Immunomodulative Effects of Chamaecyparis obtusa Essential Oil in Mouse Model of Allergic Rhinitis
Abstract
1. Introduction
2. Results
2.1. Effects of EOCO on Allergic Behaviors
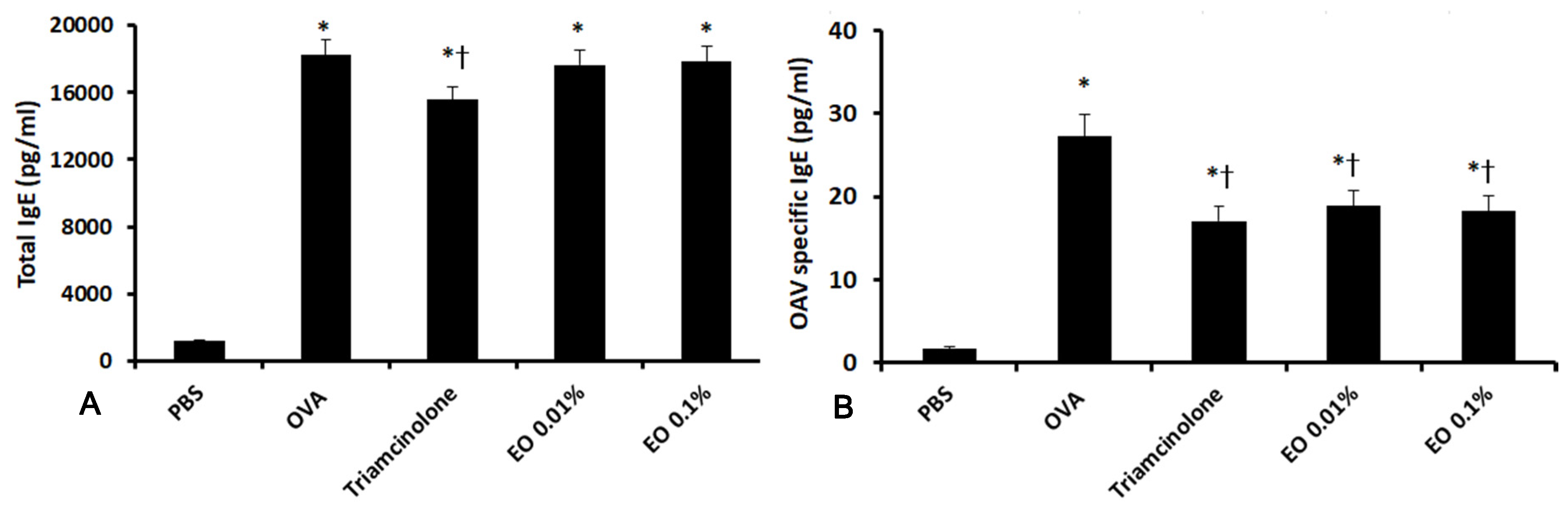
2.2. Effects of EOCO on Total Serum IgE and OVA-Specific IgE Levels
2.3. Effects of EOCO on the Inflammatory Mediator Levels in Nasal Lavage Fluid (NLF)
2.4. Effects of EOCO on the Inflammatory Mediator Production from Splenocytes
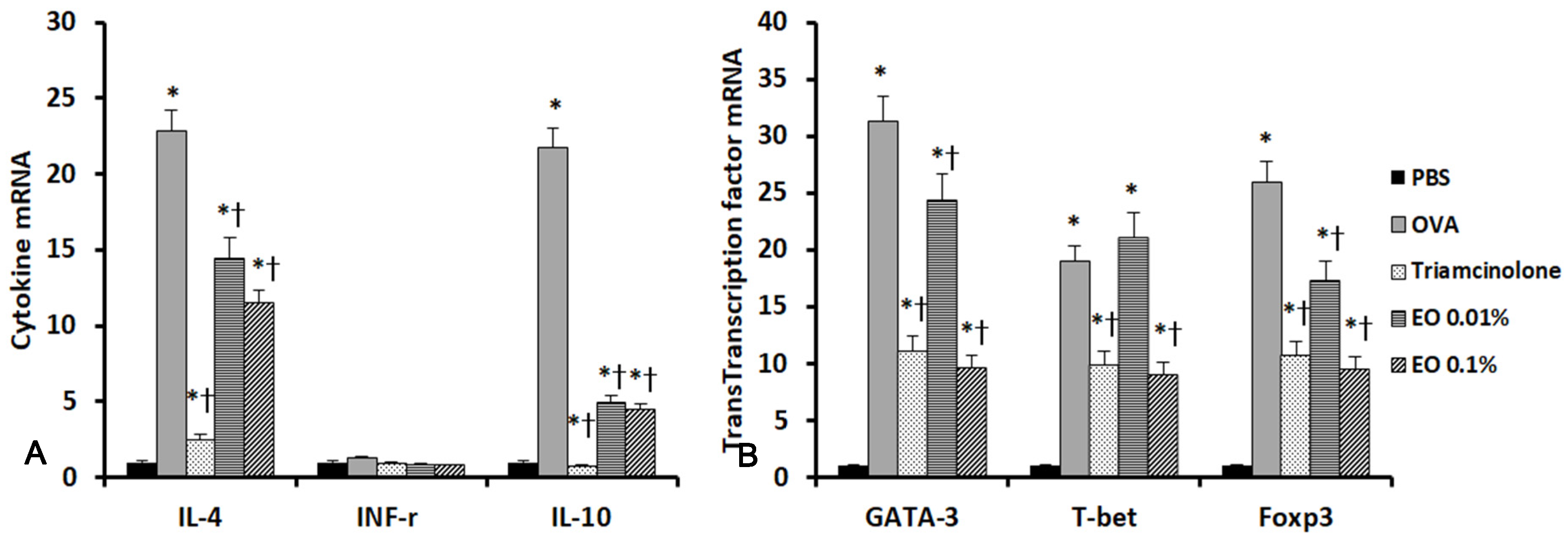
2.5. Effects of EOCO on the Expression of Inflammatory Mediators and Transcription Factors in the Sinonasal Mucosa
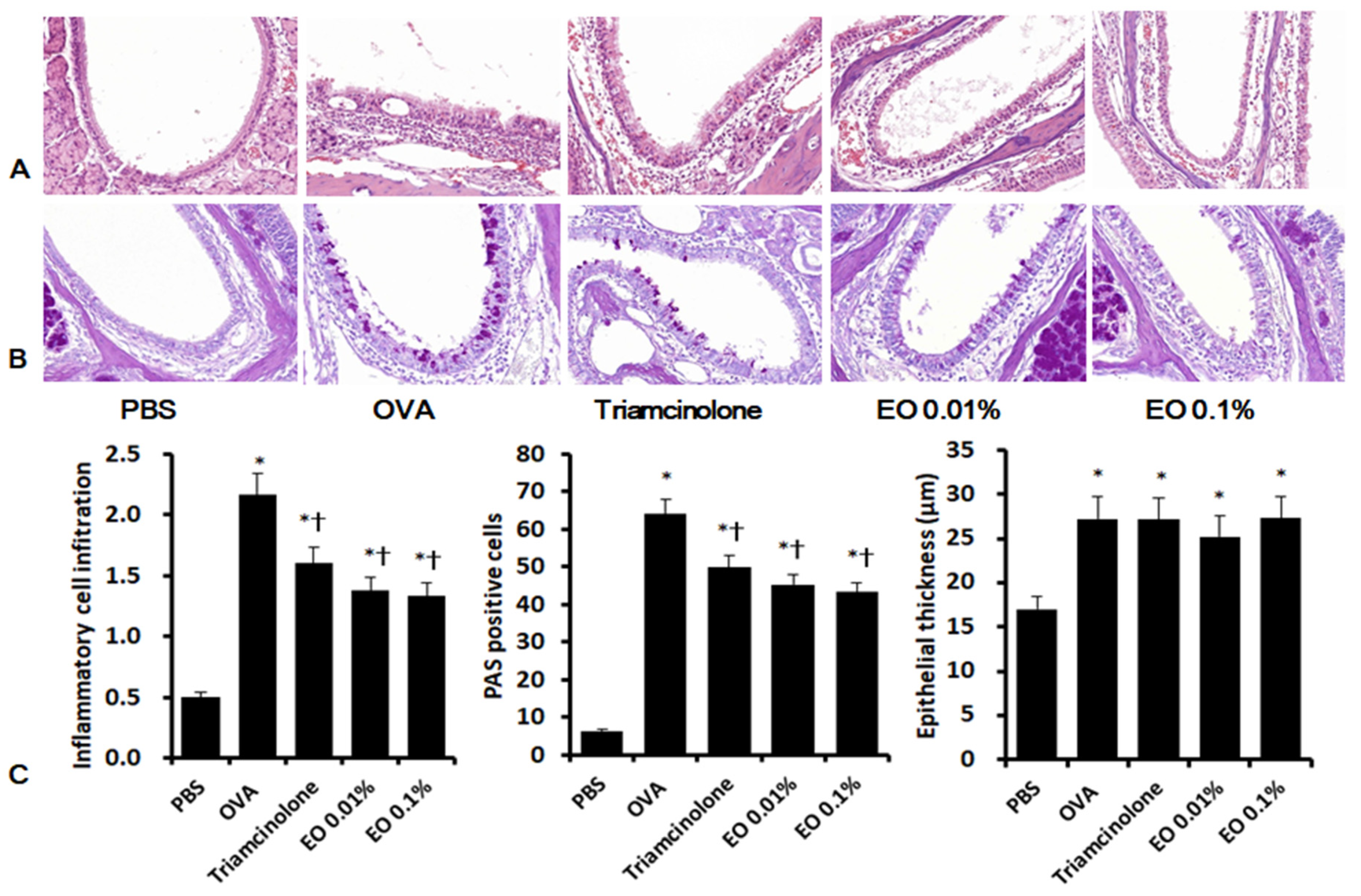
2.6. Effects of EOCO on Sinonasal Mucosal Inflammation
3. Discussion
4. Materials and Methods
4.1. Preparation of Microencapsulated EOCO
4.2. Preparation of OVA-Induced AR Mouse Model
4.3. Evaluation of Nasal Symptoms
4.4. Evaluation of Nasal Lavage Fluid (NLF)
4.5. Measurement of Serum IgE
4.6. Measurement of Cytokines and Transcription Factor mRNAs in the Sinonasal Mucosa
4.7. Measurement of Cytokines from Splenocytes with OVA
4.8. Histologic Evaluation of Sinonasal Mucosa
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bernstein, D.I.; Schwartz, G.; Bernstein, J.A. Allergic Rhinitis: Mechanisms and Treatment. Immunol. Allergy Clin. N. Am. 2016, 36, 261–278. [Google Scholar] [CrossRef]
- May, J.R.; Dolen, W.K. Management of Allergic Rhinitis: A Review for the Community Pharmacist. Clin. Ther. 2017, 39, 2410–2419. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Jung, S.M.; Yoo, S.A.; Kim, W.U.; Cho, C.S.; Park, B.J.; Woo, J.M.; Yoon, C.H. Antinociceptive and anti-inflammatory effects of essential oil extracted from Chamaecyparis obtusa in mice. Int. Immunopharmacol. 