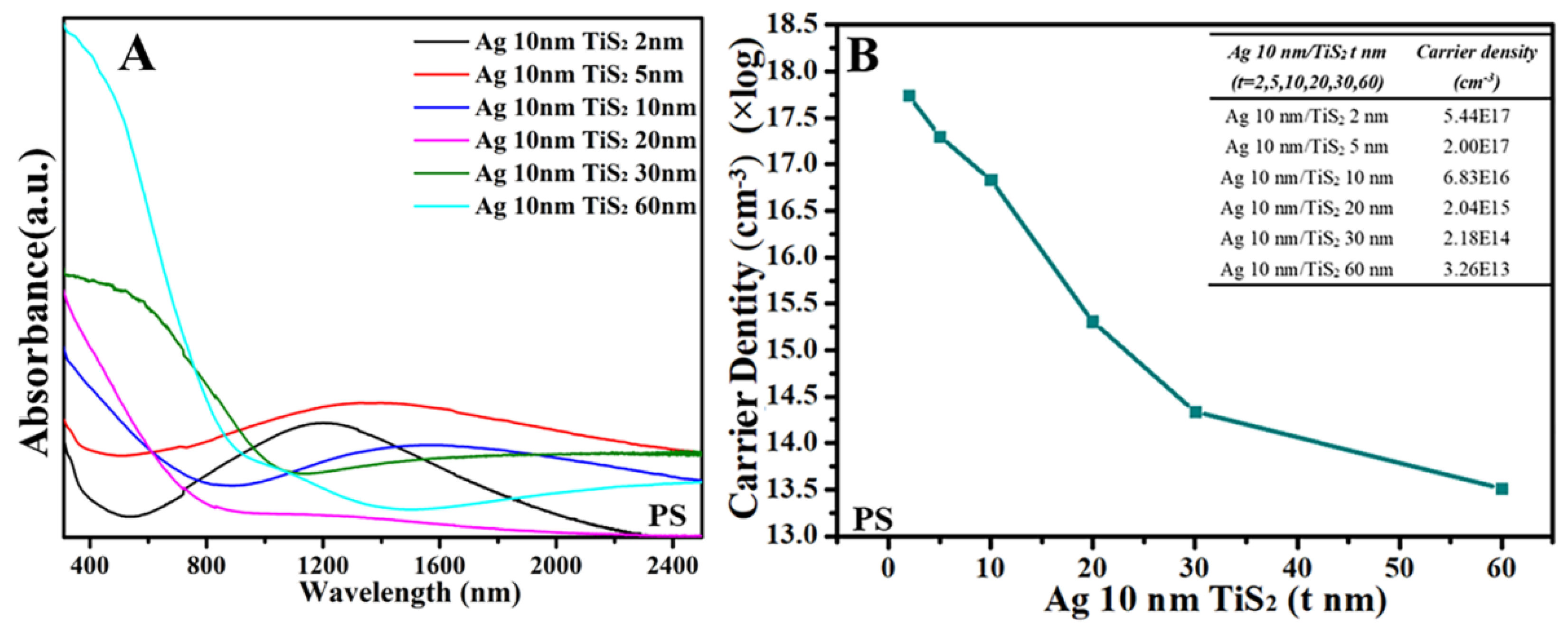
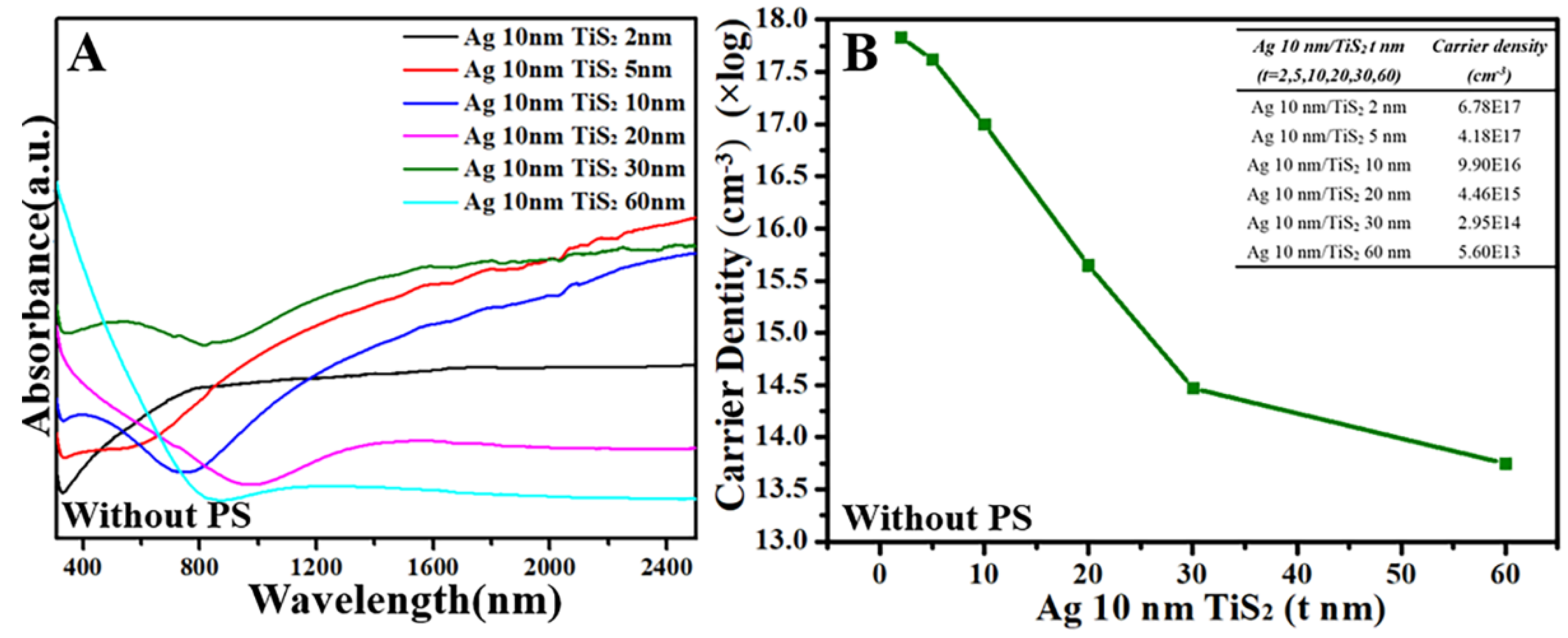
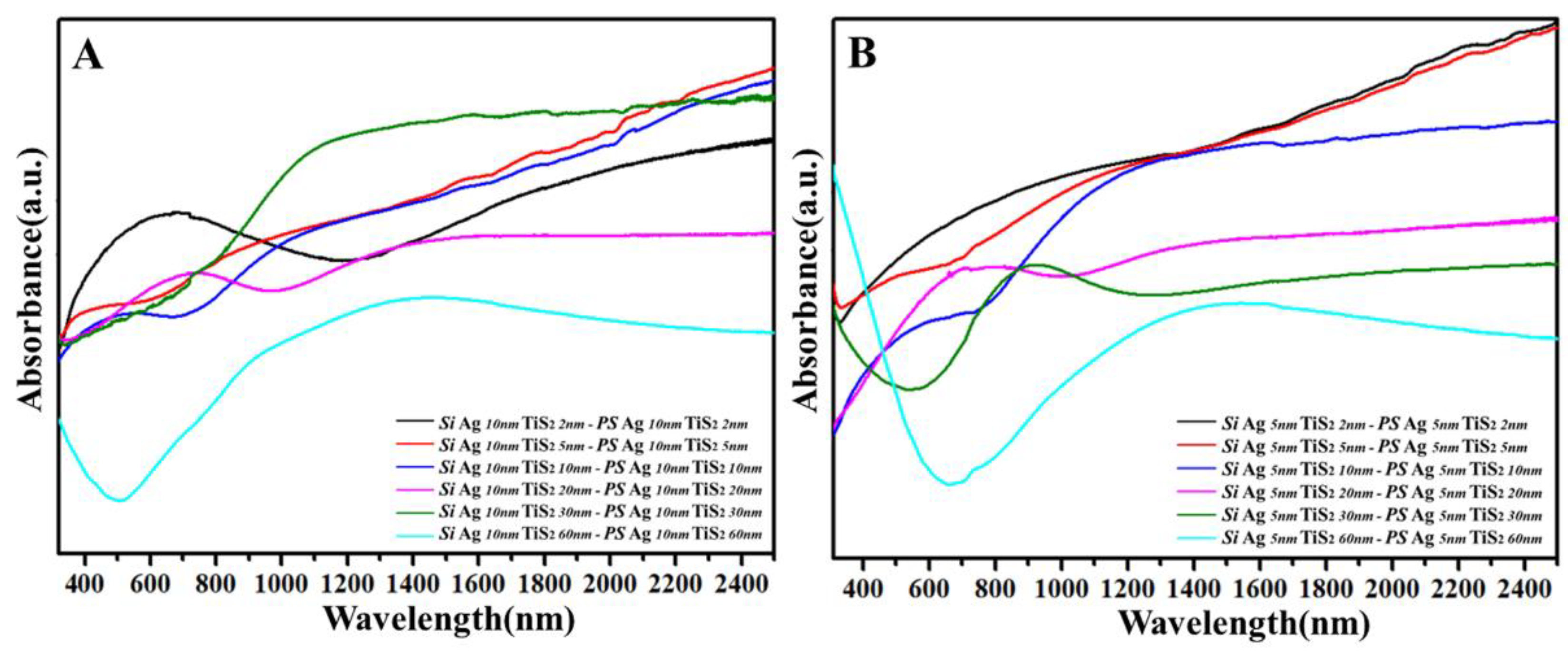
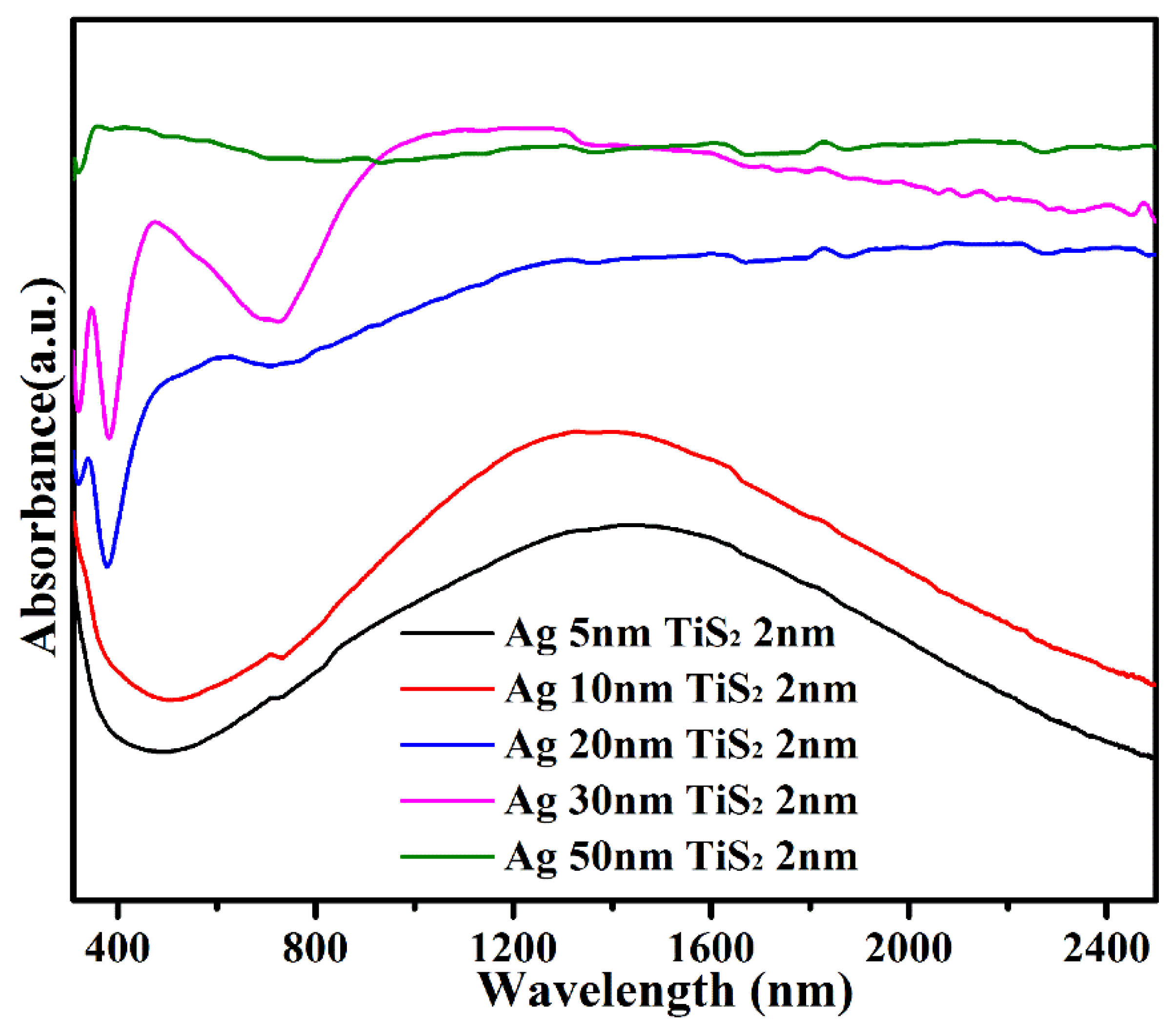
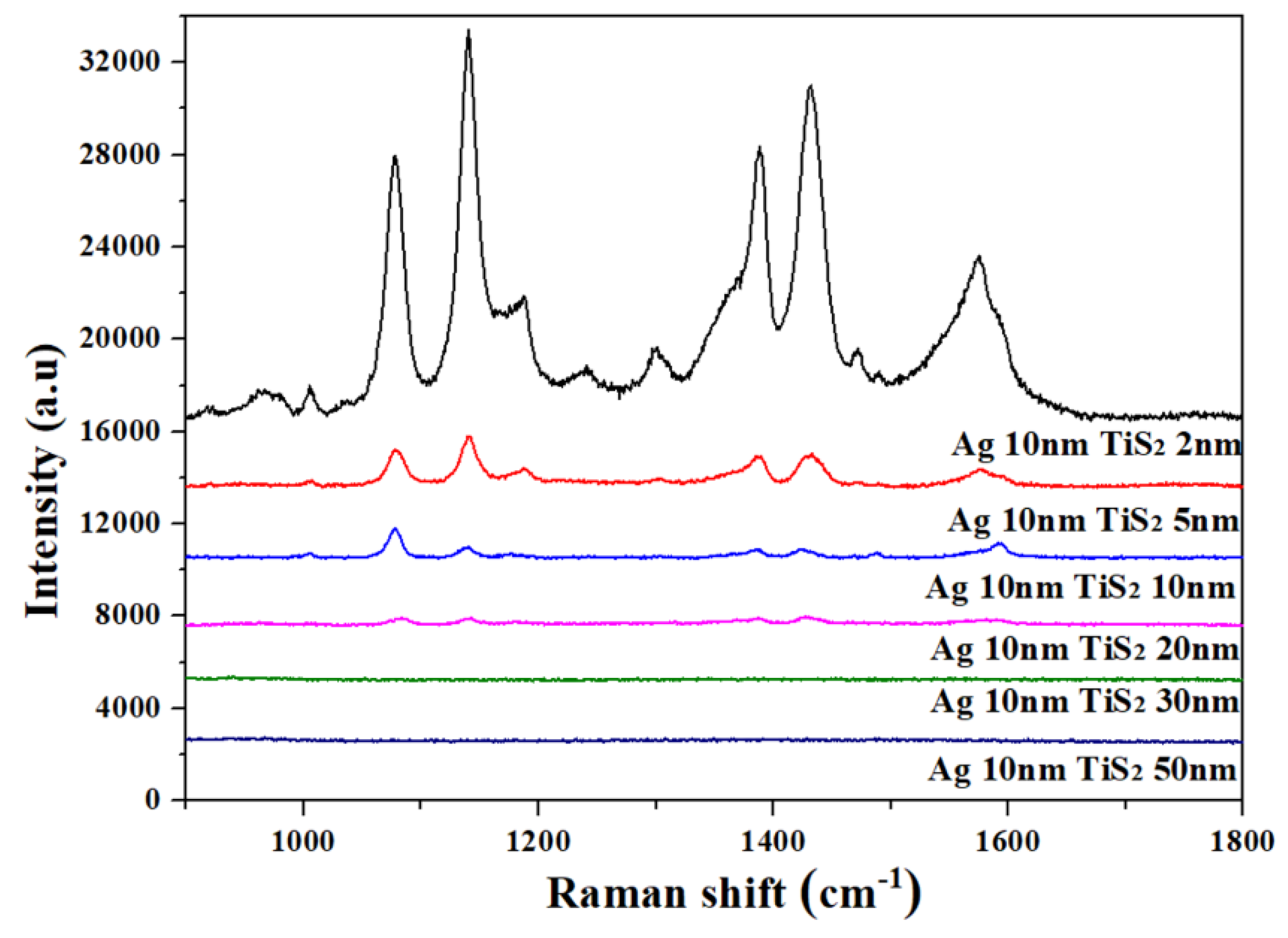
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Agrawal, A.; Cho, S.H.; Zandi, O.; Ghosh, S.; Johns, R.W.; Milliron, D.J. Localized Surface Plasmon Resonance in Semiconductor Nanocrystals. Chem. Rev. 2018, 118, 3121–3207. [Google Scholar] [CrossRef] [PubMed]
- Lesyuk, R.; Klein, E.; Yaremchuk, I.; Klinke, C. Copper Sulfide Nanosheets with Shape-Tunable Plasmonic Properties in the Nir Region. Nanoscale 2018, 10, 20640–20651. [Google Scholar] [CrossRef] [PubMed]
- Litvin, A.P.; Cherevkov, S.A.; Dubavik, A.; Babaev, A.A.; Parfenov, P.S.; Gamboa, A.L.S.; Fedorov, A.V.; Baranov, A.V. Thin Layer of Semiconductor Plasmonic Nanocrystals for the Enhancement of Nir Fluorophores. JPCC 2018, 122, 20469–20475. [Google Scholar] [CrossRef]
- Larsson, E.M.; Langhammer, C.; Zoric, I.; Kasemo, B. Nanoplasmonic Probes of Catalytic Reactions. Science 2009, 326, 1091–1094. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Tang, M.L.; Hentschel, M.; Giessen, H.; Alivisatos, A.P. Nanoantenna-Enhanced Gas Sensing in a Single Tailored Nanofocus. Nat. Mater. 2011, 10, 631–636. [Google Scholar] [CrossRef] [PubMed]
