Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach
Abstract
1. Introduction
2. Results
2.1. Total Antioxidant Capacity
2.2. Antioxidant Response Element (ARE) Activation
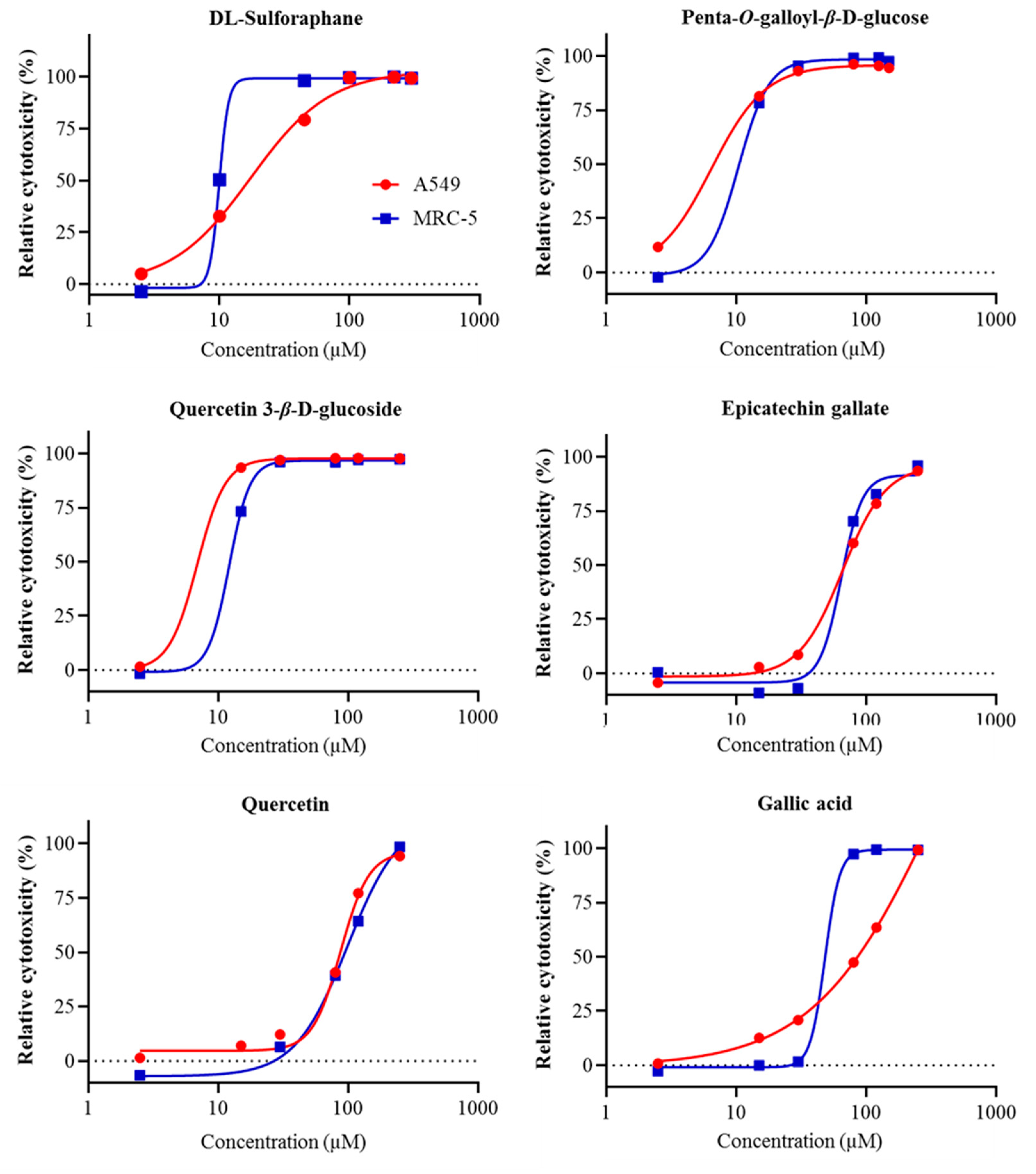
2.3. Cell Proliferation Assays
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Cell Lines
4.3. Total Antioxidant Capacity
4.4. Antioxidant Response Element (ARE) Activation
4.5. Cell Proliferation Assays
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Randolph, K.C.; Rose, A.K.; Oswalt, C.M.; Brown, M.J. Status of Black Walnut (Juglans nigra L.) in the Eastern United States in Light of the Discovery of Thousand Cankers Disease. Castanea 2013, 78, 2–14. [Google Scholar] [CrossRef]
- Coggeshall, M.V. Black Walnut: A Nut Crop for the Midwestern United States. HortScience 2011, 46, 340–342. [Google Scholar] [CrossRef]
- Nolan, J.M.; Robbins, M. Cultural Conservation of Medicinal Plant Use in the Ozarks. Hum. Organ. 1999, 58, 67–72. [Google Scholar] [CrossRef]
- Câmara, C.R.S.; Schlegel, V. A Review on the Potential Human Health Benefits of the Black Walnut: A Comparison with the English Walnuts and Other Tree Nuts. Int. J. Food Prop. 2015, 19, 2175–2189. [Google Scholar] [CrossRef]
- Ho, K.-V.; Schreiber, K.L.; Vu, D.C.; Rottinghaus, S.M.; Jackson, D.E.; Brown, C.R.; Lei, Z.; Sumner, L.W.; Coggeshall, M.V.; Lin, C.-H. Black Walnut (Juglans nigra) Extracts Inhibit Proinflammatory Cytokine Production From Lipopolysaccharide-Stimulated Human Promonocytic Cell Line U-937. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef]
- Ho, K.-V.; Lei, Z.; Sumner, L.W.; Coggeshall, M.V.; Hsieh, H.-Y.; Stewart, G.C.; Lin, C.-H. Identifying Antibacterial Compounds in Black Walnuts (Juglans nigra) Using a Metabolomics Approach. Metabolites 2018, 8, 58. [Google Scholar] [CrossRef]
