Medicinal Potential of Garcinia Species and Their Compounds
Abstract
1. Introduction
2. Garcinia Species and Bioactive Compounds
2.1. Garcinia Brasiliensis
2.2. Garcinia Gardneriana
2.3. Garcinia Pedunculata
2.4. Garcinia Cambogia
2.5. Garcinia Mangostana
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kunle, O.F.; Egharevba, H.O.; Ahmadu, P.O. Standardization of herbal medicines—A review. Int. J. Biodivers. Conserv. 2012, 4, 101–112. [Google Scholar] [CrossRef]
- Shameer, P.S.; Rameshkumar, K.B.; Mohanan, N. Diversity of garcinia in the Western Ghats. Phytochemical perspective. India Jawaharlal Nehru Trop. Bot. Gard. Res. Inst. 2016, 1–18. [Google Scholar]
- Ampofo, S.A.; Waterman, P.G. Xanthones and neoflavonoids from two Asian species of Calophyllum. Phytochemistry 1986, 25, 2617–2620. [Google Scholar] [CrossRef]
- Monache, G.D.; Monache, F.D.; Waterman, P.G.; Crichton, E.G.; De Lima, R.A. Minor xanthones from Rheedia gardneriana. Phytochemistry 1984, 23, 1757–1759. [Google Scholar] [CrossRef]
- Almeida, L.S.B.; Murata, R.M.; Yatsuda, R.; Dos-Santos, M.H.; Nagem, T.J.; Alencar, S.M.; Koo, H.; Rosalen, P.L. Antimicrobial activity of Rheedia brasiliensis and 7-epiclusianone against Streptococcus mutans. Phytomedicine 2008, 15, 886–891. [Google Scholar] [CrossRef]
- Panthong, A.; Norkaew, P.; Kanjanapothi, D.; Taesotikul, T.; Anantachoke, N.; Reutakul, V. Anti-inflammatory, analgesic, and antipyretic activies of the extract of gamboge from Garcinia hanburyi Hook. J. Ethnophamacol. 2007, 111, 335–340. [Google Scholar] [CrossRef]
- Gustafson, K.R.; Blunt, J.W.; Munro, M.H.G.; Fuller, R.W.; McKee, T.C.; Cardellina, J.H.; McMahon, J.B.; Cragg, G.M.; Boyd, M.R. The guttiferones, HIV-inhibitory benzophenones from Symphonia globulifera, Garcinia livingstonei, Garcinia ovalifolia and Clusiarosea. Tetrahedron 1992, 48, 10093–10102. [Google Scholar] [CrossRef]
- Williams, R.B.; Hoch, J.; Glass, T.E.; Evans, R.; Miller, J.S.; Wisse, J.H.; Kingston, D.G.I. A novel cytotoxic guttiferone analogue from Garcinia macrophylla from the Suriname Rainforest. Planta Med. 2003, 69, 864–866. [Google Scholar]
- Almeida-Alves, T.M.; Oliveira-Alves, R.; Romanha, A.J.; Santos, M.H.; Nagem, T.J.; Zani, C.L. Biological activities of 7-epiclusianone. J. Nat. Prod. 1999, 62, 369–371. [Google Scholar] [CrossRef]
- Nguyen, D.C.; Timmer, T.K.; Davison, B.C.; McGrane, I.R. Possible garcinia cambogia-induced mania with psychosis: A case report. J. Pharm. Pr. 2017, 32, 99–102. [Google Scholar] [CrossRef]
- Cui, J.; Hu, W.; Cai, Z.; Liu, Y.; Li, S.; Tao, W. New medicinal properties of mangostins: Analgesic activity and pharmacological characterization of active ingredients from the fruit hull of Garcinia mangostana. Pharm. Biochem. Behav. 2010, 95, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Sordat-Diserens, I.; Rogers, B.S.C.; Hostettmann, K. Prenylated xanthones from Garcinia livingstonei. Phytochemistry 1992, 31, 313–316. [Google Scholar] [CrossRef]
- Khanum, S.A.; Shashikanth, S.; Deepak, A.V. Synthesis and anti-inflammatory activity of benzophenone analogues. Bioorg. Chem. 2004, 32, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Carballo, D.; Seeber, S.; Strumberg, D.; Hilger, R.A. Novel antitumoral compound isolated from Clusiarosea. Int. J. Clin. Pharm. 2003, 41, 622–623. [Google Scholar] [CrossRef]
- Merza, J.; Aumond, M.C.; Rondeau, D.; Dumontet, V.; Le Ray, A.M.; Seraphin, D.; Richomme, P. Prenylated xanthones and tocotrienols from Garcinia virgata. Phytochemistry 2004, 65, 2915–2920. [Google Scholar] [CrossRef]
- Mundugaru, R.; Narayana, S.K.K.; Ballal, S.R.; Thomas, J.; Rajakrishnan, R. Neuroprotective activity of Garcinia pedunculata roxb ex buch ham fruit extract against aluminium chloride induced neurotoxicity in mice. Indian J. Pharm. Educ. Res. 2016, 50, 435–441. [Google Scholar] [CrossRef]
- Hay, A.E.A.; Mallet, M.C.; Dumontet, S.; Litaudon, V.; Rondeau, M.; Richomme, D. Antioxidant xanthones from Garcinia vieillardii. J. Nat. Prod. 2004, 67, 707–709. [Google Scholar] [CrossRef]
- Bennett, G.J.; Lee, H.K. Xanthones from Guttiferae. Phytochemistry 1989, 28, 967–998. [Google Scholar] [CrossRef]
- Rao, A.V.R.; Sarma, M.R.; Venkataraman, K.; Yemul, S.S. A benzophenone and xanthone with unusual hydroxylation patterns from the heartwood of Garcinia pedunculata. Phytochemistry 1974, 13, 1241–1244. [Google Scholar] [CrossRef]
- Acuña, U.M.; Dastmalchi, K.; Basile, M.J.; Kennelly, E.J. Quantitative high performance liquid chromatography photo-diode array (HPLC–PDA) analysis of benzophenones and biflavonoids in eight Garcinia species. J. Food. Compos. Anal. 2012, 25, 215–220. [Google Scholar] [CrossRef]
- Pereira, I.O.; Marques, M.J.; Pavan, A.L.R.; Codonho, B.S.; Barbiéri, C.L.; Beijo, L.A.; Doriguetto, A.C.; D’Martin, E.C.; dos Santos, M.H. Leishmanicidal activity of benzophenones and extracts from Garcinia brasiliensis Mart. Fruits. Phytomedicine 2010, 17, 339–345. [Google Scholar] [CrossRef]
- Santa-Cecília, F.V.; Vilela, F.C.; da Rocha, C.Q.; Dias, D.F.; Cavalcante, G.P.; Freitas, L.A.; dos Santos, M.H.; Giusti-Paiva, A. Anti-inflammatory and antinociceptive effectsof Garcinia brasiliensis. J. Ethnopharmacol. 2011, 133, 467–473. [Google Scholar] [CrossRef]
- Martins, F.T.; Doriguetto, A.C.; de Souza, T.C.; Souza, K.R.; Santos, M.H.; Moreira, M.E.; Barbosa, L.C. Composition, and anti-inflammatory and antioxidant activities of the volatile oil from the fruit peel of Garcinia brasiliensis. Chem. Biodivers. 2008, 5, 251–258. [Google Scholar] [CrossRef]
- Botta, B.; Mac-Quhae, M.M.; Delle-Monache, G.; Delle-Monache, F.; De Mello, J.F. Chemical investigation of the genus Rheedia. V. Biflavonoids and xanthochymol. J. Nat. Prod. 1984, 47, 1053. [Google Scholar] [CrossRef]
- Schobert, R.; Biersack, B. Chemical and biological aspects of garcinol and isogarcinol: Recent developments. Chem. Biodivers. 2019, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Saito, M.; Ariga, T.; Yoshimura, Y.; Nakazawa, H. Free radical scavenging activity and antiulcer activity of garcinol from Garcinia indica fruit rind. J. Agric. Food Chem. 2000, 48, 2320–2325. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.J.; Lemos, V.S.; Santos, M.H.; Nagem, T.J.; Cortes, S.F. Vascular effects of 7-epiclusianone, a prenylated benzophenone from Rheedia gardneriana, on the rat aorta. Phytomedicine 2006, 13, 442–445. [Google Scholar] [CrossRef]
- Ito, C.; Itoigawa, M.; Miyamoto, Y.; Onoda, S.; Sundar, R.K.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Furukawa, H. Polyprenylated benzophenones from Garcinia assigu and their potential cancer chemopreventive activities. J. Nat. Prod. 2003, 66, 206–209. [Google Scholar] [CrossRef]
- Pan, M.; Chang, W.; Lin-Shiau, S.; Ho, C.; Lin, J. Induction of apoptosis by garcinol and curcumin through cytochrome c release and activation of caspases in human leukemia HL-60 cells. J. Agric. Food Chem. 2001, 49, 1464–1474. [Google Scholar] [CrossRef]
- Piccinelli, A.L.; Cuesta-Rubio, O.; Chica, M.B.; Mahmood, N.; Pagano, B.; Pavone, M.; Barone, V.; Rastrelli, L. Structural revision of clusianone and 7-epi-clusianone and anti-HIV activity of polyisoprenylated benzophenones. Tetrahedron 2005, 61, 8206–8211. [Google Scholar] [CrossRef]