- Schlucker, S. Surface-Enhanced Raman Spectroscopy: Concepts and Chemical Applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef]
- Mejía-Salazar, J.R.; Oliveira, O.N. Plasmonic Biosensing. Chem. Rev. 2018, 118, 10617–10625. [Google Scholar] [CrossRef]
- Baia, L.; Baia, M.; Popp, J.; Astilean, S. Gold Films Deposited over Regular Arrays of Polystyrene Nanospheres as Highly Effective SERS Substrates from Visible to NIR. J. Phys. Chem. B 2006, 110, 23982–23986. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Shen, Z.X.; Fan, H.J. Highly Ordered Arrays of Particle-in-Bowl Plasmonic Nanostructures for Surface-Enhanced Raman Scattering. Small 2012, 8, 2548–2554. [Google Scholar] [CrossRef]
- Uzayisenga, V.; Lin, X.D.; Li, L.M.; Anema, J.R.; Yang, Z.L.; Huang, Y.F.; Lin, H.X.; Li, S.B.; Li, J.F.; Tian, Z.Q. Synthesis, Characterization, and 3d-Fdtd Simulation of Ag@SiO2 Nanoparticles for Shell-Isolated Nanoparticle-Enhanced Raman Spectroscopy. Langmuir 2012, 28, 9140–9146. [Google Scholar] [CrossRef]