- Vu, D.C.; Park, J.; Ho, K.-V.; Sumner, L.W.; Lei, Z.; Greenlief, C.M.; Mooney, B.; Coggeshall, M.V.; Lin, C.-H. Identification of health-promoting bioactive phenolics in black walnut using cloud-based metabolomics platform. J. Food Meas. Charact. 2019, 14, 770–777. [Google Scholar] [CrossRef]
- Reid, W.; Coggeshall, M.V.; Garrett, H.E.; Van Sambeek, J.W. Growing Black Walnut for Nut Production. Available online: http://www.centerforagroforestry.org/pubs/walnutNuts.pdf (accessed on 29 August 2020).
- Vu, D.C.; Vo, P.H.; Coggeshall, M.V.; Lin, C.-H. Identification and Characterization of Phenolic Compounds in Black Walnut Kernels. J. Agric. Food Chem. 2018, 66, 4503–4511. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Kim, S.-H.; Hagerman, A.E.; Lü, J. Anti-Cancer, Anti-Diabetic and Other Pharmacologic and Biological Activities of Penta-Galloyl-Glucose. Pharm. Res. 2009, 26, 2066–2080. [Google Scholar] [CrossRef] [PubMed]
- Actis-Goretta, L.; Romanczyk, L.J.; Rodríguez, C.A.; Kwik-Uribe, C.; Keen, C.L. Cytotoxic effects of digalloyl dimer procyanidins in human cancer cell lines. J. Nutr. Biochem. 2008, 19, 797–808. [Google Scholar] [CrossRef]
- Chen, Q.; Li, P.; Xu, Y.; Li, Y.; Tang, B. Isoquercitrin inhibits the progression of pancreatic cancer in vivo and in vitro by regulating opioid receptors and the mitogen-activated protein kinase signalling pathway. Oncol. Rep. 2014, 33, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Macarrón, R.; Banks, M.N.; Bojanic, D.; Burns, D.J.; Cirovic, D.A.; Garyantes, T.; Green, D.V.S.; Hertzberg, R.P.; Janzen, W.P.; Paslay, J.W.; et al. Impact of high-throughput screening in biomedical research. Nat. Rev. Drug Discov. 