- Abe, F.; Nagafuji, S.; Okabe, H.; Akahane, H.; Estrada-Muñiz, E.; Huerta-Reyes, M.; Reyes-Chilpa, R. Trypanocidal constituents in plants leaves of Garcinia intermedia and heartwood of Calophyllumbrasiliense. Biol. Pharm. Bull. 2004, 27, 141–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ho, P.C.; Cheng, F.; Sethi, G.; Zhi, L. Garcinol: Current status of its anti-oxidative, anti-inflammatory and anti-cancer effects. Cancer Lett. 2015, 362, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Vo, H.T.; Ngo, N.T.; Bui, T.Q.; Pham, H.D.; Nguyen, L.D. Geranylated tetraoxygenated xanthones from the pericarp of Garcinia pedunculata. Phytochem. Lett. 2015, 13, 119–122. [Google Scholar] [CrossRef]
- Tang, Z.Y.; Xia, Z.X.; Qiao, S.P.; Jiang, C.; Shen, G.R.; Cai, M.X.; Tang, X.Y. Four new cytotoxic xanthones from Garcinia nujiangensis. Fitoterapia 2015, 102, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Farombi, E.O.; Owoeye, O. Antioxidative and chemopreventive properties of Vernonia amygdalina and Garcinia biflavonoid. Int. J. Environ. Res. Public Health 2011, 8, 2533–2555. [Google Scholar] [CrossRef]
- Antia, B.S.; Pansanit, A.; Ekpa, O.D.; Ekpe, U.J.; Mahidol, C.; Kittakoop, P. α-Glucosidase inhibitory, aromatase inhibitory, and antiplasmodial activities of a biflavonoid GB1 from Garcinia kola stem bark. Planta Med. 2010, 76, 276–277. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, Y.; Lu, D.; Du, B.; Xiong, L.; Shi, J.; Yang, L.; Liu, W.; Yuan, X.; Zhang, G.; et al. Two natural products, trans-phytol and (22E)-ergosta-6,9,22-triene-3β,5α,8αtriol, inhibit the biosynthesis of estrogen in human ovarian granulosa cells by aromatase (CYP19). Toxicol. Appl. Pharm. 2014, 279, 23–32. [Google Scholar] [CrossRef]
- Castardo, J.A.; Prudente, A.S.; Ferreira, J.; Guimarães, C.L.; Delle Monache, F.; Cechinel Filho, V.; Otuki, M.F.; Cabrini, D.A. Anti-inflammatory effects of hydroalcoholic extract and two biflavonoids from Garcinia gardneriana leaves in mouse paw oedema. J. Ethnopharmacol. 2008, 118, 405–411. [Google Scholar] [CrossRef]
- Coelho, L.P.; Serra, M.F.; Pires, A.L.D.A.; Cordeiro, R.S.B.; Silva, P.M.R.; Dos Santos, M.H.; Martins, M.A. 7-Epiclusianone, a tetraprenylated benzophenone, relaxes airways mooth muscle through activation of the nitric oxide-cGMP pathway. J. Pharm. Exp. 2008, 327, 206–214. [Google Scholar] [CrossRef]
- Arwa, P.S.; Zeraik, M.L.; Ximenes, V.F.; da Fonseca, L.M.; Bolzani, V.D.A.S.; Siqueira Silva, D.H. Redox-active bioflavonoids from Garcinia brasiliensis as inhibitors of neutrophil oxidative burst and human erythrocyte membrane damage. J. Ethnopharmacol. 2015, 174, 410–418. [Google Scholar] [CrossRef]
- Gontijo, V.S.; Judice, W.A.S.; Codonho, B.; Pereira, I.O.; Assis, D.M.; Januário, J.P.; Caroselli, E.E.; Juliano, M.A.; de Carvalho-Dosatti, A.; Marques, M.J.; et al. Leishmanicidal, antiproteolytic and antioxidant evaluation of natural bioflavonoids isolated from Garcinia brasiliensis and their semi synthetic derivatives. Eur. J. Medchem. 2012, 58, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Gontijo, V.S.; de Souza, T.C.; Rosa, I.A.; Soares, M.G.; da Silva, M.A.; Vilegas, W.; Viegas, C.J.; Dos-Santos, M.H. Isolation and evaluation of the antioxidant activity of phenolic constituents of the Garcinia brasiliensis epicarp. Food Chem. 2012, 132, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Neves, J.S.; Coelho, L.P.; Cordeiro, R.S.B.; Veloso, M.P.; Silva, P.M.R.; Dos Santos, M.H.; Martins, M.A. Antianaphylactic properties of 7-epiclusianone, a tetraprenylated benzophenone isolated from Garcinia. brasiliensis. Planta Med. 2007, 73, 644–649. [Google Scholar] [CrossRef]
- Jantan, I.; Saputri, F.C. Benzophenones and xanthones from Garcinia cantleyana var. cantleyana and their inhibitory activities on human low-density lipoprotein oxidation and platelet aggregation. Phytochemistry 2012, 80, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.M.; Yatsuda, R.; Dos-Santos, M.H.; Kohn, L.K.; Martins, F.T.; Nagem, T.J.; Alencar, S.M.; Carvalho, J.E.; Rosalen, P.L. Antiproliferative effect of benzophenones and their influence on cathepsin activity. Phytother. Res. 2010, 24, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, F.J.; Claudino, A.L.R.; Cruz, J.W.; Chavasco, J.K.; Faria e Silva, P.M.; Veloso, M.P.; Dos-Santos, M.H. Antimicrobial activity of benzophenones and extracts from the fruits of Garcinia brasiliensis. J. Med. Food 2009, 12, 403–407. [Google Scholar] [CrossRef]
- Figueiredo, S.A.; Vilela, F.M.; da Silva, C.A.; Cunha, T.M.; Dos-Santos, M.H.; Fonseca, M.J. In vitro and in vivo photoprotective/photochemopreventive potential of Garcinia brasiliensis epicarp extract. J. Photochem. Photobiol. B 2014, 131, 65–73. [Google Scholar] [CrossRef]
- Sales, L.; Pezuk, J.A.; Borges, K.S.; Brassesco, M.S.; Scrideli, C.A.; Tone, L.G.; dos Santos, M.H.; Ionta, M.; de Oliveira, J.C. Anticancer activity of 7-epiclusianone, a benzophenone from Garcinia brasiliensis, in glioblastoma. BMC Complementary Altern. Med. 2015, 15, 393. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.P.; De Mattos, A.C.; Pereira, N.A.; Anchieta, N.F.; Silva, M.S.; Dias, D.F.; Silva, C.A.; Barros, G.V.; Souza, R.L.; Dos Santos, M.H.; et al. Potent schistosomicidal constituents from Garcinia brasiliensis. Planta Med. 2015, 81, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.E.C.; Natal, D.I.G.; Toledo, R.C.L.; Ramirez, N.M.; Ribeiro, S.M.R.; Benjamin, L.A.; Oliveira, L.L.; Rodrigues, D.A.; Antônio, J.D.; Veloso, M.P. Bacupari peel extracts (Garcinia brasiliensis) reduce high-fat diet-induced obesity in rats. J. Funct. Foods 2017, 29, 143–153. [Google Scholar] [CrossRef]
- Santos, M.H.; Nagem, T.J.; Oliveira, T.T.; Braz-Filho, R. 7-Epiclusianone, the new tetraprenylated benzophenone and others chemical constituents from the fruits of Rheediagardneriana. Química Nova 1999, 22, 654–660. [Google Scholar] [CrossRef]
- Ferreira, R.O.; Carvalho, M.G.; Silva, T.M.S. Ocorrência de biflavonoides em Clusiaceae: Aspectos químicos e farmacológicos. Química Nova 2012, 35, 2271–2277. [Google Scholar] [CrossRef]
- Guimarães, C.L.; Otuki, M.F.; Beirith, A.; Cabrini, D.A.A. review on the therapeutic potential of Garcinia gardneriana. Dynamis 2004, 12, 6–12. [Google Scholar]
- Asinelli, M.E.C.; de Souza, M.C.; Mourão, K.S.M. Fruit ontogeny of Garcinia gardneriana (Planch. & amp; Triana) Zappi (Clusiaceae). Acta Botbras 2011, 25, 43–52. [Google Scholar]
- Campos, P.M.; Horinouchi, C.D.S.; Prudente, A.S.; Cechinel-Filho, V.; Cabrini, D.A.; Otuki, M.F. Effect of Garcinia garderiana (Planchon and Triana) Zappi hydroalcoholic extract on melanogenesis in B16F10 melanoma cells. J. Ethnopharmacol. 2013, 148, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Solano, F.; Briganti, S.; Picardo, M.; Ghanem, G. Hypopigmenting agents: An updated review on biological, chemical and clinical aspects. Pigment Cell Res. 2006, 19, 550–571. [Google Scholar] [CrossRef]