2015, 29, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.; Lee, J.H.; Kim, J.W.; Park, M.J.; Lee, S.S.; Jeung, E.B. Alleviation effects of natural volatile organic compounds from Pinus densiflora and Chamaecyparis obtusa on systemic and pulmonary inflammation. Biomed. Rep. 2018, 9, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Bae, D.; Seol, H.; Yoon, H.G.; Na, J.R.; Oh, K.; Choi, C.Y.; Lee, D.W.; Jun, W.; Youl Lee, K.; Lee, J.; et al. Inhaled essential oil from Chamaecyparis obtuse ameliorates the impairments of cognitive function induced by injection of beta-amyloid in rats. Pharm. Biol. 2012, 50, 900–910. [Google Scholar] [CrossRef] [PubMed]
- Bae, M.S.; Park, D.H.; Choi, C.Y.; Kim, G.Y.; Yoo, J.C.; Cho, S.S. Essential Oils and Non-volatile Compounds Derived from Chamaecyparis obtusa: Broad Spectrum Antimicrobial Activity against Infectious Bacteria and MDR(multidrug resistant) Strains. Nat. Prod. Commun. 2016, 11, 693–694. [Google Scholar] [CrossRef]
- Yang, H.; Ahn, C.; Choi, I.G.; Choi, W.S.; Park, M.J.; Lee, S.S.; Choi, D.H.; Jeung, E.B. Estimation of the environmental effect of natural volatile organic compounds from Chamaecyparis obtusa and their effect on atopic dermatitis-like skin lesions in mice. Mol. Med. Rep. 2015, 12, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Ye, M.K.; Lee, D.W.; Che, M.H. Effect of microencapsulated essential oil form Chamaecyparis obtusa on monocyte-derived dendritic cell activation and CD4+ T cell polarization. PLoS ONE 2018, 13, e0201233. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S.; Hong, E.J.; Gwak, K.S.; Park, M.J.; Choi, K.C.; Choi, I.G.; Jang, J.W.; Jeung, E.B. The essential oils of Chamaecyparis obtusa promote hair growth through the induction of vascular endothelial growth factor gene. Fitoterapia 2010, 81, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Kim, Y.H.; Kim, J.K.; Park, K.K. Anti-allergic effect of bee venom in an allergic rhinitis mouse model. Biol. Pharm. Bull. 2014, 37, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Kakli, H.A.; Riley, T.D. Allergic Rhinitis. Prim. Care 2016, 43, 465–475. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Wang, C.; Zhang, L. Recent developments and highlights in allergic rhinitis. Allergy 2019, 74, 2320–2328. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Yoo, S.A.; Kim, W.U.; Cho, C.S.; Woo, J.M.; Yoon, C.H. Anti-inflammatory effects of essential oils extracted from Chamaecyparis obtusa on murine models of inflammation and RAW 264.7 cells. Mol. Med. Rep. 2016, 13, 3335–3341. [Google Scholar] [CrossRef] [PubMed]
- Kwak, B.M.; Kim, E.H.; Kim, Y.M.; Kim, H.T. Component analysis of four-part extracts from Chamaecyparis obtusa Endl. by supercritical fluid extraction and anti-inflammatory effect on RAW 264.7cells. J. Exerc. Rehabil. 2019, 15, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Kim, S.M.; Lee, H.J.; Lee, S.J.; Heo, J.D.; Venkatarame Gowda Saralamma, V.; Ha, S.E.; Kim, E.H.; Mun, S.P.; Kim, G.S. Essential oil from Korean Chamaecyparis obtusa leaf ameliorates respiratory activity in SpragueDawley rats and exhibits protection from NF-kappaB-induced inflammation in WI38 fibroblast cells. Int. J. Mol. Med. 2019, 43, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products-a review. Int. J. Cosmet. Sci 2016, 38, 109–119. [Google Scholar] [CrossRef]