2011, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Leavell, M.D.; Singh, A.H.; Kaufmann-Malaga, B.B. High-throughput screening for improved microbial cell factories, perspective and promise. Curr. Opin. Biotechnol. 2020, 62, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; McDonald, P.; Timmermann, B.N.; Gupta, M.; Chaguturu, R. Bioactivity Profiling of Plant Biodiversity of Panama by High Throughput Screening. Nat. Prod. Commun. 2019, 14, 71–74. [Google Scholar] [CrossRef]
- Dong, Z.; Kundu, J.K.; Na, H.-K. Nrf2 as a Master Redox Switch in Turning on the Cellular Signaling Involved in the Induction of Cytoprotective Genes by Some Chemopreventive Phytochemicals. Planta Med. 2008, 74, 1526–1539. [Google Scholar]
- Koh, S.-J.; Choi, Y.I.; Kim, Y.; Kim, Y.-S.; Choi, S.-W.; Kim, J.-W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor kappaB signaling in intestinal epithelial cells, and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2018, 58, 1603–1613. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.-S.; Lee, J.H.; Heo, S.C.; Lee, K.L.; Choi, S.-W.; Kim, Y. Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients 2016, 8, 439. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Ciudad, C.J.; Noé, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2015, 57, 3373–3383. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G. Suppression of Implanted MDA-MB 231 Human Breast Cancer Growth in Nude Mice by Dietary Walnut. Nutr. Cancer 2008, 60, 666–674. [Google Scholar] [CrossRef]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, J.; Blackburn, G.; Peradze, N. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 2012, 28, 67–75. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.