- Subeki, M.H.; Yamasaki, M.; Yamato, O.; Maede, Y.; Katakura, K.; Suzuki, M.; Trimurningsih, C.; Yoshihara, T. Effects of central kalimantan plant extracts on intraerythrocytic Babesia gibsoni in culture. J. Vet. Med. Sci. 2004, 66, 871–874. [Google Scholar] [CrossRef]
- Verdi, L.G.; Pizzolatti, M.G.; Montanher, A.B.P.; Brighente, I.M.C.; Smânia, J.A.; Smânia, E.F.A.; Simionatto, E.L.; Monache, F.D. Antibacterial and brine shrimp lethality tests of biflavonoids and derivatives of Rheedia gardneriana. Fitoterapia 2004, 75, 360–363. [Google Scholar] [CrossRef]
- Otuki, M.F.; Bernardi, C.A.; Prudente, A.S.; Laskoski, K.; Gomig, F.; Horinouchi, C.D.S.; Guimarães, C.L.; Ferreira, J.; Monache, F.D.; Cechinel-Filho, V.; et al. Garcinia gardneriana (Planchon and Triana) Zappi. (Clusiaceae) as a topical anti-inflammatory alternative for cutaneous inflammation. Basic Clin. Pharm. 2011, 109, 56–62. [Google Scholar] [CrossRef]
- Melo, M.S.; Quintans, J.S.; Araújo, A.A.; Duarte, M.C.; Bonjardim, L.R.; Nogueira, P.C.; Moraes, V.R.; Araújo-Júnior, J.X.; Ribeiro, E.A.; Quintans-Júnior, L.J. A systematic review for anti-inflammatory property of Clusiaceae family: A preclinical approach. J. Evid. Based Complementary Altern. Med. 2014, 2014, 960258. [Google Scholar]
- Cechinel-Filho, V. Advances and perspectives in the field of active natural products: Studies conducted at Niqfar/Univali. Quim Nova 2000, 23, 680–684. [Google Scholar]
- Luzzi, R.; Guimarães, C.L.; Verdi, L.G.; Simionatto, E.L.; Monache, F.D.; Yunes, R.A.; Floriani, A.E.O.; Oliveira, A.E.; Filho, V.C. Isolation of biflavonoids with analgesic activity from Rheedia gardneriana leaves. Phytomedicine 1997, 4, 139–142. [Google Scholar] [CrossRef]
- Cechinel-Filho, V.; da Silva, K.L.; de Souza, M.M.; Oliveira, A.E.; Yunes, R.A.; Guimarães, C.L.; Verdi, L.G.; Simionatto, E.L.; Delle-Monache, F. I3-Naringenin-II8-4′OMeeriodictyol: A new potential analgesic agent isolated from Rheedia gardneriana leaves. Z. Nat. C. 2000, 55, 820–823. [Google Scholar]
- Campos, P.M.; Prudente, A.S.; Horinouchi, C.D.; Cechinel-Filho, V.; Fávero, G.M.; Cabrini, D.A.; Otuki, M.F. Inhibitory effect of GB-2a (I3-naringenin-II8-eriodictyol) on melanogenesis. J. Ethnopharmacol. 2015, 174, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Recalde-Gil, A.M.; Klein-Júnior, L.; Salton, J.; Bordignon, S.; Cechinel-Filho, V.; Matté, C.; Henriques, A. Aromatase (CYP19) inhibition by biflavonoids obtained from the branches of Garcinia gardneriana (Clusiaceae). Z. Nat. C. J. Biosci. 2019, 10, 279–282. [Google Scholar] [CrossRef]
- Mundugaru, R.; Varadharajan, M.C.; Basavaiah, R. Hepatoprotective activity of fruit extract of Garcinia pedunculata. Bangladesh. J. Pharm. 2014, 9, 483–487. [Google Scholar]
- Sarma, R.; Devi, R. Ethnopharmacological survey of Garcinia pedunculata Roxb. Fruit six different districts of Assam, India. Int. J. Pharm. Sci. Invent. 2015, 4, 20–28. [Google Scholar]
- Kagyung, R.; Gajurel, P.R.; Rethy, P.; Singh, B. Ethnomedicinal plants used for gastro-intestinal diseases by adi tribes of dehang-debang biosphere reserve in arunachal pradesh. Indian J. Tradit. Knowl. 2010, 9, 496–501. [Google Scholar]
- Vo, H.T.; Nguyen, T.N.T.; Nguyen, H.T.; Do, K.Q.; Connolly, J.D.; Mass, G.; HeilmannWerz, J.U.R.; Pham, D.H.; Nguyen, D.L.H. Cytotoxic tetraoxygenated xanthones from the bark of Garcinia schomburgkiana. Phytochem. Lett. 2012, 5, 553–557. [Google Scholar] [CrossRef]
- Sahu, A.; Das, B.; Chatterjee, A. Polyisoprenylated benzophenones from Garcinia pedunculata. Phytochemistry 1989, 28, 1233–1235. [Google Scholar] [CrossRef]
- Ravi, M.; Febin, J.; Shrinidhi, R.; Lipika, D.; Sudhakara, B.; Ravishankar, B. Anti-inflammatory activity of aqueous extract of fruits of Garcinia pedunculata in experimental animals. Am. J. Pharma. Tech. Res. 2014, 4, 3–6. [Google Scholar]
- Ravi, M.; Senthilkumar, S.; Padmaja, U.K.; Sudhakara, B. Cardio protective activity of fruits extract of Garcinia pedunculata. Bangladesh. J. Pharm. 2016, 11, 5–9. [Google Scholar]
- Jayaprakasha, G.K.; Jena, B.S.; Sakariah, K.K. Improved liquid chromatographic method for determination of organic acids in leaves, pulp, fruits, and rinds of Garcinia. J. Aoac. Int. 2003, 86, 1063–1068. [Google Scholar] [CrossRef] [PubMed]
- Ito, C.; Itoigawa, M.; Miyamoto, Y. A new biflavonoid from Calophyllumpanciflorum with antitumor-promoting activity. J. Nat. Prod. 1999, 12, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Mudoi, T.; Deka, D.C.; Devi, R. In vitro antioxidant activity of Garcinia pedunculata, an indigenous fruit of North Eastern (NE) region of India. Int. J. Pharmtech. Res. 2012, 4, 334–342. [Google Scholar]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antibacterial activity of the extracts from the fruit rinds of Garcinia cowa and Garcinia pedunculata against food borne pathogens and spoilage bacteria. LWT-Food Sci. Technol. 2008, 41, 1857–1861. [Google Scholar] [CrossRef]
- Sarma, R.; Kumari, S.; Elancheran, R.; Deori, M.; Devi, R. Polyphenol rich extract of Garcinia pedunculata fruit attenuates the hyperlipidemia induced by high fat diet. Front Pharm. 2016, 7, 294. [Google Scholar] [CrossRef]
- Mitcheva, M.; Kondeva, M.; Vitcheva, V.; Nedialkov, P.; Kitanov, G. Effect of benzophenones from hypericum annulatum on carbon tetrachloride-induced toxicity in freshly isolated rat hepatocytes. Redox. Rep. 2006, 11, 3–8. [Google Scholar] [CrossRef]
- Hung, W.L.; Liu, C.-M.; Lai, C.S.; Ho, C.T.; Pan, M.H. Inhibitory effect of garcinol against 12-O-tetradecanoylphorbol 13- acetate-induced skin inflammation and tumorigenesis in mice. J. Funct. Foods 2015, 18, 432–444. [Google Scholar] [CrossRef]
- Mundugaru, R.; Sivanesan, S.K.; Udaykumar, P.; Joy, F.; Narayana, S.K.K.; Rajakrishnan, L.; Al Farhan, A.H.; Jacob, T.; Rajagopal, R.; Hisham, S.M. Quality standardization and nephroprotective effect of Garcinia pedunculata Roxb. Fruit extract. Indian J. Pharm. Educ. 2017, 51, 713–721. [Google Scholar] [CrossRef]
- Paul, S.; Ali, M.Y.; Rumpa, N.E.; Tanvir, E.M.; Hossen, M.S.; Saha, M.; Bhoumik, N.C.; Gan, S.H.; Khalil, M.I. Assessment of toxicity and beneficiary effects of Garcinia pedunculata on the hematological, biochemical, and histological homeostasis in rats. J. Evid. Based Complementary Altern. Med. 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Ali, M.Y.; Paul, S.; Tanvir, E.M.; Hossen, M.S.; Rumpa, N.N.; Saha, M.; Bhoumik, N.C.; Islam, M.A.; Hossain, M.S.; Alam, N.; et al. Antihyperglycemic, antidiabetic, and antioxidant effects of Garcinia pedunculata in rats. J. Evid. Based Complementary Altern. Med. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mundugaru, R.; Udaykumar, P.; Kumar, S.; Narayana, S.K.K.; Jacob, T.; AlFarhan, A.H.; Rajakrishnan, L. Protective effect of garcinia pedunculata fruit rind in acetic acid induced ulcerative colitis. Farmacia 2019, 67, 160–166. [Google Scholar] [CrossRef]
- Anu-Aravind, A.P.; Asha, K.R.T.; Rameshkumar, K.B. Phytochemical analysis and antioxidant potential of the leaves of Garcinia travancorica Bedd. Nat. Prod. Res. 2016, 30, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Vermaak, I.; Viljoen, A. A comprehensive scientific overview of Garcinia cambogia. Fitoterapia 2015, 102, 134–148. [Google Scholar] [CrossRef] [PubMed]