- Shi, Z.; Jiang, W.; Wang, M.; Wang, X.; Li, X.; Chen, X.; Qiao, L. Inhibition of JAK/STAT pathway restrains TSLP-activated dendritic cells mediated inflammatory T helper type 2 cell response in allergic rhinitis. Mol. Cell. Biochem. 2017, 430, 161–169. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, H. Protective effect of Asarum sieboldii essential oil on ovalbumin induced allergic rhinitis in rat. Biosci. Rep. 2020, 26, BSR20191370. [Google Scholar] [CrossRef]
- Fu, M.; Fu, S.; Ni, S.; Zou, L.; Liu, Y.; Hong, T. Anti-inflammatory effect of epigallocatechin gallate in a mouse model of ovalbumin-induced allergic rhinitis. Int. Immunopharmacol. 2017, 49, 102–108. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |

| NC | OVA | Triamcinolone | EOCO 0.01% | EOCO 0.1% | |
|---|---|---|---|---|---|
| IL-4 (pg/mL) | 4.08 ± 2.46 | 12.28 ± 5.24 a | 8.22 ± 2.23 a,b | 7.35 ± 1.45 a,b | 5.58 ± 1.65 b |
| IFN-r (pg/mL) | 2.65 ± 2.13 | 5.06 ± 2.65 a | 4.41 ± 0.68 | 2.50 ± 1.65 b | 4.55 ± 1.45 |
| IL-10 (pg/mL) | 4.58 ± 3.21 | 9.16 ± 3.48 a | 2.86 ± 0.77 b | 3.33 ± 1.36 b | 6.58 ± 1.82b a |
| TNF-a (pg/mL) | 5.29 ± 3.57 | 7.02 ± 3.22 | 5.81 ± 1.82 b | 5.40 ± 1.86 b | 5.35 ± 0.60 b |
| NC | OVA | Triamcinolone | EOCO 0.01% | EOCO 0.1% | |
|---|---|---|---|---|---|
| IL-4 | 5.47 ± 1.45 | 14.95 ±5.34 a | 9.43 ± 3.56 a,b | 15.55 ± 4.74 a | 9.02 ± 2.72 a,b |
| IFN-r | 6.85 ± 2.52 | 10.48 ± 5.37 a | 8.70 ± 5.12 a | 9.16 ± 2.86 a | 8.39 ± 2.94 a |