-X.; Manchester, L.C.; Korkmaz, A.; Fuentes-Broto, L.; Hardman, W.E.; Rosales-Corral, S.A.; Qi, W. A Walnut-Enriched Diet Reduces the Growth of LNCaP Human Prostate Cancer Xenografts in Nude Mice. Cancer Investig. 2013, 31, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E. Walnuts have potential for cancer prevention and treatment in mice. J. Nutr. 2014, 144, 555S–560S. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.; Marhuenda, J.; Cerdá, B.; Zafrilla, P.; Martínez-Cachá, A.; Tejada, L.; Mulero, J. HPLC-DAD determination and availability of phenolic compounds in 10 genotypes of walnuts. Int. J. Food Prop. 2016, 20, 1–33. [Google Scholar]
- Torres-León, C.; Ventura-Sobrevilla, J.; Serna-Cock, L.; Ascacio-Valdés, J.A.; Contreras-Esquivel, J.; Aguilar, C.N. Pentagalloylglucose (PGG): A valuable phenolic compound with functional properties. J. Funct. Foods 2017, 37, 176–189. [Google Scholar] [CrossRef]
- Hu, H.; Lee, H.-J.; Jiang, C.; Zhang, J.; Wang, L.; Zhao, Y.; Xiang, Q.; Lee, E.-O.; Kim, S.-H.; Lü, J. Penta-1,2,3,4,6-O-galloyl-β-d-glucose induces p53 and inhibits STAT3 in prostate cancer cells in vitro and suppresses prostate xenograft tumor growth in vivo. Mol. Cancer Ther. 2008, 7, 2681–2691. [Google Scholar] [CrossRef]
- Yu, W.S.; Jeong, S.-J.; Kim, J.-H.; Lee, H.-J.; Song, H.S.; Kim, M.-S.; Ko, E.; Lee, H.-J.; Khil, J.-H.; Jang, H.-J.; et al. The Genome-Wide Expression Profile of 1,2,3,4,6-Penta-O-Galloyl-β-d-Glucose-Treated MDA-MB-231 Breast Cancer Cells: Molecular Target on Cancer Metabolism. Mol. Cells 2011, 32, 123–132. [Google Scholar] [CrossRef]
- Huh, J.-E.; Lee, E.-O.; Kim, M.-S.; Kang, K.-S.; Kim, C.-H.; Cha, B.-C.; Dong, Z.; Kim, S.-H. Penta-O-galloyl-beta-D-glucose suppresses tumor growth via inhibition of angiogenesis and stimulation of apoptosis: Roles of cyclooxygenase-2 and mitogen-activated protein kinase pathways. Carcinogenesis 2005, 26, 1436–1445. [Google Scholar] [CrossRef]