- Klein-Junior, L.C.; Antunes, M.V.; Linden, R.; Vasques, C.A.R. Quantification of (-)hydroxycitric acid in marketed extracts of Garcinia cambogia by high performance liquid chromatography. Lat. Am. J. Pharm. 2010, 29, 835–838. [Google Scholar]
- Di-Micco, S.; Masullo, M.; Bandak, A.F.; Berger, J.M.; Riccio, R.; Piacente, S.; Bifulco, G. Garcinol and related polyisoprenylated benzophenones as topoisomerase II inhibitors: Biochemical and molecular modeling studies. J. Nat. Prod. 2019, 82, 2768–2779. [Google Scholar] [CrossRef]
- Saito, M.; Ueno, M.; Ogino, S.; Kubo, K.; Nagata, J.; Takeuchi, M. High dose of Garcinia cambogia is effective in suppressing fat accumulation in developing male Zucker obese rats, but highly toxic to the testis. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2005, 43, 411–419. [Google Scholar] [CrossRef]
- Duke, J.; Bogenschutz-Godwin, M.; DuCellier, J.; Duke, P.A. Handbook of Medicinal Herbs, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2002; p. 481. [Google Scholar]
- Iwu, M.M. Handbook of African Medicinal Plants; CRC Press: London, UK, 1993; pp. 183–184. [Google Scholar]
- Ho, C.K.; Huang, Y.L.; Chen, C.C. Garcinone E, a xanthone derivative, has potent cytotoxic effect against hepatocellular carcinoma cell lines. Planta Med. 2002, 68, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, K.; Atsumi, M.; Arakawa, T.; Oosawa, K.; Shimura, S.; Nakahata, N.; Ohizumi, Y. Inhibitions of histamine release and prostaglandin E2 synthesis by mangosteen, a Thai medicinal plant. Biol. Pharm. Bull. 2002, 25, 1137–1141. [Google Scholar] [CrossRef]
- Mahendran, P.; Sabitha, K.E.; Devi, C.S. Prevention of H-Clethanol induced gastric mucosal injury in rats by Garcinia cambogia extract and its possible mechanism of action. Indian J. Exp. Biol. 2002, 40, 58–62. [Google Scholar] [PubMed]
- Iwu, M.W.; Duncan, A.R.; Okunji, C.O. New antimicrobials of plant origin. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexandria, VA, USA, 1999; pp. 457–462. [Google Scholar]
- Chen, S.X.; Wan, M.; Loh, B.N. Active constituents against HIV-1 protease from Garcinia mangostana. Planta Med. 1996, 62, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Sripradha, R.; Magadi, S.G. Efficacy of Garcinia cambogia on body weight, inflammation and glucose tolerance in high fat fed male wistar rats. J. Clin. Diagn. Res. 2015, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, P.; Vanisree, A.J.; Devi, C.S. The antiulcer activity of Garcinia cambogia extract against indomethacin induced gastric ulcer in rats. Phytother. Res. 2002, 16, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, P.; Devi, C.S. Effect of Garcinia cambogia extract on lipids and lipoprotein composition in dexamethasone administered rats. Indian J. Physiolpharmacol. 2001, 45, 345–350. [Google Scholar]
- Kim, M.S.; Kim, J.K.; Kwon, D.Y.; Park, R. Anti-adipogeniceffects of Garcinia extract on the lipid droplet accumulationand the expression of transcription factor. Biofactors 2004, 22, 193–196. [Google Scholar] [CrossRef]
- Ishihara, K.; Oyaizu, S.; Onuki, K.; Lim, K.; Fushiki, T. Chronic (-)-hydroxycitrate administration spares carbohydrate utilization and promotes lipid oxidation during exercise in mice. J. Nutr. 2000, 130, 2990–2995. [Google Scholar] [CrossRef]
- Koshy, A.S.; Vijayalakshmi, N.R. Impact of certain flavonoidson lipid profiles-potential action of Garcinia cambogia flavonoids. Phytother. Res. 2001, 15, 395–400. [Google Scholar] [CrossRef]
- Reis, S.B.; Oliveira, C.C.; Acedo, S.C.; Miranda, D.D.; Ribeiro, M.L.; Pedrazzoli, J., Jr.; Gambero, A. Attenuation of colitis injury in rats using Garcinia cambogia extract. Phytother. Res. 2009, 23, 324–329. [Google Scholar] [CrossRef]
- Oluyemi, K.A.; Omotuyi, I.O.; Jimoh, O.R.; Adesanya, O.A.; Saalu, C.L.; Josiah, S.J. Erythropoietic and anti-obesity efects of Garcinia cambogia (bitter kola) in Wistar rats. Biotechnol. Appl. Biochem. 2007, 46, 69–72. [Google Scholar]
- Sharma, K.; Kang, S.; Gong, D.; Oh, S.H.; Park, E.Y.; Oak, M.H.; Yi, E. Combination of Garcinia cambogia extract and pear pomace extract additively suppresses adipogenesis and enhances lipolysis in 3T3-L1 Cells. Pharm. Mag. 2018, 14, 220–226. [Google Scholar]
- Sripradha, R.; Sridhar, M.G.; Maithilikarpagaselvi, N. Antihyperlipidemic and antioxidant activities of the ethanolic extract of Garcinia cambogia on high fat diet-fed rats. J. Complementary Integr. Med. 2016, 13, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Maia-Landim, A.; Ramirez, J.M.; Lancho, C.; Poblador, M.S.; Lancho, J.L. Long-term effects of Garcinia cambogia/Glucomannan on weight loss in people with obesity, PLIN4, FTO and Trp64Arg polymorphisms. BMC omplementary Altern. Med. 2018, 18, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Hayamizu, K.; Hirakawa, H.; Oikawa, D.; Nakanishi, T.; Takagi, T.; Tachibana, T.; Furuse, M. Effect of Garcinia cambogia extract on serum leptin and insulin in mice. Fitoterapia 2003, 74, 267–273. [Google Scholar] [CrossRef]
- Preuss, H.G.; Rao, C.V.; Garis, R.; Bramble, J.D.; Ohia, S.E.; Bagchi, M.; Bagchi, D. An overview of the safety and efficacy of a novel, natural (-)-hydroxycitric acid extract (HCA-SX) for weight management. J. Med. 2004, 35, 33–48. [Google Scholar] [PubMed]
- Lopez, A.M.; Kornegay, J.; Hendrickson, R.G. Serotonin toxicity associated with Garcinia cambogia over-the-counter supplement. J. Med. Toxicol. 2014, 4, 399–401. [Google Scholar] [CrossRef]
- Haber, S.L.; Awwad, O.; Phillips, A.; Park, A.E.; Pham, T.M. Garcinia cambogia for weight loss. Am. J. Health Syst. Pharm. 2018, 75, 17–22. [Google Scholar] [CrossRef]
- Jena, B.S.; Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Chemistry and biochemistry of (-)-hydroxycitric acid from Garcinia. J. Agric. Food Chem. 2002, 50, 10–22. [Google Scholar] [CrossRef]
- Stallings, W.C.; Blount, J.F.; Srere, P.A.; Glusker, J.P. Structural studies of hydroxycitrates and their relevance to certain enzymatic mechanisms. Arch. Biochem. Biophys. 1979, 193, 431–448. [Google Scholar] [CrossRef]
- Ohia, S.E.; Opere, C.A.; LeDay, A.M.; Bagchi, M.; Bagchi, D.; Stohs, S.J. Safety and mechanism of appetite suppression by a novel hydroxycitric acid extract (HCA-SX). Mol. Cell Biochem. 2002, 238, 89–103. [Google Scholar] [CrossRef]
- Asghar, M.; Monjok, E.; Kouamou, G.; Ohia, S.E.; Bagchi, D.; Lokhandwala, M.F. Super CitriMax (HCA-SX) attenuates increases in oxidative stress, inflammation, insulin resistance, and body weight in developing obese Zucker rats. Mol. Cell Biochem. 2007, 304, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Preuss, H.G.; Garis, R.I.; Bramble, J.D.; Bagchi, D.; Bagchi, M.; Rao, C.V.; Satyanarayana, S. Efficacy of a novel calcium/potassium salt of (-)-hydroxycitric acid in weight control. Int. J. Clin. Pharm. Res. 2005, 25, 133–144. [Google Scholar]
- Li, L.; Peng, M.; Ge, C.; Yu, L.; Ma, H. Hydroxycitric acid reduced lipid droplets accumulation via decreasing acetyl-coa supply and accelerating energy metabolism in cultured primary chicken hepatocytes. Cell Physiolbiochem. 2017, 43, 812–831. [Google Scholar] [CrossRef] [PubMed]