- Yang, S.; Xu, F.; Ostendorp, S.; Wilde, G.; Zhao, H.; Lei, Y. Template-Confined Dewetting Process to Surface Nanopatterns: Fabrication, Structural Tunability, and Structure-Related Properties. Adv. Funct. Mater. 2011, 21, 2446–2455. [Google Scholar] [CrossRef]
- Zhao, X.; Wen, J.; Zhang, M.; Wang, D.; Wang, Y.; Chen, L.; Zhang, Y.; Yang, J.; Du, Y. Design of Hybrid Nanostructural Arrays to Manipulate Sers-Active Substrates by Nanosphere Lithography. ACS Appl. Mater. Interfaces 2017, 9, 7710–7716. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.M.; Jain, P.K.; Ewers, T.; Alivisatos, A.P. Localized Surface Plasmon Resonances Arising from Free Carriers in Doped Quantum Dots. Nat. Mater. 2011, 10, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.W.; On, K.; Tao, A.R. Localized Surface Plasmon Resonances of Anisotropic Semiconductor Nanocrystals. J. Am. Chem. Soc. 2011, 133, 19072–19075. [Google Scholar] [CrossRef] [PubMed]
- Faucheaux, J.A.; Jain, P.K. Plasmons in Photocharged ZnO Nanocrystals Revealing the Nature of Charge Dynamics. J. Phys. Chem. Lett. 2013, 4, 3024–3030. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, X.Y.; Fan, W.Y.; Han, B.B.; Jin, S.; Park, Y.; Chen, L.; Zhang, Y.J.; Liu, Y.; Yang, J.H.; et al. Controllable Preparation of SERS-Active Ag-FeS Substrates by a Cosputtering Technique. Molecules 2019, 24, 551. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Han, D.L.; Pang, Z.Y.; Sun, Y.S.; Wang, Y.X.; Zhang, Y.J.; Yang, J.H.; Chen, L. Charge Transfer in an Ordered Ag/Cu2S/4-MBA System Based on Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2018, 122, 5599–5605. [Google Scholar] [CrossRef]
- Wang, D.S.; Fan, S.K. Microfluidic Surface Plasmon Resonance Sensors: From Principles to Point-of-Care Applications. Sensors 2016, 16, 1175. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; van Duyne, R.P. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Kim, E.; Baaske, M.D.; Schuldes, I.; Wilsch, P.S.; Vollmer, F. Label-Free Optical Detection of Single Enzyme-Reactant Reactions and Associated Conformational Changes. Sci. Adv. 2017, 3, 1603044. [Google Scholar] [CrossRef]
- Jin, J.; Zhu, S.J.; Song, Y.B.; Zhao, Z.; Zhang, H.Y.; Guo, Y.; Li, J.B.; Song, W.; Yang, B.; Zhao, B. Precisely Controllable Core−Shell Ag@Carbon Dots Nanoparticles: Application to in Situ Super-Sensitive Monitoring of Catalytic Reactions. ACS Appl. Mater. Interfaces 2016, 8, 27956. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zou, Y.; Hu, W.; Li, Y.; Gu, Y.; Cao, B.; Guo, N.; Wang, L.; Song, J.; Zhang, S.; et al. Near-Infrared Plasmonic 2D Semimetals for Applications in Communication and Biology. Adv. Funct. Mater. 2016, 26, 1793–1802. [Google Scholar] [CrossRef]