| IL-10 | 6.86 ± 1.60 | 20.20 ± 7.47 a | 25.93 ± 7.71 a | 13.84 ± 5.21 a,b | 15.52 ± 6.33 a,b |
| TNF-a | 134.69 ± 57.45 | 305.18 ± 86.73 a | 302.52 ± 60.24 a | 288.18 ± 78.54 a | 237.81 ± 112.34 a,b |
| Compound | Kovatz Index | Retention Time (min) | Peak Area (%) |
|---|---|---|---|
| α-Pinene | 928 | 8.75 | 5.96 |
| Sabinene | 966 | 10.96 | 16.72 |
| Myrcene | 989 | 11.36 | 19.45 |
| α-Terpinene | 1017 | 12.69 | 3.05 |
| γ-Terpinene | 1044 | 13.54 | 4.75 |
| Limonene | 1028 | 13.76 | 1.82 |
| Terepineol | 1095 | 15.21 | 1.26 |
| Terpinene-4-ol | 1162 | 16.23 | 2.82 |
| Bornyl acetate | 1265 | 27.53 | 9.46 |
| α -Terpinyl acetate | 1349 | 28.64 | 15.69 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, S.-H.; Ye, M.-K.; Lee, D.-W.; Che, M.-H. Immunomodulative Effects of Chamaecyparis obtusa Essential Oil in Mouse Model of Allergic Rhinitis. Molecules 2020, 25, 4517. https://doi.org/10.3390/molecules25194517
Shin S-H, Ye M-K, Lee D-W, Che M-H. Immunomodulative Effects of Chamaecyparis obtusa Essential Oil in Mouse Model of Allergic Rhinitis. Molecules. 2020; 25(19):4517. https://doi.org/10.3390/molecules25194517
Chicago/Turabian StyleShin, Seung-Heon, Mi-Kyung Ye, Dong-Won Lee, and Mi-Hyun Che. 2020. "Immunomodulative Effects of Chamaecyparis obtusa Essential Oil in Mouse Model of Allergic Rhinitis" Molecules 25, no. 19: 4517. https://doi.org/10.3390/molecules25194517
APA StyleShin, S.-H., Ye, M.-K., Lee, D.-W., & Che, M.-H. (2020). Immunomodulative Effects of Chamaecyparis obtusa Essential Oil in Mouse Model of Allergic Rhinitis. Molecules, 25(19), 4517. https://doi.org/10.3390/molecules25194517