- Nisha, V.M.; Priyanka, A.; Anusree, S.S.; Raghu, K.G. (-)-Hydroxycitric acid attenuates endoplasmic reticulum stress-mediated alterations in 3T3-L1 adipocytes by protecting mitochondria and downregulating inflammatory markers. Free Radic. Res. 2014, 48, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Pittler, M.H.; Schmidt, K.; Ernst, E. Adverse events of herbal food supplements for body weight reduction: Systematic review. Obes. Rev. 2005, 2, 93–111. [Google Scholar] [CrossRef]
- Narasimha, A.; Shetty, P.H.; Nanjundaswamy, M.H.; Viswanath, B.; Math, S.B. Hydroxycut—dietary supplements for weight loss: Can they induce mania? Aust. N. Z. J. Psychiatry 2013, 47, 1205–1206. [Google Scholar] [CrossRef] [PubMed]
- Beecheno, M.; Budd, S.; Mohan, T. Natural weight loss supplements—are they psychoactive? Aust. N. Z. J. Psychiatry 2016, 50, 700–701. [Google Scholar] [CrossRef]
- Cotovio, G.; Olivera-Maia, A.J. Hypomania induced by a Garcinia cambogia supplement. Aust. N. Z. J. Psychiatry 2016, 51, 641–642. [Google Scholar] [CrossRef]
- Crescioli, G.; Lombardi, N.; Bettiol, A.; Marconi, E.; Risaliti, F.; Bertoni, M.; Ippolito, F.M.; Maggini, V.; Gallo, E.; Firenzuoli, F.; et al. Acute liver injury following Garcinia cambogia weight-loss supplementation: Case series and literature review. Intern. Emerg. Med. 2018, 13, 857–872. [Google Scholar] [CrossRef]
- Licata, A.; Minissale, M.G. Weight-loss supplementation and acute liver failure: The case of Garcinia cambogia. Intern. Emerg. Med. 2018, 13, 833–835. [Google Scholar] [CrossRef]
- Lunsford, K.E.; Bodzin, A.S.; Reino, D.C.; Wang, H.; Basuttil, R. Dangerous dietary supplements: Garcinia cambogia-associated hepatic failure requiring transplantation. World J. Gastroenterol. 2016, 22, 10071–10076. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Akagi, E.; Njie, A.; Goyal, S.; Arsene, S.; Krishnamoorthy, G.; Ehrinpreis, M. Acute hepatitis due to Garcinia cambogia extract, an herbal weight loss supplement. Case Rep. Gastrointest. Med. 2018, 9606171. [Google Scholar]
- Hendrickson, B.P.; Shaikh, N.; Occhiogrosso, M.; Penzner, J.B. Mania induced by Garcinia cambogia: A case series. Prim. Care Companion. Cns. Disord. 2016, 18, 104088. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Han, Y.S.; Park, J.M. Ocular complications of Garcinia cambogia extract diet pills: Case report. Eur. J. Ophthalmol. 2019, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Grigos, A.; Benmoussa, J.; Sandhu, J.; Chaucer, B.; Clarke, M. Acute pancreatitis secondary to Garcinia cambogia; the unknown cost of herbal supplements. J. Pancreas. 2016, 17, 316–317. [Google Scholar]
- Bystrak, T.; Cervera-Hernandez, M.E.; Reddy, N.; King, Z.; Bratberg, J. Garcinia cambogia, Diabetic Ketoacidosis, and Pancreatitis. Rhode Isl. Med. J. 2017, 100, 48–50. [Google Scholar]
- Li, J.W.; Bordelon, P. Hydroxycitric acid dietary supplement-related herbal nephropathy. Am. J. Med. 2011, 124, 5–6. [Google Scholar] [CrossRef]
- Batcioglu, K.; Gul, M.; Uyumlu, A.B.; Esrefoglu, M. Liver lipid peroxidation and antioxidant capacity in cerulein-induced acute pancreatitis. Braz. J. Med. Biol. Res. 2009, 42, 776–782. [Google Scholar] [CrossRef]
- Corey, R.; Werner, K.T.; Singer, A.; Moss, A.; Smith, A.; Noelting, J.; Rakela, J. Acute liver failure associated with Garcinia cambogia use. Ann. Hepatol. 2016, 15, 123–126. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, M.S.; Park, Y.B.; Kim, S.R.; Lee, M.K.; Jung, U.J. Garcinia cambogia attenuates diet-induced adiposity but exacerbates hepatic collagen accumulation and inflammation. World J. Gastroenterol. 2013, 19, 4689–5701. [Google Scholar] [CrossRef]
- Ovalle-Magallanes, B.; Eugenio, D.; Pedraza-Chaverri, J. Medicinal properties of mangosteen (Garcinia mangostana L.): A comprehensive update. Food Chem. Toxicol. 2017, 109, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.F.; Godley, R.W.; Evron, J.M.; Heider, A.; Nicklas, J.M.; Thomas, M.P. Acute necrotizing eosinophilic myocarditis in a patient taking Garcinia cambogia extract successfully treated with high-dose corticosteroids. Can. J. Cardiol. 2014, 30, 1732.e13-5. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.R.M.; Mohamed, G.A.; Khayat, M.T.; Ahmed, S.; Abo-Haded, H.; Alshali, K.Z. Mangostanaxanthone VIIII, a new xanthone from Garcinia mangostana pericarps, α-amylase inhibitory activity, and molecular docking studies. Rev. Bras. Farm. 2019, 29, 206–212. [Google Scholar] [CrossRef]
- Hosakatte, N.; Dandin, V.; Dalawai, D.; Park, S.Y.; Paek, K. Bioactive compounds from garcinia fruits of high economic value for food and health. Phytochem. Spr. Nature 2018, 1, 1–28. [Google Scholar]
- Abdallah, H.M.; El-Bassossy, H.M.; Mohamed, G.A.; El-Halawany, A.M.; Alshali, K.Z.; Banjar, Z.M. Mangostana xanthones III and IV: Advanced glycation end-product inhibitors from the pericarp of Garcinia mangostana. J. Nat. Med. 2017, 71, 216–226. [Google Scholar] [CrossRef]
- Obolskiy, D.; Pischel, I.; Siriwatanametanon, N.; Heinrich, M. Garcinia mangostana L.: A phytochemical and pharmacological review. Phytother. Res. 2009, 31, 110–118. [Google Scholar] [CrossRef]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Harada, E.; Miki, A.; Tsukamoto, K.; Liang, S.; Yamahara, J. Antioxidant constituents from the fruit hulls of mangosteen (Garcinia mangostana L.) originating in Vietnam. Yakugakuzasshi. J. Pharm. Soc. Jpn. 1994, 114, 129–133. [Google Scholar] [CrossRef]
- Jung, H.-A.; Su, B.-N.; Keller, W.J.; Mehta, R.G.; Kinghorn, A.D. Antioxidant xanthones from the pericarp of Garcinia mangostana (Mangosteen). J. Agric. Food. Chem. 2006, 54, 2077–2082. [Google Scholar] [CrossRef]
- Chairungsrilerd, N.; Furukawa, K.I.; Ohta, T.; Nozoe, S.; Ohizumi, Y. Histaminergic and serotonergic receptor blocking substances from the medicinal plant Garcinia mangostana. Planta Med. 1996, 62, 471–472. [Google Scholar] [CrossRef]
- Chen, L.-G.; Yang, L.-L.; Wang, C.-C. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem. Toxicol. 2008, 46, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Chomnawang, M.T.; Surassmo, S.; Wongsariya, K.; Bunyapraphatsara, N. Antibacterial activity of Thai medicinal plants against methicillin-resistant Staphylococcus aureus. Fitoterapia 2009, 80, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Oberholzer, I.; Möller, M.; Holland, B.; Dean, O.; Berk, M.; Harvey, B. Garcinia mangostana Linn displays antidepressant-like and pro-cognitive effects in a genetic animal model of depression: A bio-behavioral study in the flinders sensitive line rat. Metab. Brain Dis. 2018, 33, 467–480. [Google Scholar] [CrossRef] [PubMed]
- Sakagami, Y.; Iinuma, M.; Piyasena, K.; Dharmaratne, H. Antibacterial activity of α-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine 2005, 12, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Iinuma, M.; Naoe, T.; Nozawa, Y.; Akao, Y. Characterized mechanism of α-mangostin-induced cell death: Caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg. Med. Chem. 2007, 15, 5620–5628. [Google Scholar] [CrossRef]
- Fang, Y.; Su, T.; Qiu, X.; Mao, P.; Xu, Y.; Hu, Z.; Zhang, Y.; Zheng, X.; Xie, P.; Liu, Q. Protective effect of alpha-mangostin against oxidative stress induced-retinal cell death. Sci. Rep. 2016, 6, 21018. [Google Scholar] [CrossRef]