- Zeng, Z.; Tan, C.; Huang, X.; Bao, S.; Zhang, H. Growth of noble metal nanoparticles on single-layer TiS2 and TaS2 nanosheets for hydrogen evolution reaction. Energy Environ. Sci. 2014, 7, 797–803. [Google Scholar] [CrossRef]
- Ma, L.; Wei, S.; Zhuang, H.L.; Hendrickson, K.E.; Hennig, R.G.; Archer, L.A. Hybrid Cathode Architectures for Lithium Batteries Based on TiS2 and Sulfur. J. Mater. Chem. A 2015, 3, 19857–19866. [Google Scholar] [CrossRef]
- Muller, G.A.; Cook, J.B.; Kim, H.S.; Tolbert, S.H.; Dunn, B. High Performance Pseudocapacitor Based on 2d Layered Metal Chalcogenide Nanocrystals. Nano Lett. 2015, 15, 1911–1917. [Google Scholar] [CrossRef]
- Wan, C.; Gu, X.; Dang, F.; Itoh, T.; Wang, Y.; Sasaki, H.; Kondo, M.; Koga, K.; Yabuki, K.; Snyder, G.J.; et al. Flexible n-type thermoelectric materials by organic intercalation of layered transition metal dichalcogenide TiS2. Nat. Mater. 2015, 14, 622–627. [Google Scholar] [CrossRef]