- Bae, S.; Kim, S.Y.; Do, M.H.; Lee, C.H.; Song, Y.-J. 1,2,3,4,6-Penta-O-galloyl-β-d-glucose, a bioactive compound in Elaeocarpus sylvestris extract, inhibits varicella-zoster virus replication. Antivir. Res. 2017, 144, 266–272. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds mentioned in this study are available from the authors. |
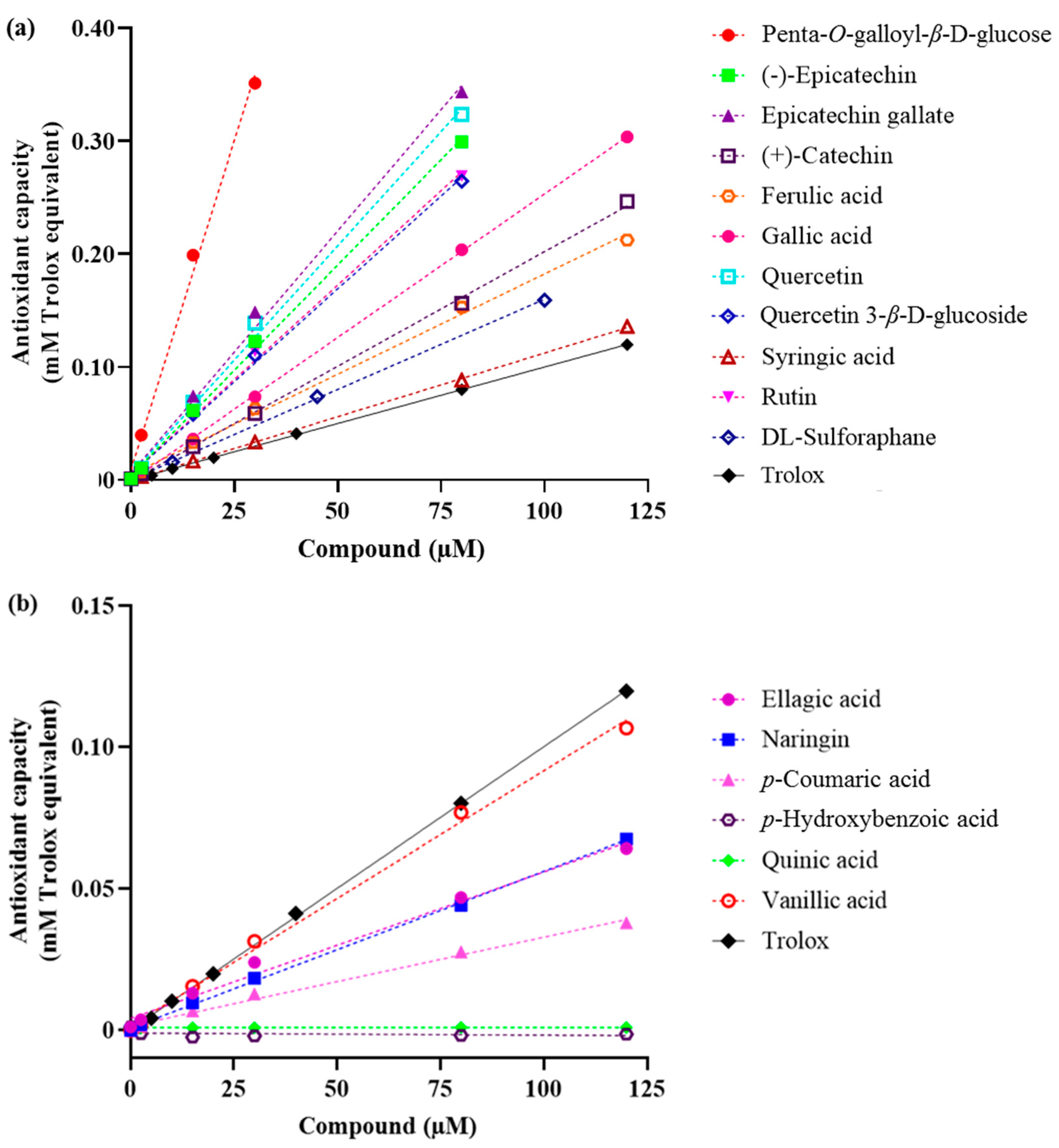
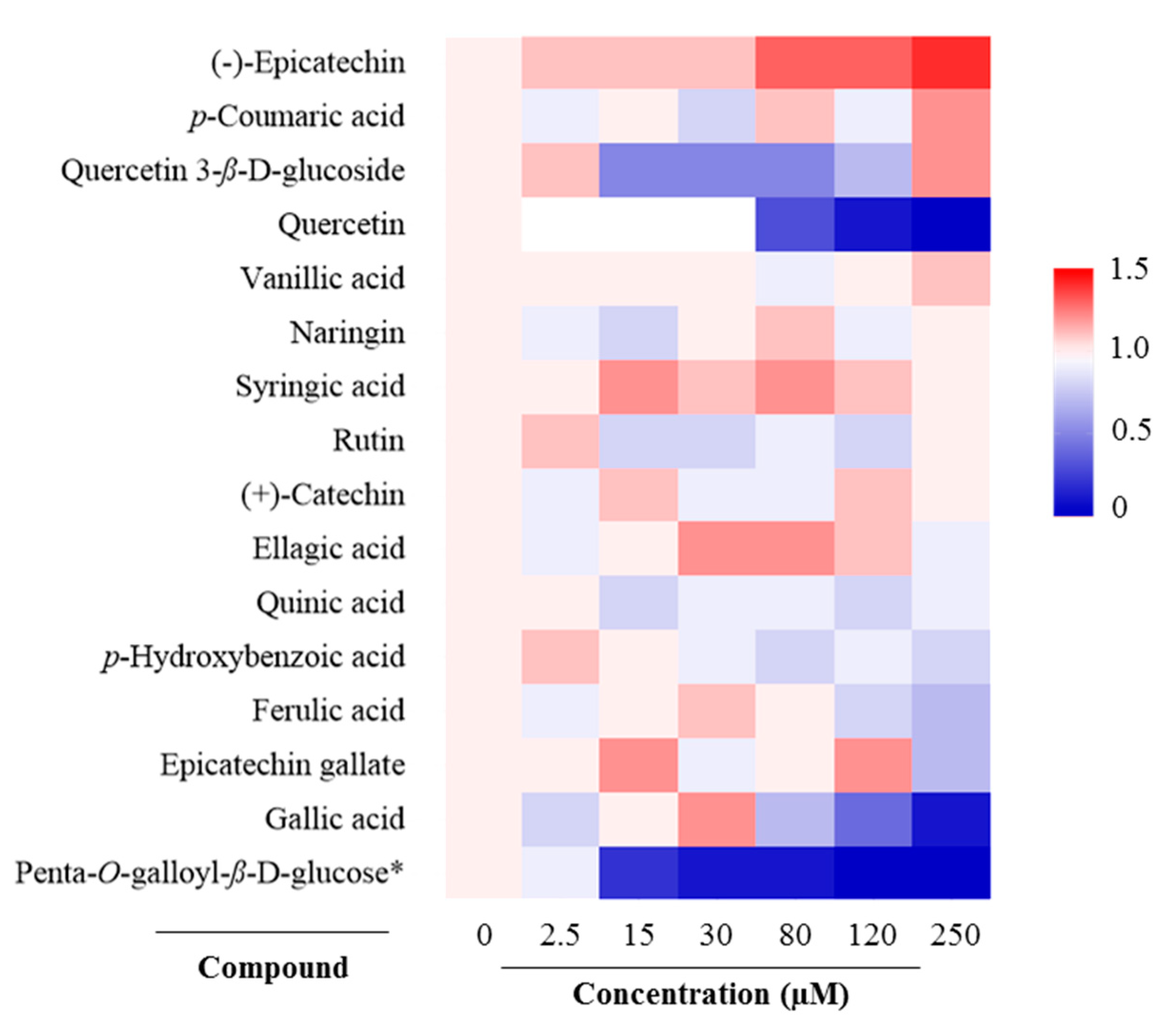
| No. | Compound | Slope (in Trolox Equivalents) | R Square | Fold-Increase Over Trolox |
|---|---|---|---|---|
| Control | ||||
| 1 | Trolox | 0.001014 ± 1.125 × 10−5 | 0.999 | 1.0 |
| Antioxidant Capacity Higher than Trolox | ||||
| 2 | Penta-O-galloyl-β-d-glucose | 0.01167 ± 5.756 × 10−4 | 0.995 | 11.5 |
| 3 | Epicatechin gallate | 0.004294 ± 1.570 × 10−4 | 0.996 | 4.2 |
| 4 | Quercetin | 0.004045 ± 1.392 × 10−4 | 0.997 | 4.0 |
| 5 | (−)-Epicatechin | 0.003729 ± 7.546 × 10−5 | 0.999 | 3.7 |
| 6 | Rutin | 0.002962 ± 1.551 × 10−4 | 0.989 | 2.9 |
| 7 | Quercetin 3-β-d-glucoside | 0.002908 ± 1.522 × 10−4 | 0.989 | 2.9 |
| 8 | Gallic acid | 0.002541 ± 1.392 × 10−5 | 0.999 | 2.5 |
| 9 | (+)-Catechin | 0.002029 ± 3.184 × 10−5 | 0.999 | 2.0 |
| 10 | Ferulic acid | 0.001775 ± 5.026 × 10−5 | 0.997 | 1.8 |
| 11 | Syringic acid | 0.001088 ± 1.057 × 10−5 | 0.999 | 1.1 |
| Antioxidant capacity lower than Trolox | ||||
| 12 | Vanillic acid | 0.0007396 ± 4.245 × 10−5 | 0.984 | 0.7 |
| 13 | Ellagic acid | 0.0005183 ± 2.860 × 10−5 | 0.988 | 0.5 |
| 14 | Naringin | 0.0005177 ± 9.755 × 10−6 | 0.998 | 0.5 |
| 15 | p-Coumaric acid | 0.0003139 ± 1.434 × 10−5 | 0.992 | 0.3 |