- Tjahjani, S.; Widowati, W.; Khiong, K.; Suhendra, A.; Tjokropranoto, R. Antioxidant properties of Garcinia mangostana L (mangosteen) rind. Procedia. Chem. 2014, 13, 198–203. [Google Scholar] [CrossRef]
- Tousian, H.; Razavi, B.M.; Hosseinzadeh, H. Alpha-mangostin decreased cellular senescence in human umbilical vein endothelial cells. DARU J. Pharm. Sci. 2019, 1–11. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Kalpravidh, R.W.; Chitchumroonchokchai, C.; Chuang, C.C.; West, T.; Kennedy, A.; McIntosh, M. Xanthones from mangosteen prevent lipopolysaccharide-mediated inflammation and insulin resistance150 in primary cultures of human adipocytes. J. Nutr. 2009, 139, 1185–1191. [Google Scholar] [CrossRef]
- Jariyapongskul, A.; Areebambud, C.; Suksamrarn, S.; Mekseepralard, C. Alpha-mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. Biomed. Res. Int. 2015, 785826. [Google Scholar] [CrossRef]
- Karim, N.; Rahman, M.A.; Changlek, S.; Tangpong, J. Short-time administration of xanthone from Garcinia mangostana fruit pericarp attenuates the hepatotoxicity and renotoxicity of type II diabetes mice. J. Am. Coll. Nutr. 2019, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, Q.; Lu, X.; Li, Z.; Wang, C.; Leung, C.-H.; Wang, Y.; Peng, C.; Lin, L. α-Mangostin remodels visceral adipose tissue inflammation to ameliorate age-related metabolic disorders in mice. Aging 2019, 23, 11084–11110. [Google Scholar] [CrossRef]
- Husen, S.A.; Winarni, D.; Khaleyla, F.; Kalqutny, S.H.; Ansori, A.N.M. Activity assay of mangosteen (Garcinia mangostana L.) pericarp extract for decreasing fasting blood cholesterol level and lipid peroxidation in type-2 diabetic mice. AIP Conf. Proc. 2017, 1888, 020026. [Google Scholar]
- Husen, S.A.; Khaleyla, F.; Ansori, A.N.M.; Susilo, R.J.K.; Winarni, D. Antioxidant activity assay of alpha-mangostin for amelioration of kidney structure and function in diabetic mice. Adv. Soc. Sci. Educ. Hum. Res. (Assehr) 2018, 98, 84–88. [Google Scholar]
- Manaharan, T.; Palanisamy, U.D.; Ming, C.H. Tropical plant extracts as potential antihyperglycemic agents. Molecules 2012, 17, 5915–5923. [Google Scholar] [CrossRef] [PubMed]
- Ansori, A.; Fadholly, A.; Hayaza, S.; Susilo, R.; Inayatilllah, B.; Winarni, D.; Husen, S. A review on medicinal properties of mangosteen (Garcinia mangostana L.). Res. J. Pharm. Technol. 2020, 13, 974–982. [Google Scholar] [CrossRef]
- Husen, S.A.; Winarni, D.; Salamun; Ansori, A.N.M.; Susilo, R.J.K.; Hayaza, S. Hepatoprotective effect of gamma-mangostin for amelioration of impaired liver structure and function in streptozotocin-induced diabetic mice. IOP Conf. Ser. Earth Environ. Sci. 2019, 217, 1–10. [Google Scholar] [CrossRef]
- Aisha, A.F.; Abu-Salah, K.M.; Ismail, Z.; Majid, A.M. In vitro and in vivo anti-colon cancer effects of Garcinia mangostana xanthones extract. BMC Complementary Altern. Med. 2012, 12, 104–112. [Google Scholar] [CrossRef]
- Matsumoto, K.; Akao, Y.; Ohguch, K.; Ito, T.; Tanaka, T.; Iinuma, M.; Nozawa, Y. Xanthones induce cell-cycle arrest and apoptosis in human colon cancer DLD-1 cells. Bioorganic Med. Chem. 2005, 21, 6064–6069. [Google Scholar] [CrossRef]
- Wang, J.J.; Sanderson, B.J.; Zhang, W. Cytotoxic effect of xanthones from pericarp of the tropical fruit mangosteen (Garcinia mangostana Linn.) on human melanoma cells. Food Chem. Toxicol. 2011, 49, 2385–2391. [Google Scholar] [CrossRef]
- Suksamrarn, S.; Komutiban, O.; Ratananukul, P.; Chimnoi, N.; Lartpornmatulee, N.; Suksamrarn, A. Cytotoxic prenylated xanthones from the young fruit of Garcinia mangostana. Chem. Pharm. Bull. 2006, 3, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Muhamad-Adyab, N.S.; Rahmat, A.; Abdul-Kadir, N.A.A.; Jaafar, H.; Shukri, R.; Ramli, N.S. Mangosteen (Garcinia mangostana) flesh supplementation attenuates biochemical and morphological changes in the liver and kidney of high fat diet-induced obese rats. BMC Complementary Altern. Med. 2019, 19, 299–344. [Google Scholar] [CrossRef] [PubMed]
- Bumrungpert, A.; Kalpravidh, R.W.; Chuang, C.C.; Overman, A.; Martinez, K.; Kennedy, A.; McIntosh, M. Xanthones from Mangosteen inhibit inflammation in human macrophages and in human adipocytes exposed to macrophage-conditioned media. J. Nutr. 2010, 140, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Sintara, M.; Chang, T.; Ou, B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: A randomized, double-blind, placebo-controlled clinical trial. Food Sci. Nutr. 2015, 3, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Raybaudi-Massilia, R.M.; Mosqueda-Melgar, J.; Martín-Belloso, O. Antimicrobial activity of malic acid against Listeria monocytogenes, Salmonella enteritidis and Escherichia coli O157:H7 in apple, pear and melon juices. Food Control. 2009, 2, 105–112. [Google Scholar] [CrossRef]
- Yang, R.; Li, P.; Li, N.; Zhang, Q.; Bai, X.; Wang, L.; Yan, J. Xanthones from the Pericarp of Garcinia mangostana. Molecules 2017, 22, 683. [Google Scholar] [CrossRef]
- Xu, T.; Deng, Y.; Zhao, S.; Shao, Z. A new xanthone from the pericarp of Garcinia mangostana. J. Chem. Res. 2016, 1, 10–11. [Google Scholar] [CrossRef]
- Wang, W.; Liao, Y.; Huang, X.; Tang, C.; Cai, P. A novel xanthone dimer derivative with antibacterial activity isolated from the bark of Garcinia mangostana. Nat. Prod. Res. 2017, 15, 1769–1774. [Google Scholar] [CrossRef]
- Mohamed, G.A.; Al-Abd, A.M.; El-Halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharm. 2017, 198, 302–312. [Google Scholar] [CrossRef]
- Tran, T.H.; Le Huyen, T.; Tran, T.M.; Nguyen, T.A.; Pham, T.B.; Nguyen Tien, D. A new megastigmanesulphoglycoside and polyphenolic constituents from pericarps of Garcinia mangostana. Nat. Prod. Res. 2016, 30, 1598–1604. [Google Scholar] [CrossRef]
- Shahat, A.A.; Ismail, S.I.; Hammouda, F.M.; Azzam, S.A.; Lemière, G.; De Bruyne, T.; Vlietinck, A. Anti-HIV activity of flavonoids and proanthocyanidins from Crataegussinaica. Phytomedicine 1998, 5, 133–136. [Google Scholar] [CrossRef]
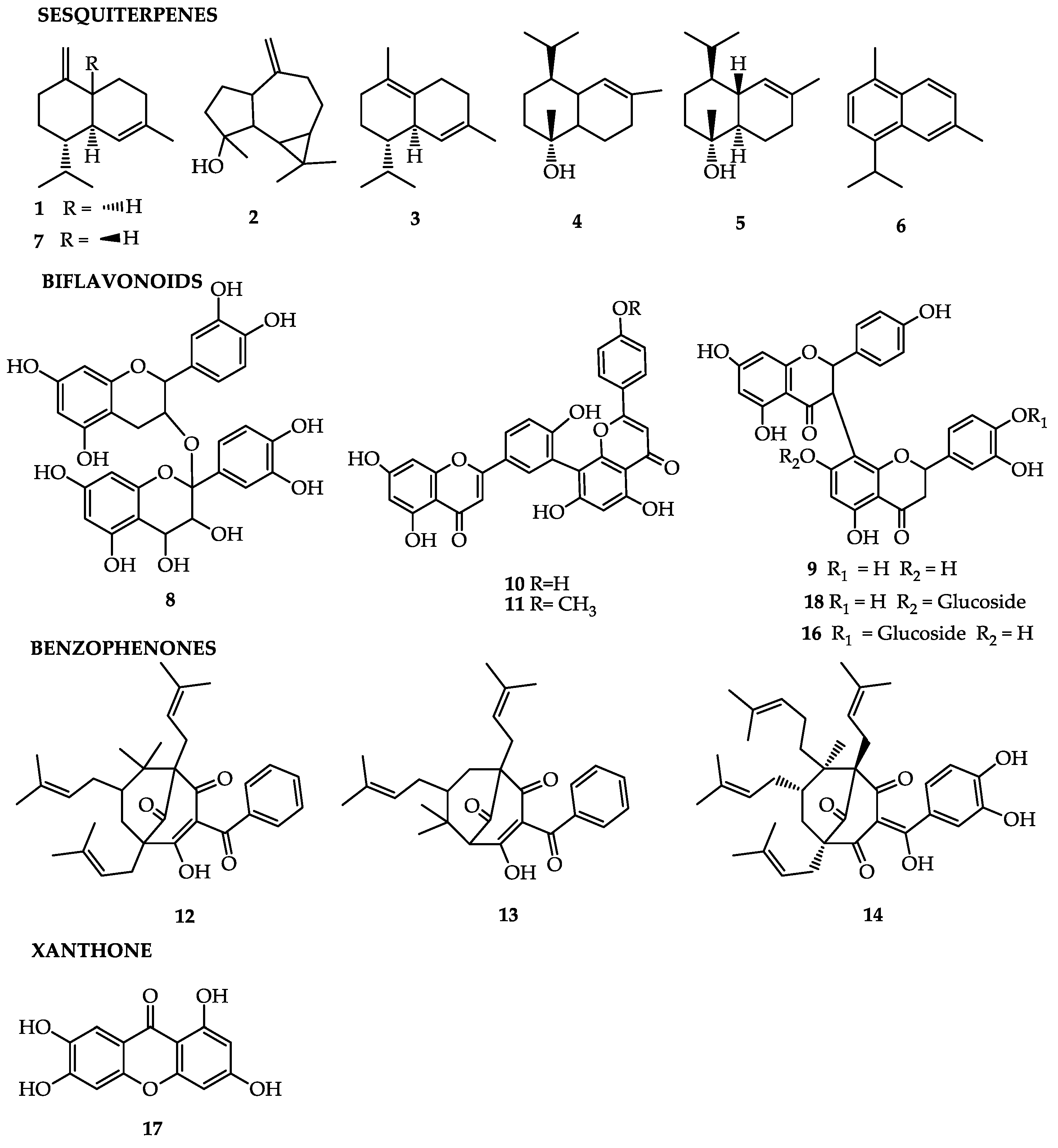
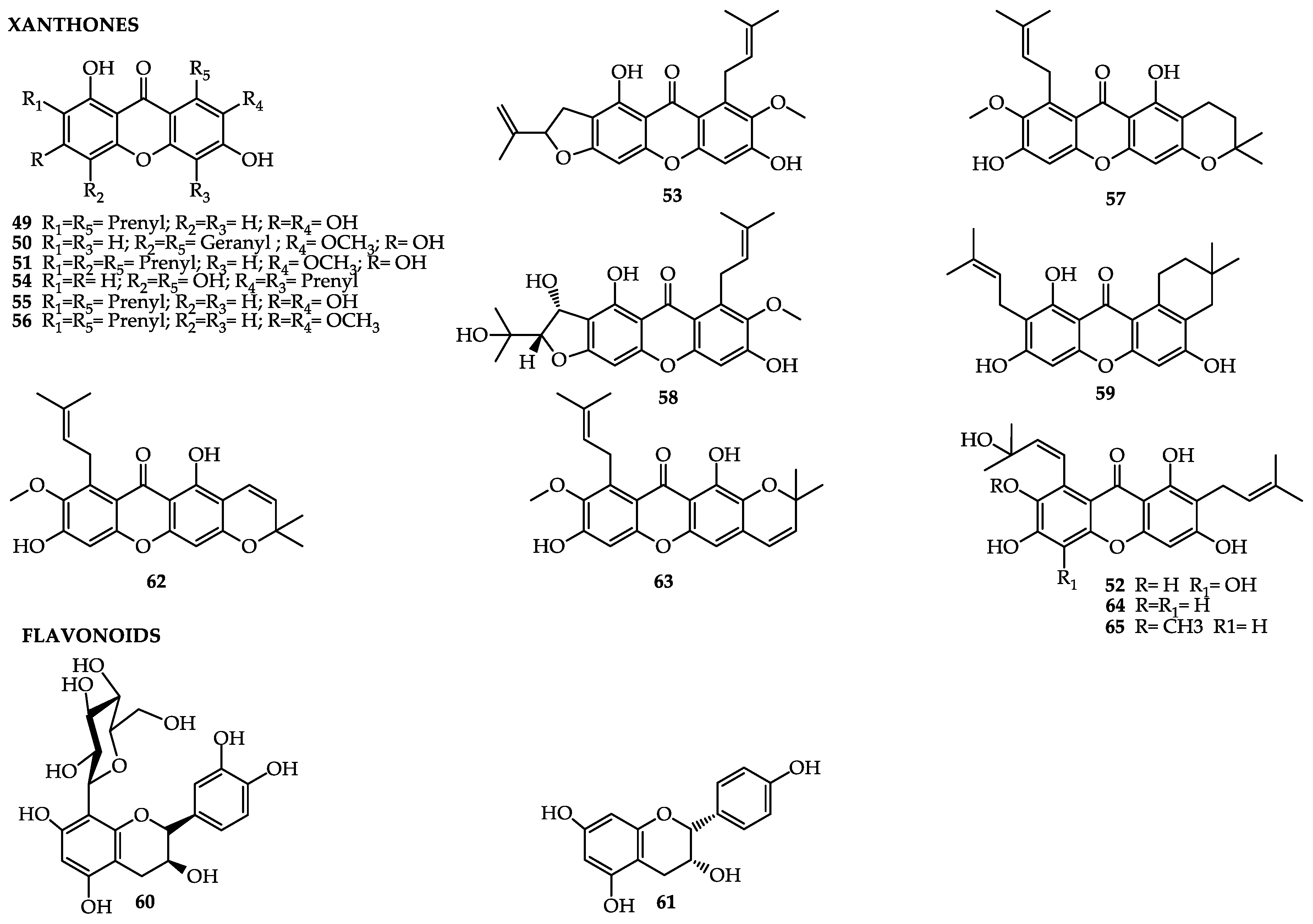
| Garcinia Brasiliensis | ||
|---|---|---|
| Sesquiterpenes | ||
| Compounds | Plant Part | Activity |
| α-Ylangene; α-Copaene; β-Bourbonene; β-Elemene; β-Caryophyllene; β-Gurjunene; Aromadendrene; α-Humulene; Drima-7,9(11)-diene; y-Muurolene-10; Germacrene D; β-Selinene; Viridiflorene; α-Muurolene; γ-Cadinene; cis-Calamenene; Cadina-1,4-diene; α-Cadinene; α-Calacorene; Longicamphenylone; Ledol; Spathulenol; Globulol; Salvial-4(14)-en-1-one; Guaiol; Viridiflorol; Humuleneepoxide II; 1,10-Diepicubenol; 1-Epicubenol; Cubenol; Cedr-8(15)-en-9a–ol; Torreyol; Selin-11-en-4a-ol; α-Cadinol; Khusinol; Cadalene; 14-Oxy-α-muurolene. | Peel [31] | Anti-inflammatory and antioxidant [21] (correlation of all compounds) |
| Biflavonoids | ||
| Fukugetin | Fruit [43] | Analgesic [52], antioxidant [43] |
| Fukugiside | Fruit [43] | Analgesic [52], antioxidant [12] |
| morelloflavone-4′’’-O-β-d-glycoside | Fruit [43] | Antioxidant [12] |
| Amentoflavone | Leaf [41] | Anti-inflammatory and antioxidant [41] |