- Lin, C.; Zhu, X.; Feng, J.; Wu, C.; Hu, S.; Peng, J.; Guo, Y.; Peng, L.; Zhao, J.; Huang, J.; et al. Hydrogen-incorporated TiS2 ultrathin nanosheets with ultrahigh conductivity for stamp-transferrable electrodes. J. Am. Chem. Soc. 2013, 135, 5144–5151. [Google Scholar] [CrossRef]
- Li, X.; Ding, X.; Li, Y.; Wang, L.; Fan, J. A TiS2 Nanosheet Enhanced Fluorescence Polarization Biosensor for Ultra-Sensitive Detection of Biomolecules. Nanoscale 2016, 8, 9852–9860. [Google Scholar] [CrossRef]
- Qian, X.; Shen, S.; Liu, T.; Cheng, L.; Liu, Z. Two-Dimensional TiS2 Nanosheets for in Vivo Photoacoustic Imaging and Photothermal Cancer Therapy. Nanoscale 2015, 7, 6380–6387. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Y.J.; Wang, Y.X.; Zhao, X.Y. Composition-dependent LSPR shifts for co-sputtered TiS2-Ag. Opt. Commun. 2020, 473, 125935. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Ozaki, Y.; Xu, Z.; Zhao, B. Effect of TiO2 on Altering Direction of Interfacial Charge Transfer in a TiO2-Ag-Mpy-Fepc System by Sers. Angew. Chem. Int. Ed. Engl. 2019, 58, 8172–8176. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.H.; Gao, R.X.; Zhu, A.N.; Hua, Z.; Chen, L.; Wang, Y.X.; Zhang, Y.J. Surface-enhanced Raman scattering from metal and transition metal nano-caped arrays. Superlattices Microstruct. 2018, 115, 59–66. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Wen, J.H.; Zhu, A.N.; Cheng, M.Y.; Zhu, Q.; Zhang, X.L.; Wang, Y.X.; Zhang, Y.J. Manipulation and Applications of Hotspots in Nanostructured Surfaces and Thin Films. Nanomaterials 2020, 10, 1667. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, K.; Moskovits, M.; Kneipp, H. Surface Enhanced Raman Scattering Physics and Applications; Topics in Applied Physics; Springer: Berlin/Heidelberg, Germany, 2006; Volume 103. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, F.; Wang, Y. Thickness-Dependent NIR LSPR of Curved Ag/TiS2 Bilayer Film. Molecules 2020, 25, 4551. https://doi.org/10.3390/molecules25194551
Zhang Y, Zhang F, Wang Y. Thickness-Dependent NIR LSPR of Curved Ag/TiS2 Bilayer Film. Molecules. 2020; 25(19):4551. https://doi.org/10.3390/molecules25194551
Chicago/Turabian StyleZhang, Yongjun, Fan Zhang, and Yaxin Wang. 2020. "Thickness-Dependent NIR LSPR of Curved Ag/TiS2 Bilayer Film" Molecules 25, no. 19: 4551. https://doi.org/10.3390/molecules25194551
APA StyleZhang, Y., Zhang, F., & Wang, Y. (2020). Thickness-Dependent NIR LSPR of Curved Ag/TiS2 Bilayer Film. Molecules, 25(19), 4551. https://doi.org/10.3390/molecules25194551