| 16 | p-Hydroxybenzoic acid | −0.000008 ± 1.260 × 10−5 | 0.086 | n/a |
| 17 | Quinic acid | −0.0000004 ± 1.034 × 10−6 | 0.032 | <0.1 |
| Compound | A549 Cell Line | MRC-5 Cell Line |
|---|---|---|
| Penta-O-galloyl-β-d-glucose | 6.11 | 10.37 |
| Quercetin 3-β-d-glucoside | 6.89 | 12.15 |
| Epicatechin gallate | 65.96 | 64.38 |
| Quercetin | 87.74 | 99.47 |
| Gallic acid | >250 | 48.18 |
| Ellagic acid | >250 | >250 |
| (−)-Epicatechin | >250 | >250 |
| Rutin | >250 | >250 |
| (+)-Catechin | >250 | >250 |
| Ferulic acid | >250 | >250 |
| Syringic acid | >250 | >250 |
| Vanillic acid | >250 | >250 |
| Naringin | >250 | >250 |
| p-Coumaric acid | >250 | >250 |
| p-Hydroxybenzoic acid | >250 | >250 |
| Quinic acid | >250 | >250 |
| dl-Sulforaphane (control) | 16.96 | 9.95 |
| Compound | Black Walnut Cultivar | English Walnut + | |||||
|---|---|---|---|---|---|---|---|
| Daniel | Hay | Jackson | Kwik Krop | Mystry | Surprise | ||
| Penta-O-galloyl-β-d-glucose | n/d | n/d | n/d | n/d | 15.2 ± 2.5 | n/d | 55.9 ± 7.7 |
| Quercetin 3-β-d-glucoside | n/d | 3.2 ± 0.1 | 1.6 ± 0.2 | n/d | 2.1 ± 0.3 | 1.8 ± 0.3 | 3.7 ± 0.2 |
| Epicatechin gallate | 13.2 ± 0.5 | 7.0 ± 0.6 | 3.6 ± 0.7 | n/d | 2.0 ± 0.2 | 6.0 ± 0.7 | 4.9 ± 1.5 |
| Gallic acid | n/d | 1.4 ± 0.2 | 0.7 ± 0.2 | 0.5 ± 0.03 | 4.3 ± 0.3 | 1.0 ± 0.03 | 8.1 ± 0.7 |
| Ellagic acid | 30.4 ± 1.0 | 40.5 ± 5.9 | 61.1 ± 3.7 | 11.4 ± 2.0 | 65.7 ± 4.8 | 72.1 ± 8.3 | 98.4 ± 20.6 |
| Rutin | n/d | n/d | n/d | 1.7 ± 0.6 | 4.2 ± 1.3 | n/d | 2.7 ± 0.3 |
| (+)-Catechin | n/d | n/d | n/d | n/d | n/d | 0.6 ± 0.01 | 47.9 ± 3.5 |
| Ferulic acid | n/d | n/d | n/d | 0.7 ± 0.04 | 0.6 ± 0.06 | 4.9 ± 0.08 | 0.9 ± 0.1 |
| Syringic acid | 7.3 ± 1.3 | n/d | 7.7 ± 1.4 | n/d | 9.5 ± 2.1 | n/d | 7.3 ± 2.2 |