| Podocarpusflavone A | Leaf [41] | Anti-inflammatory and antioxidant [41] |
| Benzophenones | ||
| Garcinol | Leaf [41] | Anti-inflammatory and antioxidant [41], anticancer, antiparasitic, action in nervous system [24] |
| 7-epiclusianone | Leaf [22]/Fruit [47] | Antinociceptive and anti-inflamatory [22], antimicrobial [47], anticarcinogenic [49], leishmanicidal [21], schistosomicidal [50] |
| Organic Acid | ||
| Galic acid | Leaf [41] | Anti-inflammatory and antioxidant [41] |
| Flavonoid | ||
| Procyanidine | Leaf [41] | Anti-inflammatory and antioxidant [41] |
| Xanthones | ||
| Guttiferone-A | Seeds [47]/Fruits [21] | Antimicrobial [47], photoprotective, and photochemopreventive [20] Leishmanicidal [21] |
| 1,3,6,7-tetrahydroxyxanthone | Fruit [43] | Antioxidant [43] |
| Garcinia Gardneriana | ||
|---|---|---|
| Biflavonoids | ||
| Compounds | Plant Part | Activity |
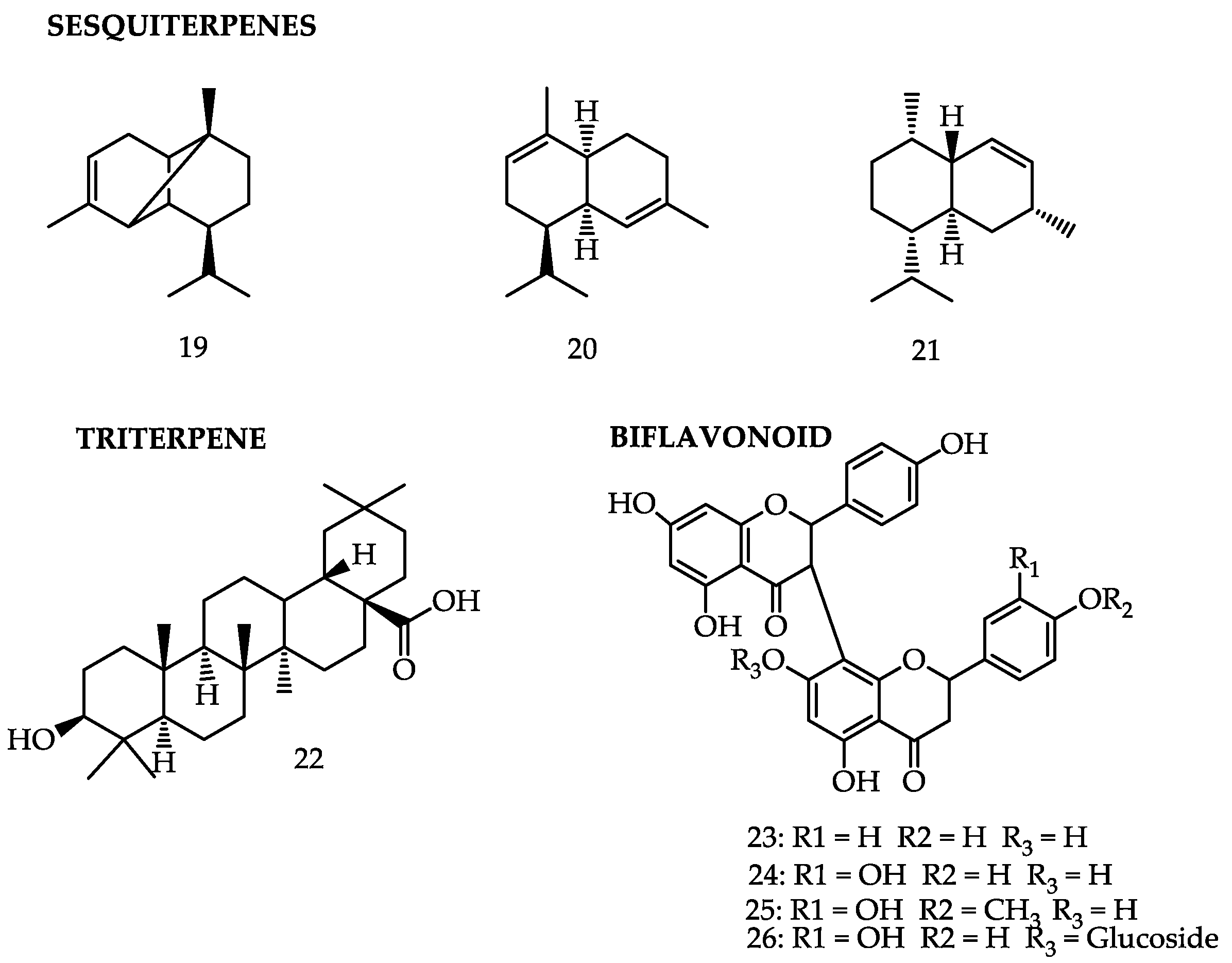
| GB-2a | Leaf [64], branches [59] | Antiedematogenic [64], anti-inflammatory [64], anticancer [65] |
| Gb-2a-7-O-glucoside | Branches [59] | Anticancer [65] |
| Volkensiflavone | Leaf [52] | Analgesic [52] |
| Fukugentin | Leaf [52] | Analgesic [52], anti-inflammatory [59], antioxidant [43] |
| Fukugiside | Leaf [52] | Analgesic [52], antioxidant [52] |
| GB-2a-II-4′-OMe | Leaf [52] | Analgesic [52] |
| Flavonoid | ||
| Compound | Plant Part | Activity |
| Epicatechin | Leaf [58] | Antibacterial [58] |
| Phytosterols | ||
| Compound | Plant Part | Activity |
| Sitosterol | Fruits [52] | Anti-inflammatory and anticancer [31] |
| stigmasterol | Fruits [52] | Anti-inflammatory and anticancer [31] |
| Benzophenones | ||
| 7-epiclusanone | Peel [52] | Antinociceptive and anti-inflammatory [22], antimicrobial [47], anticarcinogenic [49], leishmanicidal [21], schistossomicidal [50] |
| Sesquiterpenes | ||
| α-copene | Peel [52] | - |
| α-muurolene | Peel [52] | - |
| γ-cadinene | Peel [52] | - |
| Cadinene | Peel [52] | - |
| Triterpene | ||
| Oleanolic acid | Peel [52] | - |
| Garcinia Pedunculata | ||
|---|---|---|
| Xanthones | ||
| Compounds | Plant Part | Activity |
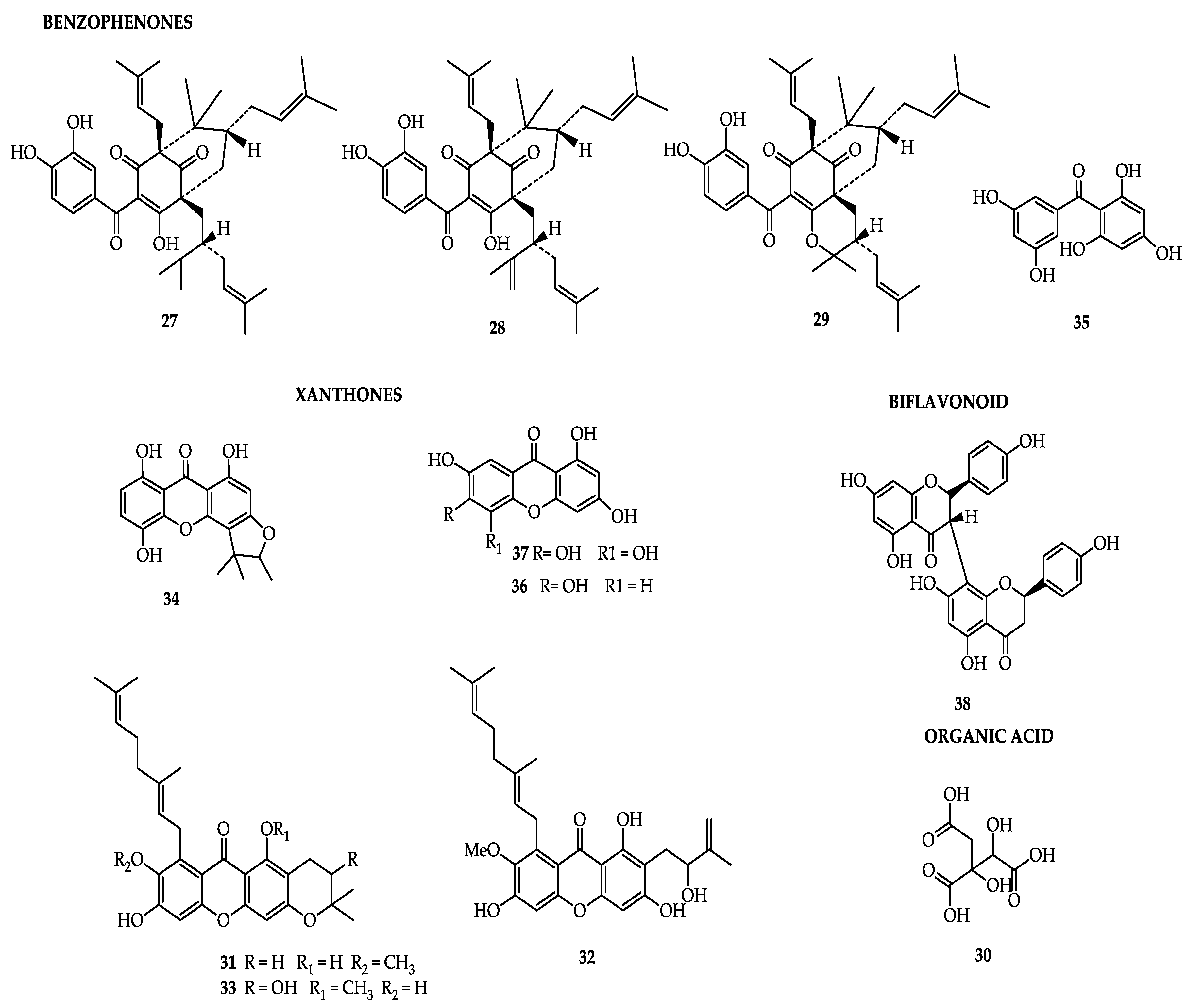
| 1,3,6,7-tetrahydroxyxanthone | Heartwood [19] | Antioxidant [43] |
| 1,3,5,7-tetrahydroxyxanthone | Heartwood [19] | Inhibits oxidation of LDL-c [45] |
| 1,5-dihydroxy-3-methoxy-6′,6′-dimethyl-2H-pyrano(2′,3′:6,7)-4-(3-methylbut-2-enyl)xanthone | Peel [69] | - |
| 1,5-dihydroxy-3-methoxy-4-(3-methylbut-2-enyl)xanthone | Peel [69] | - |
| Dulxanthone A | Peel [69] | - |
| Garbogiol | Peel [69] | Inhibition of α-glucosidade [10] |
| Pedunxanthone-A | Peel [69] | - |
| Pedunxanthone-B | Peel [69] | - |
| Pedunxanthone-C | Peel [69] | - |
| Pedunxanthone-D | Pericarp [33] | Anticancer [65] |
| Pedunxanthone-E | Pericarp [33] | Anticancer [65] |
| Pedunxanthone-F | Pericarp [33] | Anticancer [65] |
| 1,6-dihydroxy-7-methoxy-8-(3-methyl-2-butenyl)-6′,6′-dimethylpyrane-(2′,3′:3,2)-xanthone | Pericarp [33] | - |
| 6-O-demethyloliverixanthone | Pericarp [33] | - |
| Fuscaxanthone A | Pericarp [33] | Cytotoxic [16] |
| Cowanin | Pericarp [33] | Antimalarial [65] |
| Norcowanin | Pericarp [33] | Antiplasmodic [65] |
| Cowanol | Pericarp [33] | Antimalarial [65] |
| α-mangostin | Pericarp [33] | - |
| Mangostanol | Pericarp [33] | - |
| 3-isomangostin | Pericarp [33] | - |
| 1,7-dihydroxyxanthone | Pericarp [33] | - |
| Benzophenones | ||
| Pedunculol | Dry fruits [70] | Antioxidant [16] |
| Isogarcinol | Dry fruits [70] | Anticancer, anti-inflammatory, antiparasitic, action in nervous system [24] |
| Garcinol | Dry fruits [70] | Anticancer, anti-inflammatory, antiparasitic, action in nervous system [24] |
| 2,4,6,3′,5′-pentahydroxybenzophenone | Heartwood [19] | - |
| Biflavonoids | ||
| GB-1a | Heartwood [19] | Antioxidant [84] |
| volkensiflavone | Heartwood [19] | Antitumoral [74] |
| Triterpene | ||
| Oleanolic acid | Peel [69] | - |
| Garcinia Cambogia | ||
|---|---|---|
| Xanthones | ||
| Compounds | Plant Part | Activity |
| Garbogiol | Roots [85] | Inhibition of α-glucosid [10] |
| Rheedia xanthone A | Peel [85] | - |
| Oxy-guttiferone i | Fruits [85] | - |
| Oxy-guttiferone k | Fruits [85] | - |
| Oxy-guttiferone k2 | Fruits [85] | - |
| Oxy-guttiferone m | Fruits [85] | - |
| Benzophenones | ||
| garcinol | Peel [85] | Anticancer, anti-inflammatory, antiparasitic, action on nervous system [24] |
| isogarcinol | Peel [85] | Anticancer, anti-inflammatory, antiparasitic, action on nervous system [24] |
| Guttiferone i | Fruits [85] | - |
| Guttiferone n | Fruits [85] | - |
| Guttiferone j | Fruits [85] | - |
| Guttiferone k | Fruits [85] | Topoisomerase II inhibitor [87] |
| Guttiferone m | Fruits [85] | Topoisomerase II inhibitor [87] |
| Organic Acids | ||
| Heterocyclic amines | Fruits [85] | Antiobesity [137] |
| Tartaric acid | Fruits [85] | - |
| Citric acid | Fruits [85] | - |
| Malic acid | Fruits [85] | Antimicrobial [138] |
| Garcinialactone | Fruits [85] | - |
| Garcinia Mangostana | ||
|---|---|---|
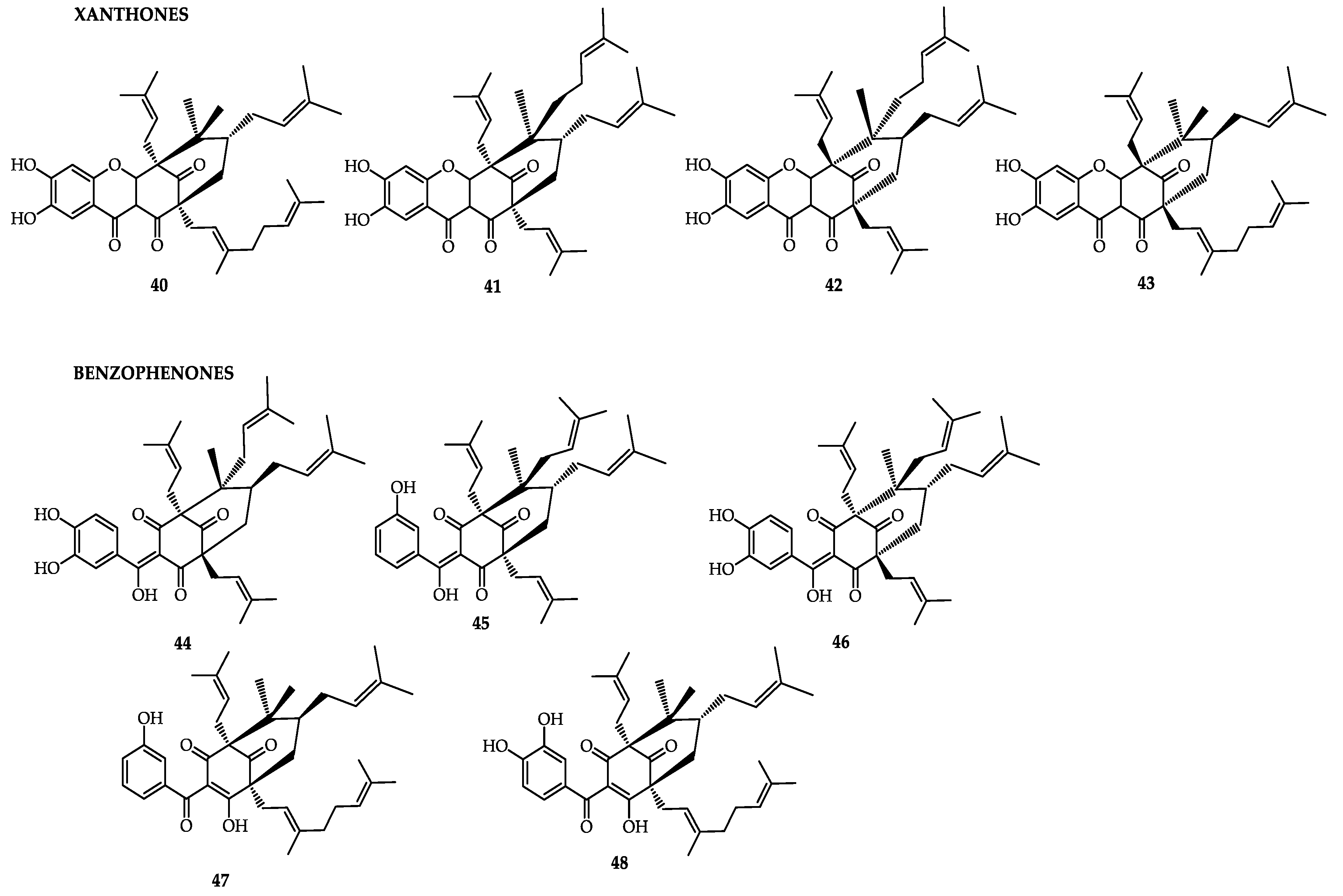
| Xanthones | ||
| Compounds | Plant Part | Activity |
| α-Mangostin | Pericarp, whole fruit, stem, arils, and seed [159] | Antibacterial, antifungal, antihistamine, antiobesity, anticancer [159], neuroprotective, antineoplastic [134], antioxidant [168] |
| β-Mangostin | Pericarp, whole fruit, stem [159] | Antiparasitic, hypoglycemic, antiobesity [134], antioxidant [168] |
| γ-Mangostin | Pericarp, whole fruit [159] | Antibacterial, anti-inflammatory, antihistamine, anticancer, hepatoprotective [159], antineoplastic, hypoglycemic, antiobesity, neuroprotective [134] |
| (16E)-1,6-Dihydroxy-8-(3-hydroxy-3-methylbut-1-enyl)-3,7-dimethoxy-2-(3-methylbut-2-enyl)-xanthone | Not stated [159] | - |
| (16E)-1-Hydroxy-8-(3-hydroxy-3-methylbut-1-enyl)-3,6,7-trimethoxy-2-(3methylbut-2-enyl)-xanthone | Whole fruit [159] | - |
| 1,2-Dihydro-1,8,10-trihydroxy-2-(2-hydroxypropan-2-yl)-9-(3-methylbut-2-enyl)furo [3,2-a]xanthen-11-one | Heartwood [159] | - |
| 1,3,6,7-Tetrahydroxy-xanthone | Pericarp [159] | - |
| 1,3,6,7-Tetrahydroxy-2,8-(3-methyl-2-butenyl)-xanthone-P1 | Pericarp [159] | - |
| 1,3,6-Trihydroxy-7-methoxy-2,8-(3-methyl-2-butenyl)-xanthone-P2 | Leaves [159] | - |
| 1,3,8-Trihydroxy-4-methyl-2,7-diisoprenylxanthone | Heartwood [159] | - |
| 1,3,7-Trihydroxy-2,8-di-(3-methylbut-2-enyl)-xanthon | Leaves [159], Pericarp [169] | - |
| 1,3-Dihydroxy-2-(2-hydroxy-3-methylbut-3-enyl)-6,7-dimethoxy-8-(3-methylbut-2-enyl)-xanthone | Heartwood [159] | - |
| 1,5-Dihydroxy-2-(3-methylbut-2-enyl)-3-methoxy-xanthone | Heartwood, stem [159] | - |
| 1,5-dihydroxy-2-isopentyl-3-methoxy xanthone | Heartwood [159] | - |
| 1,5,8-Trihydroxy-3-methoxy-2-(3-methylbut-2-enyl) xanthone | Heartwood [159], Pericarp [159] | - |
| 1,6-Dihydroxy-2-(2-hydroxy-3-methylbut-3-enyl)-3,7-dimethoxy-8-(3-methylbut-2-enyl)-xanthone | Pericarp [159] | - |
| 1,6-Dihydroxy-3-methoxy-2-(3-methyl-2-buthenyl)-xanthone | Pericarp [159] | - |
| 1,6-Dihydroxy-3,7-dimethoxy-2-(3-methylbut-2-enyl)-8-(2-oxo-3-methylbut-3-enyl)-xanthone | Whole fruit [159] | - |
| 1,6-Dihydroxy-3,7-dimethoxy-2-(3-methylbut-2-enyl)-xanthone | Heartwood [159] | - |
| 1,6-Dihydroxy-8-(2-hydroxy-3-methylbut-3-enyl)-3,7-dimethoxy-2-(3-methylbut-2-enyl)-xanthone | Heartwood [159] | - |
| 1,7-Dihydroxy-2-(3-methylbut-2-enyl)-3-methoxy-xanthone | Pericarp [159] | - |
| 1,7-dihydroxy-2-isopentyl-3-methoxy xanthone | Pericarp [159] | - |
| 11-Hydroxy-1-isomangostin | Not stated [159] | - |
| 1-Hydroxy-2-(2-hydroxy-3-methylbut-3-enyl)-3,6,7-trimethoxy-8-(3-methylbut-2-enyl)-xanthone | Heartwood [159] | - |
| 1-Isomangostin | Pericarp [159] | - |
| 1-Isomangostin hydrate | Pericarp [159] | - |
| 2-(γ,γ-Dimethylallyl)-1,7-dihydroxy-3-methoxyxanthone | Pericarp, arils [159] | - |
| 2,3,6,8-Tetrahydroxy-1-isoprenylxanthone | Not stated [159] | - |
| 2,8-bis-(γ,γ-Dimethyallyl)-1,3,7-trihydroxyxanthone | Arils [159] | - |
| 3-Isomangostin | Pericarp [159] | Hypoglycemic, antiobesity, neuroprotective [134] |
| 3-Isomangostin hydrate | Pericarp [159] | - |
| 5,9-Dihydroxy-8-methoxy-2,2-dimethyl-7-(3-methylbut-2-enyl)-2H,6Hpyrano-(3,2,6)-xanthene-6-one | Fruit hull [159] | - |
| 6-Deoxy-7-demethylmangostanin | Whole fruit [159] | |
| 6-methoxy–bis pyrano xanthone | Pericarp [159] | Antioxidant [170] |
| 6-O-Methylmangostanin | Not stated [159] | |
| 7-O-Demethyl mangostanin | Pericarp [159] | Anticancer [169] |
| 8-Deoxygartanin | Pericarp, whole fruit [159] | - |
| 8-Hydroxycudraxanthone | Pericarp [159] | - |
| 9-hydroxycalabaxanthone | Bark [171] | Neuroprotective [134] |
| BR-Xanthone-A | Pericarp [159] | - |
| BR-Xanthone B | Pericarp [159] | - |
| Calabaxanthone | Arils [159] | - |
| Cratoxyxanthone | Pericarp, stem, whole fruit [169] | - |
| Cudraxanthone | Pericarp [159] | - |
| Demethylcalabaxanthone | Whole fruit, arils, seed [159] | Antibacterial [159] |
| Dulxanthone-A | Bark [171] | Antibacterial [171] |
| Garcimangosone A | Fruit hull [159] | - |
| Garcimangosone B | Pericarp [159] | - |
| Garcimangosone C | Pericarp [159] | - |
| Garciniafuran | Heartwood [159] | Neuroprotective [134] |
| Garcinone B | Pericarp, whole fruit [159] | - |
| Garcinone C | Whole fruit [159] | Neuroprotective [134] |
| Garcinone D | Pericarp, whole fruit, stem [159] | Antibacterial [161], neuroprotective [134], antioxidant [47] |
| Garcinone E | Pericarp, whole fruit [159] | - |
| Garcinoxanthone-A | Not stated [134] | Antinociceptive, anti-inflammatory [134] |
| Garcinoxanthone-B | Not stated [134] | Antinociceptive, anti-inflammatory [134] |
| Garcinoxanthone-C | Not stated [134] | Antioxidant [46], antinociceptive, anti-inflammatory [134] |
| Garcinoxanthone-d | Not stated [134] | Antinociceptive, anti-inflammatory [134] |
| Garcinoxanthone-E | Not stated [134] | Antinociceptive, anti-inflammatory [138], antibacterial [171] |
| Garcinoxanthone-F | Not stated [134] | Antinociceptive, anti-inflammatory [134] |
| Garcinoxanthone-G | Not stated [134] | Antinociceptive, anti-inflammatory [134] |
| Garmoxanthone | Bark [171] | Antibacterial [171] |
| Gartanin | Pericarp, whole fruit [159] | Antineoplastic, hypoglycemic, antiobesity, neuroprotective [134], antioxidant [170] |
| Isogarcinol | Not stated [134] | Antinociceptive, anti-inflammatory [134], antibacterial [43] |
| Mangosharin | Stem [159] | - |
| Mangostaxanthone-I | Pericarp [133] | α-amylase inhibitor [136] |
| Mangostaxanthone-II | Pericarp [133] | α-amylase inhibitor [136] |
| Mangostaxanthone-III | Pericarp [168] | AGE* inhibitor, antioxidant [168] |
| Mangostaxanthone-IV | Fruits [172] Pericarp [164] | AGE* inhibitor, antioxidant [168] |
| Mangostaxanthone-V | Fruits [172] | - |
| Mangostaxanthone-VI | Fruits [172] | - |
| Mangostaxanthone-VII | Pericarp [136] | - |
| Mangostanaxanthone-VIIII | Pericarp [136] | α-Amylase inhibitory [136] |
| Mangostanate | Pericarp [172] | - |
| GlucosidaMangostanin | Pericarp [159] | Antibacterial [159] |
| Mangostanol | Wholefruit, stem [159] | - |
| Mangostenol | Pericarp [159] | - |
| Mangostenone A | Pericarp [159] | - |
| Mangostenone B | Pericarp [159] | - |
| MangostenoneC | Whole fruit [159] | Hypoglycemic, antiobesity [134] |
| Mangostenone D | Whole fruit [159] | Hypoglycemic, antiobesity [134] |
| Mangostenone E | Whole fruit [159] | |
| Mangostenone F | Not stated [134] | α-glucosidase inhibitor, antineoplastic [134] |
| Mangostinone | Pericarp, whole fruit [159] | - |
| Nigrolineaxanthone T | Bark [171] | - |
| Nor-mangostin | Fruits [172] | - |
| Rubraxantone | Pericarp [168] | Antioxidant [168] |
| Smeathxanthone A | Pericarp [159] | - |
| Thwaitesixanthone | Whole fruit [159] | - |
| Tovophyllin A | Pericarp [159] | - |
| Tovophyllin B | Pericarp [159] | - |
| Toxyloxanthone A (trapezifolixanthone) | Pericarp [159] | - |
| trapezifolixanthone | Pericarp [169] | - |
| 1,7-dihydroxyxanthone | Pericarp [159] | - |
| Euxanthone | Pericarp [159] | - |
| Caloxanthone A | Pericarp [159] | - |
| Macluraxanthone | Pericarp [159] | - |
| Mangostingone [7-methoxy-2-(3-isoprenyl)-8-(3-methyl-2-oxo-3-buthenyl)-1,3,6-trihydroxyxanthone | Pericarp [159] | - |
| Benzophenones | ||
| 2,4,6,3′,5′-pentahydroxybenzophenone | ||
| Garcimangosone D | Pericarp [159] | - |
| Maclurin | Pericarp, heartwood [159] | - |
| maclurin-6-O-β-d-glucopyranoside | Pericarp [134] | Hypoglycemic, antiobesity [134] |
| Kolanone | Pericarp [159] | - |
| Anthocyanidins | ||
| Chrysanthemin | Pericarp [159] | - |
| Cyanidin-3-O-glucoside | Not stated [159] | - |
| Biflavonoid | ||
| proanthocyanidin A2 | Pericarp [173] | Anti-HIV [174] |
| Flavonoid | ||
| Epicatehin | Pericarp [159] | Antidiabetic, antioxidant [173], hypoglycemic, antiobesity [134] |
| Aromadendrin-8-C-β-d-glucopyranoside | Pericarp [134] | Hypoglycemic, antiobesity [134] |
| Megastigmanesulphoglycoside | ||
| 4-O-sulpho-β-d-glucopyranosylabscisate | Pericarp [173] | Antioxidant [173] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espirito Santo, B.L.S.d.; Santana, L.F.; Kato Junior, W.H.; de Araújo, F.d.O.; Bogo, D.; Freitas, K.d.C.; Guimarães, R.d.C.A.; Hiane, P.A.; Pott, A.; Filiú, W.F.d.O.; et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules 2020, 25, 4513. https://doi.org/10.3390/molecules25194513
Espirito Santo BLSd, Santana LF, Kato Junior WH, de Araújo FdO, Bogo D, Freitas KdC, Guimarães RdCA, Hiane PA, Pott A, Filiú WFdO, et al. Medicinal Potential of Garcinia Species and Their Compounds. Molecules. 2020; 25(19):4513. https://doi.org/10.3390/molecules25194513
Chicago/Turabian StyleEspirito Santo, Bruna Larissa Spontoni do, Lidiani Figueiredo Santana, Wilson Hino Kato Junior, Felipe de Oliveira de Araújo, Danielle Bogo, Karine de Cássia Freitas, Rita de Cássia Avellaneda Guimarães, Priscila Aiko Hiane, Arnildo Pott, Wander Fernando de Oliveira Filiú, and et al. 2020. "Medicinal Potential of Garcinia Species and Their Compounds" Molecules 25, no. 19: 4513. https://doi.org/10.3390/molecules25194513
APA StyleEspirito Santo, B. L. S. d., Santana, L. F., Kato Junior, W. H., de Araújo, F. d. O., Bogo, D., Freitas, K. d. C., Guimarães, R. d. C. A., Hiane, P. A., Pott, A., Filiú, W. F. d. O., Arakaki Asato, M., Figueiredo, P. d. O., & Bastos, P. R. H. d. O. (2020). Medicinal Potential of Garcinia Species and Their Compounds. Molecules, 25(19), 4513. https://doi.org/10.3390/molecules25194513