| Vanillic acid | n/d | 9.9 ± 2.6 | 8.7 ± 1.7 | n/d | 6.9 ± 1.2 | n/d | 7.3 ± 1.8 |
| Naringin | 0.5 ± 0.1 | n/d | n/d | 0.3 ± 0.1 | 0.5 ± 0.2 | 4.9 ± 0.1 | 0.3 ± 0.04 |
| p-Coumaric acid | n/d | 0.2 ± 0.03 | 0.2 ± 0.03 | 0.2 ± 0.1 | 0.3 ± 0.02 | 0.3 ± 0.1 | 0.5 ± 0.1 |
| p-Hydroxybenzoic acid | n/d | n/d | n/d | n/d | n/d | n/d | 1.2 ± 0.5 |
| Quinic acid | 4.7 ± 0.2 | 2.4 ± 0.1 | 1.4 ± 0.2 | 4.2 ± 0.2 | 2.4 ± 0.3 | 3.9 ± 0.5 | 6.8 ± 0.4 |
| Quercetin | n/q | n/q | n/q | n/q | n/q | n/q | n/q |
| (−)-Epicatechin | n/q | n/q | n/q | n/q | n/q | n/q | 3.9 ± 0.01 |
| Biological Activity of Kernel Extracts from Black Walnuts | |||||||
| Antioxidant | ++ | + | ++ | ++ | +++ | +++ | n/c |
| Antibacterial * | . | . | . | . | +++ | +++ | n/c |
| Anti-inflammatory potential ** | + | n/a | n/a | n/a | + | +++ | n/c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, K.-V.; Roy, A.; Foote, S.; Vo, P.H.; Lall, N.; Lin, C.-H. Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach. Molecules 2020, 25, 4516. https://doi.org/10.3390/molecules25194516
Ho K-V, Roy A, Foote S, Vo PH, Lall N, Lin C-H. Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach. Molecules. 2020; 25(19):4516. https://doi.org/10.3390/molecules25194516
Chicago/Turabian StyleHo, Khanh-Van, Anuradha Roy, Sarah Foote, Phuc H. Vo, Namrita Lall, and Chung-Ho Lin. 2020. "Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach" Molecules 25, no. 19: 4516. https://doi.org/10.3390/molecules25194516
APA StyleHo, K.-V., Roy, A., Foote, S., Vo, P. H., Lall, N., & Lin, C.-H. (2020). Profiling Anticancer and Antioxidant Activities of Phenolic Compounds Present in Black Walnuts (Juglans nigra) Using a High-Throughput Screening Approach. Molecules, 25(19), 4516. https://doi.org/10.3390/molecules25194516







